Submitted:
04 October 2023
Posted:
06 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Gold Nanoparticles as next-generation delivery systems
3. MicroRNA therapies as innovative ovarian protection approach
4. Targeting the ovaries
5. Limitations and perspectives
Author Contributions
Funding
Conflicts of Interest
Additional note
References
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. The Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Pregnancy After Human Oocyte Cryopreservation. The Lancet 1986, 327, 884–886. [Google Scholar] [CrossRef]
- Brown, J.R.; Modell, E.; Obasaju, M.; Ying, Y.K. Natural Cycle In-Vitro Fertilization with Embryo Cryopreservation Prior to Chemotherapy for Carcinoma of the Breast. Human Reproduction 1996, 11, 197–199. [Google Scholar] [CrossRef]
- Demeestere, I.; Simon, P.; Emiliani, S.; Delbaere, A.; Englert, Y. Orthotopic and Heterotopic Ovarian Tissue Transplantation. Hum Reprod Update 2009, 15, 649–665. [Google Scholar] [CrossRef]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live Birth after Autograft of Ovarian Tissue Cryopreserved during Childhood. Human Reproduction 2015, 30, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A Review on Biomaterials for Ovarian Tissue Engineering. Acta Biomater 2021, 135, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Akahori, T.; Woods, D.C.; Tilly, J.L. Female Fertility Preservation through Stem Cell-Based Ovarian Tissue Reconstitution In Vitro and Ovarian Regeneration In Vivo. Clin Med Insights Reprod Health 2019, 13, 117955811984800. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How Do Chemotherapeutic Agents Damage the Ovary? Hum Reprod Update 2012, 18, 525–535. [Google Scholar] [CrossRef]
- Roness, H.; Gavish, Z.; Cohen, Y.; Meirow, D. Ovarian Follicle Burnout: A Universal Phenomenon? Cell Cycle 2013, 12, 3245–3246. [Google Scholar] [CrossRef]
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the C-Abl-TAp63 Pathway Protects Mouse Oocytes from Chemotherapy-Induced Death. Nat Med 2009, 15, 1179–1185. [Google Scholar] [CrossRef]
- Bellusci, G.; Mattiello, L.; Iannizzotto, V.; Ciccone, S.; Maiani, E.; Villani, V.; Diederich, M.; Gonfloni, S. Kinase-Independent Inhibition of Cyclophosphamide-Induced Pathways Protects the Ovarian Reserve and Prolongs Fertility. Cell Death Dis 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-Phosphate Prevents Chemotherapy-Induced Human Primordial Follicle Death. Human Reproduction 2014, 29, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Hamish, W.; Wallace, B. Toxicity of Chemotherapy and Radiation on Female Reproduction.
- Grosbois, J.; Devos, M.; Demeestere, I. Implications of Nonphysiological Ovarian Primordial Follicle Activation for Fertility Preservation. Endocr Rev 2020, 41. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide Triggers Follicle Activation and "burnout "; AS101 Prevents Follicle Loss and Preserves Fertility. Sci Transl Med 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kimura, F.; Zheng, L.; Kaku, S.; Takebayashi, A.; Kasahara, K.; Tsuji, S.; Murakami, T. Protective Effect of a Mechanistic Target of Rapamycin Inhibitor on an in Vivo Model of Cisplatin-Induced Ovarian Gonadotoxicity. Exp Anim 2018, 67, 493–500. [Google Scholar] [CrossRef]
- Hoon Jang, Younghwa Na, Kwonho Hong, Sangho Lee, Sohyeon Moon, Minha Cho, Miseon Park, Ok-Hee Lee, Eun Mi Chang, Dong Ryul Lee, Jung Jae Ko, Woo Sik Lee, Y.C. Synergistic Effect of Melatonin and Ghrelin in Preventing Cisplatin-Induced Ovarian Damage via Regulation of FOXO3a Phosphorylation and Binding to the P27Kip1 Promoter in Primordial Follicles. J Pineal Res 2017, 63, e12432. [CrossRef]
- Lambertini, M.; Horicks, F.; Del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian Protection with Gonadotropin-Releasing Hormone Agonists during Chemotherapy in Cancer Patients: From Biological Evidence to Clinical Application. Cancer Treat Rev 2019, 72, 65–77. [Google Scholar] [CrossRef]
- Lambertini, M.; Boni, L.; Michelotti, A.; Gamucci, T.; Scotto, T.; Gori, S.; Giordano, M.; Garrone, O.; Levaggi, A.; Poggio, F.; et al. Ovarian Suppression with Triptorelin during Adjuvant Breast Cancer Chemotherapy and Long-Term Ovarian Function, Pregnancies, and Disease-Free Survival a Randomized Clinical Trial. JAMA - Journal of the American Medical Association 2015, 314, 2632–2640. [Google Scholar] [CrossRef]
- Demeestere, I.; Brice, P.; Peccatori, F.A.; Kentos, A.; Dupuis, J.; Zachee, P.; Casasnovas, O.; Van Den Neste, E.; Dechene, J.; De Maertelaer, V.; et al. No Evidence for the Benefit of Gonadotropin-Releasing Hormone Agonist in Preserving Ovarian Function and Fertility in Lymphoma Survivors Treated with Chemotherapy: Final Long-Term Report of a Prospective Randomized Trial. Journal of Clinical Oncology 2016, 34, 2568–2574. [Google Scholar] [CrossRef]
- Alexandri, C.; Daniel, A.; Bruylants, G.; Demeestere, I. The Role of MicroRNAs in Ovarian Function and the Transition toward Novel Therapeutic Strategies in Fertility Preservation: From Bench to Future Clinical Application. Hum Reprod Update 2020, 26, 174–196. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angewandte Chemie - International Edition 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Stealth Nanoparticles: High Density but Sheddable PEG Is a Key for Tumor Targeting. Journal of Controlled Release 2010, 145, 178–181. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-RhTNF Nanomedicine. Clinical Cancer Research 2010, 16, 6139–6149. [Google Scholar] [CrossRef]
- Tatovic, D.; Mcateer, M.A.; Barry, J.; Barrientos, A.; Rodríguez Terradillos, K.; Perera, I.; Kochba, E.; Levin, Y.; Dul, M.; Coulman, S.A.; et al. Safety of the Use of Gold Nanoparticles Conjugated with Proinsulin Peptide and Administered by Hollow Microneedles as an Immunotherapy in Type 1 Diabetes. Immunotherapy Advances 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Lee, C.; Kim, T.W.; Oh, D.E.; Bae, S.O.; Ryu, J.; Kong, H.; Jeon, H.; Seo, H.K.; Jeon, S.; Kim, T.H. In Vivo and In Vitro Anticancer Activity of Doxorubicin-Loaded DNA-AuNP Nanocarrier for the Ovarian Cancer Treatment 2020, 12. [CrossRef]
- Banu, H.; Stanley, B.; Faheem, S.M.; Seenivasan, R.; Premkumar, K.; Vasanthakumar, G. Thermal Chemosensitization of Breast Cancer Cells to Cyclophosphamide Treatment Using Folate Receptor Targeted Gold Nanoparticles. Plasmonics 2014, 9, 1341–1349. [Google Scholar] [CrossRef]
- Xiong, X.; Arvizo R., R. R.; Saha, S.; Robertson J., D.J.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Sensitization of Ovarian Cancer Cells to Cisplatin by Gold Nanoparticles. Oncotarget 2014, 5, 6453–6465. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Mohandoss, D.K.D.; Darshakumar, R.D.; Joshi, C.M.; Katti, K. V. New Approaches in Breast Cancer Therapy through Green Nanotechnology and Nano-Ayurvedic Medicine – Pre-Clinical and Pilot Human Clinical Investigations. Int J Nanomedicine 2020, 15, 181–197. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A First-in-Human Phase 0 Clinical Study of RNA Interference-Based Spherical Nucleic Acids in Patients with Recurrent Glioblastoma. Sci Transl Med 2021, 13. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br J Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active Targeting of Gold Nanoparticles as Cancer Therapeutics. Chem Soc Rev 2020, 49, 8774–8789. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted Delivery of Gemcitabine to Pancreatic Adenocarcinoma Using Cetuximab as a Targeting Agent. Cancer Res 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Kotcherlakota, R.; Vydiam, K.; Jeyalakshmi Srinivasan, D.; Mukherjee, S.; Roy, A.; Kuncha, M.; Rao, T.N.; Sistla, R.; Gopal, V.; Patra, C.R. Restoration of P53 Function in Ovarian Cancer Mediated by Gold Nanoparticle-Based EGFR Targeted Gene Delivery System. ACS Biomater Sci Eng 2019, 5, 3631–3644. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Srinivasan, D.J.; Mukherjee, S.; Haroon, M.M.; Dar, G.H.; Venkatraman, U.; Patra, C.R.; Gopal, V. Engineered Fusion Protein-Loaded Gold Nanocarriers for Targeted Co-Delivery of Doxorubicin and ErbB2-SiRNA in Human Epidermal Growth Factor Receptor-2+ Ovarian Cancer. J Mater Chem B 2017, 5, 7082–7098. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem Soc Rev 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold Nanoparticles for Nucleic Acid Delivery. Molecular Therapy 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C.; et al. Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA in Vivo Induces Homology-Directed DNA Repair. Nat Biomed Eng 2017, 1, 889–901. [Google Scholar] [CrossRef]
- Fedoryshin, L.L.; Tavares, A.J.; Petryayeva, E.; Doughan, S.; Krull, U.J. Near-Infrared-Triggered Anticancer Drug Release from Upconverting Nanoparticles. ACS Appl Mater Interfaces 2014, 6, 13600–13606. [Google Scholar] [CrossRef]
- Stern, J.M.; Kibanov Solomonov, V. V.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int J Toxicol 2016, 35, 38–46. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc Natl Acad Sci U S A 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-Gold Nanoparticles for Atheroprotective Management of Plaques: Results of the NANOM-FIM Trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V. V. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials 2022, 15, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem Rev 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Kiernan, M.C.; Menon, P.; Huynh, W.; Rynders, A.; Ho, K.S.; Glanzman, R.; Hotchkin, M.T. Study Protocol of RESCUE-ALS: A Phase 2, Randomised, Double-Blind, Placebo-Controlled Study in Early Symptomatic Amyotrophic Lateral Sclerosis Patients to Assess Bioenergetic Catalysis with CNM-Au8 as a Mechanism to Slow Disease Progression. BMJ Open 2021, 11, e041479. [Google Scholar] [CrossRef]
- Ambros, V. The Functions of Animal MicroRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat Rev Mol Cell Biol 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Feinbaum, R.; Ambros, V.; Lee, R. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 116, 843–854. [Google Scholar]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.P.; et al. An Estimate of the Total Number of True Human MiRNAs. Nucleic Acids Res 2019, 47, 3353–3364. [Google Scholar] [CrossRef]
- Lindow, M.; Kauppinen, S. Discovering the First Microrna-Targeted Drug. Journal of Cell Biology 2012, 199, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ree, M.H.; Van Der Meer, A.J.; Van Nuenen, A.C.; De Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen Dosing in Chronic Hepatitis C Patients Results in Decreased MicroRNA-122 Levels without Affecting Other MicroRNAs in Plasma. Aliment Pharmacol Ther 2016, 43, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Inc., R.T. Inc., R.T. Regulus Announces Pipeline Updates and Advancements Available online: https://www.prnewswire.com/news-releases/regulus-announces-pipeline-updates-and-advancements-300472142.html.
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. Journal of Investigative Dermatology 2019, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Reid, G.; Pel, M.E.; Kirschner, M.B.; Cheng, Y.Y.; Mugridge, N.; Weiss, J.; Williams, M.; Wright, C.; Edelman, J.J.B.; Vallely, M.P.; et al. Restoring Expression of MiR-16: A Novel Approach to Therapy for Malignant Pleural Mesothelioma. Annals of Oncology 2013, 24, 3128–3135. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br J Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Kristen, A. V.; Ajroud-Driss, S.; Conceição, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi Therapeutic for the Treatment of Hereditary Transthyretin-Mediated Amyloidosis. Neurodegener Dis Manag 2019, 9, 5–23. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. MiRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc 2021, 4, 1–20. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Lang, M.; Wehbe, H.; Maheshwari, S.; Mendell, J.T.; Jiang, J.; Schmittgen, T.D.; Patel, T. Involvement of Human Micro-RNA in Growth and Response to Chemotherapy in Human Cholangiocarcinoma Cell Lines. Gastroenterology 2006, 130, 2113–2129. [Google Scholar] [CrossRef]
- Hummel, R.; Wang, T.; Watson, D.I.; Michael, M.Z.; Van Der Hoek, M.; Haier, J.; Hussey, D.J. Chemotherapy-Induced Modification of MicroRNA Expression in Esophageal Cancer. Oncology Reports 2011, 26, 1011–1017. [Google Scholar] [CrossRef]
- Lindholm, E.M.; Ragle Aure, M.; Haugen, M.H.; Kleivi Sahlberg, K.; Kristensen, V.N.; Nebdal, D.; Børresen-Dale, A.L.; Lingjærde, O.C.; Engebraaten, O. MiRNA Expression Changes during the Course of Neoadjuvant Bevacizumab and Chemotherapy Treatment in Breast Cancer. Molecular Oncology 2019, 13, 2278–2296. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and Drug Resistance. Cancer Gene Therapy 2010, 17, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.E.; Chung, J.H.; Verducci, J.S.; Lin, S.; Park, J.K.; Dai, Z.; Liu, C.G.; Schmittgen, T.D.; Reinhold, W.C.; Croce, C.M.; et al. MicroRNAs Modulate the Chemosensitivity of Tumor Cells. Molecular Cancer Therapeutics 2008, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, H.; Liu, X.; Evans, B.R.; Medina, D.J.; Liu, C.G.; Yang, J.M. Role of MicroRNA MiR-27a and MiR-451 in the Regulation of MDR1/P-Glycoprotein Expression in Human Cancer Cells. Biochemical Pharmacology 2008, 76, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Serguienko, A.; Grad, I.; Wennerstrøm, A.B.; Meza-Zepeda, L.A.; Thiede, B.; Stratford, E.W.; Myklebost, O.; Munthe, E. Metabolic Reprogramming of Metastatic Breast Cancer and Melanoma by Let-7a MicroRNA. Oncotarget 2015, 6, 2451–2465. [Google Scholar] [CrossRef]
- Yu, F.; Yao, H.; Zhu, P.; Zhang, X.; Pan, Q.; Gong, C.; Huang, Y.; Hu, X.; Su, F.; Lieberman, J.; et al. Let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells. Cell 2007, 131, 1109–1123. [Google Scholar] [CrossRef]
- Yin, P.T.; Pongkulapa, T.; Cho, H.Y.; Han, J.; Pasquale, N.J.; Rabie, H.; Kim, J.H.; Choi, J.W.; Lee, K.B. Overcoming Chemoresistance in Cancer via Combined MicroRNA Therapeutics with Anticancer Drugs Using Multifunctional Magnetic Core-Shell Nanoparticles. ACS Applied Materials and Interfaces 2018, 10, 26954–26963. [Google Scholar] [CrossRef]
- De Guire, V.; Robitaille, R.; Tétreault, N.; Guérin, R.; Ménard, C.; Bambace, N.; Sapieha, P. Circulating MiRNAs as Sensitive and Specific Biomarkers for the Diagnosis and Monitoring of Human Diseases: Promises and Challenges. Clinical Biochemistry 2013, 46, 846–860. [Google Scholar] [CrossRef]
- Meiri, E.; Mueller, W.C.; Rosenwald, S.; Zepeniuk, M.; Klinke, E.; Edmonston, T.B.; Werner, M.; Lass, U.; Barshack, I.; Feinmesser, M.; et al. A Second-Generation MicroRNA-Based Assay for Diagnosing Tumor Tissue Origin. The Oncologist 2012, 17, 801–812. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Cheng, C.C.; Chiang, Y.S.; Cheng, W.T.K.; Liu, I.H.; Wu, S.C. Exosomal MiR-10a Derived from Amniotic Fluid Stem Cells Preserves Ovarian Follicles after Chemotherapy. Scientific Reports 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Alexandri, C.; Stratopoulou, C.A.; Demeestere, I. Answer to Controversy: MiR-10a Replacement Approaches Do Not Offer Protection against Chemotherapy-Induced Gonadotoxicity in Mouse Model. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, C.; Stamatopoulos, B.; Rothé, F.; Bareche, Y.; Devos, M.; Demeestere, I. MicroRNA Profiling and Identification of Let-7a as a Target to Prevent Chemotherapy-Induced Primordial Follicles Apoptosis in Mouse Ovaries. Scientific Reports 2019. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, C.; Van Den Steen, G.; Demeestere, I. Let-7a Mimic Transfection Reduces Chemotherapy-Induced Damage in a Mouse Ovarian Transplantation Model. Scientific Reports 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of MiR-21 in Stem Cells Improves Ovarian Structure and Function in Rats with Chemotherapy-Induced Ovarian Damage by Targeting PDCD4 and PTEN to Inhibit Granulosa Cell Apoptosis. Stem cell research & therapy 2017, 8, 187. [Google Scholar] [CrossRef]
- Thabet, E.; Yusuf, A.; Abdelmonsif, D.A.; Nabil, I.; Mourad, G.; Mehanna, R.A. Extracellular Vesicles MiRNA-21: A Potential Therapeutic Tool in Premature Ovarian Dysfunction. Molecular Human Reproduction 2020, 26, 906–919. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MiR-144-5p Improves Rat Ovarian Function after Chemotherapy-Induced Ovarian Failure by Targeting PTEN. Laboratory Investigation 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Cao, R.C.; Lv, Y.; Lu, G.; Liu, H. Bin; Wang, W.; Tan, C.; Su, X.W.; Xiong, Z.; Ma, J.L.; Chan, W.Y. Extracellular Vesicles from IPSC-MSCs Alleviate Chemotherapy-Induced Mouse Ovarian Damage via the ILK-PI3K/AKT Pathway. Zoological Research 2023, 44, 620–635. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, B.; Zhu, Y.; Chen, M.; Huang, J.; Guo, H.; Wang, F. MicroRNA-144-3p Protects against Chemotherapy-Induced Apoptosis of Ovarian Granulosa Cells and Activation of Primordial Follicles by Targeting MAP3K9. European journal of medical research 2023, 28, 264. [Google Scholar] [CrossRef]
- Domínguez-Ríos, R.; Sánchez-Ramírez, D.R.; Ruiz-Saray, K.; Oceguera-Basurto, P.E.; Almada, M.; Juárez, J.; Zepeda-Moreno, A.; del Toro-Arreola, A.; Topete, A.; Daneri-Navarro, A. Cisplatin-Loaded PLGA Nanoparticles for HER2 Targeted Ovarian Cancer Therapy. Colloids and Surfaces B: Biointerfaces 2019, 178, 199–207. [Google Scholar] [CrossRef]
- Kumar, D.; Moghiseh, M.; Chitcholtan, K.; Mutreja, I.; Lowe, C.; Kaushik, A.; Butler, A.; Sykes, P.; Anderson, N.; Raja, A. LHRH Conjugated Gold Nanoparticles Assisted Efficient Ovarian Cancer Targeting Evaluated via Spectral Photon-Counting CT Imaging: A Proof-of-Concept Research. Journal of Materials Chemistry B 2023, 11, 1916–1928. [Google Scholar] [CrossRef]
- Khayrani, A.C.; Mahmud, H.; Ko Oo, A.K.; Zahra, M.H.; Oze, M.; Du, J.; Alam, M.J.; Afify, S.M.; Abu Quora, H.A.; Shigehiro, T.; et al. Targeting Ovarian Cancer Cells Overexpressing Cd44 with Immunoliposomes Encapsulating Glycosylated Paclitaxel. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef]
- Skubitz, A.P.N.; Taras, E.P.; Boylan, K.L.M.; Waldron, N.N.; Oh, S.; Panoskaltsis-Mortari, A.; Vallera, D.A. Targeting CD133 in an in Vivo Ovarian Cancer Model Reduces Ovarian Cancer Progression. Gynecologic Oncology 2013, 130, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Oktem, O.; Oktay, K. The Ovary: Anatomy and Function throughout Human Life. Annals of the New York Academy of Sciences 2008, 1127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Database, S. The Human Protein Atlas Available online: https://www.proteinatlas.org/.
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Molecular Cell 2018, 72, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Russo, D.D.; Drake, R.S.; Duncan, F.E.; Shalek, A.K.; Goods, B.A.; Woodruff, T.K. Single-Cell Transcriptomics of Staged Oocytes and Somatic Cells Reveal Novel Regulators of Follicle Activation. Reproduction 2022, 164, 55–70. [Google Scholar] [CrossRef]
- Kocabas, A.M.; Crosby, J.; Ross, P.J.; Otu, H.H.; Beyhan, Z.; Can, H.; Tam, W.L.; Rosa, G.J.M.; Halgren, R.G.; Lim, B.; et al. The Transcriptome of Human Oocytes. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 14027–14032. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochemistry and Biophysics Reports 2021, 26, 100991. [Google Scholar] [CrossRef]
- Poley, M.; Mora-Raimundo, P.; Shammai, Y.; Kaduri, M.; Koren, L.; Adir, O.; Shklover, J.; Shainsky-Roitman, J.; Ramishetti, S.; Man, F.; et al. Nanoparticles Accumulate in the Female Reproductive System during Ovulation Affecting Cancer Treatment and Fertility. ACS Nano 2022, 16, 5246–5257. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles : What We Have Learned so Far ? Perspective 2010, 2313–2333. [Google Scholar] [CrossRef]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected Intracellular Biodegradation and Recrystallization of Gold Nanoparticles. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F.; Murphy, C.J. Gold-Based Therapy: From Past to Present. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 22639–22648. [Google Scholar] [CrossRef] [PubMed]
- Krusius, F.E.; Markkanen, A.; Peltola, P. Plasma Levels and Urinary Excretion of Gold during Routine Treatment of Rheumatoid Arthritis. Annals of the rheumatic diseases 1970, 29, 232–235. [Google Scholar] [CrossRef] [PubMed]
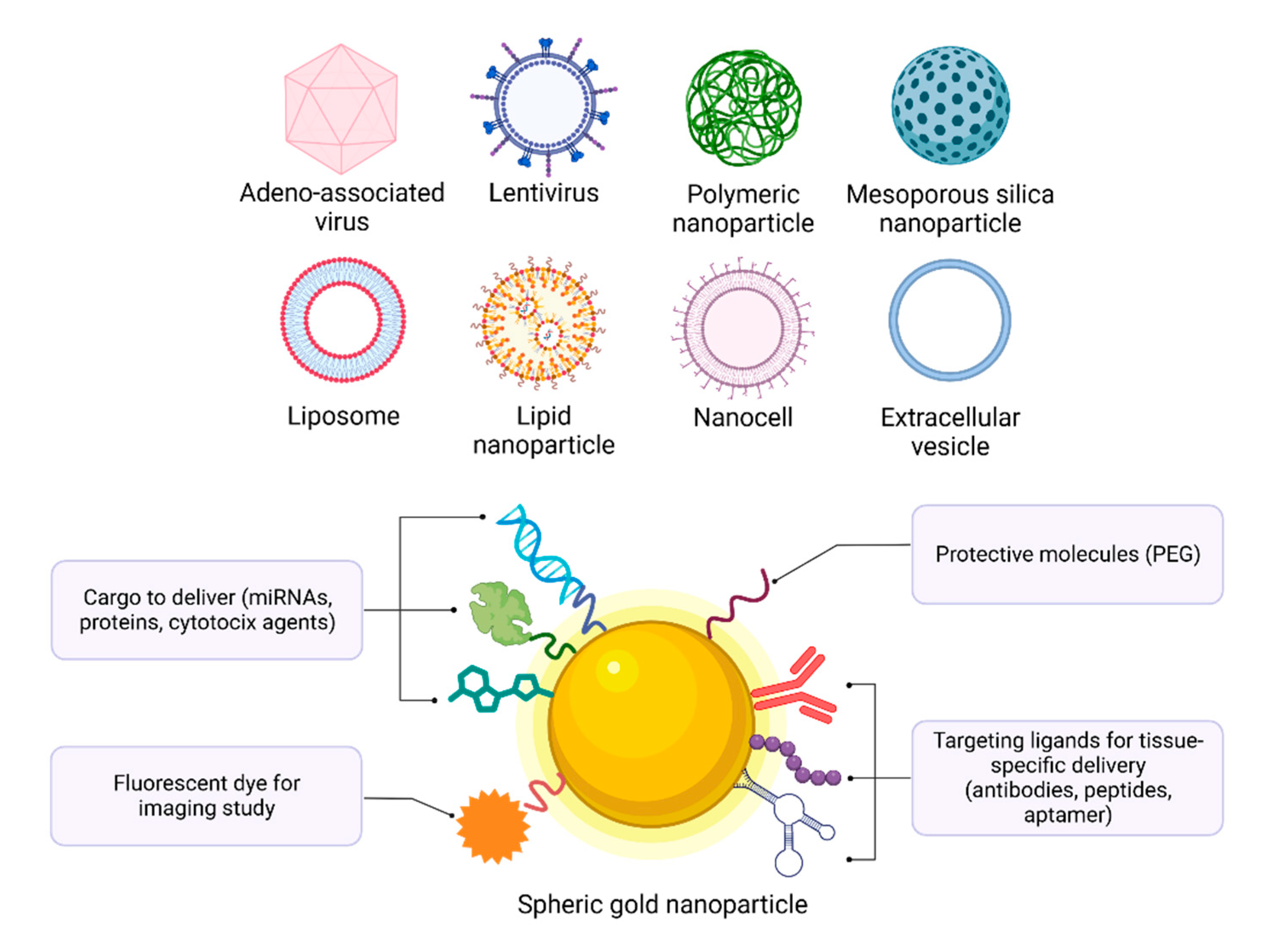
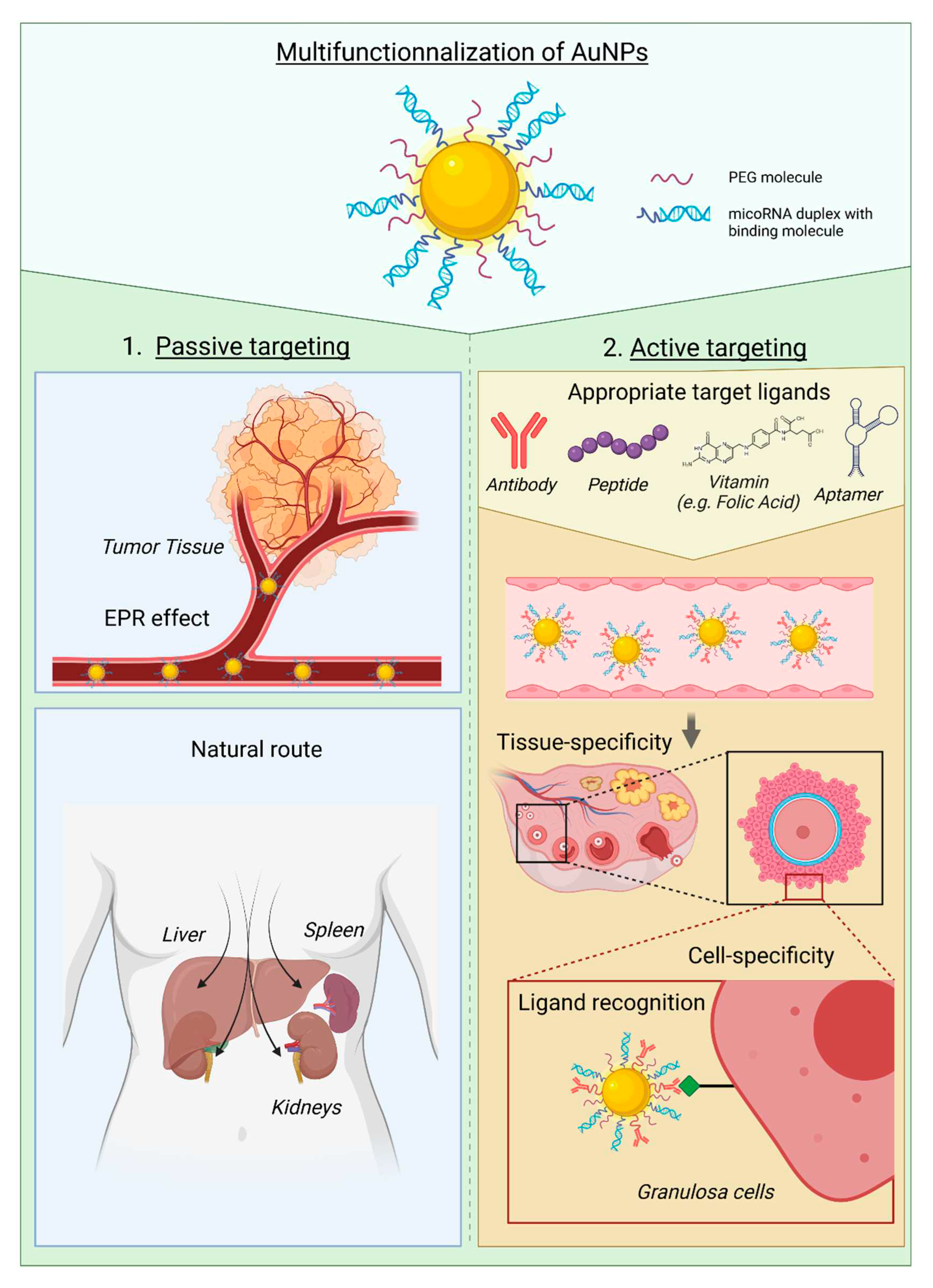
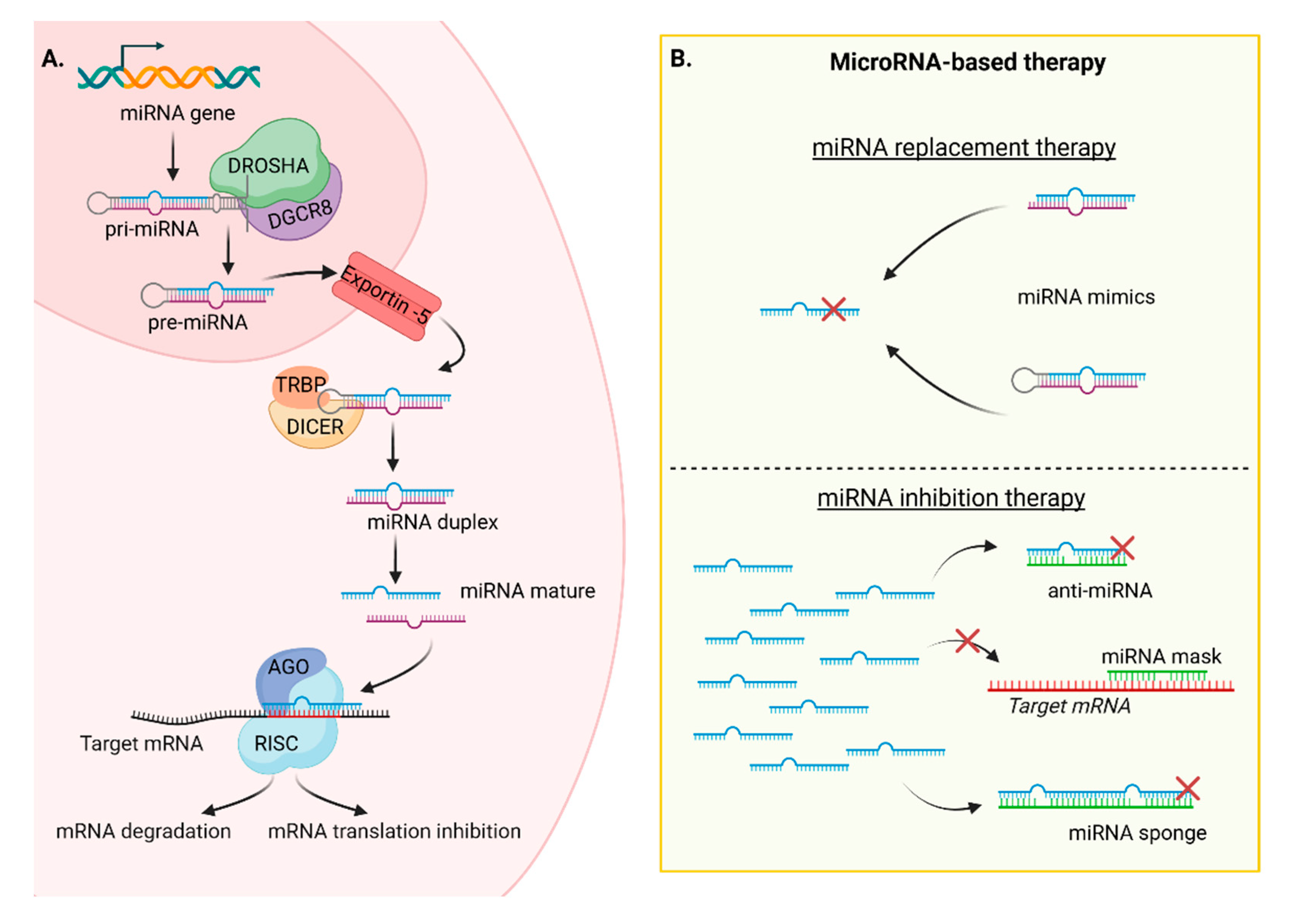
| Name | Molecule | AuNPs | Treatment | Clinical Phase |
|---|---|---|---|---|
| NU-0129 | RNAi for Bcl2L12 | 13 nm Thiolated PEG |
Gliobastoma | Phase 0 (NCT03020017) 2017-2020 |
| CYT-6091 | rhTNF | 27 nm PEGylated |
Various solid tumor |
Phase I (NCT00356980) 2006-2009 |
| C19-A3 | Proinsuline peptide | 5 nm | Type I diabetes | Phase I (NCT02837094) 2016- |
| Nano Swarna Bhasma |
Combination of phytochemicals | 35 nm | Breast cancer | Phase 0 DNA_SPN_B001_17 AYUSH |
| DengueTcP (EMX-001) |
Synthetic T cell-selective multivalent with dengue virus peptide antigens (vaccine) | 5 nm | Dengue fever | Phase I (NCT04935801) 2021- |
| Corona TcP | Betacoronavirus T cell-priming immune Vaccine |
5 nm | SARS-COV2 | Phase I (NCT05113862) 2022- |
| Name | Molecule | Delivery system | Treatment | Target | Clinical Phase |
|---|---|---|---|---|---|
| Miravirsen (RG101) |
Anti-miRNA-122 | LNA-antisense | Chronic hepatitis C |
Liver | Phase II (NCT01727934) 2012-2014 Unknown |
| MRX34 | miR-34 mimic | LNPs | Advanced solid tumors |
Tumor | Phase I (NCT02862145) 2016-2017 Withdrawn |
| MesomiR-1 | miR-16 mimic | EnGeneIC Dream Vectors | Malignant pleural mesothelioma |
Tumor expressing EGFR |
Phase I (NCT02369198) 2014-2017 Completed |
| Lademirsen (RG-012) |
Anti-miR-21 | Oligonucleotides modification |
Alport Syndrome | Kidney | Phase II Suspended |
| Cobomarsen (MRG-106) |
Anti-miR155 | LNA-antisense | Mycosis fungoides | Skin | Phase II (NCT03713320) 2019-2020 Terminated1 |
| TLV-associated adult T-cell lymphoma/leukemia, diffuse large B-cell lymphoma and chronic lymphocytic leukemia | Lymphatic system |
Phase I (NCT02580552) 2016-2020 Completed |
|||
| Remlarsen (MRG-201) |
miR-29 mimic | LNA-mimic | Fibrotic diseases | Phase II (NCT03601052) 2018-2020 Completed |
|
| Obefazimob (ABX464) |
miR-124 mimic | Capsule (oral administration) |
Active Rheumatoid Arthritis |
Immune system |
Phase II (NCT05177835) 2021- Recruiting |
| Ulcerative Colitis | Phase III (NCT05507203) 2022- Recruiting |
| Name | Abbreviation | Cell- Specificity supposed |
Model | Ovary RNA Expression1 |
Location |
|---|---|---|---|---|---|
| Alanine And Arginine Rich Domain Containing Protein |
AARD | GCs | Human | N/M | Intracellular |
| Aldehyde Dehydrogenase 1 Family Member A2 |
ALDH1A2 | GCs | Human | Endometrial stromal cells | Intracellular |
| Anti-Mullerian Hormone | AMH | GCs | Mouse and human | Granulosa cells | Secreted |
| Anti-Mullerian Hormone Receptor Type 2 |
AMHR2 | GCs | Human | Granulosa cells | Membrane, Intracellular |
| Bone Morphogenetic Protein 15 |
BMP15 | Oocytes | Human | N/M | Secreted |
| Bone morphogenetic protein receptor type 2 |
BMPR2 | Oocytes | Human | N/M | Membrane |
| Cytochrome P450 Family 11 Subfamily A Member 1 |
CYP11A1 | GCs | Mouse and human | N/M | Intracellular |
| Cytochrome P450 Family 19 Subfamily A Member 1 | CYP19A1 | GCs | Human | N/M | Membrane, Intracellular |
| Deleted In Azoospermia Like | DAZL | Oocytes | Mouse and human | Oocytes | Intracellular |
| DEAD-Box Helicase 4 | DDX4 | Oocytes | Mouse and human | Oocytes | Intracellular |
| Developmental Pluripotency Associated 3 |
DPPA3 | Oocytes | Human | Oocytes | Intracellular |
| Folliculogenesis Specific Bhlh Transcription Factor |
FIGLA | Oocytes | Human | Oocytes | Intracellular |
| Forkhead Box L2 | FOXL2 | GCs | Human | Granulosa cells, Ovarian stromal cells, Endometrial stromal cells | Intracellular |
| Follicle Stimulating Hormone Receptor |
FSHR | GCs | Human | Granulosa cells | Membrane, Intracellular |
| Follistatin | FST | GCs | Human | Granulosa cells | Secreted, Intracellular |
| Glycine Amidinotransferase | GATM | GCs | Human | Granulosa cells | Intracellular |
| Growth Differentiation Factor 9 | GDF9 | Oocytes | Human | Oocytes | Secreted, Intracellular |
| G Protein Subunit Gamma 13 | GNG13 | GCs | Human | N/M | Intracellular |
| H1.8 Linker Histone | H1FOO | OocytesGCs | HumanMouse | Oocytes | Intracellular |
| 3-Hydroxy-3-Methylglutaryl- Coa Synthase 2 |
HMGCS2 | GCs | Human | N/M | Intracellular |
| Inhibin Subunit Alpha | INHA | GCs | Mouse and human | Granulosa cells Ovarian stromal cells |
Secreted |
| Inhibin Subunit Beta A | INHBA | GCs | Mouse and human | N/M | Secreted |
| KIT Ligand | KITL | GCs | Human | N/M | Membrane, Intracellular |
| Keratin 8 | KRT8 | GCs | Human | N/M | Intracellular |
| Keratin 19 | KRT19 | GCs | Human | N/M | Intracellular |
| Luteinizing Hormone/ Choriogonadotropin Receptor |
LHCGR | GCs | Human | Ovarian stromal cells | Membrane, Intracellular |
| LIM Homeobox 8 | LHX8 | Oocytes | Mouse and human | Oocytes | Intracellular |
| NOBOX Oogenesis Homeobox | NOBOX | Oocytes | Human | Oocytes | Intracellular |
| Nucleophosmin/ Nucleoplasmin 2 |
NPM2 | Oocytes | Human | Oocytes | Intracellular |
| Oocyte-Specific Gene | OOG1 | Oocytes | Human | Oocytes | Intracellular |
| POU Class 5 Homeobox 1 | POU5F1 | Oocytes | Human | N/M | Intracellular |
| RNA Polymerase I Subunit A | RPO1 | GCs | Human | N/M | Intracellular |
| Spermatogenesis And Oogenesis Specific Basic Helix-Loop-Helix 1 |
SOHLH1 | Oocytes | Human | Oocytes | Intracellular |
| SRY-Box Transcription Factor 30 |
SOX30 | Oocytes | Mouse and human | N/M | Intracellular |
| Steroidogenic Acute Regulatory Protein |
STAR | GCs | Mouse and human | Ovarian stromal cells |
Intracellular |
| SUB1 Regulator Of Transcription |
SUB1 | Oocytes | Human | N/M | Intracellular |
| Synaptonemal Complex Protein 3 |
SYCP3 | Oocytes | Mouse and human | Oocytes | Intracellular |
| TATA-Box Binding Protein Associated Factor 7 Like |
TAF7L | Oocytes | Human | N/M | Intracellular |
| Transforming growth factor beta receptor 1 |
TGFBR1 | Oocytes | Human | N/M | |
| Uroplakin 3B | UPK3B | GCs | Human | N/M | Membrane, Intracellular |
| Y-Box Binding Protein 2 | YBX2 | Oocytes | Mouse and human | N/M | Intracellular |
| Zygote Arrest 1 | ZAR1 | Oocytes | Mouse and human | Oocytes | Intracellular |
| Zona Pellucida Glycoprotein 2 |
ZP-2 | Oocytes | Mouse and human | N/M | Secreted, Membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




