1. Introduction
Membrane bioreactors (MBRs), combining biological treatment and membrane filtration processes, allow the efficient removal of suspended solids, organic matter, nutrients (such as nitrogen and phosphorus), and even micropollutants [
1,
2]. Moreover, MBRs have a smaller footprint and enable a more efficient and stable operation compared to conventional wastewater treatments. However, one of the main issues affecting the operation of MBRs is membrane biofouling, which is associated with biofilm formation [
3]. Biofilm formation on the membrane surface can lead to fouling, which reduces the permeability of the membrane and thus the efficiency of the system. Fouling can result in increased transmembrane pressure, which requires more frequent cleaning and maintenance, resulting in higher operational costs [
4,
5]. Extensive research has been conducted over the past three decades to address the challenge posed by biofouling. However, because it is nearly impossible to completely prevent biofilm formation, it is important to develop a technology that can effectively minimize and control this process.
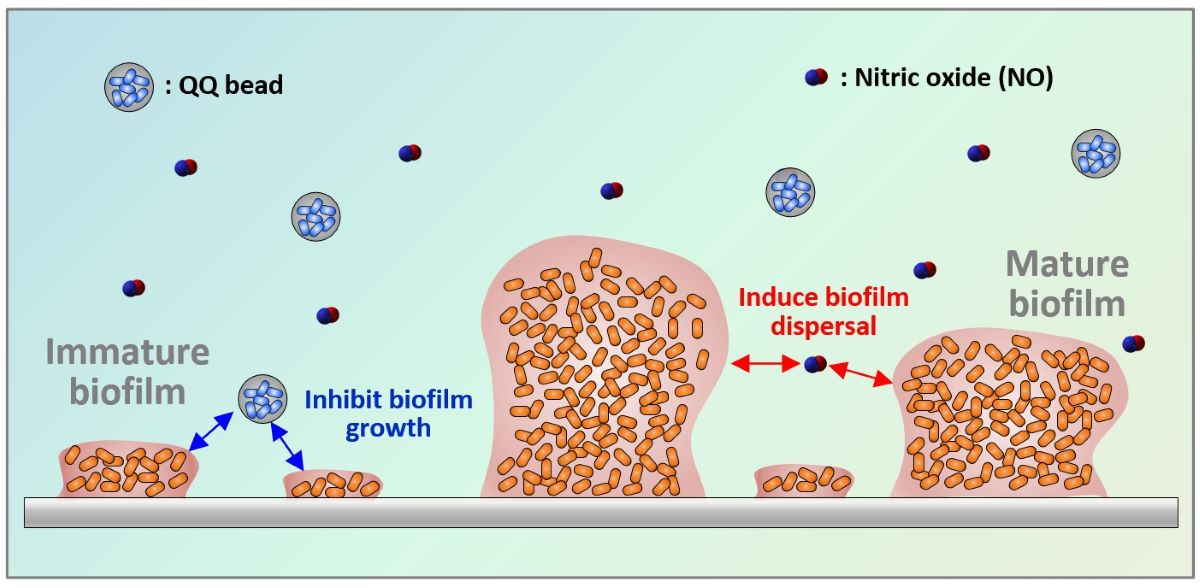
Having recognized this limitation, a recently emerged research direction focuses on the modulation of cell–cell signaling between microorganisms. Recent studies have highlighted the contribution of nitric oxide (NO) to regulating the expression of specific genes and signaling pathways related to biofilm dispersal [
6,
7]. Understanding the mechanisms underlying NO signaling in biofilm dispersions holds great promise for developing novel strategies to control and mitigate biofilm-related issues in various applications, including water treatment, medical devices, and industrial processes. Bacteria sense NO through a signal response pathway, which stimulates the intracellular phosphodiesterase activity. This activity leads to the degradation of cyclic diguanylate monophosphate (c-di-GMP) and induces changes in gene expression that favor the planktonic state [
6,
8,
9]. Thus, NO plays a crucial role in the transition of microorganisms from attached to suspended growth. Owing to its unstable gas nature with a very short half-life of 2–6 s in the radical state, injecting an NO donor that gradually releases NO into water at a neutral pH can achieve efficient biofilm dispersion [
10,
11,
12].
Quorum sensing (QS) is another important bacterial signaling mechanism in biofilm formation. QS is a cell-to-cell communication mechanism used by bacteria to regulate gene expression based on their density, thus coordinating various group behaviors including biofilm formation. This includes the production and sensing of signaling molecules known as autoinducers, such as
N-acyl homoserine lactones (AHLs), autoinducer-2, and autoinducing peptides [
13]. Disrupting bacterial QS, known as quorum quenching (QQ), can be an effective method to inhibit biofilm formation. In particular, extensive research on controlling biofouling in membrane processes using QQ bacteria that disrupt the QS process of AHLs has been carried out in the past decade [
14].
Biofilm formation involves a series of processes, including initial attachment, growth, and dispersal [
15]. While QS is known to influence the initial attachment and growth, NO has been reported to play a role in biofilm dispersal. Therefore, it is anticipated that combining QQ and NO-based approaches could provide a more effective control of biofilms; however, no information is currently available on the effects of their combination. Therefore, this study aims to investigate the combined effect of QQ and NO signaling mechanisms on biofilm control. In batch tests using
Pseudomonas aeruginosa as a model biofilm-forming bacterium, we applied combinations of two QQ bacteria (
Rhodococcus sp. BH4 and genetically modified
Escherichia coli) with various types of NONOates to investigate their efficiency in controlling biofilms. The results of this study illustrate the potential and applicability of combining the two signaling-based approaches in overcoming the challenge of inhibiting biofilm formation.
2. Materials and Methods
2.1. Microorganisms
The strains, plasmids, and culture conditions used in the present experiments are listed in
Table 1. All strains were cultured in Luria–Bertani (LB) broth (BD Difco, USA). To investigate the regulation of biofilm formation via QS, we cultured
P. aeruginosa PAO1, which is an aerobic gram-negative bacterium known to produce biofilms through the QS mechanism. The suppression of the QS of AHL-signaling molecules was confirmed using
Rhodococcus sp. BH4 and
E. coli TOP10-AiiO.
E. coli TOP10-Empty was employed as a negative control for
E. coli TOP10-AiiO. Furthermore,
Agrobacterium tumefaciens A136 (Ti−)(pCF218)(pCF372) was utilized as biosensor to detect the presence of
N-(3-oxo-hexanoyl)-L-homoserine lactone (OHHL).
2.2. NO Donors
Various NONOates (chemical formula: R1R2N[N(O)NO]−), which can release NO spontaneously at ambient temperatures, were used as NO donors. (Z)-1-[N-(3-aminopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate (PAPA NONOate, half-life: 15 min), (Z)-1-[N-[3-aminopropyl]-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate (Spermine NONOate, half-life: 39 min), (Z)-1-[N-(3-aminopropyl)-N-(3-ammoniopropyl) amino]diazen-1-ium-1,2-diolate (DPTA NONOate, half-life: 3 h) were purchased from Cayman Chemical (Ann Arbor, MI, USA). Stock solutions of the NO donors were prepared by dissolving them in 10 mM NaOH to prevent NO release before the experiment.
2.3. Preparation of QQ Beads
Two kinds of hydrogel beads, alginate and polyvinyl alcohol (PVA)/alginate were used to immobilize QQ bacteria at an OD
600 of 3. The alginate beads were prepared by a previously reported method, in which a 1% (w/v) alginate–bacterial cell mixture was dropped into a 4% (w/v) calcium chloride solution [
18]. A modified version of a previous method was used for preparing the PVA/alginate beads: a 10% (w/v) PVA (Sigma-Aldrich, USA) and 1% (w/v) alginate–bacterial cell mixture was dropped onto a 7% (w/v) boric acid and 4% (w/v) calcium chloride solution, followed by incubation for 1 h for the first cross-linking process [
20] . After that, the beads were transferred to a 0.5 M sodium sulfate solution and incubated for 8 h for the second cross-linking process.
Figure 1 shows photographs of the prepared beads.
2.4. Biofilm Formation Assay
The impact of NO and/or QQ on the biofilm formation of PAO1 was assessed through 24-well microtiter plate assays, following previously reported procedures [
21]. The model biofilm-forming bacterium
P. aeruginosa PAO1 was grown in LB broth overnight and centrifuged at 4,500 rpm for 10 min. Then, the concentration of PAO1 was adjusted to 0.03 of OD
600 using M9 minimal medium (containing 9 mM NaCl
2, 22 mM KH
2PO
4, 48 mM Na
2HPO
4, 19 mM NH
4Cl, 2 mM MgSO
4, 100 μM CaCl
2, and 0.4% glucose, pH 7.0). The bacterial solutions (1 mL) were placed in the wells of a 24-well microtiter plate and incubated for 6 h at 30 °C with shaking (180 rpm). After the 6 h culture, 10 mM NaOH (Control) or the prepared NO donor stock solution was added to each well, to obtain final concentrations of 50 and 100 μM. The plate was incubated with shaking for an additional 30 min after injection of the NO donor.
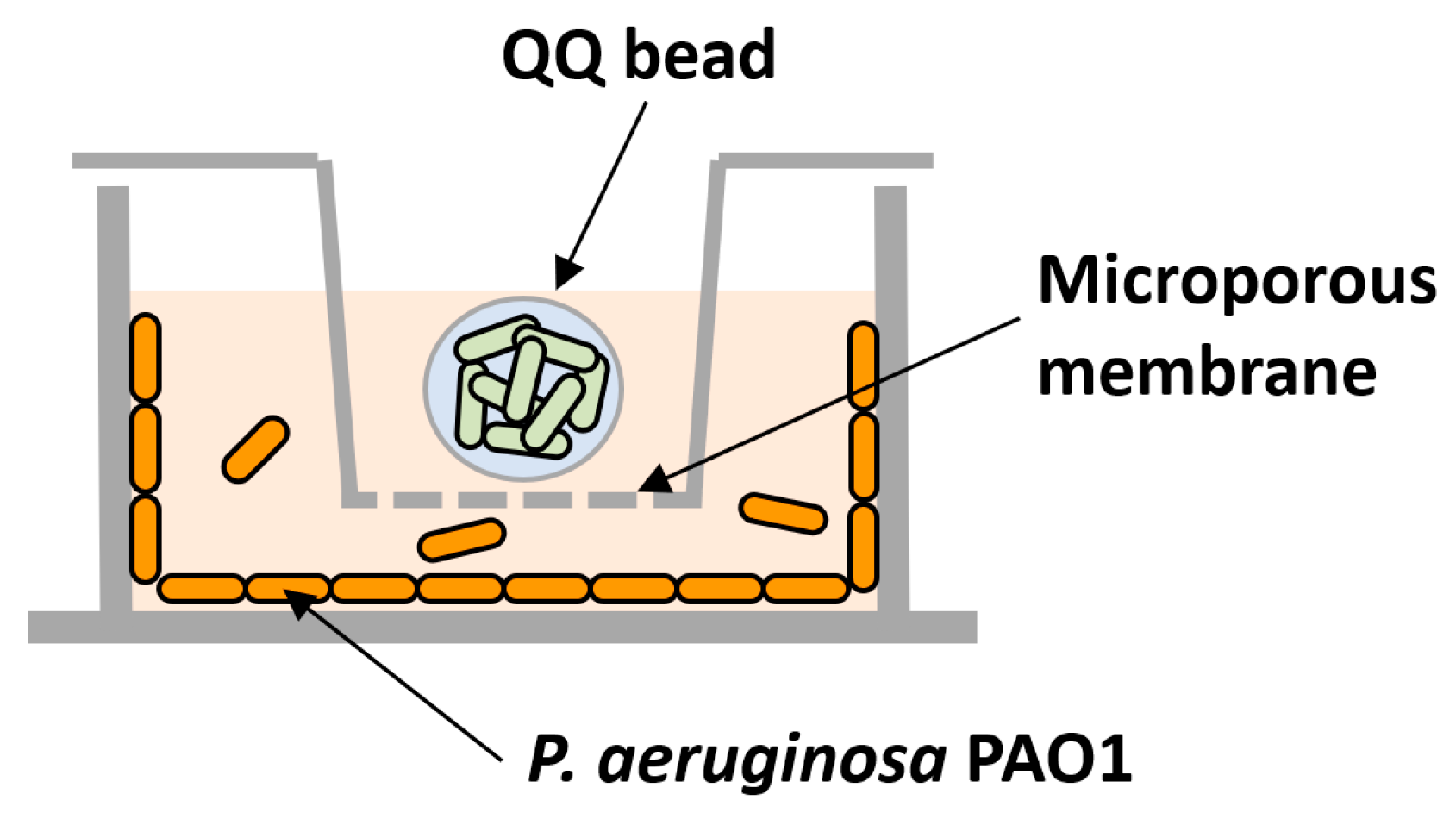
For testing the individual or combined effects of QQ, transwells (6.5 mm with 8.0 μm polycarbonate membrane inserts, Coster) were used to prevent the QQ beads from directly contacting the 24-well plates (
Figure 2). Following the inoculation of PAO1 as described above, transwells were installed in the 24-well plate; then, a QQ and a control bead (immobilizing E. coli TOP10) were placed into the corresponding transwell.
The biofilms formed on the well surface were quantified using crystal violet (CV) assays. The biofilm remaining in the wells was stained using 0.1% (w/v) CV for 20 min and washed two times with phosphate-buffered saline buffer. The wells were then destained with 99.9% ethanol, and the quantity of biofilm was determined by measuring the absorbance at a wavelength of 550 nm using a microtiter plate reader (Gen 5, Biotek, Winooski, VT, USA).
The expected combined effect of biofilm reduction was estimated as [
22]:
where R
exp, R
QQ, and R
NO denote the expected biofilm reduction (%) by the combination of QQ and NO, the biofilm reduction (%) by QQ, and the biofilm reduction (%) by NO, respectively.
2.5. AHL Bioassay
AHLs were quantified using a luminescence-based bioassay method described in a previous study [
22]. The samples (5 μL) and the reporter strain A136 (95 μL) were mixed into a 96-well plate and then incubated at 30 °C for 90 min. Then, 30 μL of Beta-Glo assay system (Promega, Madison, WI) was added, and the plate was kept at 25 °C for 35 min. The luminescence intensity was measured by a microplate reader (Synergy HTX, Biotek, USA). OHHL was dissolved in a 50 mM Tris-HCl buffer to prepare standard solutions at concentrations of 12.5, 25, 50, 100, and 200 nM.
2.6. QQ Activity Test
QQ activity tests were conducted to confirm the AHL degradation capability of the QQ beads. Twenty milliliters of 200 nM OHHL were placed into a conical tube along with 50 QQ beads. The mixture was cultured at 30 °C and 100 rpm, and 500-μL samples were collected at reaction times of 0, 1, 10, 30, 60, 120, and 180 min. These samples were centrifuged at 13,500 rpm for 3 min, and 200 μL of the supernatant was treated at 95 °C for 2 min before storing it at −20 °C. Then, the residual OHHL concentrations at each time point were measured using the AHL bioassay method described in
Section 2.5.
3. Results and Discussion
3.1. Effect of QQ Beads on Biofilm Formation
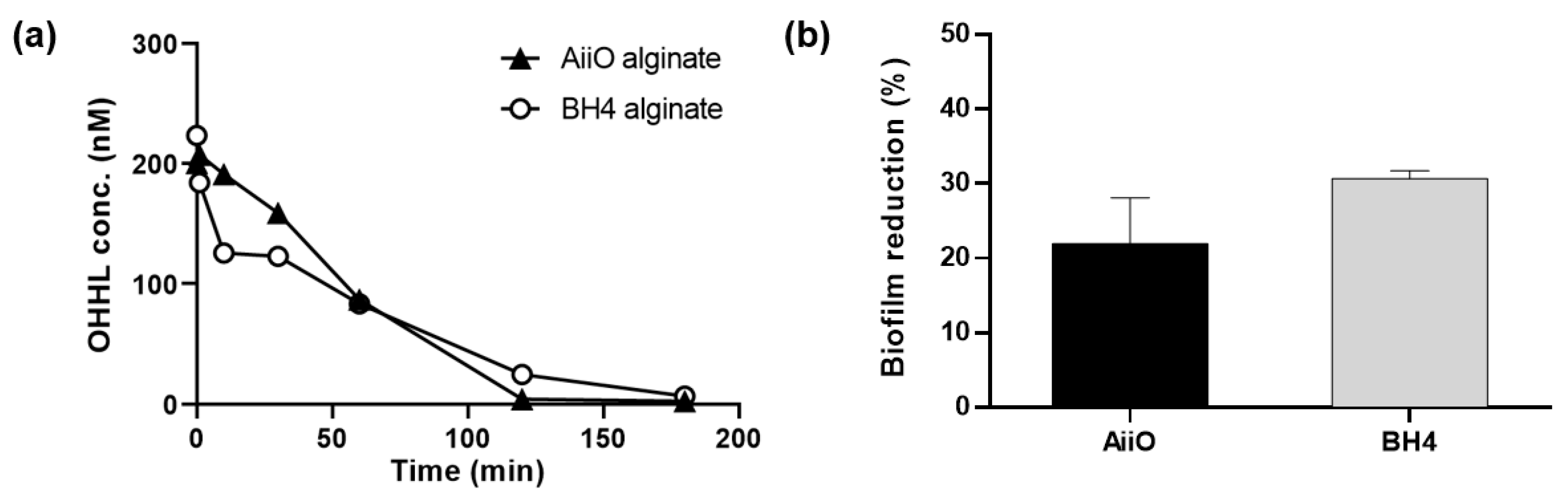
The QQ activity of alginate beads entrapping AiiO or BH4 strains was measured (Figure 3a). In the case of the AiiO alginate beads, almost all OHHL was decomposed in 120 min, while the complete decomposition with the BH4 alginate beads required 180 min. During the initial 60 min, a higher OHHL decomposition rate was observed for the BH4 alginate beads; however, after that a slightly higher decomposition rate was found for the AiiO alginate beads. Overall, the difference in QQ activity between the two types of beads was small, and both efficiently decomposed AHL. Although the rate of AHL degradation may decrease when microbial cells are confined within hydrogel beads compared to when they are in a suspended state, the protection of QQ bacteria can provide a safer environment for continuous process applications [
23].
To evaluate the efficacy of biofilm reduction by the QQ beads, a biofilm test was conducted using a microplate setup with a transwell, as shown in
Figure 2. As shown in
Figure 3b, the addition of AiiO alginate beads led to a 21.9% biofilm reduction compared to the addition of Empty alginate beads (i.e., alginate beads entrapping
E. coli TOP10-Empty). BH4 alginate beads also showed a substantial biofilm reduction (30.7%) compared to the vacant beads (i.e., alginate beads without bacteria). In any case, the results confirmed that quenching AHL using alginate beads with immobilized AiiO or BH4 can mitigate the biofilm formation of PAO1.
3.2. Dispersal of Biofilm by Addition of NO Donors
To assess the NO-induced dispersal of biofilm cells, we selected three specific NO donors: PAPA NONOate (PAPA), Spermine NONOate (Spermine), and DPTA NONOate (DPTA). These compounds have half-lives in water ranging from several minutes to a few hours, which are neither too short nor too long [
6]. Thus, they are considered to strike an appropriate balance in terms of the rate and long-term stability of NO emissions.
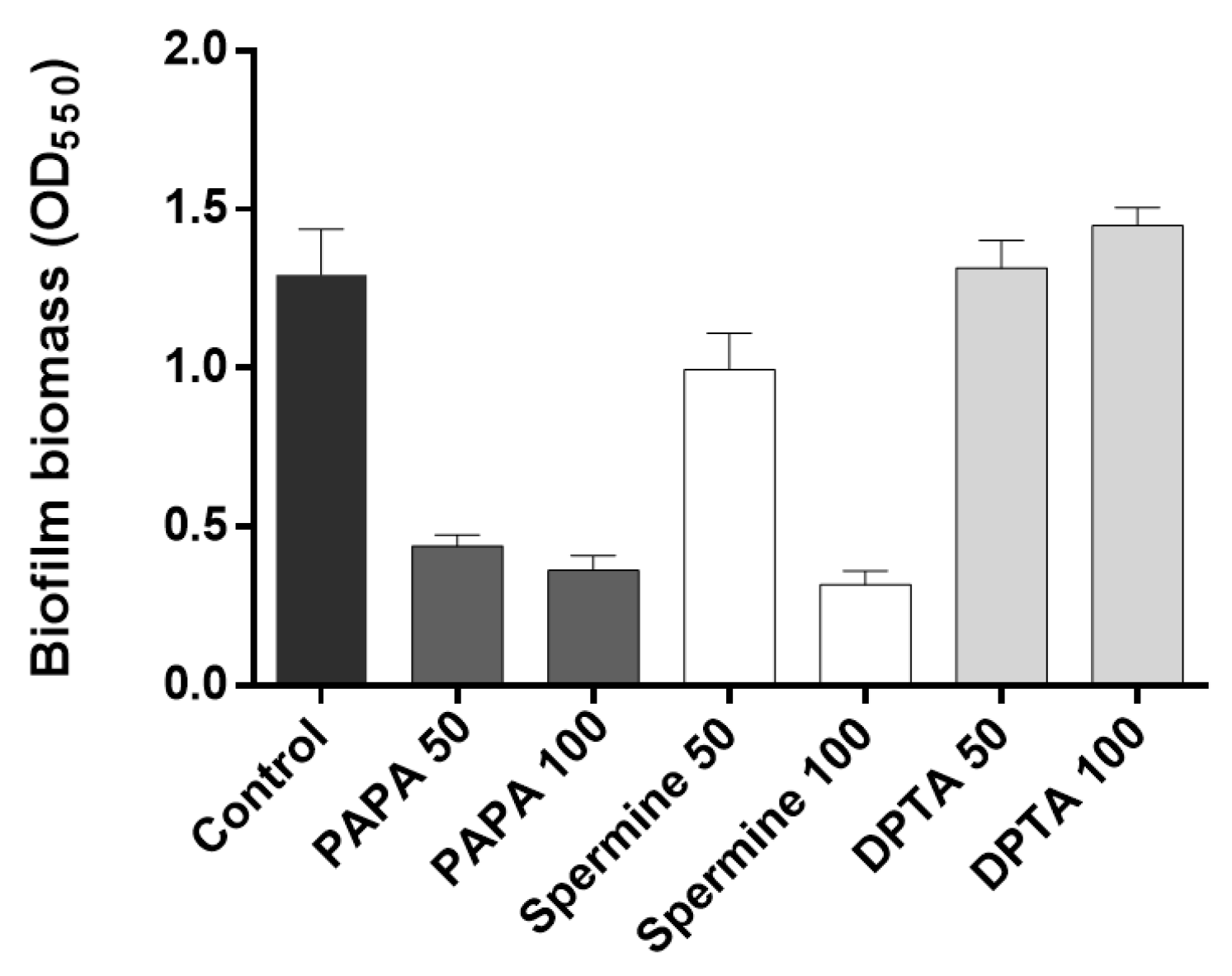
The effectiveness of different types and concentrations of NO donors in mitigating biofilm formation was assessed by quantifying the percentage biofilm reduction following a 30-min incubation period with NO donors. The results showed that both 50 μM and 100 μM PAPA additions led to substantial reductions (65.9% and 71.8%, respectively) in biofilm formation (
Figure 4). In the case of Spermine, a 50 μM injection resulted in 23% biofilm reduction, while a 100 μM injection achieved a significantly improved biofilm reduction of 75.3%. In contrast, DPTA showed no reduction in biofilm formation at either concentration during the 30-min incubation period, consistent with previous findings [
6,
10]. Overall, PAPA demonstrated superior biofilm reduction effects at 50–100 μM injections compared to the other two NO donors and was thus chosen for the subsequent experiments.
3.3. Combination of NO Treatment and QQ Alginate Beads for Biofilm Reduction
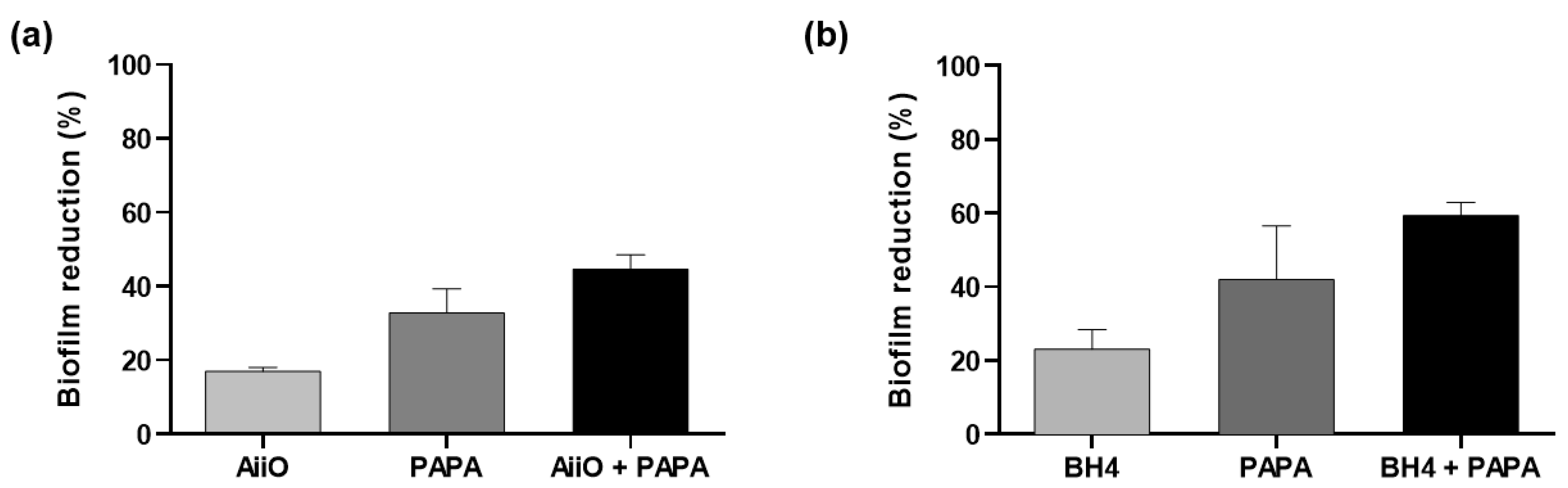
After confirming the biofilm reduction effects of the individual QQ and NO treatments, we investigated their combined inhibitory effect on biofilm formation. As shown in
Figure 5a, when AiiO alginate beads and 50 μM PAPA were applied separately, the biofilm removal rates were approximately 16.9% and 32.8%, respectively. The expected reduction efficiency by the combination of these two treatments can be estimated as 44.2% using Eq. 1. An experiment with AiiO alginate beads applied together with 50 μM PAPA showed a 44.6% reduction in biofilm formation (
Figure 5a). This value was close to the theoretical prediction, indicating that no synergistic or antagonistic effect was observed using the combination of the two treatments.
When BH4 alginate beads and 50 μM PAPA were applied separately, they showed 23.1% and 42.0% reductions in biofilm formation, respectively (
Figure 5b). When combined, the treatments resulted in a 59.4% reduction in biofilm formation, which is slightly lower than the expected rate of 64.2%. Therefore, we can also conclude that no synergistic effect was present between the BH4 alginate beads and 50 μM PAPA.
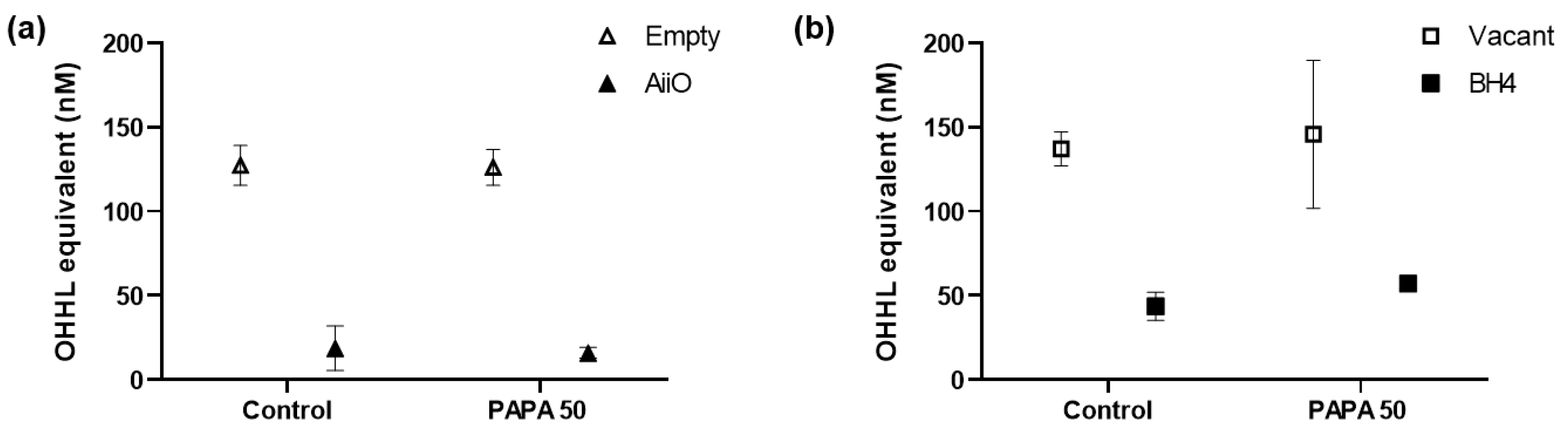
Figure 6 shows the amount of AHL QS signaling molecules that were degraded by QQ in the biofilm reduction experiments.
P. aeruginosa PAO1 is known to produce more than two types of AHLs.
The biosensor A136 used in this experiment can respond to various types of AHLs; therefore, bioassays using this biosensor cannot determine which AHLs were generated and degraded. However, the luminescence values obtained from the sample can be represented as OHHL equivalent values using the standard curve established with OHHL [
18]. In the absence of PAPA injection (control), an 85.3% reduction in AHL levels was observed upon injection of AiiO alginate beads compared to the well with Empty alginate beads (
Figure 6a). This confirms that AHLs produced by PAO1 within the transwell microplate setup were effectively degraded by the AiiO beads. The injection of PAPA did not have any impact on either the production or degradation of AHLs. In the case of BH4 alginate beads, 68.2% of AHLs were found to be degraded compared to the well where vacant beads were injected (
Figure 6b, control). Although this degradation rate was somewhat lower than that observed with AiiO beads, it is expected to have a significant impact on reducing biofilm formation by degrading a substantial amount of AHLs. The injection of PAPA also had no effect on the AHL production and degradation in the experiment with BH4 beads. Despite the high AHL removal rate (68.2–85.3%), the efficiency of biofilm reduction by the QQ beads (16.9–23.1%) was lower than expected.
This discrepancy could be attributed to PAO1 utilizing an extracellular signal molecule called Pseudomonas quinolone signal (PQS) in addition to AHL. PQS influences the synthesis of quorum sensing-dependent extracellular products such as pyocyanin and elastase, which are associated with biofilm formation [
24]. Moreover, while AHL was effectively removed from the supernatant, the possibility that AHL remained in high concentrations within the extracellular polymeric substance (EPS) matrix of the biofilm cannot be ruled out.
In summary, QQ beads effectively degraded AHLs produced by PAO1, thus inhibiting biofilm formation, although the reduction rate was limited (16.9–23.1%). However, when NO was applied together with QQ beads, the reduction rate increased to 44.6–59%, indicating that the combination of the two techniques can achieve a more effective biofilm reduction.
3.4. Combination of NO Treatment and QQ PVA/Alginate Beads
Despite their high biocompatibility and ease of preparation, alginate beads have the disadvantages of low physical strength and stability [
25,
26,
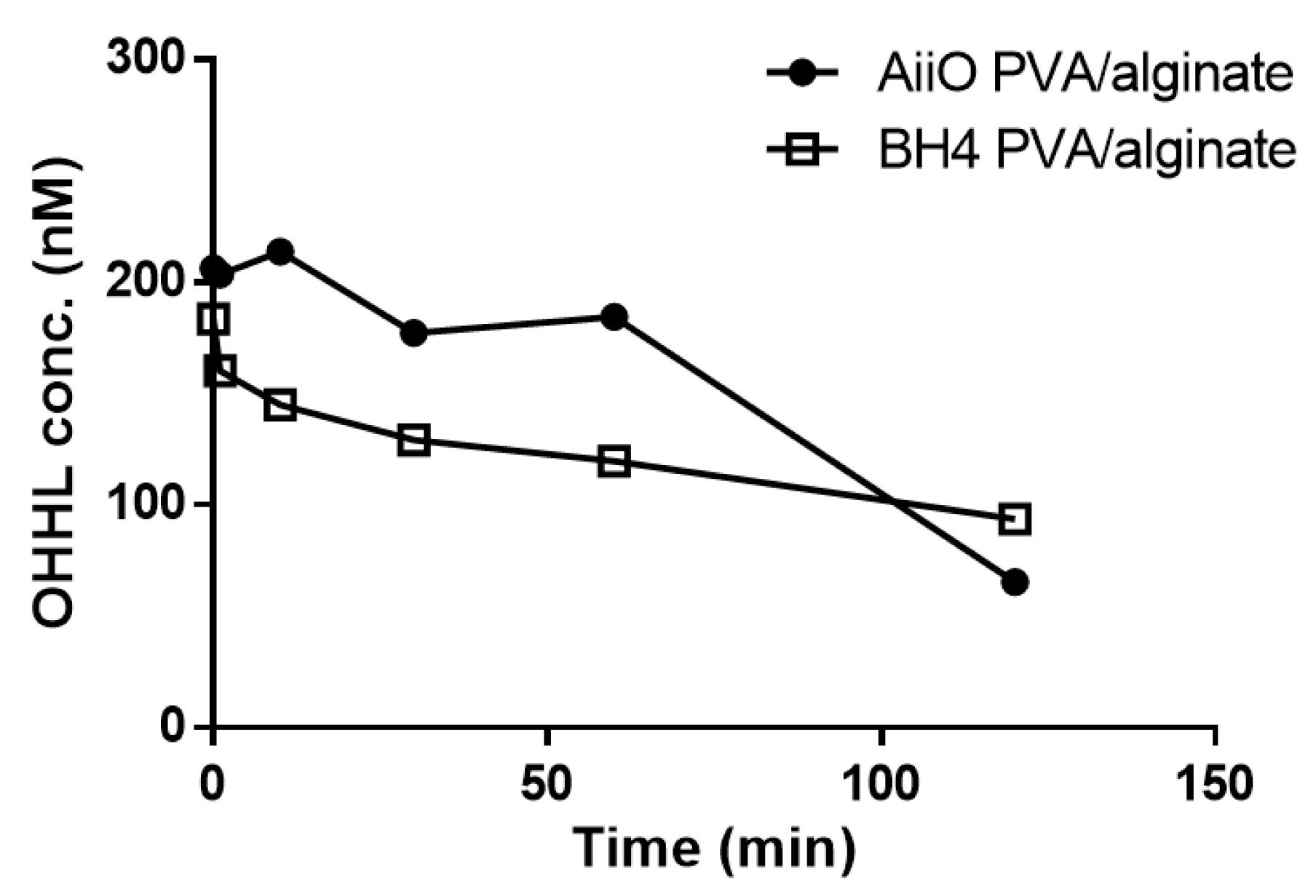
27]. Therefore, to improve the durability, we produced beads immobilizing two QQ bacteria using a mixture of PVA and alginate. As expected, the addition of PVA resulted in beads with higher physical strength and stability, but their AHL degradation ability showed a slight decrease (
Figure 7). Over 120 min, BH4 and AiiO PVA/alginate beads degraded approximately 48.5% and 65.7% of OHHL, respectively. Similar to the alginate beads (
Figure 3a), the BH4 beads showed superior degradation ability during the initial 60 min, but AiiO exhibited a faster degradation afterward.
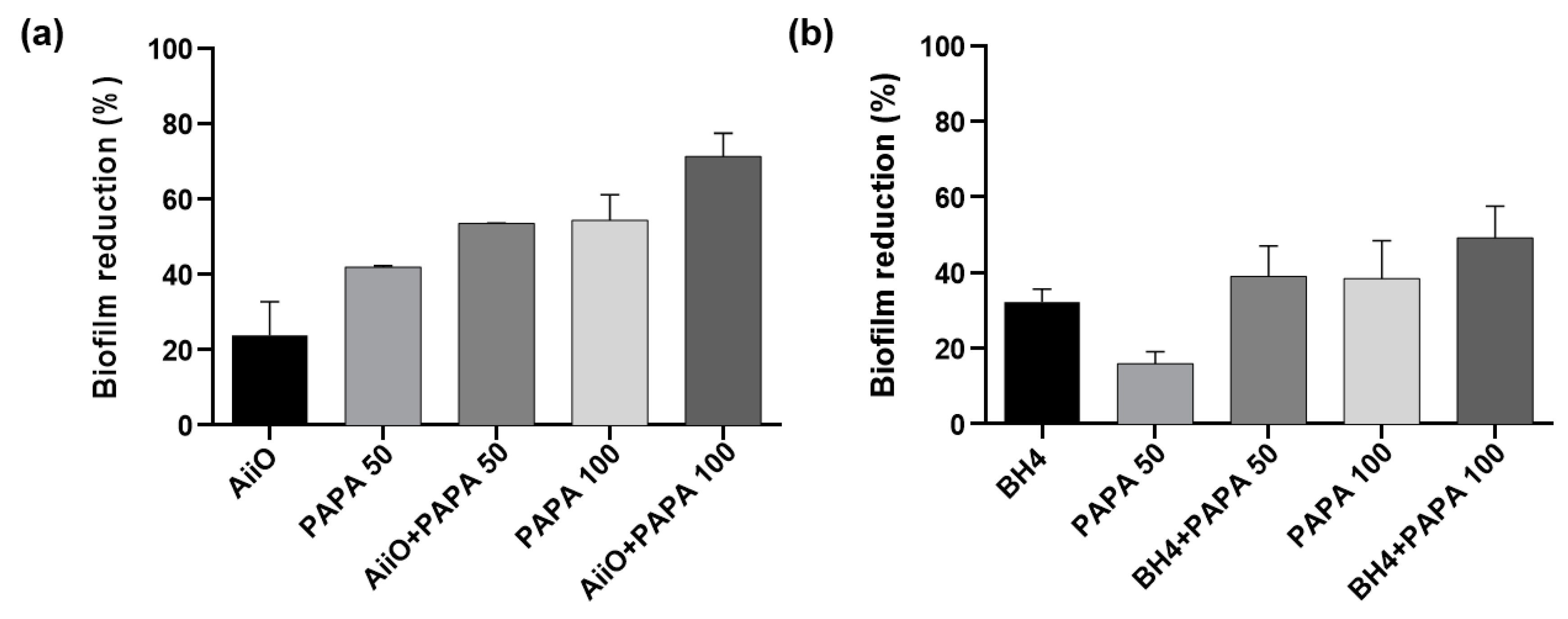
As shown in
Figure 8, a similar trend to those observed with alginate beads was obtained when PVA/alginate beads were combined with PAPA NONOate. The combination of AiiO and 50 μM PAPA resulted in a 53.5% reduction in biofilm formation (
Figure 8a), which was nearly identical to the expected reduction of 55.9% calculated based on the combination using Eq. (1). Similarly, a 39.0% reduction in biofilm formation was observed when BH4 PVA/alginate beads and 50 μM PAPA were applied together (
Figure 8b), which was comparable to the expected reduction of 43.1%. To achieve even higher biofilm reduction rates, the dosage of PAPA was increased to 100 μM. Its combination with AiiO led to a biofilm reduction of up to 71.3%, whereas that with BH4 yielded a reduction of up to 49.2%. These results confirmed that, using durable PVA/alginate with potential applications in MBR processes for wastewater treatment, the combination of immobilized QQ bacteria with an NO donor can further enhance the biofilm reduction effect.
4. Conclusions
This study investigated the combination of quorum quenching and NO signaling for effective biofilm control. First, biofilm tests using a microplate equipped with a transwell confirmed that biofilm reduction occurred when QQ bacteria-immobilized beads and NO donors were applied separately. Then, when these two approaches were combined, they resulted in an improved biofilm reduction compared to those corresponding to their individual applications. These results highlight the promising prospects of the combination of these two technologies for more efficient biofilm inhibition in various processes requiring biofilm control, such as membrane filtration processes and cooling towers.
Author Contributions
Conceptualization, Y.K., H.-S.O.; methodology, Y.K., H.-S.O.; validation, P.A.; data curation, H.K.; visualization, P.A., H.K.; writing – original draft preparation, Y.K., P.A., H.K.; writing – review and editing, P.A., H.-S.O.; Supervision, H.-S.O.; Funding acquisition. P.A., H.-S.O. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This study was financially supported by Seoul National University of Science and Technology.
References
- Feng, L.; Wu, Z.; Yu, X. Quorum Sensing in Water and Wastewater Treatment Biofilms. J Environ Biol 2013, 34, 437–444. [Google Scholar] [PubMed]
- Huang, L.; Lee, D.J. Membrane Bioreactor: A Mini Review on Recent R&D Works. Bioresour Technol 2015, 194, 383–388. [Google Scholar]
- Lee, W.N.; Chang, I.S.; Hwang, B.K.; Park, P.K.; Lee, C.H.; Huang, X. Changes in Biofilm Architecture with Addition of Membrane Fouling Reducer in a Membrane Bioreactor. Process Biochemistry 2007, 42, 655–661. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.Y.; Shu, L. Treatment of Textile Wastewater with Membrane Bioreactor: A Critical Review. Bioresour Technol 2016, 204, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Oh, H.S.; Constancias, F.; Ramasamy, C.; Tang, P.Y.P.; Yee, M.O.; Fane, A.G.; McDougald, D.; Rice, S.A. Biofouling Control in Reverse Osmosis by Nitric Oxide Treatment and Its Impact on the Bacterial Community. J Memb Sci 2018, 550, 313–321. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should We Stay or Should We Go: Mechanisms and Ecological Consequences for Biofilm Dispersal. Nat Rev Microbiol 2012, 10, 39–50. [Google Scholar] [CrossRef]
- Cai, Y. ming; Webb, J.S. Optimization of Nitric Oxide Donors for Investigating Biofilm Dispersal Response in Pseudomonas Aeruginosa Clinical Isolates. Appl Microbiol Biotechnol 2020, 104, 8859–8869. [Google Scholar] [CrossRef]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric Oxide Signaling in Pseudomonas Aeruginosa Biofilms Mediates Phosphodiesterase Activity, Decreased Cyclic Di-GMP Levels, and Enhanced Dispersal. J Bacteriol 2009, 191, 7333–7342. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y. Biological Control of Microbial Attachment: A Promising Alternative for Mitigating Membrane Biofouling. Appl Microbiol Biotechnol 2010, 86, 825–837. [Google Scholar] [CrossRef]
- Heil, J.; Vereecken, H.; Brüggemann, N. A Review of Chemical Reactions of Nitrification Intermediates and Their Role in Nitrogen Cycling and Nitrogen Trace Gas Formation in Soil. Eur J Soil Sci 2016, 67, 23–39. [Google Scholar] [CrossRef]
- Barraud, N.; Storey, M. V.; Moore, Z.P.; Webb, J.S.; Rice, S.A.; Kjelleberg, S. Nitric Oxide-Mediated Dispersal in Single- and Multi-Species Biofilms of Clinically and Industrially Relevant Microorganisms. Microb Biotechnol 2009, 2, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm Formation and Inhibition Mediated by Bacterial Quorum Sensing. Appl Microbiol Biotechnol 2022, 106, 6365–6381. [Google Scholar] [PubMed]
- Anburajan, P.; Kim, Y.; Rice, S.A.; Oh, H.S. Bacterial Signaling and Signal Responses as Key Factors in Water and Wastewater Treatment. Journal of Water Process Engineering 2021, 44, 102434. [Google Scholar] [CrossRef]
- Passos da Silva, D.; Schofield, M.C.; Parsek, M.R.; Tseng, B.S. An Update on the Sociomicrobiology of Quorum Sensing in Gram-Negative Biofilm Development. Pathogens 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Evans, L.R.; Linker, A. Production and Characterization of the Slime Polysaccharide of Pseudomonas Aeruginosa. J Bacteriol 1973, 116, 915–924. [Google Scholar] [CrossRef]
- Ryu, D.H.; Lee, S.W.; Mikolaityte, V.; Kim, Y.W.; Jeong, H.; Lee, S.J.; Lee, C.H.; Lee, J.K. Identification of a Second Type of Ahllactonase from Rhodococcus Sp. BH4, Belonging to the α/β Hydrolase Superfamily. J Microbiol Biotechnol 2020, 30, 937–945. [Google Scholar] [CrossRef]
- Oh, H.S.; Tan, C.H.; Low, J.H.; Rzechowicz, M.; Siddiqui, M.F.; Winters, H.; Kjelleberg, S.; Fane, A.G.; Rice, S.A. Quorum Quenching Bacteria Can Be Used to Inhibit the Biofouling of Reverse Osmosis Membranes. Water Res 2017, 112, 29–37. [Google Scholar] [CrossRef]
- Fuqua, C.; Winans, S.C. Conserved Cis-Acting Promoter Elements Are Required for Density-Dependent Transcription of Agrobacterium Tumefaciens Conjugal Transfer Genes; 1996; Vol. 178. [Google Scholar]
- Lee, K.; Kim, Y.W.; Lee, S.; Lee, S.H.; Nahm, C.H.; Kwon, H.; Park, P.K.; Choo, K.H.; Koyuncu, I.; Drews, A.; et al. Stopping Autoinducer-2 Chatter by Means of an Indigenous Bacterium (Acinetobacter Sp. DKY-1): A New Antibiofouling Strategy in a Membrane Bioreactor for Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 6237–6245. [Google Scholar] [CrossRef]
- Noori, A.; Kim, H.; Kim, M.H.; Kim, K.; Lee, K.; Oh, H.S. Quorum Quenching Bacteria Isolated from Industrial Wastewater Sludge to Control Membrane Biofouling. Bioresour. Technol. 2022, 352, 127077. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of Synergism in Drug Combinations and Reference Models for Future Orientations in Oncology. Current Research in Pharmacology and Drug Discovery 2022, 3, 100110. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R.; Mehmood, M.A. Recent Advances on Bacterial Quorum Quenching as an Effective Strategy to Control Biofouling in Membrane Bioreactors. Bioresour Technol Rep 2021, 15, 100745. [Google Scholar] [CrossRef]
- Diggle, S.P.; Winzer, K.; Chhabra, S.R.; Worrall, K.E.; Cámara, M.; Williams, P. The Pseudomonas Aeruginosa Quinolone Signal Molecule Overcomes the Cell Density-Dependency of the Quorum Sensing Hierarchy, Regulates Rhl-Dependent Genes at the Onset of Stationary Phase and Can Be Produced in the Absence of LasR. Mol. Microbiol. 2003, 50, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Zain, N.A.M.; Suhaimi, M.S.; Idris, A. Development and Modification of PVA-Alginate as a Suitable Immobilization Matrix. Process Biochem. 2011, 46, 2122–2129. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Ahmed, M.M.; Akhdhar, A.; Sulaiman, M.G.M.; Khan, Z.A. Recent Advances in Alginate-Based Adsorbents for Heavy Metal Retention from Water: A Review. Desalin. Water Treat. 2022, 272, 50–74. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, W. The Neutral Protease Immobilization: Physical Characterization of Sodium Alginate-Chitosan Gel Beads. Appl. Biochem. Biotechnol. 2022, 194, 2269–2283. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).