Introduction
Cultured marine fish species represent a considerable economical source in the Mediterranean area (Lanza et al. 2017; Massa et al., 2017; FAO 2020). However, infectious diseases are the main impediment to the development of aquaculture and are often the most significant cause of economic losses (Lafferty et al. 2014; Behringer et al. 2020).
The European seabass Dicentrarchus labrax (Linnaeus, 1758) is the most important cultured marine fish species raised in Corsica, which is the first French region to produce adult fish from mariculture. The production cycle of D. labrax is well known and differs from the other countries. All stages of the fattening cycle are done in marine cages and fishes receive no antibiotic treatment during their lifetime (Mare & Stagni Corsi 2011). Corsican fish farmers use marine cage farming and have chosen to adhere to a sustainable aquaculture policy since 2006 (low densities, no antibiotic treatments, longer breeding time). Marine cage culture has several advantages compared to intensive farming because fish evolve in an environment close to the natural environment. However, disease outbreaks caused by pathogens, including monogenean flatworms, can occur extremely rapidly, resulting in a reduction of fish health or significant mortalities over a very short time. Massive infections have already been observed in fish farms from the Mediterranean basin and heavy mortalities were also reported in Corsican fish farms (Lia et al. 2007; Merella et al. 2009; Ternengo et al. 2010). These phenomena also pose a threat to wild fish stocks. Marine cages allow free movement of pathogens between wild and cultured fish and parasitic transfers can occur. Sea cages hold fishes for months in the same location and parasite populations may grow exponentially and release pathogens back into the environment shared with wild fish (Bergh 2007; Costello 2009).
Earlier studies have shown that D. labrax can be a long-term host of the genus Diplectanum, especially Diplectanum aequans (Wagener, 1857) Diesing 1858 (Monogenea, Diplectanidae). It was identified on the gills of cultured fish from 48 Mediterranean countries (Bragoni et al. 1984; Dezfuli et al. 2007; Antonelli 2010; Antonelli and Marchand 2012: Muniesa et al. 2020). Diplectanum aequans are known to have negative effects on their hosts (Cecchini et al. 1998; Massimo et al. 2022), and can be responsible for extensive mucus hypersecretion and swelling, especially on the apical portions of the secondary gill lamellae. This effect is revealed by hyperplasia and haemorrhage of the gill tissue at the site of helminth attachment (Bragoni et al. 1984; Merella et al. 2006). In addition, chronic infections make fish more vulnerable to environmental conditions and more susceptible to secondary pathogens. Some individuals, weakened by parasitic infection, may provide a breeding ground for secondary bacterial infections or viruses. Monogeneans can also be responsible for a mechanical blockage of water flow and gas exchange due to the entangled mass of flatworms causing severe respiratory disturbances leading to fish death (Ternengo et la. 2010). In our previous studies, we highlighted seasonal variations in D. aequans distribution and host-age-dependent occurrences of parasites. Diplectanum aequans had the highest biological parasitic indices in winter and fall, and the oldest fish appeared to be more parasitized (Antonelli 2010; Antonelli et al. 2012).
This work is important for sustainable aquaculture and the objectives are to exchange knowledge and pool research efforts with Corsica fish farmers, contributing to the development of a blue economy, respectful of ecosystems and their biodiversities. Specifically, the objectives are: (i) to establish a comparison between two parasitological surveys with a ten-year interval to understand the variation of D. aequans populations, (ii) to analyse the organisation of monogenean populations, (iii) to study gill repartition of monogeneans and determine gill micro-habitat according to preferential gradients and (iiii) to purpose a predictive tool that would reduce sampling effort and allow optimal exploitation for fish farmers.
Materials and Methods
Fish Sampling and Parasite Examination
Two separate parasitological surveys were conducted in 2007–2008 and 2019–2020 on three Corsican fish farms. Among the fish intended for sale and consumption, we recovered a total of 430 common sea bass
D. labrax for analysis. We sampled ten specimens per season from farm 1 and farm 2, and five to ten specimens from farm 3. This sample was valid for both study periods. The names of the fish farms are not included for confidentiality; each farm is designated by a number (
Figure 1). The fish were collected from the marine cages of the fish farms and immediately killed by fish farmers before utilization. Approval from the Ethics Committee of the Research Ministry was not necessary because the fish collected were fishery products for sale and they were not specifically killed for the study. The fish were placed individually in plastic bags after fishing and maintained on ice until dissection.
Before dissection, biometrical measures (weight, length), sex and maturity stage of each specimen were recorded. Body surface (skin, fins) and gill cavities were observed.
Gill arches were carefully removed and studied in fresh conditions. Arches were separated (four right, four left), immersed in Petri dishes containing 37‰ NaCl solution and individually examined. Once the general observation was complete, the gill filaments were “combed” using fine pliers to remove mucus. This mucus was then distributed in the petri dish to facilitate the search for parasites. To have consistent and significant results we collected our samples from the same cages at each farm throughout the study. This allowed us to have regular monitoring of the same cage during the seasons. Parasites were examined alive, fixed in 75% ethanol solution, labelled and stored in the laboratory collection. Parasite determination was made using a light microscope according to morphological descriptions done in previous studies (Oliver 1976; Antonelli 2010).
Measurements of water temperature were collected daily from fish farmers (public data). A seasonal mean temperature was calculated for each farm (
Table 1).
Statistical Analyses
Parasite distribution was studied by season for all sampling years. The levels of parasite infection expressed as prevalence (proportion of infected hosts), abundance (mean number of parasites of infected fish) and mean intensity (mean number of parasites of infected hosts) were calculated and applied according to Bush et al. (1997). Comparison of prevalence values according to seasons were performed with a chi-squared test (χ2) using R software. A non-parametric Mann–Whitney U test (two groups) and Kruskal–Wallis test (more than two groups) were used to test statistical significance differences in mean abundance and mean intensity of parasites according seasons to determine the influence of host weight (and length) on D. aequans distribution and to compare parasite distribution on gill arches (Dagnelie 1975; Sprent 1992). Four weight classes (g) were determined according to dispersion measures: WFS1 (100–250), WFS2 (260–450), WFS3 (460–650), WFS4 (> 650). They include fish from the lightest (WFS1) to the heaviest (WFS4). The four quartiles are constituted by 25% (107 or 108 specimens) of the total number of fish (430 specimens), and parasitic indices were compared.
The level of significance for all statistical tests was set at p<0.05. Calculations were performed using Systat 12 and Xlstat 2021.2.2.
Linear models and convergence equations were used to describe differences in parasite loading between gill arches using Python 3.5.2 (numpy 1.18.1, pandas 0.24.2).
Results
During the study, we collected a total of 8,609 monogeneans. Diplectanum aequans was the only monogenean species identified on gill arches of cultured sea bass used for this work. Parasites occurred in all farms during all the years studied.
Distributions of D. aequans in Host Populations
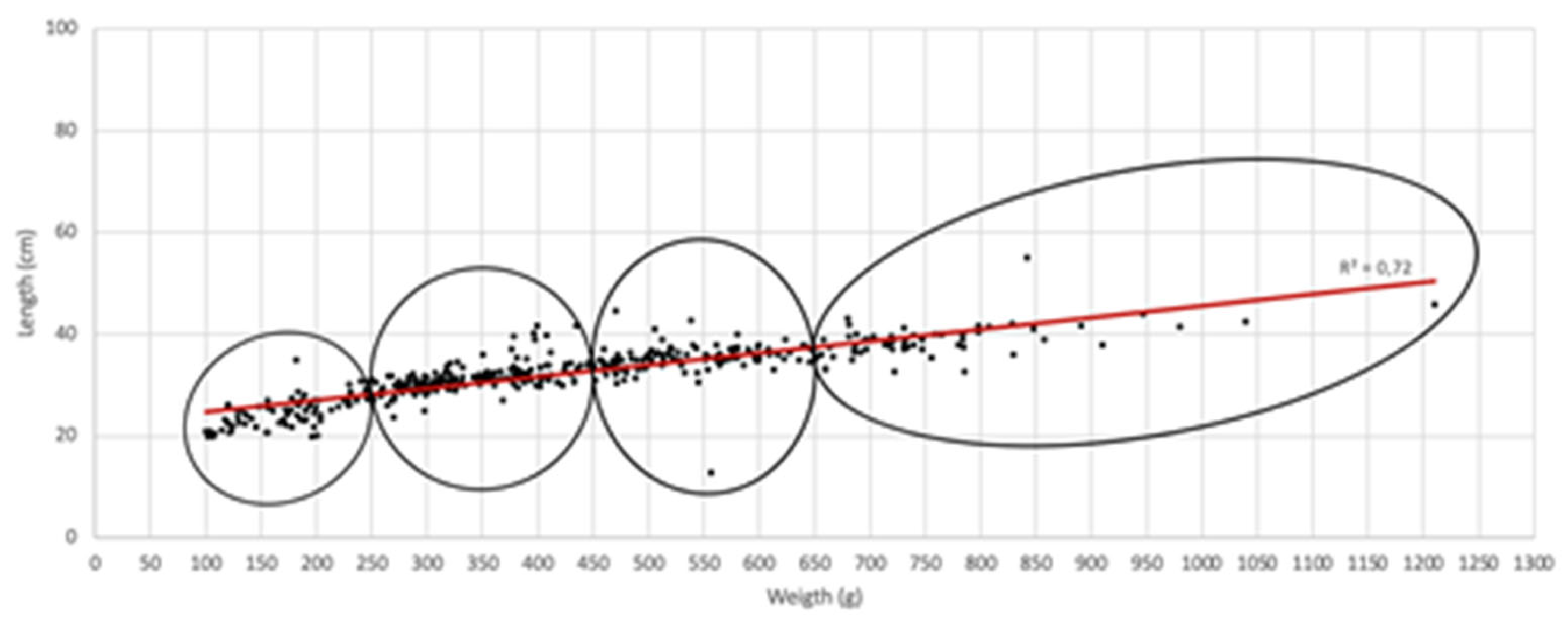
First, we highlight the link between the weights of fish and the parasite load. A linear curve model allowed us to distinguish four quartiles based on fish length and weight (
Figure 2).
Prevalence values of parasitized fish are more important in WFS2 and WFS3 (
Table 2). However, abundance and mean intensity values revealed an increase from the group constituted by the lightest fish (WFS1) to the group constituted by the heaviest fish (WFS4) despite a large standard deviation (
Table 2).
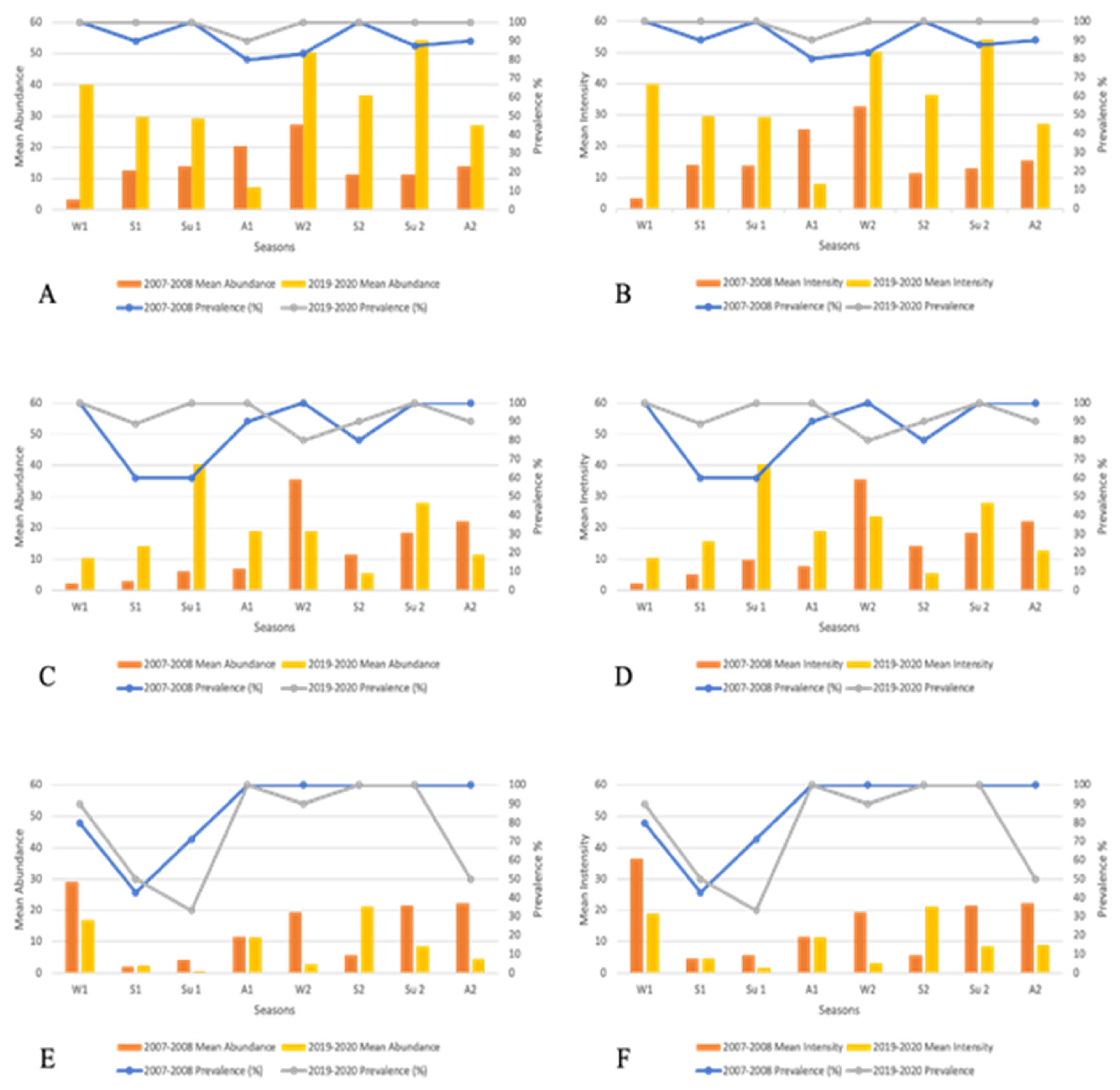
A comparison of the level of parasitic infection was made between the 2007–2008 and 2019–2020 periods for each farm. The following results show greater numbers of parasites during the second period (2019–2020) except for farm 3.
Farm 1 (
Figure 3A,B). Values of prevalence of infected fish varied from 80 to 100% during all seasons of survey periods. These values were significantly higher during the 2019–2020 period with the increase being statistically significant (χ
2, p<0.05) (
Table 3). During the first period (2007–2008), the highest mean abundance and intensity values were recorded during late autumn and winter months, with a peak infection occurring every other winter. In contrast, the second period highlights the highest rates of infection in winter and summer. The Mann-Whitney
U test showed significant differences between the two survey periods (p<0.05) (
Table 3).
Farm 2 (
Figure 3C,D). Cumulative prevalence values over the two survey periods ranged from 60 to 100 and followed the same trend with no statistical difference (χ
2, p>0.05) (
Table 3). The highest mean abundance and intensity values were recorded during the winter months in 2007–2008, while results of the second period revealed the highest rates of infection during the summer, but these differences were insignificant (Mann-Whitney
U test, p>0.05) (
Table 3). The values observed in summer 2019, with a higher peak, are higher than in summer 2020, and show variability in intensity from one year to another.
Farm 3 (
Figure 3E,F). Prevalence values ranged from 33 to 100%, with curves that followed almost the same trend except for spring 2019–2020. Significant differences between prevalence values of the two periods were revealed (χ
2, p<0.005) (
Table 3). The highest mean abundance and intensity values were observed in autumn and winter during the first period (2007–2008) where the lowest were recorded in spring. The second period follows the same trend with a parasitic peak shifted from winter to spring in the second year. No statistical differences were observed between the two periods of the survey (Mann-Whitney
U test, p>0.05).
Parasite Distribution on Gills
Differences between gill arches were tested on the data from the three farms during the two periods. The differences were not found to be significant between the number of parasites on the left and right gill arches from same fish.
Diplectanum aequans showed an equivalent distribution with no statistically significant difference in infection (Mann-Whitney
U test, p=0.68) (
Table 4).
However, finer analysis at the level of each gill arch revealed that the distribution of the number of parasites varied according to the total number of parasites and host weight (
Table 5). When parasites are numerous (on the biggest fish, fish groups 3 and 4), the individuals were distributed on the gill arches according to an anterior-posterior gradient. Gill arches 1 (GA1) and 2 (GA2) had the highest parasite abundances and the population spread on a decreasing gradient. The number of parasites decreased from GA1 to GA4. In contrast, the parasites appeared to be randomly distributed on the four arches on smaller fish with low rates of infection (fish groups 1 and 2). These differences in distribution were statistically significant (Mann-Whitney U test, p<0.05).
Our observations also revealed a centrifugal migration of individuals in the gill arch as they mature. The post-larvae and the first stages development of parasites were preferentially localized at the base of the gill filaments and were isolated, while adults were mainly localized at the ends of the filaments and aggregated in groups of four to five individuals. Our data allow us to observe that regardless of the groups and treatments performed, the numbers of parasites present on the left and right gill arches was equivalent. These results, while clear at the scale of a sample set, can be highly variable at the individual level.
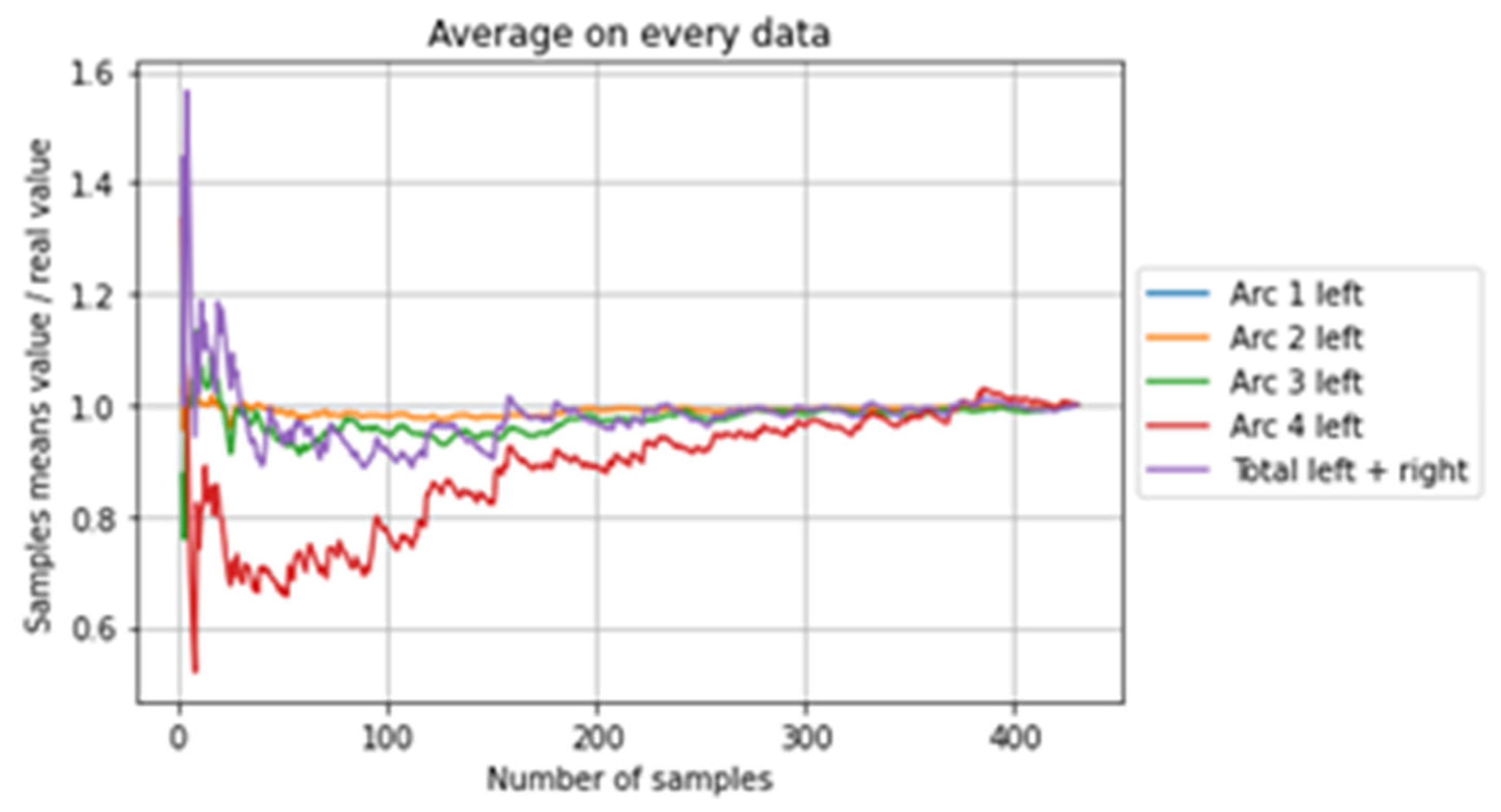
Figure 4 shows the change in the ratio of the mean numbers of parasites in the sub-sample to the mean of all the samples based on the number of samples selected for all farms and years (totals and on the left arcs only for legibility). A value of 1 therefore corresponds to equivalent results between total population and sub-sample. For about 300 samples out of 430 all the results were very close to the results obtained from all the samples.
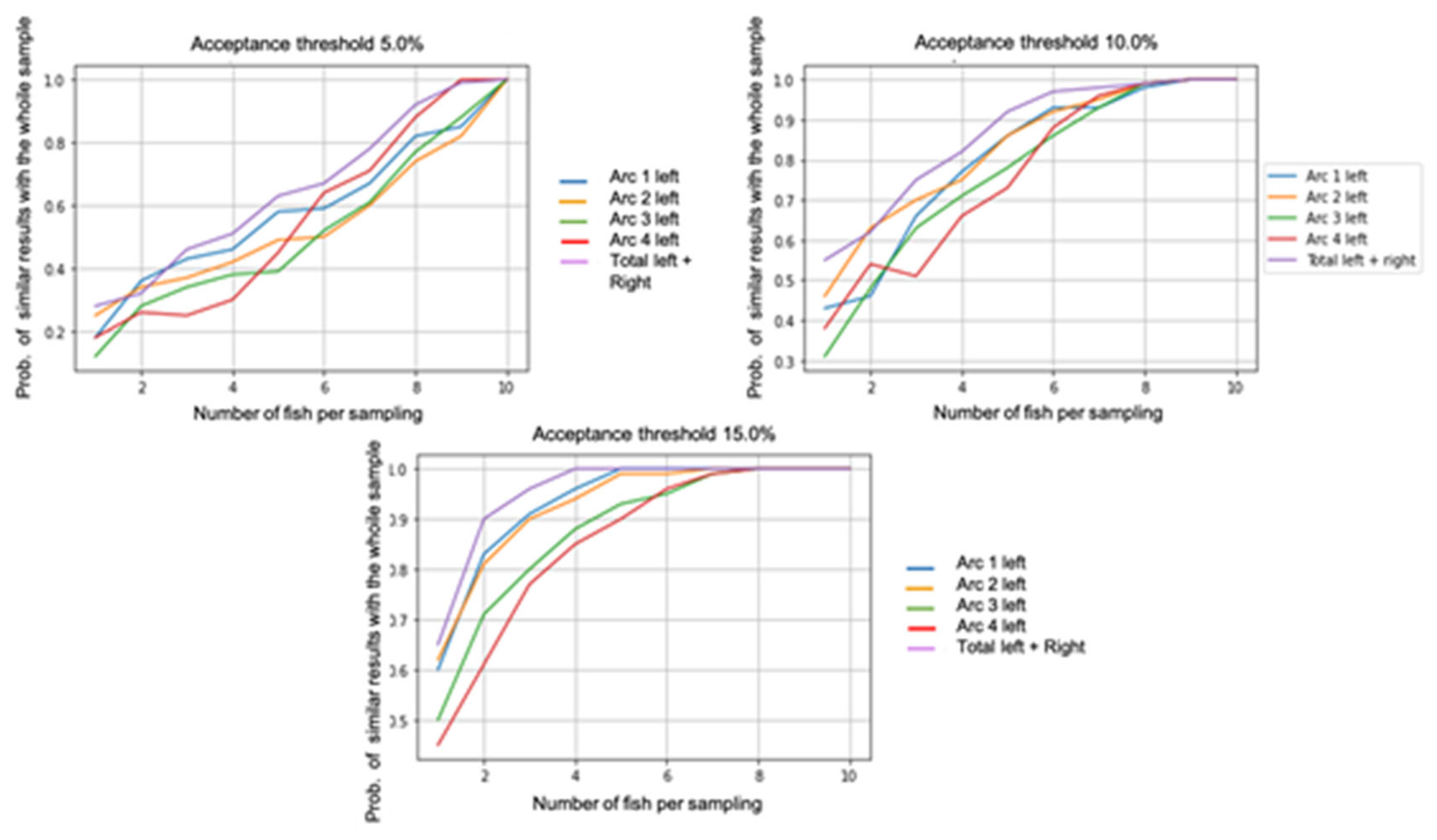
Figure 5 shows the results obtained with different threshold values set at 5% (
Figure 5a), 10% (
Figure 5b) and 15% (
Figure 5c). With a sample of nine fish per day, it is safe to obtain the same value as the number of total parasites to within 5% (i.e., for an X value, there is a 100% chance of getting a value between [0.95 X, 1.05 X]). However, the values observed on arcs 1, 2 and 3 were equivalent to about 5% between 80% and 85% of the time.
Discussion
Distribution of D. aequans in Host the Population
Our results showed that larger fish were parasitized more. This positive correlation between fish length (and therefore age) and parasitic abundance has already been demonstrated in various ectoparasite studies (Poulin and Rodhe 1997; Cable and Van Oosterhout 2007). In addition, large mortalities in older cultured seabass caused by pathogens have been observed in the Mediterranean basin (Alvarez-Pellitero and Sitjà-Bobadilla 1993). However, there appears to be no minimum weight for D. labrax to develop a parasitic infection. Experiments conducted by Silan and Maillard (1989) demonstrated that 15-day alevins could be parasitized by D. aequans larvae.
Similar to other organisms, aquatic parasites have specific temperature optima to complete their life cycles and various patterns of parasitic dynamics in response to seasonal factors have been reported (Marcogliese 2001; Winger et al 2001; Antonelli et al. 2010a; Antonelli et al. 2010b; Antonelli et al 2016). In the 2007–2008 survey, trends showed that D. aequans had the highest mean intensity values during late autumn and winter for all farms studied, corresponding with a decrease in water temperature. Minimum values were recorded during spring months. Similar results with parasitic infection occurring during the winter season were also revealed in Spain (Gonzalez-Lanza et al. 1991). However, results for 2019–2020 showed that mean intensity values of D. aequans do not follow the same trend, with maximum values recorded during spring and summer months. These fluctuations in rates of infections showed that water temperature, which generates a definite seasonal cycle, has a great influence on the population dynamics of parasites. Previous studies highlighted the influence of temperature on incubation time. Diplectanum aequans eggs subjected to different temperatures (between 15 and 23 °C) showed different hatching times. The time is on the order of 7–15 days at 15–16 °C versus 5–7 days at 20–21 °C. Four to five days are sufficient beyond 21 °C (Silan and Maillard 1989; Mladineo 2004). These results have been confirmed by Cecchini et al. (1998), which mentioned that all developmental stages of D. aequans are temperature-dependent. As the temperature increases, the time to reach sexual maturity becomes shorter. In our study, the range extension and high prevalence of D. aequans followed seasonally warm winters in Corsica, associated with a warmer sea surface temperature that permitted overwinter survival. This may be accentuated by having several generations. If the optimal conditions are extended in the season, they are likely to provide a longer window for parasitic infections and this may lead to more generations of parasites over the seasonal cycle (Callaway et al. 2012; Karvonen et al. 2020). Changes in water temperature over the past five years can explain this phenomenon (Gobert et al. 2020). The Mediterranean has been indicated among the region most sensitive to climate change (Giorgi 2006; Adloff et al. 2015; Piroddi et al. 2017; Bianchi et al. 2018). The temperature increase measured in the coastal regions during the last decades, especially in Corsica, is somewhat greater than at the global scale, with a global increase of 1.1 °C in 27 years (0.04 °C per year) (Scavia et al. 2002; Mladineo 2004; Parry et al. 2007; Kapsenberg et al. 2017). The results for 2019–2020 also showed that high rates of infection with D. aequans are still observed in winter. Seasonal changes in populations of D. aequans could be also a sign of parasitic adaptation (Adloff et al 2015; Antonelli et al. 2016). Climate warming can affect host–pathogen interactions by increasing pathogen development rates, transmission and number of generation times per year, directly through the ambient environment or indirectly via effects on host parameters (Harvell et al. 2002; Lloret at al. 2004; Marcogliese 2008; Studer et al. 2010; Callaway et al. 2012; Duvallet 2015; Godwin et al. 2020).
The northwest of the Mediterranean Sea is becoming saltier; the salinity of the deep waters of the Gulf of Lion is increasing at a rate of 0.007 psu/decade (Godwin et al. 2020). Salinity is an important environmental factor involved on D. aequans development (Mladineo 2004). Less saline locations near rivers have considerably lower prevalence and abundances values. This can explain our results for farm 3, which is located in a zone of shallow depth, near a freshwater supply. The contributions of continental freshwater generate vertical stratifications that can modify the parameters of temperature and salinity rates. Moreover, the presence of the copepod Lernanthropus kroyeri Van Beneden, 1851, only at farm 3 can suggest direct interspecific competition explaining lower rates of D. aequans. Salt concentration is less important for copepods than monogeneans (Aminot and Kerouel 2004; Devreker et al. 2009).
Many authors have shown that high density living conditions and cages close together can facilitate parasite infection (Hudson et al. 2001; Krkosek 2010; Farhaduzzaman et al. 2020). Corsican fish farmers have made the choice to have fewer fish in the cages to produce better quality fish; densities in cages rarely exceed 20 kg/m3. Hudson et al. (2001) also reported that the rate of encounter between hosts and pathogens can be lower with low densities, resulting in slower rates of infection. However, the fact that the cage remains a confined habitat could still explain the high prevalence observed throughout the year in both studies (Raveni 1983; Buchmann et al. 2006; Rohde et al. 1995).
Diplectanum aequans appear to be highly specific to seabass (Mladineo 2005; Dezfuli et al. 2007) and our first study highlighted that wild fish collected around cages were not parasitized by this species (Antonelli 2010). We can therefore rule out the hypothesis of a parasitic transfer resulting from the presence of wild fish around fish cages. We can assume that the alevins were already infested with parasites when they were transferred to the open sea cages. This has already been highlighted in Italian fish-farms. Ravenni (1983) demonstrated that larvae and alevins of D. labrax can be infected with D. aequans in hatcheries with closed water circuit systems.
Parasite Distribution on Gills
The difference between the numbers of parasites on the right and left gills was not significant. Our results are consistent with previous studies on D. aequans distribution on fish gills (Lambert and Maillard 1975; Cognetti-Varriale et al. 1992). All gill arches were colonized in all groups of fish (smaller and larger). Diplectanum aequans occurs in four gills arches but its distribution is not uniform, depending on abundance of parasites.
Our results revealed that the repartition of the number of parasites varied according to the total number of parasites and host weight. The parasites appear to be randomly distributed on the four arches on smaller fish with low rates of infection, while in larger fish parasites are mainly reported on gill arches 1 and 2 according to an anterior-posterior gradient (1>2>3>4). There is a migration of parasites in the centrifugal direction, from the gill arch to the ends of the gill filaments according to their stage of maturity. This provision suggests that the invasion of the gills by post-larvae is through the roots of the gill arches (Silan and Maillard 1989). Microhabitat distribution appears to be intensity-dependent (Yang et al. 2006).
In fact, previous studies reported that the highest parasite abundances were more frequently identified on the first and middle gill arches and lowest on the fourth gill arch, which is the smallest one (Bagge et al. 2005). Some authors revealed that monogenean distribution is correlated with the area on some gill arches that allows more parasites to attach to them (Koskivaara et al. 1991; Tombi and Bilong Bilong 2004). Our study revealed that for smaller fish (with lower rates of infection), parasites have an equal statistical distribution, with a random pattern on the four gill arches. Simková et al. (2006) explained that such low levels of infection result from the fact that niches are always available on the gill biotope. In contrast, the specific gradient identified on bigger fish (with higher rates of infection) showed that arches 1 and 2 were most infested due to the large colonizing gill surface. This gradient also may be the result of parasite interactions. It can be explained by the fact that D. aequans could have interspecific competition for space, food and reproduction on the available gill surface (Morand et al. 2002).
Some authors showed that the spatial distribution of Monogenea is also determined by the differential action of water flow through the gill arches (Oliva and Luque 1998; Tombi et al. 2014). Also, differences in water current over all the four gills have been considered important in determining the distribution of parasites on the gills (Arme and Halton 1972; Gutiérrez and Martorelli 1994; Hanek and Fernando 2011). Parasites were distributed along a specific gradient to promote exposure of egg clutches to water flow Gobbin et al. 2021).
The results obtained to reduce sampling effort and allow optimal exploitation for fish farmers show many things. However, due to the random nature of sub-sampling, these results may vary. It is then necessary to perform many replicates to calculate the probability of observing these results correctly according to the number of samples. Our sampling campaign consisted of a sampling of 10 fish per farm per sampling date. Thus, in the following analysis, we propose testing the reduction in sampling effort by reducing the number of fish taken per sampling day (from 1 to 10, that is, the present value of the sampling plan). We found that by accepting a 10% difference, eight fish per sample is sufficient to obtain results similar to those of a full study. However, it is important to ensure that a reduction in sampling effort does not impact the results of the different sites and years of observation. A careful optimization of fish sampling can ensure high quality of collected data with limited sampling effort. To automate the likelihood judgment of replicates, we also propose setting different threshold values (5%, 10% and 15%), thus allowing the probability of obtaining the same results at this threshold value according to the number of fish collected to be calculated.
On the other hand, having equivalent intensity values between the left and right gills shows that only one side could be analysed to reduce the sampling effort. Other statistical tests must therefore be carried out to confirm our hypothesis. It should also be considered that even if the results are equivalent, the reduction in the number of samples inevitably leads to a lower robustness of the statistical analyses. It is therefore up to the scientist to judge the relevance of reduction of sampling effort in relation to these observations. In fact, sampling efforts may vary depending on the survey objectives.
It does not seem possible to determine the number of parasites present on the right side of a given fish by knowing the number of parasites present on the left side and conversely. A set of machine learning algorithms was also tested for this purpose, but the results were inconclusive in this study.
Conclusions
The long-term monitoring of D. aequans showed changes in the cycle of infection, with seasonal variation that has changed over time. Comparing the results obtained in 2019–2020 with data from 2007–2008 highlighted that the increase of sea surface temperature provided a longer window for parasitic infections, leading more generations of D. aequans per year. Parasites occurred throughout the year and were present in all times and locations studied. The parasite distribution showed also that D. aequans is randomly distributed between the two sides of the gills. There was no preference found in the distribution on the gill arches between left and right sides of the hosts. However, they preferred specific gill arches (anterior-posterior gradient) in the biggest fish. Concerning reduction of sampling effort, it seems interesting for future campaigns to determine whether the number of samples can be reduced while maintaining similar results. We therefore propose recalculating the previous analyses from sub-samples to compare them with the results of the total sample and to judge the number of samples that would have been necessary to obtain the latter.
Author Contributions
L.M-A and Q.G designed this study. L.M-A wrote the main manuscript text. L. M-A and Q.G have done parasitic analysis. L. M-A prepared figures and tables. N. P-G and P-A. B have carried out statistical analysis. B.M and Y.Q reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Syndicat des Aquaculteurs Corses, Mare & Stagni Corsi, as part of a research collaboration with the University of Corsica.
Institutional Review Board Statement
Ethical review and approval were not necessary since the fish used in this study were dead and obtained from the aquaculture sector, destined for human consumption.
Data Availability Statement
The raw data that supports this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to Corsican fish farmers for providing us fish for sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adloff F, Somot S, Sevault F, Jordà G, Aznar R et al. (2015) Mediterranean Sea response to climate change in an ensemble of twenty first century scenarios. Clim Dyn 45:775–2802. [CrossRef]
- Alvarez-Pellitero P, Sitjà-Bobadilla A, Franco-Sierra A (1993) Protozoan parasites of wild and cultured sea bass, Dicentrarchus labrax (L.), from the Mediterranean area. Aqua Res 24:101–108.
- Aminot A, Kerouel R (2004) Hydrologie des écosystèmes marins: paramètres et analyses. Collection Méthodes d'analyse en milieu marin IFREMER.
- Antonelli L (2010) Impact du parasitisme sur la pisciculture en Corse. Suivi des parasitoses et étude des transferts de parasites depuis la faune sauvage vers les poissons élevés en mer ouverte. Thèse, Université de Corse, Pasquale Paoli.
- Antonelli L, Quilichini Y, Marchand B (2010a) Sparicotyle chrysophrii (Van Beneden and Hesse 1863) (Monogenea: Polyopisthocotylea) parasite of cultured Gilthead sea-bream Sparus aurata (Linnaeus 1758) (Pisces: Teleostei) from Corsica: ecological and morphological study. Parasitol Res 107:389–398. [CrossRef]
- Antonelli L, Quilichini Y, Marchand B (2010b) Biological study of Furnestinia echeneis Euzet and Audouin 1959 (Monogenea: Monopisthocotylea: Diplectanidae), parasite of cultured Gilthead sea-bream Sparus aurata (Linnaeus 1758) (Pisces: Teleostei) from Corsica. Aquaculture 307:179–186. [CrossRef]
- Antonelli L, Marchand B (2012) Metazoan parasites of the European sea bass Dicentrarchus labrax (Linnaeus 1758) (Pisces: Teleostei) from Corsica. In: Health and Environment in Aquaculture. Edited by Edmir Daniel Carvalho, Gianmarco Silva David and Reinaldo J. Silva, InTech, pp 43–62.
- Antonelli L, Foata J, Quilichini Y, Marchand B (2016) Influence of season and site location on European cultured sea bass parasites in Corsican fish farms using indicator species analysis (IndVal). Parasitol Res 115:561–568. [CrossRef]
- Arme C, Halton DW (1972) Observations on the occurrence of Diclidophora merlangi (Trematoda: Monogenea) on the gills of whiting, Gadus merlangus. J of Fish Biol 4:27–32. [CrossRef]
- Bagge AM, Sasal P, Valtonen ET, Karvonen A (2005) Infracommunity level aggregation in the monogenean communities of crucian carp (Carassius Carassius). Parasitology 131:367–372. [CrossRef]
- Behringer DC, Wood CL, Krkosek M, Bushek D (2020) Disease in fisheries and aquaculture. In: Marine disease ecology. Edited by Donald C. Behringer, Brian R. Silliman and Kevins Lafferty, Oxford University Press, pp 183–210.
- Bergh O (2007) The dual myths of the healthy wild fish and the unhealthy farmed fish. Dis Aquat Organ 75:159–164. [CrossRef]
- Bianchi CN, Caroli F, Guidetti P, Morri C (2018) Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J Mar Biol Ass UK 98:1–12. [CrossRef]
- Bragoni G, Romestand B, Trilles JP (1984) Parasitoses à Cymothoidiens chez le loup Dicentrarchus labrax Linnaeus (1758) en élevage. 1. Ecologie parasitaire dans le cas de l’étang de Diana (Haute-Corse) (Isopoda, Cymothoidae). Crustaceana 44:47–51.
- Buchmann K, Bresciani J (2006) Monogenea (Phylum Platyhelminthes). In: Fish Diseases and Disorders: Protozoan and Metazoan Infections. Oxfordshire: Wallingford, UK, Volume 1, pp 297–344.
- Bush AO, Lafferty KD, Lotz JM, Shotstak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. [CrossRef]
- Cable J, Van Oosterhout C (2007) The impact of parasites on the life history evolution of guppies (Poecilia reticulate): the effects of host size on parasite virulence. Int J Parasitol 13:1449-1458.
- Callaway R, Shinn AP, Grenfell SE, Bron JE, Burnell G, Cool EJ, Crumlish M, Culloty S, Davidson K, Ellis RP, Flynn KJ et al. (2012) Review of climate change impacts on marine aquaculture in the UK and Ireland. Aquat Conserv: Mar Freshw Ecosyst 22:389–421. [CrossRef]
- Cecchini S, Saroglia M, Berni P, Cognetti-Varriale AM (1998) Influence of temperature on the life cycle of Diplectanum aequans (Monogenea, Diplectanidae), parasitic on sea bass, Dicentrarchus labrax (L.). J Fish Dis 21:73–75. [CrossRef]
- Cognetti-Varriale AM, Castelli A, Cecchini S, Saroglia M (1992) Distribution of Diplectanum aequans (Monogenea) on the gills of intensively reared seabass (Dicentrarchus labrax, L.). Bull Eur Ass Fish Pathol 13:13.
- Costello MJ (2009) How Sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be threat to fishes elsewhere. Proc R Soc B 276:3385–3394. [CrossRef]
- Dagnelie P (1975) Analyses statistiques à plusieurs variables, Presses agronomiques de Gembloux, Bruxelles.
- Devreker D, Souissi S, Winkler G, Forget-Leray J, Leboulenger F (2009) Effects of salinity, temperature and individual variability on the reproduction of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: A laboratory study. J Exp Mar Biol Ecol 368:113–123.
- Dezfuli BS, Giari L, Simoni E, Menegatti R, Shinn AP, Manera M (2007) Gill histopathology of cultured European sea bass, Dicentrarchus labrax (L.), infected with Diplectanum aequans (Wagener 1857) Diesing 1958 (Diplectanidae: Monogenea). Parasitol Res 100:107–113. [CrossRef]
- Duvallet G (2015) Changement climatique et écologie vectorielle. Bull Acad vét Fr 168(2):116. [CrossRef]
- FAO (2020) The state of world fisheries and aquaculture. Published by FAO. Rome, p. 244.
- Farhaduzzaman AM, Hanif A, Khan S, Osma MH, Shovon NH, Rahman K, Ahmed SB (2020) Perfect stocking density ensures best production and economic returns in floating cages aquaculture system. J of Aquac Res Development 11:1–7.
- Gonzalez-Lanza C, Alvarez-Pellitero P, Sitjà-Bobadilla A (1991) Diplectanidae (Monogenea) infestations on sea bass, Dicentrarchus labrax (L.), from the Spanish area. Histopathology and population dynamics under culture conditions. Parasitol Res 77:307–314. [CrossRef]
- Giorgi F (2006) Climate change hot-spots. Geophys Res Lett 33-L08707:1–4. [CrossRef]
- Gobbin TP, Vanhove PM, Seehausen O, Maan ME (2021) Microhabitat distributions and species interactions of ectoparasites on the gills of cichlid fish in Lake Victoria, Tanzania. Int J Parasitol 51:201–214.
- Gobert S, Fullgrabe L, Lejeune P, Marengo M (2020) Climate change and fisheries: the case study of Corsica, an ideal reference station in the Mediterranean Sea. Aquac Fish Stud 2:1–2. [CrossRef]
- Godwin SC, Fast MD, Kuparinen A, Medcalf KE, Hutchings JA (2020) Increasing tempeartures accentuate negative fitness consequences of a marine parasite. Sci rep 10:18467. [CrossRef]
- Gutiérrez PA, Martorelli SR (1994) Seasonality, distribution, and preference sites of Demidospermus valenciennesi Gutiérrez et Suriano, 1992 (Monogenea: Ancyrocephalidae) in catfish. Res Rev Parasitol 54:259–261.
- Hanek G, Fernando CH (2011) Spatial distribution of gill parasites of Lepomis Gibbosus (L.) and Ambloplites rupestris (Raf.). Can J Zool 56:1235–1240. [CrossRef]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD (2002) Climate warming and diseases risks for terrestrial and marine biota. Science 5576:2158–2162. [CrossRef]
- Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP (2001) The ecology of wildlife diseases. Oxford University Press, Oxford.
- Kapsenberg L, Alliouane S, Gazeau F, Mousseau L, Gattuso JP (2017) Costal Ocean acidification and increasing total alkalinity in the Northwestern Mediterranean Sea. Ocean Sci 13:411–426. [CrossRef]
- Karvonen A, Rintamäki P, Jokela J, Valtonen ET (2010) Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. Int J Parasitol 40:1483–1488.
- Koskivaara M, Valtonen ET, Vuori KM (1991) Microhabitat distribution and coexistence of Dactylogyrus species (Monogenea) on the gills of roach. Parasitology 104:273–281. [CrossRef]
- Krkosek M (2010) Host densities thresholds and disease control for fisheries and aquaculture. Aquacul Environ Interact 1:21–32.
- Lafferty KD, Harvell CD, Conrad JM, Friedman CS, Kent ML, Kuris AM, Powell EN, Rondeau D, Saksida SM (2014 Infectious diseases affect marine fisheries and aquaculture economics. Ann Rev Ma Sci 7:471–496. [CrossRef]
- Lia RP, Zizzo N, Tinelli A, Lionetti C, Cantacessi C, Otranto D. (2007) Mass mortality in wild greater amberjack (Seriola dumerili) infected by Zeuxapta seriolae (Monogenea: Heteraxinidae) on the Ionian Sea. Bull Eur Ass Fish Pathol 27:108–111.
- Lambert A, Maillard C (1975) Gill repartition of two Monogenea: Diplectanum aequans (Wagener 1857) Diesing 1958 and D. laubieri Lambert and Maillard 1974 (Monogenea, Monopisthocotylea) simultaneous parasites of the sea bass Dicentrarchus labrax. Ann Parasitol Hum Comp 50:691–9. [CrossRef]
- Lanza GR, Wilda KM, Bunluesin S, Panich-Pat T (2017) Green Aquaculture: designing and developing aquaculture systems integrated with phytoremediation treatment options. Phytoremediation 5:307–323. [CrossRef]
- Lloret J, Palomera I, Salat J, Solé I (2004) Impact of fresh-water input and wind on landings of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) in shelf-waters surrounding the Ebre (Ebro) River delta (north-western Mediterranean). Fish Oceanogr 13:102−110. [CrossRef]
- Marcogliese DJ (2001) Implications of climate change for parasitism of animals in the aquatic environment. Can J Zool 79:1331−1352. [CrossRef]
- Marcogliese DJ (2008) The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Off Int Epizoot 27:467−84.
- Mare & Stagni Corsi (2011) Syndicat des Aquaculteurs Corses. Cahier des charges Label Rouge LA 01-11« Bar d’aquaculture marine ».
- Massa F, Onofri L, Fezzardi D (2017) Aquaculture in the Mediterranean and the Black Sea: A Blue Growth perspective. Nunes PALD, Svensson LE, Markandy A (Eds). Handbook on the Economics and Management of Sustainable Oceans, Edward Elgar Publishing, Cheltenham and Northampton, UK, pp 93–123.
- Massimo M, Volpatti D, Galeotti M, E Bron J, Beraldo P (2022) New insights into the host parasites interactions of Amyloodiniosis in European sea-bass: a multi modal approach. Pathogens 11:62. [CrossRef]
- Merella P, Garippa G, Salati F (2006) Parasites of cage cultured European sea bass Dicentrarchus labrax and gilthead sea bream Sparus aurata from Sardinia (western Mediterranean): first results. Parassitologia 48:290.
- Merella P, Cherchi S, Garippa G, Fioravanti ML, Gustinelli A, Salati F (2009) Outbreak of Sciaenacotyle panceri (Monogenea) on cage-reared meagre Argyrosomus regius (Osteichtyes) from the western Mediterranean Sea. Dis of Aquat Org 86:169–173. [CrossRef]
- Mladineo M (2004) Monogenean parasites in Adriatic cage-reared fish. Acta Adriat 45:65–73.
- Mladineo M (2005) Parasites communities of Adriatic cage reared fish. Dis Aquat Organ 64(1):77-83. [CrossRef]
- Morand S, Simková A, Matějusová I, Plaisance L, Verneau O, Desdevises Y (2002) Investigating patterns may reveal processes: evolutionary ecology of ectoparasitic monogeneans. Int J Parasitol 32:111−119. [CrossRef]
- Muniesa A, Basurco B, Aguilera C, Furones D, Reverté C, Sanjuan-Vilaplana A, Jansen MD, Brun E, Tavorpanich S (2020) Mapping the knowledge of the main diseases affecting sea bass and sea bream in Mediterranean. Transbound. Emerg Dis 67:1089−1100. [CrossRef]
- Oliva ME, Luque JL (1998) Distribution patterns of Microcotyle nemadactylus (Monogenea) on gill filaments of Cheilodatylus variegatus (Teleostei). Mem Ins Oswaldo Cruz 93:477−478.
- Oliver G (1976) Study of Diplectanum aequans (Wagener, 1957) Diesing, 1858 (Monogenea, Monopisthocotylea, Diplectanidae) with a scanning electronic microscope. Z Parasitenkd 51:91–98. [CrossRef]
- Parry ML, Canziani OF, Palutokof JP, Van der Linden PJ, Hanson CE IPCC (2007). Climate change 2007: Impacts, Adapatation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmantal Panel on Climate Change. Cambrdige University Press, Cambridge, UK.
- Piroddi C, Coll M, Liquete C, Macias D, Greer K, Buszowski J, Steenbeek J, Danovaro R Christensen V (2017) Historical changes of the Mediterranean Sea ecosystem: modelling the role and impact of primary productivity and fisheries changes over time. Sci rep 7:44491. [CrossRef]
- Poulin R, Rohde K (1997) Comparing the richness of metazoan ectoparasite communities of marine fishes: controlling for host phylogeny. Oecologia 110:278–28. [CrossRef]
- Ravenni G (1983) Infestation by Diplectanum aequans (Wagener 1857) Diesing 1858 in cultured sea bass (Dicentrarchus labrax L.). Riv Ital Piscic-Ittiopatol:167–176.
- Rohde K, Hayward C, Heap M (1995) Aspects of the ecology of metazoan ectoparasites of marine fishes. Int J Parasitol 25:945–970. [CrossRef]
- Scavia D, Field JC, Boesch DF, Buddemeier RW, Burkett V, Cayan DR, Fogarty M, Harwell M et al. (2002) Climate change impacts on the US coastal and marine ecosystems. Estuaries 25:149−196. [CrossRef]
- Silan P, Maillard C (1989) Biologie comparée du développement et discrimination des Diplectanidae ectoparasites du Bar (Teleostei). Ann Sci Nat Zool Biol Ani 10:31−45.
- Simková A, Verneau O, Gelnar M, Morand S (2006) Specificity and specialization of congeneric monogenans parasiting Cyprinid fish. Evolution 60:1023−1037. [CrossRef]
- Sprent P (1992) Pratique des statistiques non paramétriques, Institut National de la Recherche Agronomique, Paris.
- Studer A, Thieltges DW, Poulin R (2010) Parasites and global warming: net effects of temperature on an intertidal host-parasite system. Mar Ecol Prog Ser 415:11−22. [CrossRef]
- Ternengo S, Agostini S, Quilichini Y, Euzet L, Marchand B (2010) Intensive infestations of Sciaenocotyle pancerii (Monogenea, Microcotylidae) on Argyrosomus regius (Asso) under fish farming conditions. J Fish Dis 33:89–92. [CrossRef]
- Tombi J, Bilong Bilong CF (2004) Distribution of gill parasites of the freshwater fish Barbus martorelli Roman, 1971 (Teleostei: Cyprinidae) and tendency to inverse intensity evolution between Myxosporidia and Monogenea as a function of the host age. Rev élev méd vét Pays trop 57(1-2):71−76.
- Tombi J, Akoumba JF, Bilong Bilong, CF (2014) The monogenean community on the gills of Oreochromis niloticus from Melen fish station in Yaounde, Cameroon. Int J Mod Biol Res 2:16−23.
- Winger AC, Kanck M, Kristoffersen R, Knudsen R (2008) Seasonal dynamics and persistence of Gyrodactylus salaris in two riverine anadromous Arctic charr populations. Environ Biol Fish 83:117−123. [CrossRef]
- Yang T, Liu J, Gibson DI, Dong A (2006) Spatial distributions of two species of monogeneans on the gills of Siganus Fuscescens (Houttuyn) and their seasonal dynamics in caged versus wild caught-hosts. J Parasitol 95:933−940. [CrossRef]
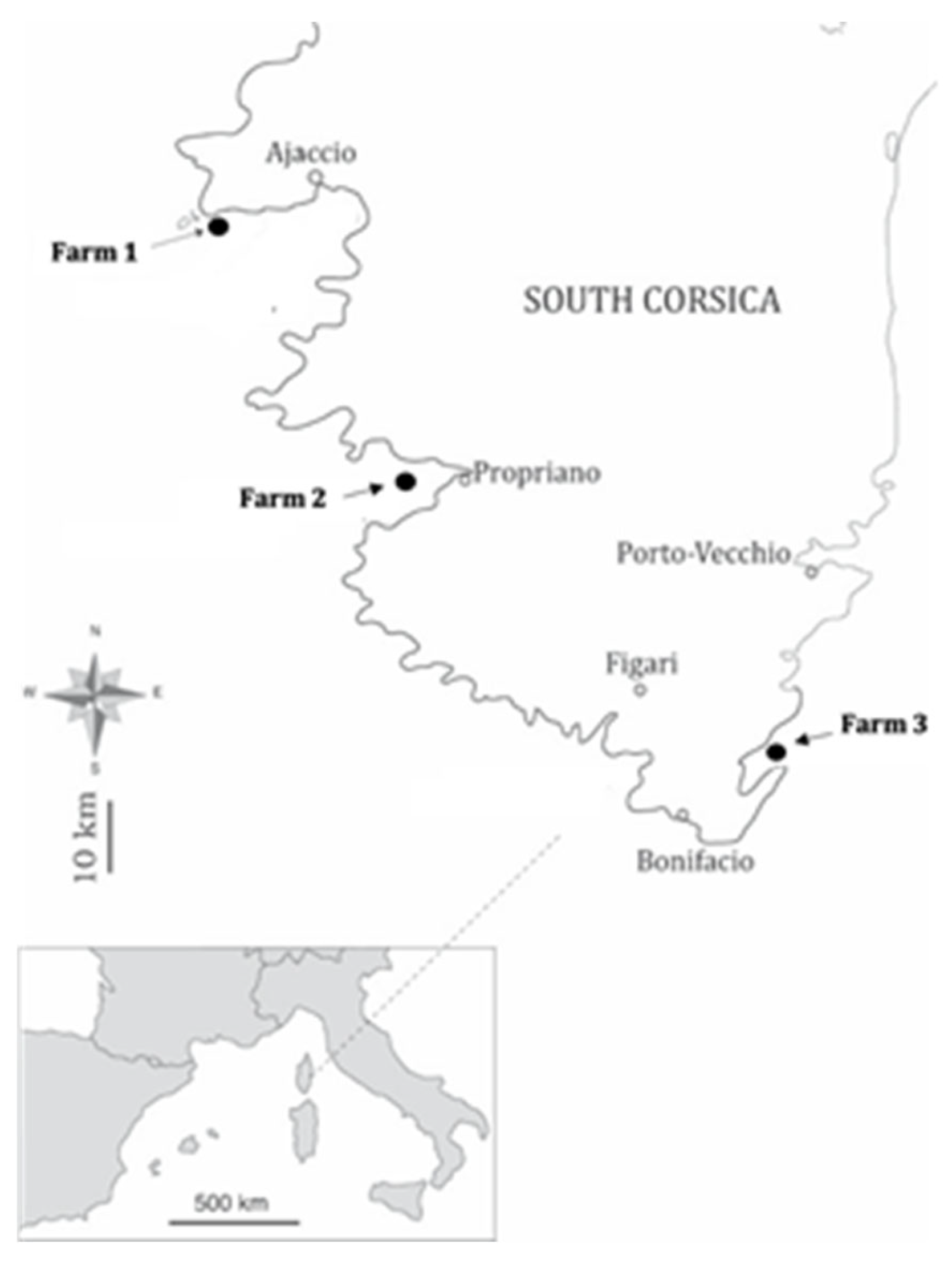
Figure 1.
Locations of Corsican fish farms studied.
Figure 1.
Locations of Corsican fish farms studied.
Figure 2.
Relationship between fish weight (g) and length (cm).
Figure 2.
Relationship between fish weight (g) and length (cm).
Figure 3.
Seasonal comparison of prevalence, mean abundance and mean intensity values of D. aequans between two parasitological surveys of fish farms. A, B: farm 1; C, D: farm 2; E, F: farm 3. W: winter, S: spring, Su: summer, A: autumn, 1: first parasitological study (2007–2008), 2: second parasitological study (2019–2020).
Figure 3.
Seasonal comparison of prevalence, mean abundance and mean intensity values of D. aequans between two parasitological surveys of fish farms. A, B: farm 1; C, D: farm 2; E, F: farm 3. W: winter, S: spring, Su: summer, A: autumn, 1: first parasitological study (2007–2008), 2: second parasitological study (2019–2020).
Figure 4.
Change in the ratio of the mean number of parasites in the subsample to the mean of all samples based on the number of samples selected for all farms and years (totals and on the left arches).
Figure 4.
Change in the ratio of the mean number of parasites in the subsample to the mean of all samples based on the number of samples selected for all farms and years (totals and on the left arches).
Figure 5.
Reduction of sampling effort test by reducing the number of fish taken per sampling day (from 1 to 10) at different threshold values (5%, 10% and 15%), Prob.: probability.
Figure 5.
Reduction of sampling effort test by reducing the number of fish taken per sampling day (from 1 to 10) at different threshold values (5%, 10% and 15%), Prob.: probability.
Table 1.
Mean seasonal temperatures (T) recorded during parasitological surveys (°C). W: winter, S: spring, Su: summer, A: autumn.
Table 1.
Mean seasonal temperatures (T) recorded during parasitological surveys (°C). W: winter, S: spring, Su: summer, A: autumn.
| |
|
Farm 1 |
Farm 2 |
Farm 3 |
Mean T (°C) |
| 2007 |
W |
14.2 |
14.1 |
14.1 |
14.13 |
| |
S |
17.1 |
20.3 |
20.6 |
19.33 |
| |
Su |
25.5 |
22.4 |
24.3 |
24,1 |
| |
A |
17.7 |
21.7 |
21.5 |
20.3 |
| Mean T (°C) |
|
18.63 |
19.63 |
20.13 |
|
| 2008 |
W |
13.7 |
13.5 |
13.3 |
13.5 |
| |
S |
18.8 |
20.5 |
20.8 |
20.03 |
| |
Su |
24.8 |
24.9 |
24.8 |
24.83 |
| |
A |
21 |
20.4 |
22.6 |
21.33 |
| Mean T (°C) |
|
19.6 |
19.8 |
20.4 |
|
| 2019 |
W |
14 |
14.5 |
14 |
14.2 |
| |
S |
15 |
18.1 |
19.8 |
17.63 |
| |
Su |
26 |
26.2 |
28.2 |
26.8 |
| |
A |
21 |
23.1 |
24 |
22.7 |
| Mean T (°C) |
|
19 |
20.5 |
21.5 |
|
| 2020 |
W |
14 |
14 |
15.7 |
14.6 |
| |
S |
18 |
19 |
19.6 |
18.9 |
| |
Su |
25 |
28 |
27 |
26.7 |
| |
A |
18 |
24 |
23.8 |
21.9 |
| Mean T (°C) |
|
18.8 |
21.3 |
21.5 |
|
Table 2.
Parasitological indices of D. aequans found on D. labrax of Corsican fish farms.
Table 2.
Parasitological indices of D. aequans found on D. labrax of Corsican fish farms.
| Fish group (Weight (g)) |
N fish/group (%) |
Prevalence (%) |
Mean abundance
(± SD) |
Mean intensity
(± SD) |
| WFS4 (> 650) |
108 (25%) |
75 |
21.4 (± 21.7) |
24.1 ± (22.8) |
| WFS3 (451–650) |
107 (25%) |
81 |
19.5(± 21.7) |
21.3 ± (22.6) |
| WFS2 (251–450) |
107 (25%) |
79 |
19.1 (± 21.8) |
21.1 ± (22.8) |
| WFS1 (100–250) |
108 (25%) |
66 |
14.6 (± 19.8) |
17.2 ± (21.3) |
Table 3.
Ecological indices of D. aequans for fish farms. χ2: chi-square test, p: significance level, SD: standard deviation, U test: Mann-Whitney U test.
Table 3.
Ecological indices of D. aequans for fish farms. χ2: chi-square test, p: significance level, SD: standard deviation, U test: Mann-Whitney U test.
| |
|
|
Prevalence |
Abundance |
Intensity |
| |
Mean (%) |
±SD |
χ2
|
(p) |
Mean |
±SD |
U test (p) |
Mean |
±SD |
U test (p) |
| Farm 1 |
|
|
|
|
|
|
|
|
|
|
| 2007–2008 |
91.4 |
7.89 |
4.74 |
0.029 |
16.07 |
4.93 |
0.026 |
18.24 |
5.87 |
0.02 |
| 2019–2020 |
98.8 |
3.53 |
33.52 |
15.8 |
33.63 |
15.58 |
| Farm 2 |
|
|
|
|
|
|
|
|
|
|
| 2007–2008 |
87.5 |
15.81 |
2.09 |
0.148 |
14.96 |
11.47 |
0.38 |
16.17 |
10.55 |
0.46 |
| 2019–2020 |
86,2 |
17.68 |
19.67 |
11.58 |
20.77 |
11.36 |
| Farm 3 |
|
|
|
|
|
|
|
|
|
|
| 2007–2008 |
81.2 |
22.32 |
4.66 |
0.031 |
11.48 |
8.27 |
0.26 |
12.35 |
7.3 |
0.32 |
| 2019–2020 |
86.8 |
20.94 |
7.34 |
7.22 |
8.5 |
6.6 |
Table 4.
Mean abundance of D. aequans according to the distribution on gill arches on all fish analysed. GA: gill arches, SD: standard deviation, U test: Mann-Whitney U test.
Table 4.
Mean abundance of D. aequans according to the distribution on gill arches on all fish analysed. GA: gill arches, SD: standard deviation, U test: Mann-Whitney U test.
| |
Mean abundance (± SD) |
| |
Left (L) |
Right ® |
| GA1 |
3.37 (± 5,02) |
3.17 (± 4,55) |
| GA2 |
2.91 (± 4.07) |
2.81 (± 4.13) |
| GA3 |
2.07 (± 3.21) |
1.81 (± 2.8) |
| GA4 |
1.37 (± 2.75) |
1.14 (± 2.35) |
| Total |
9.71 (± 12.28) |
8.99 (± 11.45) |
|
U test |
p=0.68 |
Table 5.
Prevalence values of D. aequans (%) on D. labrax gill arches.
Table 5.
Prevalence values of D. aequans (%) on D. labrax gill arches.
| |
|
Distribution of parasites (%) |
| Fish group* |
N fish/group (%) |
GA*1 |
GA2 |
GA3 |
GA4 |
| 4 |
108 (25%) |
34.5 |
31.4 |
20.4 |
13.8 |
| 3 |
107 (25%) |
38.1 |
27.7 |
20.3 |
14.1 |
| 2 |
107 (25%) |
28 |
33.6 |
25.5 |
12.9 |
| 1 |
108 (25%) |
40.8 |
31.3 |
16.5 |
22.6 |
| Total |
430 (100%) |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).