Preprint
Article
Change in the Physiological Aspects of Soybean Caused by Infestation by Bemisia tabaci MEAM1
Altmetrics
Downloads
91
Views
30
Comments
0
A peer-reviewed article of this preprint also exists.
















This version is not peer-reviewed
Submitted:
05 October 2023
Posted:
06 October 2023
You are already at the latest version
Alerts
Abstract
The whitefly leads extensive damage to plants through direct feeding, honeydew secretion, plant physiological disorders, and vectoring plant viruses. This study aimed to evaluate the physiological characteristics of susceptible and resistant soybean cultivars to B. tabaci. The experiments were conducted in a greenhouse. Eleven soybean cultivars were selected and infested with 100 adults of B. tabaci at the V3 stage. The evaluation of photosynthetic parameters, such as photosynthetic rate, leaf transpiration, stomatal conductance, and internal CO2 concentration, revealed that B. tabaci infestation influenced gas exchange in soybean plants. The photosynthetic rate was higher in cultivars AS3810 and M8349 during the V6 stage. Infestations led to alterations in photosynthetic parameters, suggesting increased energy demand to maintain photosynthetic activity. However, the response to infestation varied among different cultivars, indicating varying levels of resistance and tolerance to the whitefly's damage. Additionally, the impact of infestation was more significant during the vegetative phenological. In conclusion, B. tabaci infestation affects soybean plants' physiology, leading to changes in gas exchange parameters and water use efficiency. The response to infestation varied among soybean cultivars, suggesting potential differences in resistance to the pest. The study highlights the importance of evaluating the physiological impact of whitefly infestations on soybean.
Keywords:
Subject: Environmental and Earth Sciences - Environmental Science
1. Introduction
The Middle East–Asia Minor 1 (MEAM1) whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), is a devastating cosmopolitan sap-sucking insect that poses a significant threat to various economically important crops, including soybean. As the second most widespread and economically important arthropod pest globally, B. tabaci has been responsible for substantial yield losses in soybean crops [1,2,3]. The economic impact of this pest reaches hundreds of millions of US dollars annually across diverse agricultural production systems worldwide [3,4,5].
Bemisia tabaci is a cryptic species complex consisting of at least 40 morphologically indistinguishable species belonging to eleven genetically distinct well-defined groups [6,7,8]. The damage caused by this whitefly is extensive and multifaceted, including direct feeding on vegetables [9,10], secretion of honeydew [11], induction of plant physiological disorders, and transmission of plant viruses [12,13,14].
Studies have revealed that whitefly infestations can deplete the energy reserves of plants, diminish primary production, and exert direct phytotoxic effects [15]. Whitefly infestations have been shown to reduce chlorophyll levels in various plant species, including tomato, zucchini, eggplant, and soybean [15,16,17,18]. This damage extends to the photosynthetic apparatus, particularly PSII and PSI, due to decreased stability in the oxygen-evolving complex and reaction centers of PSII, as well as a decline in electron transport [12]. Whitefly-infested tomato plants also display reduced parameters such as liquid photosynthesis, stomatal conductance, apparent carboxylation efficiency, and maximum efficiency of PSII [13].
Given the significant impact of B. tabaci MEAM1 on soybean crops, the objective of this study was to assess the physiological characteristics of susceptible and resistant soybean cultivars to B. tabaci. Understanding the specific physiological changes induced by the infestation can provide valuable insights into the mechanisms of damage and potential strategies for managing this destructive pest in soybean cultivation. B. tabaci infestation affects soybean plants’ physiology, leading to changes in gas exchange parameters and water use efficiency.
2. Materials and methods
2.1. Rearing and maintaining the whitefly population
The experiments were carried out at the Federal University of Piauí (UFPI-CPCE) (9°05004.4″S, 44°19037.5″W, 270 m). Bioassays were conducted in a greenhouse from March to July 2021. Whitefly nymphs were collected 3 months before the start of the experiments in tomato fields (9°01030.9′′S, 44°23021.2′′W, 353 m) under insecticide-free growing conditions. The insects were then brought to a laboratory to develop a rearing population. B. tabaci was housed in cages in the greenhouse. To prevent insects from escaping, PVC pipe construction cages (0.5m x 0.5m x 0.8m) were coated with white voile fabric. The greenhouse had a shaded ceiling made of cloth and a transparent canvas that was closed laterally with an antiaphid screen (50 mesh). Leaf cabbage (Brassica oleracea L. var. sabellica, Brassicaceae) was used for whitefly breeding. It was grown in 5-L pots and monitored daily to eliminate other insects. The cabbage plants were grown on a substrate consisting of soil, washed sand, and bovine manure (1:1:1), fertilized as recommended based on soil analysis.
2.2. Identification of biotype B, Middle East-Asia Minor 1 (MEAM1)
For identification of the biotype, pumpkin plants were placed inside cages with the whiteflies and checked for the presence of the characteristic symptom of B. tabaci MEAM1 infestation, when the leaves become silvery. Silvery leaves are a typical response to the physiological disturbance caused by feeding of the insect on this culture. For confirmation, adults were sent to the Entomology sector at Embrapa Arroz e Feijão (Santo Antônio de Goias, GO, Brazil), where molecular characterization was performed, and it was verified that the biotype of the whitefly used in the study was Middle East-Asia Minor 1 (MEAM1).
2.3. Soybean cultivars
The eleven soybean cultivars were chosen based on prior research, which demonstrated their adaptability to the northeastern Brazilian environment and their ability to yield well. These cultivars can be considered valuable sources of resistance against B. tabaci MEAM1 for breeding programs aimed at developing resistant soybean cultivars [19,20] (Table 1).
2.4. Conducting the experimente
The indicators for the chemical characterization of the samples, necessary for the recommendation of liming and fertilization in soybean crop, were obtained through the interpretation of soil analysis. Soybean plants were cultivated in pots containing 10 kg of substrate, composed of soil and cattle manure in a 3:1 ratio. In each pot, five seeds were planted, treated with insecticide and fungicide (Belure® + Vitavax®-Thiram 200 SC), and inoculated with bacteria of the genus Rhizobium.
Thinning was carried out after seed emergence, leaving only one plant per pot. Irrigation was performed according to the water requirements of the plants, with special attention given to irrigating at the base of the plant rather than wetting the leaf area to prevent the growth of phytopathogens. Six plants of each cultivar were used, where three were infested with whitefly and three were left free of infestation, resulting in a total of 66 (sixty-six) experimental units.
When the soybean reached the V3 stage, 100 (one hundred) adults of B. tabaci were captured from the rearing cabbage plants, placed in glass test tubes, and released at the base of each soybean plant, allowing the whitefly to have a chance to choose the plant for infestation.
Two evaluations were carried out for each physiological parameter, with 6 readings per treatment. The photosynthetic rate (A μmol m−2 s−1), leaf transpiration (E mmol m−2 s−1), stomatal conductance (gs mol m-2 s-1), and internal CO2 concentration (Ci μmol mol-1) were measured using an infrared gas analyzer (IRGA, Portable Gas Exchange Fluorescence System® GFS-3000) coupled with artificial light using blue and red-light emitting diodes (LEDs) with an intensity of 1,200 μmol m−2 s−1. One reading per plant was performed in the morning (7:00 to 10:00 am) to determine gas exchange, using a fully expanded middle third leaf.
From the gas exchange data, the following relationships were calculated: instantaneous water use efficiency (EUA = A/E μmol CO2/mmol−1 H2O), intrinsic water use efficiency (EIUA = A/gs μmol CO2/mmol −1 H2O), and instantaneous carboxylation efficiency (A/Ci μmol m−2 s−1/ μmol mol−1). The evaluations were carried out in four periods. The first and second readings occurred when the plant was in the V3 stage, one and two days after infestation, respectively. The third reading was performed when the plants reached the V6 stage after fifteen days, and the last one at the R1 stage, twenty days after infestation.
2.5. Statistical analysis
The physiological data were subjected to a triple factorial analysis of variance, involving 11 (eleven) cultivars and five evaluation periods. As a triple interaction was detected for all the evaluated parameters, an analysis of canonical variables was conducted, considering the seven physiological parameters resulting from the interaction between the 11 (eleven) cultivars and five evaluation periods. The statistical procedures were performed using GENES software [21].
3. Results
According to the analysis of variance, significant interactions are observed in the photosynthetic parameters of soybean plants infested with B. tabaci (Table 2). However, in the interaction between the factors, whitefly infestation versus evaluation period, there was no significant effect (p < 0.05) on the photosynthetic rate (A), leaf transpiration (E), stomatal conductance (gs), and internal CO2 concentration (ci). Conversely, in the evaluations for photosynthetic rates, leaf transpiration, and gas exchanges, significant effects were observed in plants infested by B. tabaci, in the interaction between soybean cultivars and infestation (Table 2).
The evaluation performed for Photosynthesis (A) revealed that the photosynthetic rate was altered in cultivars under infestation. However, such results were significant only for three cultivars, namely: AS 3810 IPRO, M8808 and M8349, being the cultivars AS3810 and M8808, resistant to B. tabaci, due to antixenosis and antibiosis. We perceive that for these cultivars the V6 phenological stage was the photosynthetically relevant phase, where the photosynthetic rate (A μmol m-2 s-1) was higher in the cultivars AS3810 and M8349 the evaluation period V6, in the infested plants and in the cultivar M8808 it was lower (Figure 1).
Regarding the transpiration rate (E) parameter, during the evaluation periods V3-2 and V6, corresponding to 48 h and fifteen days of infestation with B. tabaci, there were statistically significant differences when comparing infested and non-infested plants (Table 2 and Figure 2). It was observed that, for the cultivars BRS9280 and DOMÍNIO, there was an increase in the transpiration rate at the R1 phenological stage in de infested plants.
In Figure 3, it can be observed that the stomatal conductance in soybean cultivars under B. tabaci infestation increased significantly. Specifically, in the vegetative stage, there was a significant increase in stomatal conductance in the cultivars EXTREMA, M8349, M8644, AS3810, M8644, and M8808. Additionally, in the reproductive stage, the cultivars DOMINIO, EXTREMA, and BRS9280 also showed a significant increase in stomatal conductance (Figure 3).
In the AS3810 cultivar, during the evaluation period V6, all the photosynthetic parameters were significantly altered when comparing infested and non-infested plants, with higher activity observed in the infested plants (Figure 1, Figure 2 and Figure 3). The blooming phase (R1) was identified as the phenological stage with the most significant changes in activity when subjected to infestation, based on the examination of instantaneous carboxylation efficiency (A/Ci), water use efficiency (WUE), and instantaneous water use efficiency (IWUE) (supplementary material).
Regarding the photosynthetic parameter, internal concentration of CO2 (Ci), in the cultivar M8808, at all evaluation periods, the phenological stage showed significantly higher values in the treatment of infested plants. For the cultivars Bonus, Domínio, FTR 3190, M8349, M8644, and M8808, the significant difference was observed only in the evaluation period R1 (supplementary material).
Regarding the instantaneous rate (USA) and intrinsic rate of water use (EIUA) parameters, the significant differences observed between infested and non-infested cultivars were evident in the evaluation periods of the vegetative phenological stage, with higher values in the treatment of non-infested plants. However, in cultivars BRS 8383, BRS 9280, Bonus, Domínio, FTR 3190, and M8349, the significant difference was observed in the evaluation period R1 (supplementary material).
Regarding the efficiency of carboxylation (A/Ci), for the cultivars BRS 9280, Domínio, FTR 3190, M8349, M8644, and M8808, the significant difference was observed in the evaluation period R1. In the case of the cultivar M8644, significant differences were found in the periods V3-1, V3-2, and V6, corresponding to the vegetative phenological stage. For the cultivar AS 3810, the significant difference was observed only in the period V6 (supplementary material).
As for water use efficiency (EUA), infested plants showed lower values in most of the studied cultivars, while the intrinsic rate of water use (EIUA) was similar among the cultivars (supplementary material).
The photosynthetic rate (A) in infested plants was higher, while the internal CO2 concentration (Ci) was proportionally lower. There was no consistent grouping pattern between cultivars regarding infested and non-infested plants, leading to different responses in the four phenological periods. The physiological parameters exhibited distinct characteristics for each cultivar in the presence of the herbivore, potentially contributing to varying degrees of resistance and/or tolerance to herbivore damage.
Cultivars Extrema, at the phenological stages V3.1, V6, and R1, and BRS9280, across all phenological stages, showed similar patterns when comparing infested and non-infested plants. Cultivars Bonus and M8808 exhibited similarities between the treatments, indicating a response to the presence of the herbivore, possibly resulting in alterations in physiological parameters (Figure 4).
On the other hand, the GCS cultivar demonstrated a distinct response in the dispersion analysis between infested and non-infested plants. Unlike the previously mentioned cultivars, GCS showed a clear separation between the control and infested forms in the V6 and R1 stages, as well as the other cultivars. This cultivar displayed different physiological parameter behavior in plants without infestation compared to other cultivars in the V6 and R1 phenological stages (Figure 4).
Similarly, cultivar Dominio consistently occupied opposite quadrants in the vegetative stages, indicating significant alteration of physiological responses in the presence of the fly. However, the distance between the forms decreased relatively closer in the R1 stage, suggesting that the physiological parameter differences between infested and non-infested plants decreased in the later phenological stages.
For the FTR3190 cultivar, the photosynthetic parameters showed similarities between treatments in the initial phenological stages but differed in the final phenological stages.
Regarding the cultivar M8808i, it consistently exhibited positive dispersion in both canonical variables, except for the negative dispersion observed in the R1 stage, which brought it closer to the other cultivars. This indicates a distinct physiological behavior under infestation compared to other cultivars in the vegetative phenological stage, but a similar behavior when entering the reproductive cycle.
Notably, transgenic cultivars displayed a consistent pattern of behavior in infested plants, while this behavior was not replicated when observing non-infested plants (Figure 4).
There was no grouping pattern between cultivars in terms of infested and non-infested cultivars, so that in the four phenological periods they behaved differently. Demonstrating that the physiological characters regarding the response to infestation must be different in each genotype and thus may cause a greater or lesser degree of resistance to the insect (Figure 4).
Some cultivars showed a pattern of behaving similarly when infested or not, and the two forms were dispersed in the same quadrant, they are Extrema in V3.1, V6 and R1, BRS9280 for all stages, Bonus that despite not being in the same quadrant whenever the control and infested are close and M8808 always being in the same or close quadrant in the infested and control forms. These cultivars show a lack of reaction to infestation by the pest insect, implying that their physiological behavior is not altered by the presence of the whitefly.
In opposition to these cultivars, we have those that demonstrate a well-differentiated physiological behavior between the control and the infested, and we can mention the GCS that despite being in the same quadrant in the V6 and R1 stages, the control form is completely separated from the infested form, as well as the other cultivars. Demonstrating that this cultivar has a physiological behavior without infestation different from most other cultivars tested in phases V6 and R1. Another example is the cultivar Dominio, which in the vegetative stages is always found in opposite quadrants, implying that the presence of the fly considerably alters the physiological response, but the distance between the forms decreases as the culture advances until it is relatively close at the R1 stage (Figure 4).
Suggesting that this cultivar has a high physiological response to infestation at first and that this decreases as the plant grows. Unlike the Dominio cultivar, FTR3190 presents an initial behavior similar to that of the control and infested, which differs over time.
It is important to note that the Monsoy cultivars have a similar pattern of behavior when infested and this behavior is not repeated for the control. Since the cultivar M8808 was always dispersed in the positive field for both canonical variables, with the exception of the R1 stage, which was negative and closer to the other cultivars. Demonstrating a physiological behavior under infestation differentiated from other materials in the vegetative phase and similar when it enters the reproductive cycle.
4. Discussion
In our study, soybean plants were infested during the vegetative stage, and the nymphal density increased during the reproductive stage. This suggests that during the vegetative stage, more nutrients are available in the leaves as the plant allocates photosynthate to promote growth, providing favorable conditions for B. tabaci development [13,18]. The results indicated an initial increase in photosynthetic parameters upon infestation, suggesting an increase in energy allocation for maintaining photosynthetic functions.
The observation that resistant cultivars, which exhibit antixenosis or antibiosis against B. tabaci, show increased photosynthetic parameters, while susceptible cultivars consistently exhibit reductions in these parameters, suggests that the resistance mechanisms play a crucial role in maintaining the photosynthetic efficiency of soybean plants under whitefly infestations. The term “antixenosis” refers to a resistance mechanism where the plant’s physical or chemical characteristics deter the insect from settling or feeding on it. “Antibiosis,” on the other hand, involves resistance mechanisms that negatively impact the insect’s growth, development, or survival when it feeds on the plant.
The increase in photosynthetic parameters in resistant cultivars could be attributed to several factors, reduced feeding, antixenosis and antibiosis mechanisms could lead to reduced feeding by B. tabaci on resistant cultivars. As a result, there is less damage to the plant’s vascular system, allowing for better nutrient and water transport, which supports enhanced photosynthetic activity. The resistance mechanisms may help mitigate the stress caused by whitefly feeding. This can help the plant maintain optimal physiological conditions for photosynthesis. Resistant cultivars may be better able to maintain a balance between energy allocation for defense mechanisms and energy allocation for growth and photosynthesis. This balance can contribute to higher photosynthetic efficiency. Resistant cultivars might produce secondary metabolites in response to whitefly feeding, and some of these compounds could have positive effects on photosynthesis and overall plant health [19,20].
In contrast, the reduction in photosynthetic parameters observed in susceptible cultivars could be due to factors such as, direct feeding damage, these susceptible cultivars likely experience greater feeding by B. tabaci, leading to physical damage to the vascular system and a disruption in nutrient and water transport. Susceptible plants may experience more stress due to infestation, leading to alterations in hormone levels that negatively impact photosynthesis.
It is known that in healthy plants, photosynthetic parameters are typically lower compared to plants subjected to stress [22]. Feeding by B. tabaci induces stress, as evidenced by changes in photosynthetic parameters in soybean cultivars. This implies that plants also depend on efficient photosynthetic performance to maintain their health and, paradoxically, may become more suitable hosts for B. tabaci.
Whiteflies are phloem-feeders, and phloem is responsible for transporting photoassimilates, including sugars [23]. Sucrose and glucose are primary sugars associated with high occurrences of whiteflies, promoting their survival, adult longevity, fecundity, feeding, oviposition preference, and immature development [24]. These sugars are closely linked to photosynthesis, and therefore, a negative relationship between B. tabaci infestation and photosystem activity would be expected. However, in our study, a positive relationship between B. tabaci infestation and photosystem activity was observed.
Quantifying the damage caused by B. tabaci presents challenges due to its indirect effects on productivity and potential confounding impacts from other pests during the soybean production cycle. Evaluating the effects of B. tabaci feeding on photosynthesis can serve as an alternative approach to indirectly assess the damage caused by this pest. Previous studies have reported reductions in the photosynthetic rate caused by B. tabaci infestations in various crops [17,25,26,27].
Our findings indicate that B. tabaci feeding affects the plant’s physiology, as reflected in the relationships among photosynthetic parameters, as well as the levels of sugars and starch. The most significant impacts of insect infestation occurred during the vegetative phenological stage, when plants were more suitable for B. tabaci development, leading to plant stress that may have consequences on productivity [15].
In plants infested with herbivores, a reduction in the photosynthetic rate and changes in other variables associated with photosynthesis, such as CO assimilation (Ci), may occur. Our study observed an increase in photosynthetic rate in cultivars M8808 and M8349 at the end of the V6 growth stage, which may be associated with CO2 assimilation with rubisco. This indicates that the increase in photosynthetic rate in these cultivated plants is associated with the CO2 assimilation phase.
Schutze et al. (2022) observed higher chlorophyll content in the vegetative stage for all levels of B. tabaci infestation. In our study, where plants were infested at the beginning of the vegetative period and remained infested throughout the phenological cycle, we also noted an increase in photosynthetic parameters in infested plants. Yee et al. (1996) found similar results, reporting higher photosynthetic rates and stomatal conductance in cotton plants infested with B. argentifolii. These findings indicate that B. tabaci feeding significantly impacts the plant’s physiology, particularly related to photosynthetic parameters.
Overall, our study highlights the importance of considering the timing of insect infestation, as the greatest impacts on plant physiology occurred during the vegetative phenological stage. This information is valuable for understanding the effects of B. tabaci on soybean plants and may have implications for productivity. The findings highlight the importance of plant resistance in maintaining photosynthetic activity and overall plant health when faced with insect infestations. Understanding these mechanisms can provide valuable insights for breeding and selecting soybean cultivars that are more resilient to B. tabaci and other insect pests, ultimately contributing to more sustainable and productive agricultural practices.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, L.B.S., R.S.M. and B.E.P.; Data curation, B.E.P., L.S.F., L.C.A., C.M.P.S., G.S.R., J.V.S.M., A.F.T.S., H.M. and N.S.P.; Formal analysis, L.C.A., R.R.B., R.S.M., V.M.G.C.L., T.R.O., R.H.F.R., M.C.F.S., L.B.S. and H.M.; Funding acquisition, L.B.S. and R.S.M.; Investigation, L.CA., M.C.F.S., T.R.O. and R.H.F.R.; Methodology, B.E.P., L.S.F., L.C.A., C.M.P.S., G.S.R., J.V.S.M., A.F.T.S., H.M. and N.S.P.; Resources, Software, B.E.P., R.R.B., L.B.S., R.H.F.R., V.M.G.C.L., N.S.R., and L.S.F.; Supervision, L.B.S, R.R.B., R.S.M and L.S.F; Visualization, B.E.P., L.S.F., L.C.A., C.M.P.S., G.S.R., J.V.S.M., A.F.T.S., H.M. and N.S.P.; Writing—original draft, B.E.P., R.R.B., L.B.S., R.H.F.R., V.M.G.C.L., N.S.R., and L.S.F.; Writing—review and editing, B.E.P., R.R.B., L.B.S., R.H.F.R., V.M.G.C.L., N.S.R., G.S.R., J.V.S.M. and T.R.O.; authors have read and agreed to the published version of the manuscript.
Funding
National Council for Scientific and Technological Development — CNPq–004/2020; Coordination for the Improvement of Higher Education Personnel (CAPES);–scholarship Piauí State Research Foundation (FAPEPI) 008/2018.
Acknowledgments
We thank the National Council for Scientific and Technological Development — CNPq — Brazil for financial support. We also thank the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil, the Piauí State Research Foundation (FAPEPI) for the scholarships and resources provided, and the Federal University of Piauí, Brazil, for providing logistical support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors agree with the publication.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Padilha, G.; Pozebon, H.; Patias, L.S.; Ferreira, D.R.; Castilhos, L.B.; Forgiarini, S.E.; Donatti, A.; Bevilaqua, J.G.; Marques, R.P.; Moro, D.; et al. Damage assessment of Bemisia tabaci and economic injury level on soybean. Crop. Prot. 2021, 143, 105542. [Google Scholar] [CrossRef]
- Tay, W.T.; Elfekih, S.; Polaszek, A.; Court, L.N.; Evans, G.A.; Gordon, K.H.J.; De Barro, P.J. Novel molecular approach to define pest species status and tritrophic interactions from historical Bemisia specimens. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Economic impact of exotic insect pests in Brazilian agriculture. J. Appl. Èntomol. 2013, 137, 1–15. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Legg, J.P; Stansly, P.A.; Naranjo, S.E.; Brown, J.K.; Rami Horowitz, A.; Legg, J.P.; Polston, J.E.; Gerling, D. and Lapidot, M. 2010. “Bemisia: Bionomics and Management of a Global Pest.” In Bemisia: Bionomics and Management of a Global Pest, edited by Philip A. Stansly and Steven E. Naranjo, 1–540. Dordrecht: Springer Netherlands. [CrossRef]
- Li, Y.; Mbata, G.N.; Punnuri, S.; Simmons, A.M.; Shapiro-Ilan, D.I. Bemisia tabaci on Vegetables in the Southern United States: Incidence, Impact, and Management. Insects 2021, 12, 198. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Èntomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined Global Analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) Mitochondrial Cytochrome Oxidase 1 to Identify Species Level Genetic Boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Jiang, Z.; Zhang, F.; Liu, Y.; Li, Z.; Zhang, Z. New putative cryptic species detection and genetic network analysis of Bemisia tabaci (Hempitera: Aleyrodidae) in China based on mitochondrial COI sequences. Mitochondrial DNA Part A 2018, 29, 474–484. [Google Scholar] [CrossRef]
- Stansly, P.A., and Naranjo, S.E. 2010. Bemisia: Bionomics and Management of a Global Pest. Edited by Philip A. Stansly and Steven E. Naranjo. Springer. Vol. 1999. Dordrecht: Springer Netherlands. [CrossRef]
- Pollard, D.G. FEEDING HABITS OF THE COTTON WHITEFLY, BEMISIA TAB AC I GENN. (HOMOPTERA: ALEYRODIDAE). Ann. Appl. Biol. 1955, 43, 664–671. [Google Scholar] [CrossRef]
- Hequet, E.F.; Henneberry, T.J. and Nichols, R.L. 2007. “Sticky Cotton: Causes, Effects, and Prevention.” United States Department of Agriculture, no. 1915: 1–219.
- Li, Q.; Tan, W.; Xue, M.; Zhao, H.; Wang, C. Dynamic changes in photosynthesis and chlorophyll fluorescence in Nicotiana tabacum infested by Bemisia tabaci (Middle East–Asia Minor 1) nymphs. Arthropod-Plant Interactions 2013, 7, 431–443. [Google Scholar] [CrossRef]
- Toledo, C.A.d.L.; Ponce, F.d.S.; Oliveira, M.D.; Aires, E.S.; Júnior, S.S.; Lima, G.P.P.; de Oliveira, R.C. Change in the Physiological and Biochemical Aspects of Tomato Caused by Infestation by Cryptic Species of Bemisia tabaci MED and MEAM1. Insects 2021, 12, 1105. [Google Scholar] [CrossRef]
- Jones, D.R. Plant Viruses Transmitted by Whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Islam, T.; Shunxiang, R. Effect of sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infestation on eggplant (Solanum melongena L.) leaf. J. Pest Sci. 2009, 82, 211–215. [Google Scholar] [CrossRef]
- Buntin, D.G.; Gilbertz, D.A.; Oetting, R.D. Chlorophyll Loss and Gas Exchange in Tomato Leaves After Feeding Injury by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Èntomol. 1993, 86, 517–522. [Google Scholar] [CrossRef]
- McAuslane, H.J.; Chen, J.; Carle, R.B.; Schmalstig, J. Influence of Bemisia argentifolii (Homoptera: Aleyrodidae) Infestation and Squash Silverleaf Disorder on Zucchini Seedling Growth. J. Econ. Èntomol. 2004, 97, 1096–1105. [Google Scholar] [CrossRef]
- Schutze, I.X.; Yamamoto, P.T.; Malaquias, J.B.; Herritt, M.; Thompson, A.; Merten, P.; Naranjo, S.E. Correlation-Based Network Analysis of the Influence of Bemisia tabaci Feeding on Photosynthesis and Foliar Sugar and Starch Composition in Soybean. Insects 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.H.F.; Silva, L.B.; Almeida, L.F.O.; da Silva, S.R.; Sobrinho, N.; Maggioni, K.; Rodrigues, T.F.; Pavan, B.E. Vertical distribution of Bemisia tabaci on soybean and attractiveness of cultivars. Èntomol. Exp. et Appl. 2021, 169, 610–622. [Google Scholar] [CrossRef]
- e Silva, M.C.F.; Rodrigues, A.d.S.; Rodrigues, R.H.F.; Pavan, B.E.; Silva, L.B. Performance of Bemisia tabaci MEAM1 on soybean and resistance traits of cultivars. J. Asia-Pacific Èntomol. 2023, 26. [Google Scholar] [CrossRef]
- Cruz, C.D. (2013). <b>GENES–a software package for analysis in experimental statistics and quantitative genetics</b>–doi: 10.4025/actasciagron.v35i3.21251. Acta Scientiarum. Agronomy, 35(3), 271-276. [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. 2000. Probing Photosynthesis: Mechanisms, Regulation and Adaptation. CRC Press; Boca Raton, FL, USA: Analysis of the Fluorescence The fluorescence transiet as a tool to characterize and screen photosynthetic samples; pp. 445–483.
- Walker, G.P.; Perring, T.M. and Freeman, T.P. 2009. “Life History, Functional Anatomy, Feeding and Mating Behavior.” In Bemisia: Bionomics and Management of a Global Pest, 109–60. Dordrecht: Springer Netherlands. [CrossRef]
- Hasanuzzaman, A.T.M.; Islam, N.; Liu, F.-H.; Cao, H.-H.; Liu, T.-X. Leaf Chemical Compositions of Different Eggplant Varieties Affect Performance of Bemisia tabaci (Hemiptera: Aleyrodidae) Nymphs and Adults. J. Econ. Èntomol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Xue, M.; Zhao, H. Dynamic changes in energy metabolism and electron transport of photosystem II in Nicotiana tabacum infested by nymphs of Bemisia tabaci (Middle East-Asia Minor 1). Arthropod-Plant Interactions 2018, 12, 505–515. [Google Scholar] [CrossRef]
- Lin, T.-B.; Schwartz, A.; Saranga, Y. Non-stomatal factors limit cotton photosynthesis under silverleaf whitefly stress. Physiol. Plant. 1999, 107, 303. [Google Scholar] [CrossRef]
- Buntin, D.G.; Gilbertz, D.A.; Oetting, R.D. Chlorophyll Loss and Gas Exchange in Tomato Leaves After Feeding Injury by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Èntomol. 1993, 86, 517–522. [Google Scholar] [CrossRef]
- Yee, W.L.; Toscano, N.C.; Chu, C.-C.; Henneberry, T.J.; Nichols, R.L. Bemisia argentifolii (Homoptera:Aleyrodidae) Action Thresholds and Cotton Photosynthesis. Environ. Èntomol. 1996, 25, 1267–1273. [Google Scholar] [CrossRef]
Figure 1.
Heat map showing the photosynthetic CO2 assimilation (A) of soybean cultivars in non-infested and infested plants. *Asterisks indicate statistically significant differences; P ≤ 0.05. The analyzed cultivars were AS 3810 IPRO1; BONUS IPRO2; BRS 9280RR, FTR 3190 IPRO, M 8349 IPRO; M 8644 IPRO; M 8808 IPRO; BRASMAX EXTREMA IPRO®; BRASMAX DOMÍNIO; PRO®; GSC F07 BT; BRS 8383 IPRO. The data represent the change in A (µ mol CO2) of infested plants at phenological stages V3-1; V3-2; V6, and R1 compared to their respective control conditions, non-infested plants. In the heat map, blue and orange colors represent up-regulated and down-regulated genes, respectively. Statistical analyses are shown in Supplementary Table S1.
Figure 1.
Heat map showing the photosynthetic CO2 assimilation (A) of soybean cultivars in non-infested and infested plants. *Asterisks indicate statistically significant differences; P ≤ 0.05. The analyzed cultivars were AS 3810 IPRO1; BONUS IPRO2; BRS 9280RR, FTR 3190 IPRO, M 8349 IPRO; M 8644 IPRO; M 8808 IPRO; BRASMAX EXTREMA IPRO®; BRASMAX DOMÍNIO; PRO®; GSC F07 BT; BRS 8383 IPRO. The data represent the change in A (µ mol CO2) of infested plants at phenological stages V3-1; V3-2; V6, and R1 compared to their respective control conditions, non-infested plants. In the heat map, blue and orange colors represent up-regulated and down-regulated genes, respectively. Statistical analyses are shown in Supplementary Table S1.
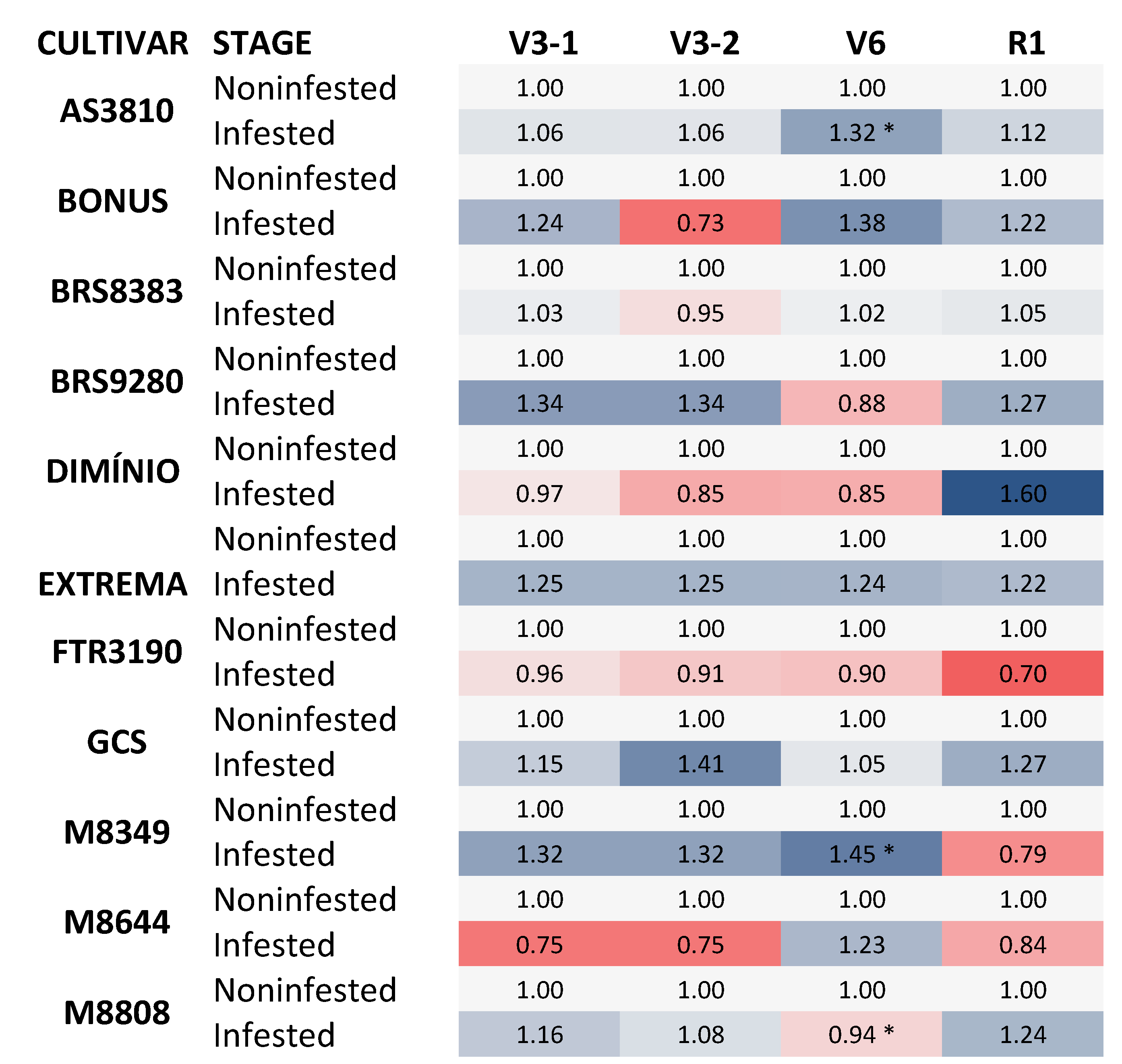
Figure 2.
Heat map showing the transpiration (E) of soybean cultivars in non-infested and infested plants. *Asterisks indicate statistically significant differences; P ≤ 0.05. The analyzed cultivars were AS 3810 IPRO1; BONUS IPRO2; BRS 9280RR, FTR 3190 IPRO, M 8349 IPRO; M 8644 IPRO; M 8808 IPRO; BRASMAX EXTREMA IPRO®; BRASMAX DOMÍNIO; PRO®; GSC F07 BT; BRS 8383 IPRO. The data represent the change in E (mmol H2O m−2 S−1) of infested plants at phenological stages V3-1; V3-2; V6, and R1 compared to their respective control conditions, non-infested plants. In the heat map, blue and orange colors represent up-regulated and down-regulated genes, respectively. Statistical analyses are shown in Supplementary Table S1.
Figure 2.
Heat map showing the transpiration (E) of soybean cultivars in non-infested and infested plants. *Asterisks indicate statistically significant differences; P ≤ 0.05. The analyzed cultivars were AS 3810 IPRO1; BONUS IPRO2; BRS 9280RR, FTR 3190 IPRO, M 8349 IPRO; M 8644 IPRO; M 8808 IPRO; BRASMAX EXTREMA IPRO®; BRASMAX DOMÍNIO; PRO®; GSC F07 BT; BRS 8383 IPRO. The data represent the change in E (mmol H2O m−2 S−1) of infested plants at phenological stages V3-1; V3-2; V6, and R1 compared to their respective control conditions, non-infested plants. In the heat map, blue and orange colors represent up-regulated and down-regulated genes, respectively. Statistical analyses are shown in Supplementary Table S1.
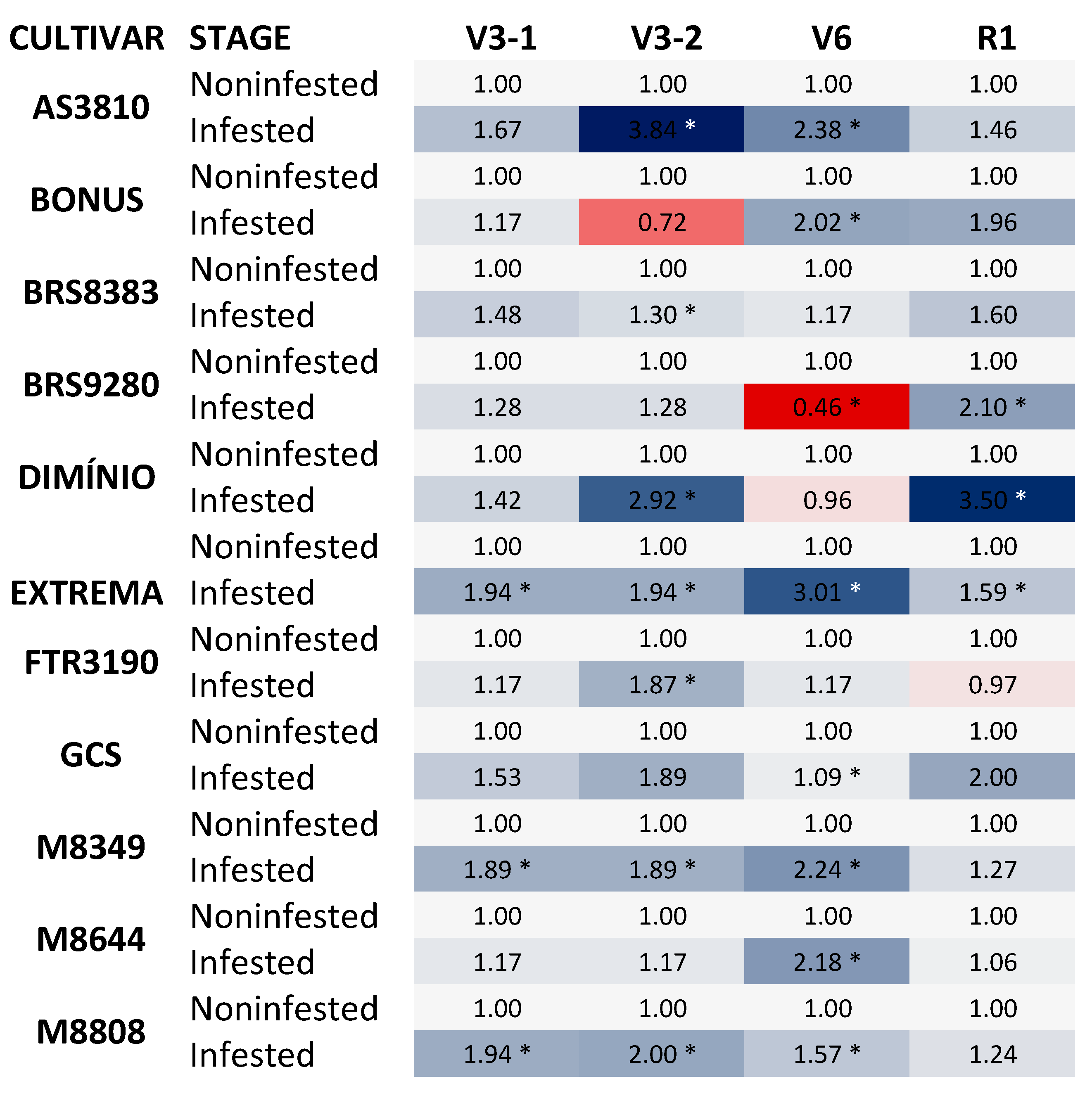
Figure 3.
Heat map showing the stomatal conductance to water vapour (Gs) of soybean cultivars in non-infested and infested plants. *Asterisks indicate statistically significant differences; P ≤ 0.05. The analyzed cultivars were AS 3810 IPRO1; BONUS IPRO2; BRS 9280RR, FTR 3190 IPRO, M 8349 IPRO; M 8644 IPRO; M 8808 IPRO; BRASMAX EXTREMA IPRO®; BRASMAX DOMÍNIO; PRO®; GSC F07 BT; BRS 8383 IPRO. The data represent the change in E (mmol H2O m−2 S−1) of infested plants at phenological stages V3-1; V3-2; V6, and R1 compared to their respective control conditions, non-infested plants. In the heat map, blue and orange colors represent up-regulated and down-regulated genes, respectively. Statistical analyses are shown in Supplementary Material.
Figure 3.
Heat map showing the stomatal conductance to water vapour (Gs) of soybean cultivars in non-infested and infested plants. *Asterisks indicate statistically significant differences; P ≤ 0.05. The analyzed cultivars were AS 3810 IPRO1; BONUS IPRO2; BRS 9280RR, FTR 3190 IPRO, M 8349 IPRO; M 8644 IPRO; M 8808 IPRO; BRASMAX EXTREMA IPRO®; BRASMAX DOMÍNIO; PRO®; GSC F07 BT; BRS 8383 IPRO. The data represent the change in E (mmol H2O m−2 S−1) of infested plants at phenological stages V3-1; V3-2; V6, and R1 compared to their respective control conditions, non-infested plants. In the heat map, blue and orange colors represent up-regulated and down-regulated genes, respectively. Statistical analyses are shown in Supplementary Material.
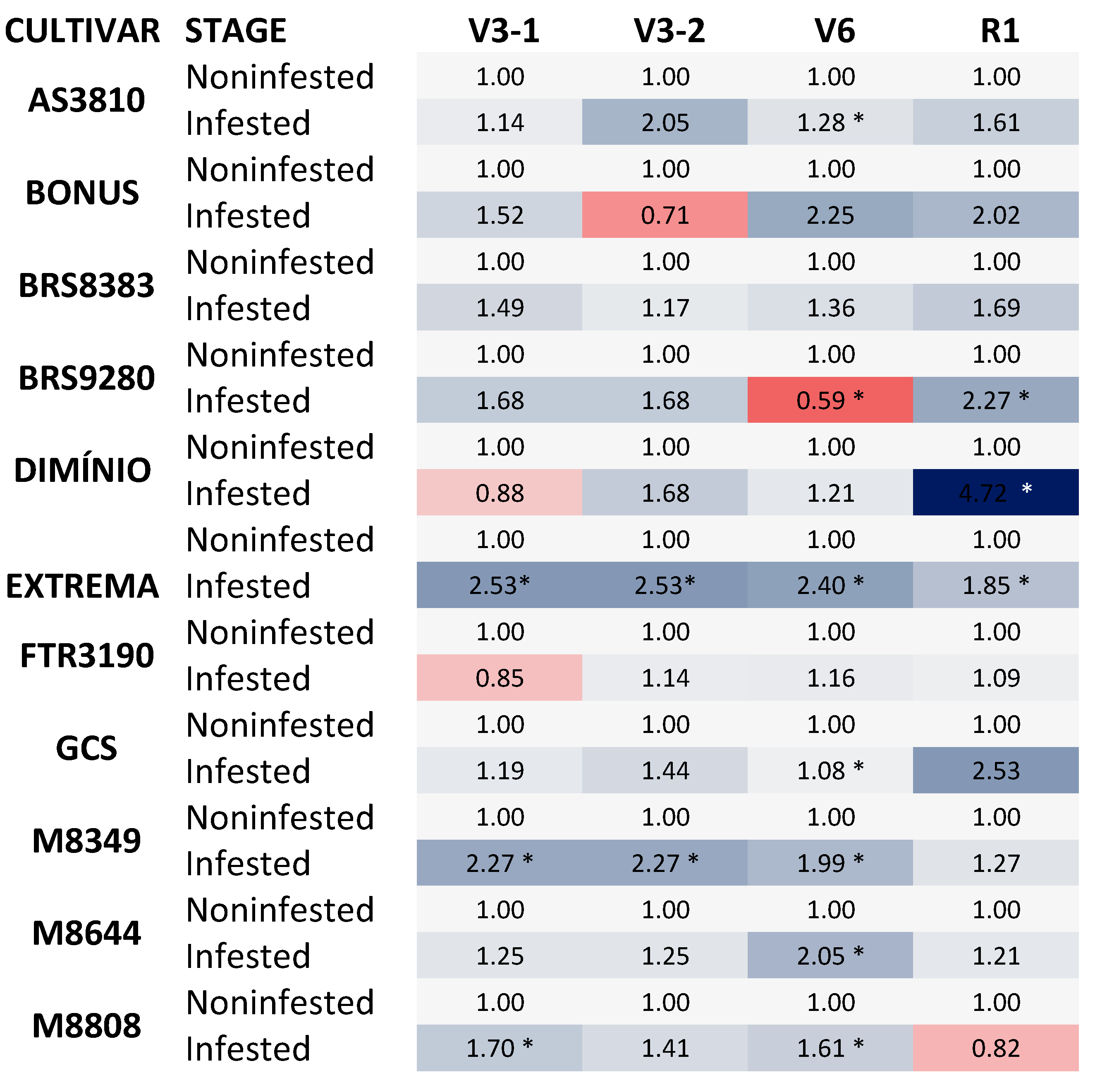
Figure 4.
Dispersion
of soybean cultivars obtained by analysis of canonical variables of soybean
physiological characters under whitefly attack in four phenological stages V3.1
(A), V3.2 (B), V6 (C) and R1 (D).
Figure 4.
Dispersion
of soybean cultivars obtained by analysis of canonical variables of soybean
physiological characters under whitefly attack in four phenological stages V3.1
(A), V3.2 (B), V6 (C) and R1 (D).

Table 1.
Soybean cultivars used in the experiments.
| Nº | Cultivar | Resistance history |
|---|---|---|
| 1 | AS 3810 IPRO1 | Resistance–Antibiosis [20] |
| 2 | BONUS IPRO2 | Susceptible [19,20] |
| 3 | BRS 9280RR | Resistance–Antixenosis and Antibiosis [19,20] |
| 4 | FTR 3190 IPRO | -- |
| 5 | M 8349 IPRO | -- |
| 6 | M 8644 IPRO | Antixenosis [19] |
| 7 | M 8808 IPRO | Resistance–Antixenosis and Antibiosis [19,20] |
| 8 | BRASMAX EXTREMA IPRO® | -- |
| 9 | BRASMAX DOMÍNIO IPRO® | -- |
| 10 | GSC F07 BT | -- |
| 11 | BRS 8383 IPRO | Resistance–Antibiosis [20] |
Table 2.
Photosynthetic response, leaf transpiration (E), stomatal conductance (gs), photosynthetic rate (A), internal CO2 concentration (Ci), instantaneous carboxylation efficiency (A/Ci), instantaneous water use efficiency (EUA) and intrinsic water use efficiency (EIUA) of soybean leaves subjected to injury from whitefly.
Table 2.
Photosynthetic response, leaf transpiration (E), stomatal conductance (gs), photosynthetic rate (A), internal CO2 concentration (Ci), instantaneous carboxylation efficiency (A/Ci), instantaneous water use efficiency (EUA) and intrinsic water use efficiency (EIUA) of soybean leaves subjected to injury from whitefly.
| Source of variation | df | Medium Squares | ||||||
|---|---|---|---|---|---|---|---|---|
| E | gs | A | ci | A/Ci | EUA | EIUA | ||
| Cultivars (C) | 10 | 2.476** | 58472** | 22,34** | 21421** | 0.033** | 2.18** | 0.017** |
| Infestation (I) | 1 | 167.8** | 449097** | 98,45** | 53924** | 0.037** | 218.2** | 0.076** |
| EP (E) | 3 | 8.06** | 142370** | 129,9** | 103422** | 0.114** | 11,62** | 0.066** |
| C x I | 10 | 4.38** | 8836** | 23,64** | 5738** | 0.031** | 5,20** | 0.003** |
| C x E | 30 | 3.85** | 28115** | 19,85** | 17693** | 0.030** | 7,89** | 0.009** |
| I x E | 3 | 1,18ns | 976,1ns | 4,86ns | 267,7ns | 0.017* | 2,61** | 0.004** |
| C x I x E | 30 | 3.10** | 895,6 ns | 15,2* | 5207** | 0.015** | 4.29** | 0.002** |
| error | 176 | 0.70 | 3389 | 9.22 | 2096 | 0.005 | 0.64 | 0.0003 |
| Mean | 3.99 | 210,3 | 16,50 | 214,6 | 0.106 | 4.63 | 0.102 | |
| CV% | 20.96 | 27.68 | 18,41 | 21,33 | 65.5 | 17.36 | 17.33 | |
*, **, ns significant to 5, 1 % and not significant respectively by the F test. EP Evaluation period. A: photosynthetic rate (μmol m−2 s−1), E: leaf transpiration (mmol m−2 s−1), gs: stomatal conductance (mol m−2 s−1), Ci: internal CO2 concentration (μmol mol−1), EUA: instantaneous water use efficiency (A/E μmol CO2/mmol−1 H2O), EIUA: intrinsic water use efficiency (A/gs μmol CO2/mmol −1 H2O) and A/Ci: instantaneous carboxylation efficiency (μmol m−2 s−1/ μmol mol−1).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





