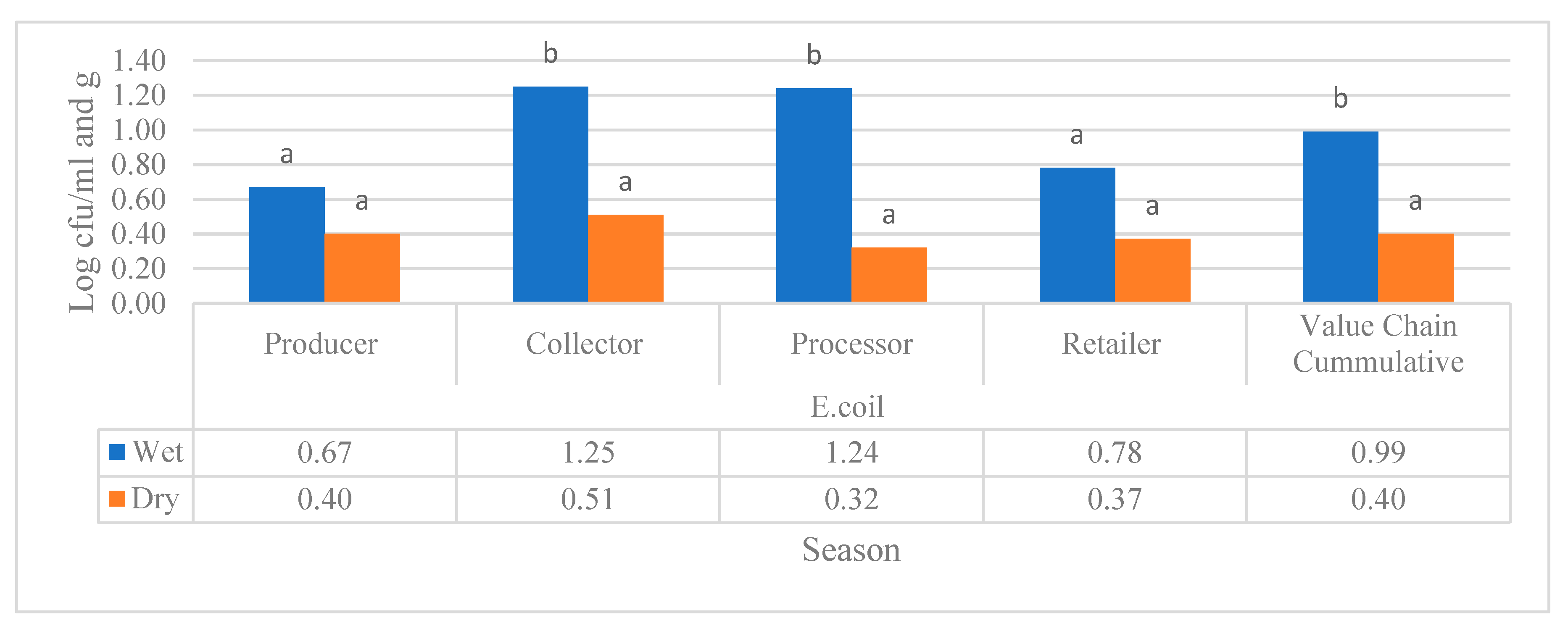1. Introduction
Dairy products, especially fresh milk, are regarded as a complete diet because they contain all the necessary nutrients (1). Besides, milk has a number of active chemicals that are important for both nutrition and health protection, as well as being a source of macro and micronutrients (2.). The main solid components of milk, such as fat and protein, are what make it valuable both commercially and nutritionally (3.). Among the environmental factors, the type and safety of feeds the milking cows consume and the season of the year have a considerable influence on the safety of milk (4). Foodborne illnesses, including milk-borne diseases, are a substantial public health concern for individuals and nations in the developed world and developing nations. This is because milk and other dairy products are produced in unclean circumstances and using subpar production techniques (5). Human diseases caused by milk-borne bacteria range from gastrointestinal disorders characterized by diarrhea and vomiting to other, more widespread, and even fatal foodborne infections. This issue extends beyond just public health issues and includes economic issues as well (6).
The circumstances under which milk is collected, transported, processed, and stored impact not just its quality and shelf stability but also its microbial safety. This is because milk is highly nutritious and is particularly sensitive to microbial deterioration (7). Milk contamination occurs due to mal sanitary practice that occurs in all milk contact surfaces, including milker’s hands, milk containers, and bulk tanks containers (8). Environmental condition in the rainy season the ground will become muddy which favor the proliferation and transmission of pathogens it is easier to keep the cows clean during the dry period (9). In addition, in the wet season, prolonged precipitation occurs it is mostly associated with the dissemination of microbes from contaminated areas including, water reservoirs like groundwater (10). Unmanaged and spring groundwater is characterized by the absence of standard safety measures for restricting any foreign material, including contaminant dirt, from entering (11) most Ethiopian dairy farmers’ uses for washing purposes of teats of cows and milk storage utensils according our survey. During the dry season temperature increases, there will create a shortage of water, more specifically in rural parts of developing countries (12, 13), since the water readily available to the farmers may not be enough to clean the equipment and the farm environment.
In Ethiopia, generally, there are three distinct seasons. From February to May, there is a short rainy season called the Belg. The long rainy season, known as the Kiremt, which lasts from late June to mid-September, comes next. The Bega, which typically lasts from October to January, is distinguished by relatively dry conditions across the majority of the nation and wet conditions and a secondary peak in rainfall over the south (14). The survival and reproduction of microorganisms in food products are significantly influenced by seasonal fluctuations (15, 16.). Seasonal variation influences both the quality and accessibility of food (17). As a result, there are significant economic, societal, public, and environmental effects. Numerous studies have demonstrated how seasonal variations in temperature and humidity have an adverse impact on food safety and local and global food commerce (18). Summer’s higher temperatures and humidity favor the development of bacteria and fungi in food products (19). Different groups of microorganisms require different temperature ranges for optimum growth and multiplication. According to reports, bacteria, particularly those found in food, prefer a temperature range of 32 to 43 °C for growth (20). In a recent study, several foodborne illnesses are more common in the summer than in the winter (21). The study done in the dry season by Mengstu et al., (2023) revealed that 86% and 90% of raw and pasteurized milk samples collected from major milk shade of Ethiopia Oromia, SNNP, and Amhara region were substandard for microbiology requirement, based upon ESA standards (22).
Since total bacteria, total coliforms, and E. coli count are typical hygienic indicator parameters, the hygienic condition of the dairy environment is highly influenced by the fluctuation of seasons, geographical distribution, and farm practices. Seasonal microbial load variation in dairy products, environment, and value chain actors in Ethiopia is very minimal studying this uncovered problem very crucial to establishing strategic interventions and predicting outbreaks. The objective of the current study was to detect and quantify the seasonal variation in microbial indicators of milk hygiene for raw milk, pasteurized milk, and cottage cheese across the value chain in the Oromia, SNNP, and Amhara regional states of Ethiopia.
2. Materials and Methods
2.1. Study Area and Study Design
The investigation of seasonal variation of total aerobic mesophilic bacteria count, TCC, and generic E.coli was conducted using a cross-sectional study using a longitudinal study design. Three representative locations were selected from the three regions, namely Debre-Zeit, Hawassa, and Bahir Dar from Oromia, SNNP, and Amhara areas, respectively. The wet season from May to June and dry season from January to March were considered when designing seasonal variance.
2.2. Sample Collection, Transportation, and Storage
A random sampling technique was used to collect milk samples from producers and a purposeful sampling technique was used for milk collectors, processors, and retailers. A total of 228 samples were collected for wet season sampling from Debre-Zeit (n = 120), Hawassa (n = 60%), and Bahir Dar (n = 48%) (June, July, and August). Data from the dry season was used for the seasonal comparison (22). The samples which were collected in the wet season from producers, collectors, processors, and retailers were the participant that was involved in the dry season study (January, February, March, and April) in the three study sites. Dairy product samples were collected aseptically using sterile containers and transported and a portable fridge was maintained at 4℃. The samples arrived at Addis Ababa University Center for Food Science Nutrition (CFSN) microbiology laboratory for microbial analysis.
2.3. Assessment of Microbiological quality and safety of milk and cottage cheese
2.3.1. Total aerobic mesophilic bacteria count (APC)
The pour plate method with plate count agar (Oxoid, UK) was utilized, according to the FDA (2000). The presence of mesophilic bacteria in one mL of diluted milk and cottage cheese samples is tested using this approach. One ml of milk sample was serially diluted in 9 ml of Butterfield’s phosphate-buffered dilution water (ratio of 1:10) up to five and seven dilutions for pasteurized and raw milk, respectively. The dilution index was used to designate sterile duplicate glass Petri dishes of 90 ml. One ml of each dilution was aseptically withdrawn using a sterile measuring pipette and added into the center of the sterile Petri dish, and then closed. The same was done for a duplicate Petri dish. This was repeated till all the dilutions were pipetted into their corresponding plates up to 10−5 dilutions. This was followed by pouring about 15-20 ml of standard plate count agar. The aliquot and the medium were gently mixed by alternate clock and anti-clockwise rotations and left to solidify on the bench for about 30 min. The plates were inverted and incubated at 32+/-1°C for 48 hr. Plates inoculated with sample dilution yielding between 25 and 250 colonies were counted after incubation. The number of bacteria in a milliliter of milk was determined using the FDA (2000) method after colony counts were performed with a colony counter (23).
Where:
C = s the sum of colonies on all plates counted
V = s the volume applied to each plate
n= s the number of plates counted at first dilution.
n2= s the number of plates counted at the second dilution,
d = s the dilution from which the first count was obtained.
N= s the average plate count
2.3.2. Enumeration of Coliform and E. coil
The protocols for counting total coliforms and E. coli counts in milk samples were followed according to 3M Food Safety, 2017. Ten milliliters of raw and pasteurized milk were serially diluted in 90 milliliters of saline up to five and three dilutions, respectively. Before applying the 1mL diluent to the E. Coli-Coliform count plate Petri film for quantification of coliforms and E. coli, the diluent was vortexed to homogenize the serial dilution. For serial dilution, 1 mL of milk was withdrawn from each stock sample, and 10 g of cottage cheese was weighed and homogenized with 90 mL of PBS in the stomacher bag. One mL of diluent was taken from the supernatant for serial dilution. Then, the top film was lifted, and 1 mL of sample suspension was dispensed onto the inoculation area. The Petri film plates were incubated in a horizontal position at 35 °C ± °C for 24 hours ± 2 hours. The plates were incubated for an additional 24 hours ± 2 hours (48 hours ± 4 hours total) until colonies of sufficient size to count were observed. The total coliform count consisted of both red and blue colonies associated with gas, and blue colonies with a gas bubble was counted as E. coli (24)
Where:
∑C s the sum of the colonies counted on the two Petri films
V s the volume of culture Plates
d s the dilution corresponding to the first dilution retained d = when the undiluted liquid product (test sample) is retained (24).
2.4. Statistical analysis
Independent t-test were employed to assess the significant variation between microbial loads of hygienic indicators (total bacteria, total coliforms, and E. coli) in the dry and wet seasons. SPSS version 22 was used and α= 0.05 was considered to be significant. For quantification data, sample type served as the experimental unit (raw milk, pasteurized milk, and cottage cheese) and data were analyzed utilizing a general linear mixed model with three ×three factorial (region × value chain) design. The response variable was log cfu/mL for (raw milk and pasteurized milk) or log cfu/g (for cottage cheese), and the linear predictors included the fixed effects of the region (SNNP, Oromia, Amhara), value chain (producer, collector, retailer) and all two-way interactions.
3. Results
3.1. Total aerobic mesophilic bacteria count (APC) in milk and cottage cheese collected from the study sites
APC of milk and cottage cheese samples collected from three regions during wet and dry seasons according to Ethiopian Standard Authority (ESA,2021) for milk and Kenya Bureau of Standard (KEBS,2018) for cottage cheese as put forth in
Table 1 (25,26). The wet season investigation for APC count revealed that 100% of the collected samples from the three regions failed to meet the standard set by ESA (2021) and KEBS (2018)((25,26). Whereas, in our prior work Mengistu et al., 2023, during dry season in Oromia region 14.58% (n =7) of the collected samples complied with the standards as shown in
Table 1 (21).
The APC in log cfu/ml of the pasteurized milk was significantly lower than that of raw milk in the three study sites, as shown in
Table 2. During the wet season higher values of APC of raw milk, pasteurized milk, and cottage cheese were observed in the SNNP region 9.67±0.52 log10 cfu/ml, 6.976.17±0.63 log10 cfu/ml, and 7.34±0.32 log10 cfu/ml, respectively comparing to remaining study region as mentioned in
Table 2.
Mean comparison of APC raw, pasteurized milk and cottage cheese samples collected during dry and wet seasons is presented in
Table 2. Seasonal variation of APC in raw milk samples that were collected from the SNNP region showed statistically higher than during dry season (10.80±0.67 log10 cfu/ml), whereas, in the remaining study regions, APC value was higher during wet season as shown in the
Table 2. APC value in pasteurized milk that was collected in Oromia and SNNP regions demonstrated, microbial counts was statistically higher during the dry season. Whereas, no significant variation has been observed in those samples that were gathered in the dry and wet seasons in the Amhara region. Seasonal variation in APC value in the cottage cheese showed the microbial load was significantly higher during the dry season in all regions of the study as shown in
Table 2.
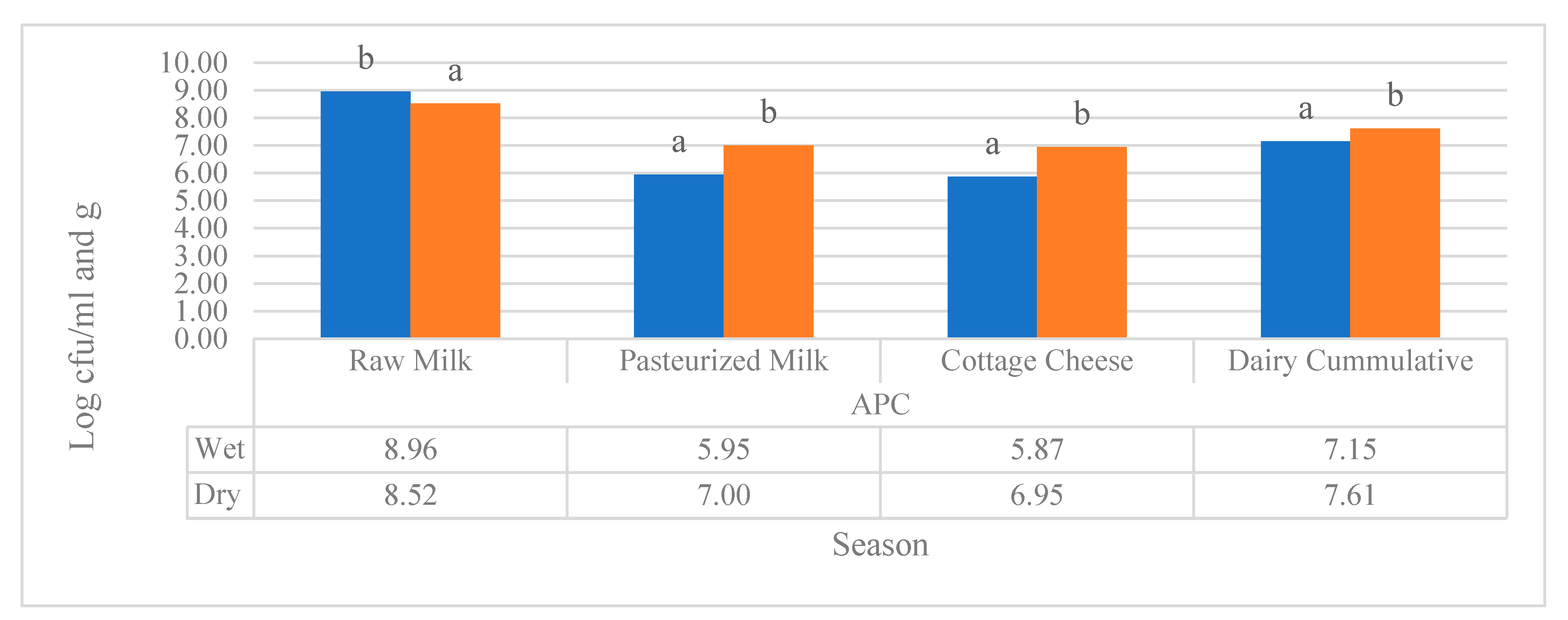
Figure 1 illustrates a seasonal comparison of APC in milk (log cfu/ml) and cottage cheese (log cfu/g) samples collected from the three study sites. Except for raw milk sample, the APC load in pasteurized milk and cottage cheese was found to be statistically higher in the dry season. Furthermore, the commutative APC load of the three-sample type still showed that the APC load significantly higher in the dry season at p<0.05.
3.2. Total coliforms in milk and cottage cheese collected from the study sites
TCC of raw milk revealed that the samples that were gathered from the SNNP region (54.17%) mostly fit the standard stated by Ethiopian Standard Authority (ESA, 2021) comparing to Oromia (33.3%) and Amhara (5%) regions in wet season investigation. Whereas, the samples that were collected in Oromia region majorly fit the standard during dry season regions as shown in
Table 3. The level of TCC in pasteurized milk that was collected in Oromia region during wet season revealed majority of the samples (97.92%) comply with the ESA, (2021) compared to the SNNP (20.83%) and Amhara region (0%) as mentioned in
Table 3. The majority of cottage samples that were collected in the wet season were shown to be complied the ESA, (2021) in all study regions. While in dry season, 58.33% of cottage cheese samples failed to comply with the ESA, (2021) as it was reported in our prior work Mengstu et al., (2023) as shown in
Table 3 (22,25).
Mean comparisons of total coliform counts of raw milk, pasteurized milk and cottage cheese samples during wet and dry seasons collected from the study region indicated in
Table 4. The total coliform count was significantly (p<0.05) higher in raw milk compared to pasteurized milk in all-study region during the wet season. The total coliform counts were found to be higher in raw milk (5.67±0.90 log cfu/ml) and pasteurized milk (3.71±0.86 log cfu/ml) in Amhara region compared to Oromia and SNNP regions. The total coliform count in cottage cheese that was collected from the Oromia region (3.29±3.18 log cfu/g) had a higher load compared to that of the SNNP and Amhara region during wet season.
Like raw milk the pasteurized milk samples that was collected during the dry season (4.50±2.1 log cfu/ml) showed strongly significantly higher TCC compared to the wet season (0.04±0.1 log cfu/ml) in Oromia region. Pasteurized milk that was collected during the wet season (3.71±0.86 log cfu/ml) had a significantly higher TCC level than the dry season in Amhara region. The TCC level variability in pasteurized milk due to season still had no significant impact on the samples that were collected from the SNNP region. The variation in TCC in wet and dry seasons in cottage cheese samples had a similar pattern to that of raw and pasteurized milk in the three- regions as shown in
Table 4. The variation in TCC levels in raw milk samples that were collected during wet and dry seasons did not vary significantly in the three regions (
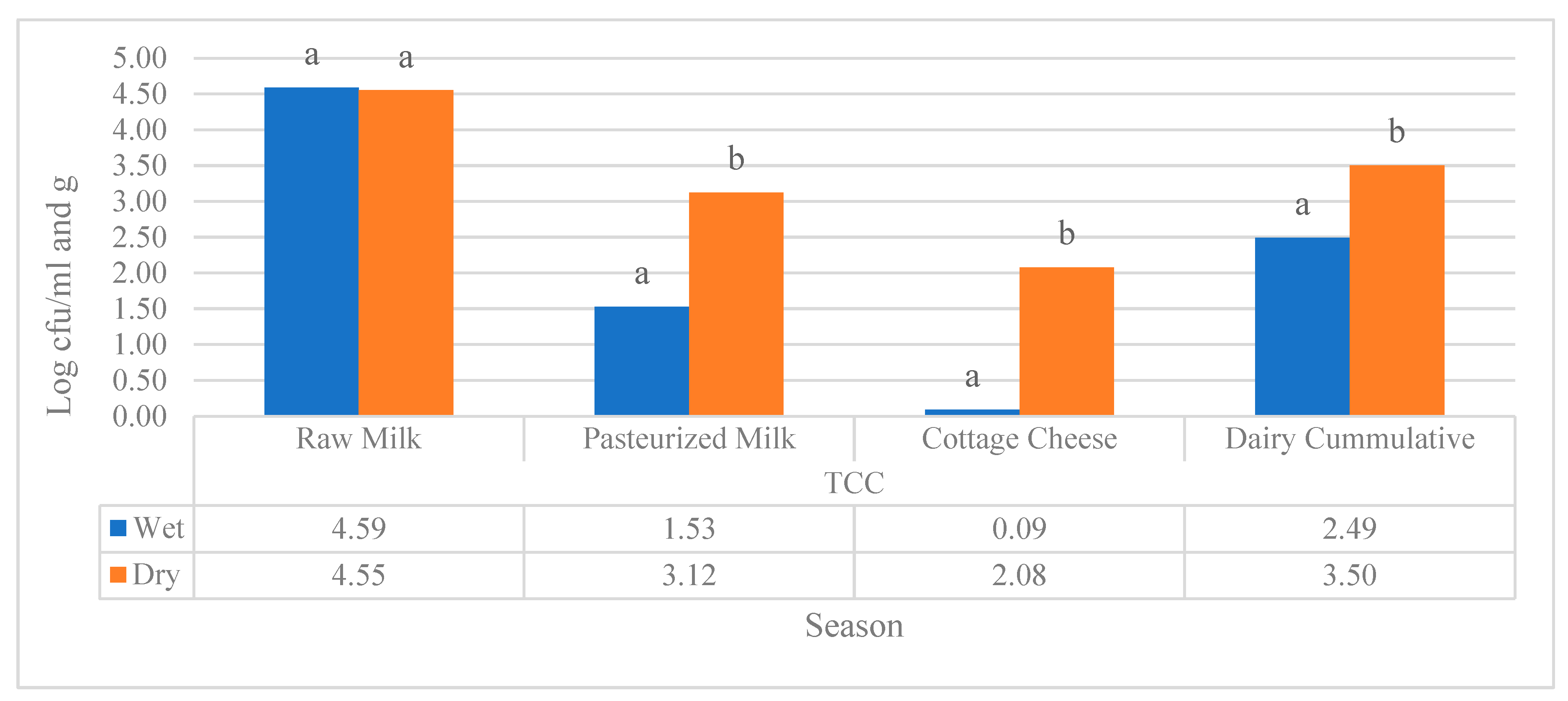
Figure 2). Whereas, pasteurized milk and cottage samples collected during dry season showed a statistically significant difference at (p<0.05) as compared to that of wet season.
3.3. Seasonal comparison of generic Escherichia coli count in milk and cottage cheese samples collected from three regions
Seasonal comparison of Escherichia coli count in milk and cottage cheese samples collected from three regions is presented in
Table 5. The E. coli counts for wet season investigation revealed that 56.3% (n = 27) raw milk, 60.42% (n = 29) pasteurized and 100% (n=24) cottage cheese from the Oromia region, 41.6% (n = 10) raw milk, 66.67% (n =16) pasteurized milk and 100% (n =12) cottage cheese from SNNP region, and 85% (n=17) raw milk, 35% (n=7) pasteurized milk and 100% (n = 8) from the Amhara region comply with the safety recommendation of the ESA, (2021), which is to be nil in marketable dairy products (25).
Table 5 demonstrates the seasonal comparison of Escherichia coli (log cfu/ml a g) count in milk and cottage cheese samples collected from the three regions. The E. coli counts of raw and pasteurized milk samples collected from SNNP and Amhara regions during wet season significantly different at (p<0.05). In the SNNP region, raw milk had a higher E. coli count (1.48±1.48 log cfu/ml), whereas, in the Amhara region, pasteurized milk (1.77±1.44 log cfu/ml) had a significantly higher load of E. coli bacteria. The counts of raw and pasteurized milk samples collected from the Oromia region did not vary significantly from each other.
Seasonal attribution in E. coli level was found to be significant in raw and pasteurized milk, as
Table 5 demonstrated. The raw milk samples that were collected in Oromia region only found to have significant seasonal variation in E. coli load compared to the remaining study regions. The E.coli count significantly higher during wet season than dry season. The E. coli count in pasteurized milk had the same pattern in Oromia and SNNP regions as that of raw milk. The E. coli level in both study sites was significantly higher during wet season 0.9±1.23 log cfu/ml and 0.12±0.63 log cfu/ml respectively, than in the dry season. The result of E. coli counts in cottage cheese samples that were collected from the three regions did not exhibit significant differences due to seasonal fluctuation.
Pasteurized milk samples that were collected from the three study sites had significant variation in the E. coli counts, as
Figure 3 illustrated due to seasonal fluctuation. Unlike the cumulative TBC and TCC seasonal variation these findings E. coli load found to be higher during in wet season.
3.4. Total aerobic mesophilic bacteria count (APC), total coliform count (TCC), and generic E. coli in milk and cottage cheese across the dairy value chain of study regions
APC load was significantly higher in wet season in both producer (8.62± 0.35 log cfu/ml) and collector (8.55 ±0.38 log cfu/ml) value chain of raw milk than dry season which was 7.53±1.24 log cfu/ml and 7.31±0.91 log cfu/ml respectively in the Oromia region. Contrary to the raw milk value chain actors, the APC was significantly higher during dry season in both processers (5.85±0.52 log cfu/ml) and retailers (6.44±0.52 log cfu/ml) of the pasteurized milk value chain compared to the wet season sample collection from processors (5.46±0.55 log cfu/ml) and retailers (5.31±0.44 log cfu/ml).
The TCC load variation due to season among the samples that were collected in the dairy value chain of Oromia region showed the TCC significantly higher in dry season in both milk types (raw and pasteurized) value chain actors. The TCC load on those samples that were collected from raw milk value chain was found to be 4.35±2.09 log cfu/ml and 4.06±2.7 log cfu/ml from producer and collector value chains, respectively during wet season. Whereas, the TCC load was found to be 6.27±1.89 log cfu/ml (producer) and 5.77±2.2 log cfu/ml (collector) during dry season. The pasteurized milk that was collected during the wet season had 0.11±0.44 log cfu/ml TCC from the processor value chain and none of the samples that were collected from retailer value were found to have TCC. Whereas, in the dry season 3.38±1.89 log cfu/ml (processor) and 2.24±2.5 log cfu/ml (retailer) total coliform count was found. Unlike to total coliform count in the dairy value chain, the E. coli load of the samples was significantly higher during wet season. The raw milk that was collected from producers (0.78±1.04 log cfu/ml) and collectors (1.23±1.39 log cfu/ml) had E. coli in wet season study. In dry season 0.13±.61 log cfu/ml and 0.27±0.93 log cfu/ml E. coli load from the sample collected from producer and collector value chain, respectively. E. coli counts in processor (0.91±1.15 log cfu/ml) and retailer (0.90±1.34 log cfu/ml) was significantly higher during wet season than dry season 0.08±0.41 log cfu/ml and 0.15±0.52 log cfu/ml, respectively.
The dairy value chain of this study, which was located in the SNNP region, revealed that APC was found to be significantly higher during dry season in both sample types (raw and pasteurized) of value chain actors. The APC level in raw milk value chain was found to be 11.05±0.47 log cfu /ml and 10.56±0.76 log cfu/ml in the producer and collector value chains during dry season, respectively. Whereas, during wet season 9.56±0.51 log cfu/ml and 9.79±0.53 log cfu/ml in producer and collector, respectively. Furthermore, the APC load processer and retailer was found to be 7.0±1.15 log cfu/ml and 6.95±0.37 log cfu/ml, respectively. While, in dry season 9.35±1.72 log cfu/ml and 9.39±1.7 log cfu/ml APC was found in processor and retailer value chains, respectively. Seasonal attribution in TCC level the samples that were collected from collector value chain of raw milk and the processor value chain of pasteurized milk revealed the TCC load significantly higher during wet season. The total coliform count was found to be 5.31±0.92 log cfu/ml in the collector and 7.01±1.15 log cfu/ml in the processer value chain during wet season. While, in dry season, TCC was found to be 3.00±2.84 log cfu/ml and 4.5±0.85 collector and processor value chain, respectively. Producers (3.69±0.67 log cfu/ml) and retailer (2.33±2.39 log cfu/ml) value chain had not had significant variation in total coliform count during wet season compared to dry season which was 4.10±1.89 log cfu/ml and 1.11±1.7 log cfu/ml, respectively. The raw milk samples that were gathered from the producer (0.75±.1.13 log cfu/ml) value chain and the pasteurized milk from the processer (1.17±.0.96 log cfu/ml) only showed a significant difference in E. coli count due to seasonal fluctuation compared to the other remaining value chain actors. The E. coli load was found to be higher in dry season for the producer value chain while, for processer value chains it was wet season.
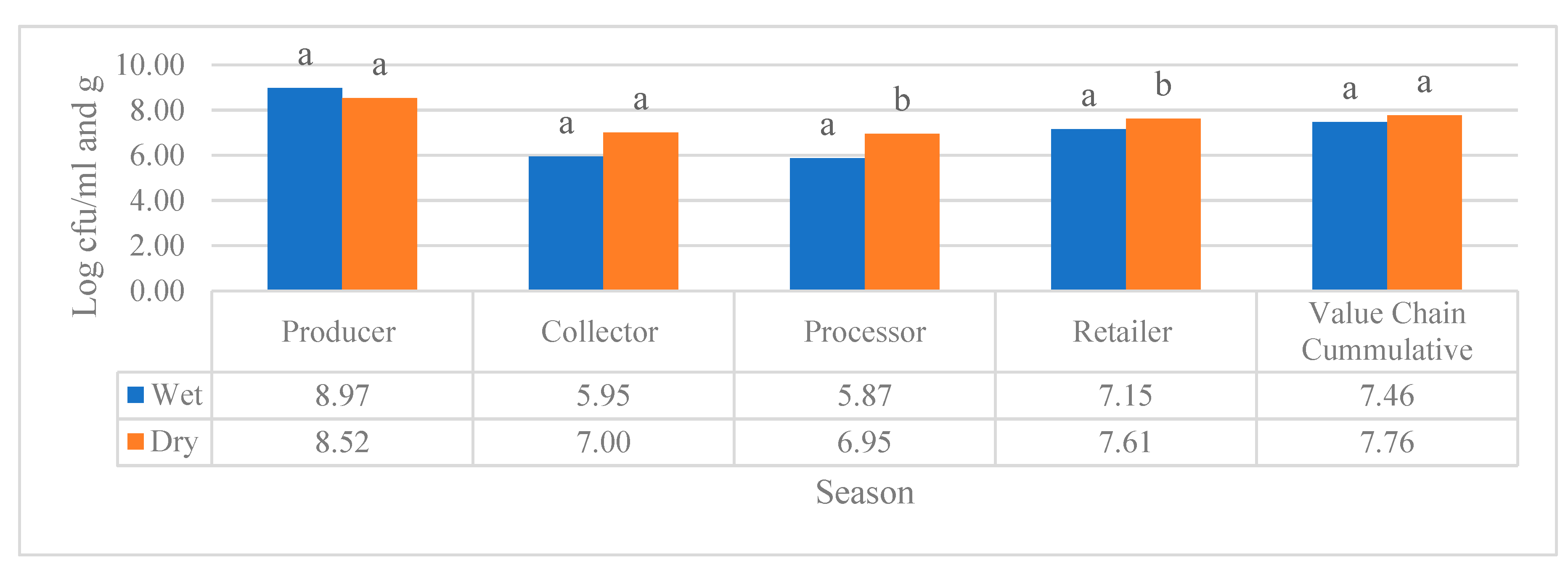
Producer (9.11±0.67 log cfu/ml) value chain of raw milk and retailer (9.39±1.7 log cfu/ml) value chain of pasteurized milk in Amhara region was found to have statistically significant higher APC during the wet season than dry season 8.2±0.67 log cfu/ml and 5.9±0.18 log cfu/ml, respectively. The total bacterial count from the sample collected from the collector (8.88±0.21 log cfu/ml) and processor (5.71±0.49 log cfu/ml) value chain had not have a significant variation in APC when compared to the dry season 8.72±0.83 log cfu/ml and 5.85±0.16 log cfu/ml, respectively. All of the dairy value chains in Amhara region for both milk sample types (raw and pasteurized) were found to have significantly higher TCC during wet season sample collection compared to dry season sample collection. High level of the TCC was observed from the sample that was collected from a collector (5.91±0.99 log cfu/ml) and producer (5.41±75 log cfu/ml) compared to the processor (3.97±1.04 log cfu/ml) and retailer 3.45±0.58 log cfu/ml) during wet season. The same is true for dry season higher TCC were found in those samples that were collected from producer (2.61±3.41 log cfu/ml) and collector (1.98±1.07 log cfu/ml) than that of retailer (0.40±1.27 log cfu/ml) and processor which was nil. The E. coli load in the diary value chains of Amhara region had no significant variation during wet and dry season sample collection. Total aerobic mesophilic bacteria count in the dairy value chain actors of the raw and pasteurized milk, located in the three study regions, revealed only the samples that were collected from processer and retailer value chains had significant variation in both value chains, the APC was higher during dry season. As per the remaining value chains of the three regions no significant difference was observed as shown in
Figure 4 in addition the cumulative APC in all value chains of raw and pasteurized milk showed no significant variation during wet and dry seasons.
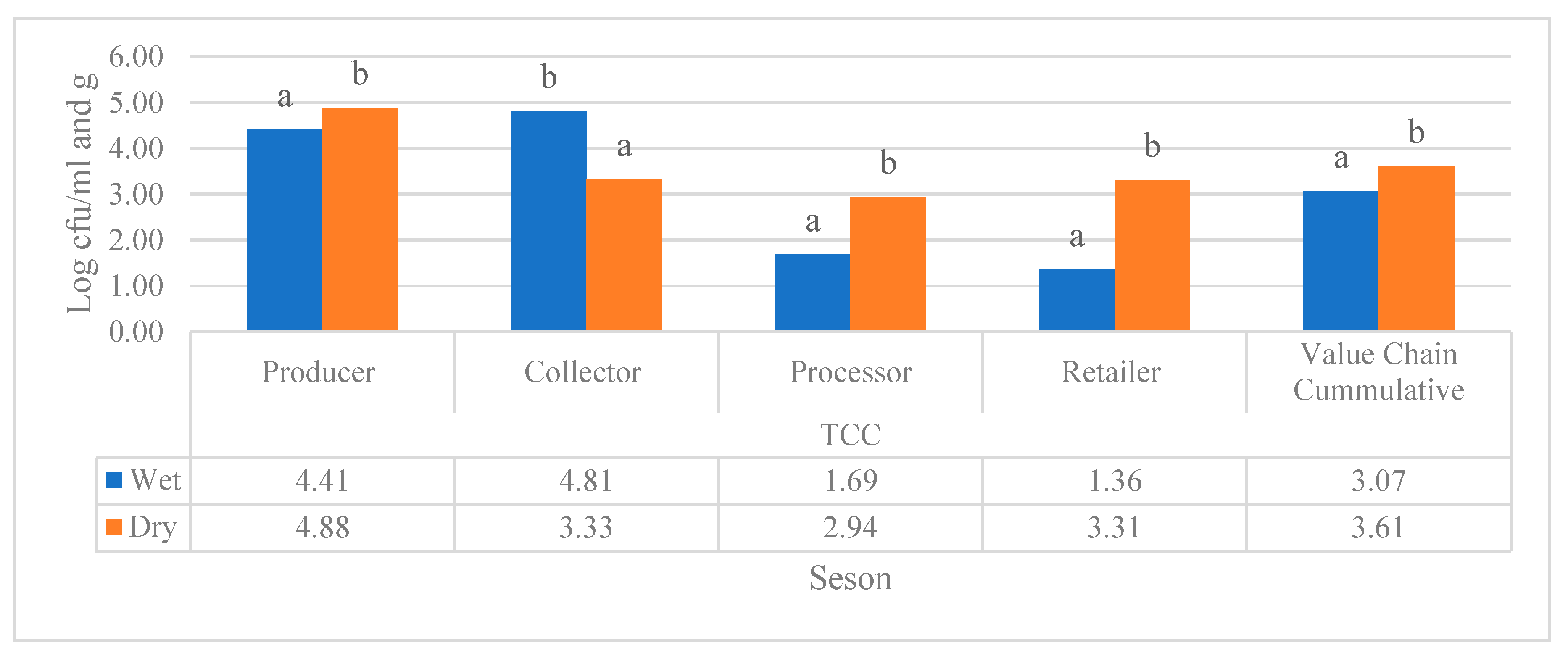
Figure 5 demonstrated that total coliform count varied significantly during dry and wet seasons in samples collected from all value chain actors of raw and pasteurized milk of the study regions. The samples that were collected in the collector value chain only shown to have significantly higher TCC in the wet season, while the remaining value chain and the cumulative TCC of the all-value chain were shown to have a statistically higher TCC in the dry season as it put forth in
Figure 5.
Seasonal comparison of E. coli count during wet and dry seasons in both milk types (raw and pasteurized) value chains revealed the samples that were gathered from the collector value chain of the raw milk and the processer value chain of pasteurized milk only found to have a significant difference in the E. coli load during wet and dry season from the study regions as shown in
Figure 6. Furthermore, the cumulative E. coli load of all the diary value chains of raw and pasteurized milk value chains was significantly higher during wet season, as indicated in
Figure 6.
The cottage cheese value chain as shown in Table (9), the seasonal variation of APC, collected during dry season from both producers (6.05±0.19 log cfu/g) and retailers (6.210.55 log cfu/g) in Oromia region, retailer (9.0±51 log10 cfu/g, (6.88±0.2 log cfu/g) in the SNNP and Amhara regions respectively, had significantly higher APC than wet season. The cottage cheese that was collected from producers and retailer in Oromia (2.24±2.59 log cfu/g, 4.33±3.48 log cfu/g) and Amhara (1.08±1.25 log cfu/g, 2.09±151 log cfu/g) regions was shown to have significantly higher TCC in dry season compared to wet season. Contrary to APC and TCC findings, no significant difference has been observed in E. coli level during dry and wet season sample collections as put forth in
Table 6.
The cumulative APC in cottage cheese collected from the value chains from the three-region found to have significant variation. In both value chains of the cottage cheese, APC statistically higher in dry season. Furthermore, APC was shown to be higher during dry season in the cumulative count of producer and retailer value chain of cottage cheese. The total coliform count is significantly higher during the dry season in both value chains of the cottage cheese and also in the cumulative count of TCC of both value chains. Unlike APC and TCC result, no significant variation has been observed in E. coli load of the cottage since none of the cottage cheese that has been gathered in both value chains was found to have E. coli.
4. Discussion
The cumulative APC in raw milk collected from the three regions during the wet season (8.9 log cfu /ml) higher than some previously reported study in Ethiopia. The reports from Debre Zeit by Solomon et al., (2013) showed that 7.07 log cfu/ml overall mean, in addition, Yirsaw et al., (2004) revealed that APC was found to be 8.13 log cfu/ml in the milk that was taken from the storage tank (27,28). The studies by Weleregay et al. (2012) and Habtamu et al. (2018) from Hawassa also reported 7.28 log cfu/ml and 6.83 log cfu/ml TBC load in raw milk, respectively (29,30). The TBC in raw milk that was gathered from Bahir Dar showed 7.61 and 8.12 log cfu/ml in individual farms and cooperatives, respectively, according to Tassew and Sefu (2012) (31). Yeserah et al. (2019) and Takliye and Gizaw (2017) also reported 7.1 log cfu/ml and 6.87 log cfu/ml APC in raw samples that were collected in Bahir Dar (32,33). A similar report from the Oromia region by Gemechu (2016) also revealed 8.2 log cfu/ml APC in raw milk samples (34). The higher APC value in the current study might be attributed to the variation in the sample size; variations in milking and hygienic practices followed in each region, the present study covered three regions of the country and collected a large number of samples.
The cumulative APC of pasteurized milk samples collected from three-study regions of 5.95% log cfu/ml for wet season was found to be lower compared to previously published works in different parts of Ethiopia by Mikru et al. (2021) (Hawassa) and Aberra., (2010) (Addis Ababa) who reported 6.14 log cfu/ml and 6.33 log cfu/ml (35,36). While, higher than a report of Tamirat.,(2018) who found 2.42 log cfu/ml APC in pasteurized milk samples (37). The higher APC load in pasteurized milk might be attributed to the absence of a cold chain, as the milk that was collected by the cooperative unions transported without cooling through long distances to delivered to processors, in addition, value chain effects since the processer received the milk from both the collector and directly from the farmers. As this study showed that the APC load in raw milk is much higher, the pasteurization method may not be effective since the raw milk has too much APC. The other possible reasons could be that there might be faulty pasteurization or poor pasteurization efficiency to kill the bacteria. During the risk factory survey, it was observed that most of the milk processing companies do not calibrate processing equipment and do not have certifications in good manufacturing practices.
The cumulative APC load in pasteurized milk in wet and dry seasons did not comply with the standard set by East African requirements (EAS 69:2006) and the Food and Drug Administration (FDA) the standards has declared the acceptable limit of TBC in pasteurized milk to be less than 3 × 104 cfu/ml (4.47 log cfu/ml) and 2 × 104 cfu/ml (4.3 log cfu/ml), respectively (38, 23). Cottage samples that were collected during wet season in the three study sites were found to have a 5.87 log cfu/g APC. Comparative reports have been reported by Ashenafi (2006) (5.38 log CFU/g) and Solomon and Ketema (2011) (6.13 log CFU/g) (39, 40). Zelalem et al. (2005) and Mamo et al. (2016) also reported 8.8 log cfu/g, and 6.5 log cfu/g APC in cottage cheese, respectively, from different areas in Ethiopia (41,42). The overall mean of APC in cottage cheese in both seasons was above the tolerable limit for cooked cheese (4.7 log CFU/g) within the markets as it was mentioned by Al-Khatib and Al-Mitwalli, (2009) (43).
Seasonal variation of APC in raw milk samples found to be significantly higher during wet season. While, some of the works from abroad reports APC in raw milk found to be higher during wet season. Botton et al., (2019) and Simioni et al., (2014) from Brazil reported the APC level was shown to be higher during the winter season compared to other seasons (44, 45). In another study by Scano and Caboni (2022) (Italy), APC in raw milk was higher during winter than spring and summer season (46). Margatho et al. (2018) from Portugal found APC count was higher in wet season: winter (2.65) and autumn (2.62) compared to summer (2.56) and spring (2.50) (47). Even though microbes’ multiplication in most cases increases in warm and humid environments conditions, in the rainy and cold seasons, cattle housed in intensive and semi-confinement systems arrive at the milking parlor with larger amounts of dirt on the udder (48). This eventually increases the risk of cross-contamination of milk while milking (49). A higher APC level during dry season in raw milk has been reported by Elmoslemany et al. (2010) (Canada) who found the APC level in summer was 0.33 and 0.21 times higher than in fall and winter (50). Hajmohamnadi et al. (2021) found the total bacteria count during summer (6.25 log cfu/ml) significantly higher than winter (6.10 log cfu/ml) in raw milk collected from different parts of Iran (51). In another similar study from Italy by Bertocchi et al. (2014), they revealed the APC load in raw milk collected during summer (9.99 log cfu/ml) and spring (9.87 log cfu/ml) was higher than in fall (9.84 log cfu/ml) and winter (9.83 log cfu/ml) (52). In warm and humid environments, the growth and number of environmental bacteria in cows bedding material increase owing to favorable temperature and humidity (51). The bacteria most probably enter into dairy products in dairy farms that utilize traditional practices in countries like Ethiopia (53).
The TCC in raw milk samples collected from the three regions during wet season (4.59 log cfu/ml) was found to be lower compared some work in Ethiopia by Habtamu et al. (2018), Gemechu (2016) and Amakelwu et al. (2015) who reported 6.94 log cfu/ml, 8.58 log cfu/ml and 5.47 log cfu/ml of TCC, respectively, but higher than Mirku et al. (2020) Tamirat, (2018) and Mesfine et al. (2015), who found 4.09 log cfu/ml, 3.59 log cfu/ml and 4.03 log cfu/ml, and 1.82 log cfu/ml TCC, respectively (30,34,55, 35,37,54). The wet season cumulative TCC load in pasteurized milk (1.53 log cfu/ml) was found to be much lower than most of the previous reports in Ethiopia. Mikru et al. (2021), Habtamu et al. (2018), Asaminew and Eyasu, (2011) and Aberra (2010) reported 6.20 log cfu/ml, 5.38 log cfu/ml, 6.05 log cfu/ml and 5.49 log cfu/ml, respectively (35,30,56,36). The reason for this decrement in TCC may be attributed to environmental factors since the above-mentioned study was done during the dry season. It may have contributed to the increment of total coliform load in pasteurized milk since, in developing countries cold chain transport and backup generators in pasteurized milk retailers aren’t common. Thus, such kind of risk factors may have facilitated the multiplication of coliforms (57).
The TCC in cottage cheese samples that were collected from the three regions (0.09 log cfu/gram) was found to be lower than the earlier reports by Mamo et al. (2016), Yilma (2012), Ashenafi (2006), Zelalem et al., (2005) 3.6 log cfu/g, 4.42 log cfu/g, 4.4 log cfu/g and 5.58 log cfu/g, respectively (42,58,41,39).The overall mean of TCC in cottage cheese of this study is below the standard sated by Kiiyukia (2003), which states the TCC of heat-treated food should not be above (log10 cfu/g) (60). Seasonality of total coliform count is not observed in raw milk; it’s only observed in pasteurized milk and cottage cheese samples that were collected from the three regions. Seasonality of TCC in raw milk by Elmoslemany et al. (2016) from Saudi Arabia, revealed that TCC levels was higher from November to January compared to other months of the year (50). A similar study from Egypt by Zeinhom et al. (2016) indicated TCC load significantly precedes in the months of summer (June-September) than in autumn (October-November) and winter (December-April) (61). Zucali et al. (2011) also demonstrated TCC significantly higher in hot season (2.41 log cfu/ml) than in the months that have mild (1.92 log cfu/ml) and cold (1.6 log cfu/ml) temperature (62). The coliform counts in raw milk samples that were collected during summer (47.8%) had higher levels of coliforms than during winter (43.7) as reported by Salmn and Hamad (2011) from Sudan (63). Contrary reports to the mentioned works have been released by Chanda et al. (2008). They found the coliform load was higher in the rainy months (June-August) 4.84 x105 than summer (4.11x105), autumn (3.85x105) and winter (2.75 x 105) (64). High rates of coliform load in dry season compared to the wet season due to the bacteria’s growth requirements. Since, coliforms are generally recognized due to their ability to ferment lactose to form gas and acid within 48 hours at 32-370C and, more specifically, thermophilic ones can grow and ferment lactose at 44-450C (65), so they prefer the warm and humid environmental conditions of the dry season. The higher TCC load during dry season in pasteurized milk might have arisen due to the absence of calibration of processing equipment and absence of food safety plans in most milk processing factories. The risk of biofilm production in processing equipment, especially in the process lines and joint parts, (66) since most of pasteurized milk processing factories in Ethiopia doesn’t calibrate the equipment according to our survey. In addition, the absence of cold chain transport might have also aggravated the problem since in the dry season, humid and warm temperatures prevail.
One of the most common methods of detecting facial contamination is the E. coli count in dairy products (64). The detection of E. coli during wet season from the sample collection of three study regions was found to be higher in raw milk, than that in pasteurized milk. A similar finding was reported by Habtamu et al. (2018) they discovered 26% of raw and 18.5% of pasteurized milk was tainted by E. coli (30). In another report by Weleregay et al. (2012), who found 25% of pasteurized milk was contaminated by E. coli, which is lower than Oromia (35.98%) and Amhara (35%) regions’ (29). Demme and Abegaz (2015) also found 18.6% of raw milk detected E. coli (67). Higher detection of E. coli has been reported by Mikrus et al. (2021), Megersa et al. (2019) and Aberra (2010), who reported 60% in raw milk, 42% in raw milk and 60% in pasteurized milk, respectively (35,68,36). The pattern of E. coli detection in the Amhara region showed the bacterium predominantly detected in pasteurized milk than raw milk. This finding witnessed that insufficient pasteurization, post-pasteurization problems, lack of cold chain during transportation, and lack of backup generators for pasteurized milk retail, worsened the problem of safety of dairy products. The detection of E. coli in pasteurized milk also reported from Iran by Vahedi et al. (2013) from 100 pasteurized milk samples, 9 (9%) were positive for E. coli (69).
Variability of E. Coli count due to seasons in the dairy product has been reported by Zeinhom et al., (2016) who found E.coli detection higher during summer (June-September) than autumn (October–November) and winter (December -April) (61). According to Zucali et al. (2011), E. coli contamination was higher in mild and hot temperature seasons than cold temperature seasons (62). Nevertheless, the present study founds E. coli load significantly higher during wet season than dry season (
Figure 6). In the rainy season, cows were significantly dirtier and soiled as compared to the dry season; this was probably due to the difficulty in keeping cow beddings and passageways dry and clean during wet seasons, and the consequent increasing amounts of manure on the hindquarters, flanks, and udders (62). Unhygienic condition could be the main risk factor for the introduction of E. coli into dairy products, which are produced at the farm level, most importantly raw milk, since E. coli is an indicator of fecal contamination as it is mostly found in the intestines of warm-blooded animals (65). Prolonged precipitation during wet season mostly associated with the dissemination of microbes from contaminated areas to water reservoirs like groundwater that have unmanaged well, which are characterized mostly by the absence of standard safety measures for restricting any foreign material, including dirt entering into them (10, 11). During the risk factor survey and milk sampling, it was observed that most milk producers, collectors, and some milk processors use groundwater for cleaning, milking, storing, and processing equipment. Hence, in rainy seasons, this groundwater and farm surfaces will receive microbes due to this dissemination and anthropogenic activities. This eventually will worsen the problem by increasing the microbial load, including pathogenic ones, on dairy products (70, 71, and 72).
APC of raw milk in producer (8.97 log cfu/ml) and collector (8.95 log cfu/ml) value chains of the wet season study showed a resemblance with Adugna et al., (2015) who found APC value 7.6±0.12 log cfu/ml and 7.56±0.13 log cfu/ml from dairy farms (producer) and cooperatives (collector), respectively, from Bahir Dar, Zuria, and Mecha (73). Contrary reports have been released by Berhe et al. (2020) from Tigray, which revealed the APC mean value of raw milk collected from non-producers (non-farmers) value chains like cafeterias and hospitals (7.42±0.272 log cfu/ml) was higher than from producers (farmers) 7.35±0.18 log cfu/ml (74). Welearegay et al. (2012) demonstrated APC of raw milk collected from distribution containers (collectors) (10.28 log cfu/ml) was higher than farms (5.93 log cfu/ml) (29). Similar work by Amakelew et al. (2015) showed raw milk that was collected from producers had lower APC (6.88 ± 0.3 log cfu/ml) than that of the collector (7.10 ± 0.79 log cfu/ml) (55). This finding points out that the higher APC load in the dairy value chain has no seasonal effect and is most likely a systemic problem.
The present study also demonstrates that the cumulative TCC value of raw milk in wet season that was gathered from the collectors (4.81 4.81 log cfu/ml) higher than producer (4.41 log cfu/ml) this finding supported by Amakelew (2015), who reported TCC in collector value chain had 5.63 ± 0.56 log cfu/m mean value than producer (5.57 ± 0.22 log cfu/ml) value chain (55). Similarly, Berhanu et al. (2020) reported TCC load was lower in distribution center (collector) 4.3±0.5 log cfu/ml than farm 4.4±0.5 log cfu/ml (59). In addition, Habtamu et al. (2018) reported the TCC of raw milk collected from dairy farms (6.63 ± 0.19 log cfu/ml) was found to be higher than raw milk collected from urban areas (7.07 ± 0.23 log cfu/ml). Habtamu et al. (2018) also reported that the TCC of pasteurized milk collected from the retailer shop was 5.87 ± 0.19 log cfu/ml, which is much higher than the cumulative TCC load in pasteurized milk that was collected from retailers from the three regions during the wet season (1.36 log cfu/ml) (30). The higher TCC load in the collector value chain than producer may be attributed to the value chain effect since the collector value chain received raw milk from different farmers with different hygienic practices. This will create more chains to recover the microbes in those samples that were collected in the collection center. In the current study, the TCC was significantly higher during dry season except for the collection centers. This may be because the coliforms belong to the enterobacteria family, which are recognized as mesophilic bacteria that prefer warm and humid conditions for growth and multiplication (65). In addition, during dry season, there is a shortage of water, more specifically in rural areas of Ethiopia, used to clean milk equipment and the farm environment (12, 13). The current result also demonstrated that the cumulative E. coli contamination in the dairy value chain of raw and pasteurized milk was significantly higher during wet season. The cottage cheese value chain predominantly reserves total aerobic mesophilic bacteria and total coliform during dry season. This is due to unhygienic practices during open field production and retail, most importantly, the open market, which is exposed to dust and insects. In addition, the environmental factors of humidity and warm temperature, are more conducive to the growth and multiplication of microbes.
5. Conclusions
This study aimed to determine the seasonal influence of microbial safety on raw milk, pasteurized milk, and cottage cheese along the dairy value chain in three regions of Ethiopia by using the most common microbial quality and hygienic indicator tests (total bacterial count, total coliforms, and E. coli count) on the collected samples. Among sample types, raw milk samples were shown to have a significantly higher total bacterial load during the wet season. This justified proper heat treatment before the consumption of raw milk. The pasteurized milk and cottage significantly contained total aerobic mesophilic bacteria, total coliform, and E. coli during dry season. It is mostly due to faulty pasteurization, improper handling, and the absence of a cold chain, in addition to the humid warm temperature of dry season that is conducive to the growth of most bacteria. This stresses the fact of maintaining standard safety procedures along the dairy value chain, most importantly during dry seasons. Significantly higher hygienic indicators load was recovered from the collector (APC and TCC) and processer (TBC) value chain in wet season. The risk factors like the source of water must be according to good hygiene like tap water (warm) with detergent in the collector value chain and the establishment and follow-up of good manufacturing practices in the processer value chain must be employed. The retailer value chain of pasteurized milk and the producer value chain of raw milk significantly harbor total coliform in the dry season. Proper storage temperature conditions must be followed while storing the milk most importantly in dry season where warm and humid are more common which creates a conducive growth condition for coliforms.
Author Contributions
HN: Conceptualization; Investigation; Formal analysis; Data Collection; Data Analysis; Writing-original draft, Writing-review & Editing; AT: Conceptualization, Data collection, Supervision; Data Analysis Writing-review & Editing; TS: Conceptualization; Methodology; Supervision; Writing-review & Editing; JK: Conceptualization; Methodology; Supervision; Fund Acquisition; Writing-review & Editing; JV: Conceptualization; Methodology; Supervision, Fund Acquisition; Writing-review & Editing; AZ: Conceptualization; Data Curation; Fund Acquisition; Supervision; Writing-review & editing.
Funding
This work was supported by the Bill & Melinda Gates Foundation and The Foreign, Commonwealth and Development Office of UK (Grant Number of INV-008459).
Data Availability Statement
All raw data used in the analyses presented here are available in the Supplementary Material.
Acknowledgments
The project is supported by the Bill and Melinda Gates Foundation and the Foreign, Commonwealth & Development Office of UK through the ENSURE project grant number of INV-008459 awarded to Addis Ababa University. We would like to extend our sincere appreciation to the respondents across the dairy value chain in rural and peri-urban areas of central Oromia, Southern Nations Nationalities and Peoples, and Amhara regions for their willingness to provide their milk and cottage cheese samples. The agricultural development agents (DAs) in each study sites also deserve our sincere gratitude for their facilitation of screening study participants and help during the administration of the questionnaires and sampling. We also thank Mrs. Zenebech Aytenew and Mrs. Alemitu Beyene who supported the laboratory analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hossain, M. B., & Dev, S. R. (2013). Physiochemical characteristics of various raw milk samples in a selected dairy plant of Bangladesh. International Journal of Engineering, 1(3), 2305-8269.
- Ceballos, L. S., Morales, E. R., Martínez, L. P., Extremera, F. G., & Sampelayo, M. R. S. (2009). Utilization of nitrogen and energy from diets containing protein and fat derived from either goat milk or cow milk. Journal of dairy research, 76(4), 497-504. [CrossRef]
- Negash, F., Tadesse, E., Aseffa, E., Yimamu, C., & Hundessa, F. (2012). Production, handling, processing, utilization and marketing of milk in the Mid Rift Valley of Ethiopia. Livestock Research for Rural Development, 24(9), 1-12.
- Smith, B. A., Meadows, S., Meyers, R., Parmley, E. J., & Fazil, A. (2019). Seasonality and zoonotic foodborne pathogens in Canada: relationships between climate and Campylobacter, E. coli and Salmonella in meat products. Epidemiology & Infection, 147.
- Carbas, B., Cardoso, L., & Coelho, A. C. (2013). Investigation on the knowledge associated with foodborne diseases in consumers of northeastern Portugal. Food Control, 30(1), 54-57. [CrossRef]
- Quigley, L., O’Sullivan, O., Stanton, C., Beresford, T. P., Ross, R. P., Fitzgerald, G. F., & Cotter, P. D. (2013). The complex microbiota of raw milk. FEMS microbiology reviews, 37(5), 664-698. [CrossRef]
- Keba, A., Rolon, M. L., Tamene, A., Dessie, K., Vipham, J., Kovac, J., & Zewdu, A. (2020). Review of the prevalence of foodborne pathogens n milk and dairy products in Ethiopia. nternational Dairy Journal, 104762.
- Perin, L. M., Pereira, J. G., Bersot, L. S., & Nero, L. A. (2019). The microbiology of raw milk. In Raw milk (pp. 45-64). Academic Press.
- Deddefo, A., Mamo, G., Asfaw, M., & Amenu, K. (2023). Factors affecting the microbiological quality and contamination of farm bulk milk by Staphylococcus aureus in dairy farms in Asella, Ethiopia. BMC microbiology, 23(1), 1-13. [CrossRef]
- Stephen, D. M., & Barnett, A. G. (2016). Effect of temperature and precipitation on salmonellosis cases in South-East Queensland, Australia: an observational study. BMJ open, 6(2), e010204. [CrossRef]
- Kapembo, M. L., Mukeba, F. B., Sivalingam, P., Mukoko, J. B., Bokolo, M. K., Mulaji, C. K., ... & Poté, J. W. (2022). Survey of water supply and assessment of groundwater quality in the suburban communes of Selembao and Kimbanseke, Kinshasa in Democratic Republic of the Congo. Sustainable Water Resources Management, 8, 1-13. [CrossRef]
- Komba, E. V., Komba, E. V., Mkupasi, E. M., Mbyuzi, A. O., Mshamu, S., Mzula, A., & Luwumba, D. (2012). Sanitary practices and occurrence of zoonotic conditions in cattle at slaughter in Morogoro Municipality, Tanzania: implications for public health. Tanzania Journal of Health Research, 14(2). [CrossRef]
- Ismaili, M. A., Saidi, B., Zahar, M., Hamama, A., & Ezzaier, R. (2019). Composition and microbial quality of raw camel milk produced in Morocco. Journal of the Saudi Society of Agricultural Sciences, 18(1), 17-21. [CrossRef]
- Walker, B. (2016). Seasonal Weather Assessment for Ethiopia during March–July 2016. London: Government of the United Kingdom.
- Nalepa, B., Olszewska, M. A., & Markiewicz, L. H. (2018). Seasonal variances in bacterial microbiota and volatile organic compounds in raw milk. International journal of food microbiology, 267, 70-76. [CrossRef]
- Suriyasathaporn, W., & Nakprasert, W. (2012). Seasonal patterns of aflatoxin M1 contamination in commercial pasteurised milk from different areas in Thailand. Food Additives and Contaminants: Part B, 5(2), 145-149.
- Zimba, T. R., Mwangwela, A. M., Mtimuni, B., & Chisala, P. W. (2019). Seasonality of food availability influences dietary patterns in two farming districts of Malawi. bioRxiv, 607606.
- Ali, S., Liu, Y., Ishaq, M., Shah, T., Ilyas, A., & Din, I. U. (2017). Climate change and its impact on the yield of major food crops: Evidence from Pakistan. Foods, 6(6), 39. [CrossRef]
- Koluman, A., Dikici, A., Kahraman, T., & İncili, G. K. (2017). Food safety and climate change: Seasonality and emerging food borne pathogens. Journal of Gastroenterology Research, 1(1), 24-29.
- Mercier-Bonin, M., & Chapot-Chartier, M. P. (2017). Surface proteins of Lactococcus lactis: bacterial resources for muco-adhesion in the gastrointestinal tract. Frontiers in microbiology, 8, 2247. [CrossRef]
- Burkart, K., Khan, M. H., Krämer, A., Breitner, S., Schneider, A., & Endlicher, W. R. (2011). Seasonal variations of all-cause and cause-specific mortality by age, gender, and socioeconomic condition in urban and rural areas of Bangladesh. International journal for equity in health, 10(1), 1-10. [CrossRef]
- Mengstu, B., Tola, A., Nahusenay, H., Sisay, T., Kovac, J., Vipham, J., & Zewdu, A. (2023). Evaluation of microbial hygiene indicators in raw milk, pasteurised milk and cottage cheese collected across the dairy value chain in Ethiopia. International Dairy Journal, 136, 105487. [CrossRef]
- Food and Drug Administration. (2001). Center for Food Safety and Applied Nutrition, Bacteriological Analytical Manual.
- Food Safety. (2017). 3M Petri film E. coli/Coliform Count Plate Interpretation Guide. 3M Food Safety United States.
- Ethiopian Standard Authority (ESA). (2021). Bacteriological standards for raw and pasteurized milk.
- Kenya Bureau of Standard (KEBS).(2018). Cottage Cheese Specification.
- Solomon, M., Mulisa, M., Yibeltal, M., Desalegn, G., & Simenew, K. (2013). Bacteriological quality of bovine raw milk at selected dairy farms in Debre Zeit town, Ethiopia. Journal of Food Sciences and Technology Research, 1(1), 1-8.
- Yirsaw, A. W. (2004). Bacteriological quality of bovine milk in small holder dairy farms in Debrezeit, Ethiopia. An MSC Thesis Submitted to the Faculty of Veterinary Medicine, Addis Ababa University in Partial Fulfillment of the Requirement of the Degree of Master of Science in Tropical Veterinary Medicine.
- Welearegay, H., Yilma, Z., & Tekle-Giorgis, Y. (2012). Hygienic practices and microbiological quality of raw milk produced under different farm size in Hawassa, southern Ethiopia. Agricultural Research and Review, 1(4), 1132-142.
- Habtamu, K., Ajebu, N., & Edessa, N. (2018). Microbiological quality and safety of milk production and marketing in Hawassa district, Ethiopia. African Journal of Microbiology Research, 12(25), 587-594. [CrossRef]
- Tassew, A., & Seifu, E. (2011). Microbial quality of raw cow’s milk collected from farmers and dairy cooperatives in Bahir Dar Zuria and Mecha district, Ethiopia. Agriculture and Biology Journal of North America, 2(1), 29-33. [CrossRef]
- Yeserah, B., Tassew, A., & Mazengia, H. (2019). Microbiological Quality of Raw Cow’s Milk In and Around Bahir Dar City, Ethiopia.
- Tekliye, M., & Gizaw, M. (2017). Handling practices, evaluation of adulteration and microbial quality of raw cow milk around Bahir Dar, Ethiopia. Food Science and Quality Management, 61, 1-9.
- Gemechu, A. T. (2016). Assessment of safety and quality of raw whole cow milk produced and marketed by smallholders in central highlands of Ethiopia. Assessment, 49.
- Mikru, A., Adane, M., & Dobo, B. (2021). Microbial Hazard Analysis in the Pasteurized Milk Production Value Chain at a Commercial Dairy Plant in.
- Abera M., Demie B., Aragaw K., Regassa F. and Regassa A. (2010): Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. Journal of Veterinary Medicine and Animal Health, 2: 29–34.
- Tamirat, T. (2018). Microbiological quality analysis of raw and pasteurized milk samples collected from Addis Ababa and its surrounding in Ethiopia. Approaches in Poultry, Dairy and Veterinary Sciences, 4(5), 374-381.
- East African Community. East African Standard; EAS 69:2006, ICS 67.100; specification for pasteurized milk. East African Community, Arusha, Tanzania; 2006.
- Ashenafi, M. (2006). A review on the microbiology of indigenous fermented foods and beverages of Ethiopia. Ethiopian Journal of Biological Sciences, 5(2), 189-245. [CrossRef]
- Solomon, T., & Ketema, T. (2011). Microbiological safety of street vended ayib in Jimma town, southwest Ethiopia. Ethiopian Journal of Education and Sciences, 7(1), 81-91.
- Zelalem, Y., & Bernard, F. J. E. J. o. A. P. (2005). Handling and microbial load of cow’s milk and ergo-fermented milk collected from different shops and producers in central highlands of Ethiopia. 6(2), 67-82.
- Mamo, J., Kumera, B., & Asmamaw, M. (2016). Evaluation of microbiological quality of raw milk, homemade Ergo and homemade Ayib in North Shoa District, Amhara, Ethiopia. Pakistan Journal of Food Science, 26(2), 83-9.
- Al Khatib, I. A., & Al Mitwalli, S. M. (2009). Microbiological quality and sample collection policy for dairy products in Ramallah and Al-Bireh district, Palestine. EMHJ-Eastern Mediterranean Health Journal, 15 (3), 709-716, 2009. [CrossRef]
- Botton, F. S., Alessio, D. R. M., Busanello, M., Schneider, C. L. C., Stroeher, F. H., & Haygert-Velho, I. M. P. (2019). Relationship of total bacterial and somatic cell counts with milk production and composition-multivariate analysis. Acta Scientiarum. Animal Sciences, 41.
- Simioni, F. J., Lopez, L. S., Nespolo, C. R., Stefani, L. M., Bordignon, R., & Bittelbrun, M. S. (2014). Season influence on milk physico-chemical and microbiological aspects in Western Santa Catarina. Semina: Ciências Agrárias, 35(4), 2033-2046. [CrossRef]
- Scano, P., & Caboni, P. (2022). Seasonal Variations of Milk Composition of Sarda and Saanen Dairy Goats. Dairy, 3(3), 528-540. [CrossRef]
- Margatho, G., Rodríguez-Estévez, V., Medeiros, L., & Simões, J. (2018). Seasonal variation of Serrana goat milk contents in mountain grazing system for cheese manufacture. Rev. Med. Vet, 169, 157-165.
- Lal, A., Hales, S., French, N., & Baker, M. G. (2012). Seasonality in human zoonotic enteric diseases: a systematic review. PLoS one, 7(4), e31883.
- Fernández, D., Rodriguez, E. M., Arroyo, G. H., Padola, N. L., & Parma, A. E. (2009). Seasonal variation of Shiga toxin-encoding genes (stx) and detection of E. coli O157 in dairy cattle from Argentina. Journal of Applied Microbiology, 106(4), 1260-1267.
- Elmoslemany, A. M., Keefe, G. P., Dohoo, I. R., Wichtel, J. J., Stryhn, H., & Dingwell, R. T. (2010). The association between bulk tank milk analysis for raw milk quality and on-farm management practices. Preventive veterinary medicine, 95(1-2), 32-40. [CrossRef]
- Hajmohammadi, M., Valizadeh, R., Ebdalabadi, M. N., Naserian, A., & OLIVEIRA, C. A. F. D. (2021). Seasonal variations in some quality parameters of milk produced in Khorasan Razavi Province, Iran. Food Science and Technology, 41, 718-722. [CrossRef]
- Bertocchi, L., Vitali, A., Lacetera, N., Nardone, A., Varisco, G., & Bernabucci, U. (2014). Seasonal variations in the composition of Holstein cow’s milk and temperature–humidity index relationship. Animal, 8(4), 667-674. [CrossRef]
- Tegegne, B., & Tesfaye, S. (2017). Bacteriological milk quality: possible hygienic factors and the role of Staphylococcus aureus in raw bovine milk in and around Gondar, Ethiopia. International Journal of Food Contamination, 4(1), 1-9. [CrossRef]
- Mesfine, S., Feyera, T., & Mohammed, O. (2015). Microbiological quality of raw cow’s milk from four dairy farms in Dire Dawa City, Eastern Ethiopia. World Journal of Dairy & Food Sciences, 10(1), 09-14.
- Amakelew, S., Eshetu, M., Animut, G., & Gebeyew, K. (2015). Microbial quality of cow milk in Dawa Chefa district, Amhara Region, Ethiopia. Advances in Dairy Research, 1-4.
- Asaminew, T., & Eyassu, S. (2011). Microbial quality of raw cow’s milk collected from farmer and dairy cooperatives in Bahir Dar Zuria and Mecha district.
- Ali, A., & Erenstein, O. (2017). Assessing farmer use of climate change adaptation practices and impacts on food security and poverty in Pakistan. Climate Risk Management, 16, 183-194. [CrossRef]
- Yilma, Z. (2012). Microbial properties of Ethiopian marketed milk and milk products and associated critical points of contamination: An epidemiological perspective. Epidemiology insights, 20, 297.
- Berhanu, L., Gume, B., Kassa, T., Dadi, L. S., Tegegne, D., Getnet, M., ... & Mereta, S. T. (2021). Microbial quality of raw cow milk and its predictors along the dairy value chain in Southwest Ethiopia. International Journal of Food Microbiology, 350, 109228. [CrossRef]
- Kiiyukia, C. (2003). Laboratory manual of food microbiology for Ethiopian health and nutrition research institute. UNIDO project (YA/ETH/03/436/11-52), 1-197.
- Zeinhom, M. M., Aziz, R. L. A., Mohammed, A. N., & Bernabucci, U. (2016). Impact of seasonal conditions on quality and pathogens content of milk in Friesian cows. Asian-Australasian Journal of Animal Sciences, 29(8), 1207. [CrossRef]
- Zucali, M., Bava, L., Tamburini, A., Brasca, M., Vanoni, L., & Sandrucci, A. (2011). Effects of season, milking routine and cow cleanliness on bacterial and somatic cell counts of bulk tank milk. Journal of Dairy Research, 78(4), 436-441. [CrossRef]
- Salman, A. M., & Hamad, I. M. (2011). Enumeration and identification of coliform bacteria from raw milk in Khartoum State, Sudan. Journal of cell and Animal Biology, 5(7), 121-128.
- Chanda, G. C., Uddin, G. M. N., Deb, A., Bilkis, T., Chowdhury, S., & Uddin, M. B. (2008). Microbiological profile of the traditionally collected industrial raw milk from the milk pocket zones of Bangladesh. Bangladesh Journal of Microbiology, 25(1), 17-20. [CrossRef]
- Martin, N. H., Trmčić, A., Hsieh, T. H., Boor, K. J., & Wiedmann, M. (2016). The evolving role of coliforms as indicators of unhygienic processing conditions in dairy foods. Frontiers in microbiology, 7, 1549.
- Weber, M., Liedtke, J., Plattes, S., & Lipski, A. (2019). Bacterial community composition of biofilms in milking machines of two dairy farms assessed by a combination of culture-dependent and–independent methods. PLOS One, 14(9), e0222238.
- Demme, B., & Abegaz, S. (2015). Isolation and identification of major bacterial pathogen from clinical mastitis cow raw milk in Addis Ababa, Ethiopia. Academic Journal of Animal Diseases, 4(1), 44-51.
- Megersa, R., Mathewos, M., & Fesseha, H. (2020). Isolation and identification of Escherichia coli from dairy cow raw milk in Bishoftu town, Central Ethiopia. Archives of Veterinary and Animal Sciences, 1.
- Vahedi, M., Nasrolahei, M., Sharif, M., & Mirabi, A. M. (2013). Bacteriological study of raw and unexpired pasteurized cow’s milk collected at the dairy farms and super markets in Sari city in 2011. Journal of preventive medicine and hygiene, 54(2), 120.
- Ahmed, W., Vieritz, A., Goonetilleke, A., & Gardner, T. (2010). Health risk from the use ofroof-harvested rainwater in Southeast Queensland, Australia, as potable or nonpotable water, determined using quantitative microbial risk assessment. Applied and Environmental Microbiology, 76(22), 7382-7391.
- Sidhu, J. P., Ahmed, W., Gernjak, W., Aryal, R., McCarthy, D., Palmer, A., ... & Toze, S. (2013). Sewage pollution in urban stormwater runoff as evident from the widespread presence of multiple microbial and chemical source tracking markers. Science of the Total Environment, 463, 488-496.
- Mazhar, I., Hamid, A., & Afzal, S. (2019). Groundwater quality assessment and human health risks in Gujranwala District, Pakistan. Environmental Earth Sciences, 78, 1-12. [CrossRef]
- Adugna, M., Asresie, A., & Adigrat, E. (2015). A review on microbiological quality of Ethiopian raw bovine milk. Food Sci Qual Manage, 35, 17-34.
- Berhe, G., Wasihun, A. G., Kassaye, E., & Gebreselasie, K. (2020). Milk-borne bacterial health hazards in milk produced for commercial purpose in Tigray, northern Ethiopia. BMC Public Health, 20, 1-8. [CrossRef]
Figure 1.
Seasonal comparison of APC (log cfu/ml and g) in milk and cottage cheese samples collected from the three study sites. Different superscripts letters above of the bar graph are significantly different at (P<0.05).
Figure 1.
Seasonal comparison of APC (log cfu/ml and g) in milk and cottage cheese samples collected from the three study sites. Different superscripts letters above of the bar graph are significantly different at (P<0.05).
Figure 2.
Seasonal comparison of TCC of milk (log cfu/ml) and cottage cheese log cfu/g) samples collected from the three regions. Different superscripts letters above of the bar graph are significantly different at (P<0.05).
Figure 2.
Seasonal comparison of TCC of milk (log cfu/ml) and cottage cheese log cfu/g) samples collected from the three regions. Different superscripts letters above of the bar graph are significantly different at (P<0.05).
Figure 3.
Seasonal comparison of E. coli (log cfu/ml and log cfu/g) of milk and cottage cheese samples, respectively in three regions. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 3.
Seasonal comparison of E. coli (log cfu/ml and log cfu/g) of milk and cottage cheese samples, respectively in three regions. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 4.
Cumulative APC (log cfu/ml) raw and pasteurized milk value chains located in all study sites. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 4.
Cumulative APC (log cfu/ml) raw and pasteurized milk value chains located in all study sites. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 5.
Cumulative TCC (log10 cfu/ml) raw and pasteurized milk value chains located in all study sites. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 5.
Cumulative TCC (log10 cfu/ml) raw and pasteurized milk value chains located in all study sites. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 6.
Mean comparison of E. coli (log cfu/ml) raw and pasteurized milk value chains located in all study regions. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Figure 6.
Mean comparison of E. coli (log cfu/ml) raw and pasteurized milk value chains located in all study regions. Different superscripts letters above the bar graph are significantly different at (P<0.05).
Table 1.
Total aerobic mesophilic bacteria count (APC) was established by the Ethiopian Standard Authority (ESA) for milk and the Kenya Bureau of Standard (KEBS) for cottage cheese during wet and dry seasons.
Table 1.
Total aerobic mesophilic bacteria count (APC) was established by the Ethiopian Standard Authority (ESA) for milk and the Kenya Bureau of Standard (KEBS) for cottage cheese during wet and dry seasons.
| Region |
Quality |
Raw milk |
Pasteurized milk |
Cottage Cheese |
| Pass |
Fail |
Pass |
Fail |
Pass |
Fail |
| Log cfu/ml and g |
0–6
n (%) |
>6
n (%) |
0-4
n (%) |
>4
n (%) |
0-4.3
n (%) |
>4.3
n (%) |
| Oromia |
TBC Wet |
0(%) |
48(100%) |
0 (0%) |
48(100%) |
0(100%) |
24(100%) |
| TBC Dry |
7(14.58%) |
41(85.42%) |
0 (0%) |
48(100%) |
0(100%) |
24(100%) |
| SNNP |
TBC Wet |
0(0%) |
24(100%) |
0 (0%) |
24(100%) |
0(100%) |
12(100%) |
| TBC Dry |
0(0%) |
24(100%) |
0 (0%) |
24(100%) |
0(100%) |
12(100%) |
| Amhara |
TBC Wet |
0(0%) |
20(100%) |
0 (0%) |
20(100%) |
0(100%) |
8(100%) |
| TBC Dry |
0(0%) |
20(100%) |
0 (0%) |
20(100%) |
0(100%) |
8(100%) |
Table 2.
Mean comparison of total aerobic mesophilic bacteria count log cfu/ml in raw, pasteurized milk and Cottage cheese (log cfu/g) samples collected from the three regions during dry and wet seasons.
Table 2.
Mean comparison of total aerobic mesophilic bacteria count log cfu/ml in raw, pasteurized milk and Cottage cheese (log cfu/g) samples collected from the three regions during dry and wet seasons.
| Study Sites |
Season |
APC |
| Raw milk |
Pasteurized milk |
Cottage Cheese |
| Oromia |
Wet |
8.58±0.37bB |
5.48±0.48aA |
5.17±0.33a |
| Dry |
7.41±1.08aB |
6.17±0.63bA |
6.19±0.34b |
| SNNP |
Wet |
9.67±0.52aB |
6.97±0.83aA |
7.34±0.32a |
| Dry |
10.80±0.67bB |
9.37±1.65bA |
8.73 ±1.35b |
| Amhara |
Wet |
8.99±0.19bB |
5.88±0.17aA |
5.69 ±0.28a |
| Dry |
8.46±0.78aB |
6.16±0.73aA |
6.58±0.48b |
Table 3.
Total coliforms count (TCC) established by the Ethiopian Standard Authority (ESA) for milk and cottage cheese during wet and dry seasons.
Table 3.
Total coliforms count (TCC) established by the Ethiopian Standard Authority (ESA) for milk and cottage cheese during wet and dry seasons.
| Regions |
Quality |
Raw milk |
Pasteurized milk |
Cottage Cheese |
| Pass |
Fail |
Pass |
Fail |
Pass |
Fail |
| Log cfu/ ml and g |
0 –4
n (%) |
>4
n (%) |
0-1
n (%) |
>1
n (%) |
0-2
n (%) |
>2
n (%) |
| Oromia |
TCC Wet |
16(33.3%) |
32(66.7%) |
47(97.92%) |
1(2.08%) |
24(100%) |
0(0%) |
| TCC Dry |
11(22.92%) |
37(77.08%) |
4 (8.3%) |
44(91.7%) |
10(41.6%) |
14(58.33%) |
| SNNP |
TCC Wet |
13(54.17%) |
11(45.83%) |
5(20.83%) |
19(79.16%) |
11(91.67%) |
1(8.33%) |
| TCC Dry |
0(0%) |
24(100%) |
2 (8.3%) |
22(91.7%) |
12(100%) |
0(0%) |
| Amhara |
TCC Wet |
1(5%) |
19(95%) |
0 (0%) |
20(100%) |
8(100%) |
0(0%) |
| TCC Dry |
20(100%) |
0(0%) |
7(35%) |
13(65%) |
8(100%) |
0(0%) |
Table 4.
Mean comparison of total bacterial count in log cfu/ml in raw, pasteurized milk and Cottage cheese (log cfu/g) samples collected from the three regions during dry and wet seasons.
Table 4.
Mean comparison of total bacterial count in log cfu/ml in raw, pasteurized milk and Cottage cheese (log cfu/g) samples collected from the three regions during dry and wet seasons.
| Study Sites |
Season |
TCC |
| Raw milk |
Pasteurized milk |
Cottage Chasse |
| Oromia |
Wet |
4.20±2.41aB
|
0.04±0.1aA
|
3.29±3.18b
|
| Dry |
5.98±2.04bB
|
4.50±2.19bA
|
NDa
|
| SNNP |
Wet |
4.47±1.12aB
|
2.67±1.71aA
|
0.34±0.81a
|
| Dry |
3.55±2.43aA
|
2.80±2.21aA
|
NDa
|
| Amhara |
Wet |
5.67±0.90bB
|
3.71±0.86bA
|
NDa
|
| Dry |
2.28±2.48aB
|
0.2±0.9aA
|
1.59±1.39b
|
Table 5.
Seasonal comparison of Escherichia coli (log cfu/ml) count in milk and cottage cheese samples collected from three regions.
Table 5.
Seasonal comparison of Escherichia coli (log cfu/ml) count in milk and cottage cheese samples collected from three regions.
| Study Sites |
Season |
E. Coli |
| Raw milk |
Pasteurized milk |
Cottage Chasse |
| Oromia |
Wet |
1.00±1.23bA
|
0.9±1.23bA
|
NS |
| Dry |
0.21±0.78aA
|
0.12±0.47aA
|
NS |
| SNNP |
Wet |
1.48±1.48aB
|
0.12±0.63bA
|
0.34±0.81 |
| Dry |
0.76±1.13aB
|
0.13±0.63aA
|
NS |
| Amhara |
Wet |
0.25±0.65aA
|
1.77±1.44aB
|
NS |
| Dry |
0.7±1.28aA
|
1.07±1.14aA
|
NS |
Table 6.
Mean comparison of total bacteria, total coliforms, and E. coli in cottage cheese samples.
Table 6.
Mean comparison of total bacteria, total coliforms, and E. coli in cottage cheese samples.
| Indicator |
Season |
Cottage Cheese |
| Oromia |
(SNNP) |
(Amhara) |
| Producer |
Retail |
Producer |
Retail |
Producer |
Retail |
| APC |
Wet |
5.4±0.26a
|
4.9±0.2a
|
7.60±0.16a
|
7.20±32a
|
5.8±28a
|
5.59±0.26a
|
| Dry |
6.05±0.19b
|
6.21±0.55b
|
8.49±20a
|
9.0±51b
|
6.28±0.52a
|
6.88±0.2b
|
| TCC |
Wet |
ND |
ND |
0.69±1.10a
|
ND |
ND |
ND |
| Dry |
2.24±2.59b
|
4.33±3.48b
|
ND |
ND |
1.08±1.25b
|
2.09±151b
|
| E. coli |
Wet |
ND |
ND |
ND |
ND |
ND |
ND |
| Dry |
ND |
ND |
ND |
ND |
ND |
ND |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).