1. Introduction
Parkinson's disease (PD) was defined as a complex neurodegenerative disorder characterized by dopaminergic neuronal cell death in the substantia nigra [
1]. It is the second most common neuropathological condition after Alzheimer’s disease associated with significant disability and poor quality of life [
2]. Many factors such as aging, smoking and alcohol consumption are thought to be related to the initiation and progression of PD [
3,
4].
It was suggested that the effect of the aging process on the pathogenesis of PD was partially mediated by mitochondrial dysfunction [
5]. With aging, mitochondria become sensitive to environmental factors, so the possibility of mitochondrial dysfunction increases. A variety of mtDNA mutations lead to reduction of ATP production, increase free radicals and oxidative stress and cause mitochondrial dysfunction [
6]. As mitochondrial respiration is linked to ATP production, neurons and differentiated neuronal cells are dependent on oxidative phosphorylation and display a considerable mitochondrial respiratory capacity, to meet the requirements of increased functional demand and stress [
7]. Strong evidence for altered mitochondrial metabolism in human neurodegenerative diseases, and especially in PD patients, is still missing.
The implementation of reliable secreted biomarkers associated with motor or cognitive PD subtypes could allow accurate prediction of clinical outcomes. Recently, research on novel biomarkers in PD is expanding and data about prognostic cerebrospinal fluid (CSF) and blood biomarkers for prognosis are reported. They could help clinicians predict reliably patients’ survival and to determine the best choice of therapy. Furthermore, the potential advantage of biomarkers as drug targets in clinical trials is also discussed.
Chitinase-3-like protein 1 (CHI3L1/YKL-40) is considered as a marker for macrophage and microglial differentiation and activation [
8]. It is an extracellular matrix glycoprotein belonging to glycoside hydrolase family 18 [
9]. Because of the amino acid substitutions in the active site of chitinases, YKL-40 has no catalytic activity against chitinase substrates [
10]. Protein is normally expressed by multiple cell types such as macrophages, chondrocytes, and vascular smooth muscle cells. However, increased YKL-40 levels are detected in several solid tumors and chronic inflammatory diseases [
9,
11,
12].
Elevated CSF YKL-40 levels were reported in different infectious and noninfectious diseases of the CNS [
13]. Previous studies found that the YKL-40 protein may have a potential role as a promising biomarker reflecting the severity of inflammation in PD [
14]. There is no data available on YKL-40 levels and mitochondrial metabolism in PD patients.
Due to the complex nature of this disease, biomarkers with diagnostic and prognostic value are needed to reliably identify the early stages of PD and to stratify patients into clinical subtypes, to allow individualization and more effective application of therapy. In this context, in the present study, we aimed to search for a possible relationship between cellular bioenergetics and YKL-40 gene and protein levels.
2. Results
2.1. Patients
Eighteen newly diagnosed according to MDS-PD criteria, treatment naïve patients (aged 42 to 79) were included in this study. They had UPDRS score between 20 and 48, mean 33.39±8.261. According to Hoen – Yahr scale the degree of disability in the majority of the patients (15/18, 83.33%) was 1.5 (unilateral signs and involvement of the axial muscles), and 3 out of 18 (16.67%) had 1.0 (unilateral parkinsonian signs only).
2.2. Mitochondrial activity
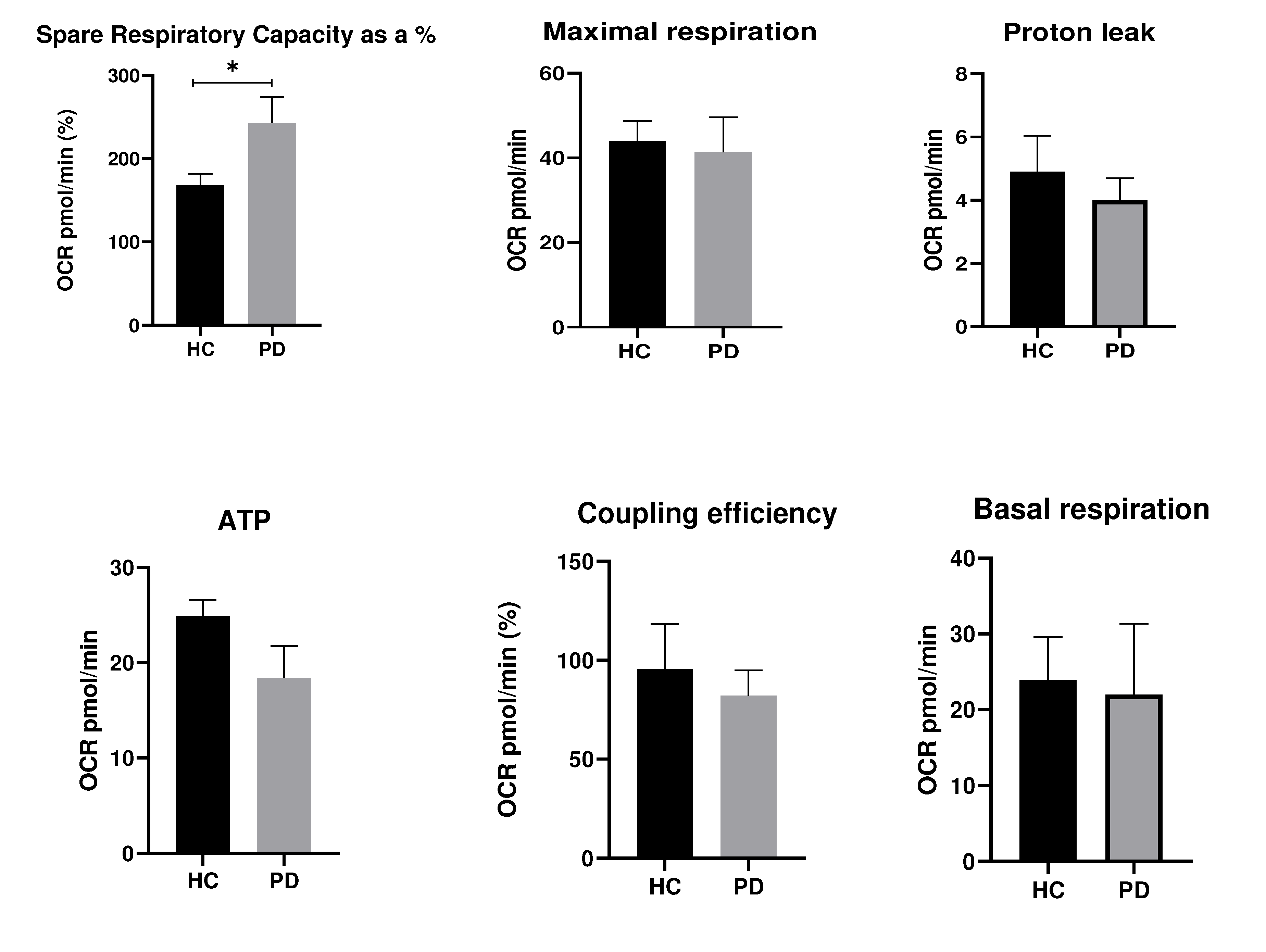
After measuring mitochondrial function in PD patients and healthy controls (HC), we obtained data on five metabolic parameters that best describe different factors influencing the ability of PBMCs to meet cell energy needs.
Our results show an almost two-fold increased spare respiratory capacity in PD patients compared to HC. This difference was statistically significant (P=0.038) and depicted the characteristic respiration pattern of PD PBMC (
Figure 1). The studied indicator in patients averaged 243%, while for HC it was 168%. However, the rest of the bioenergetic data showed a tendency for decreased values in PD. The difference in ATP-coupled respiration (p=0.194), coupling efficiency (p=0.161), maximal respiration (p=0.680) and proton leak (p=0.445) compared to HC, was not statistically significant. In addition, both PD PBMCs and HC PBMCs had a similar mean basal respiration without any statistical significance (p= 0.794), indicating the homogenous onset and the same conditions in studying the target groups. The results are shown in
Figure 1.
2.3. Gene and protein expression of YKL-40
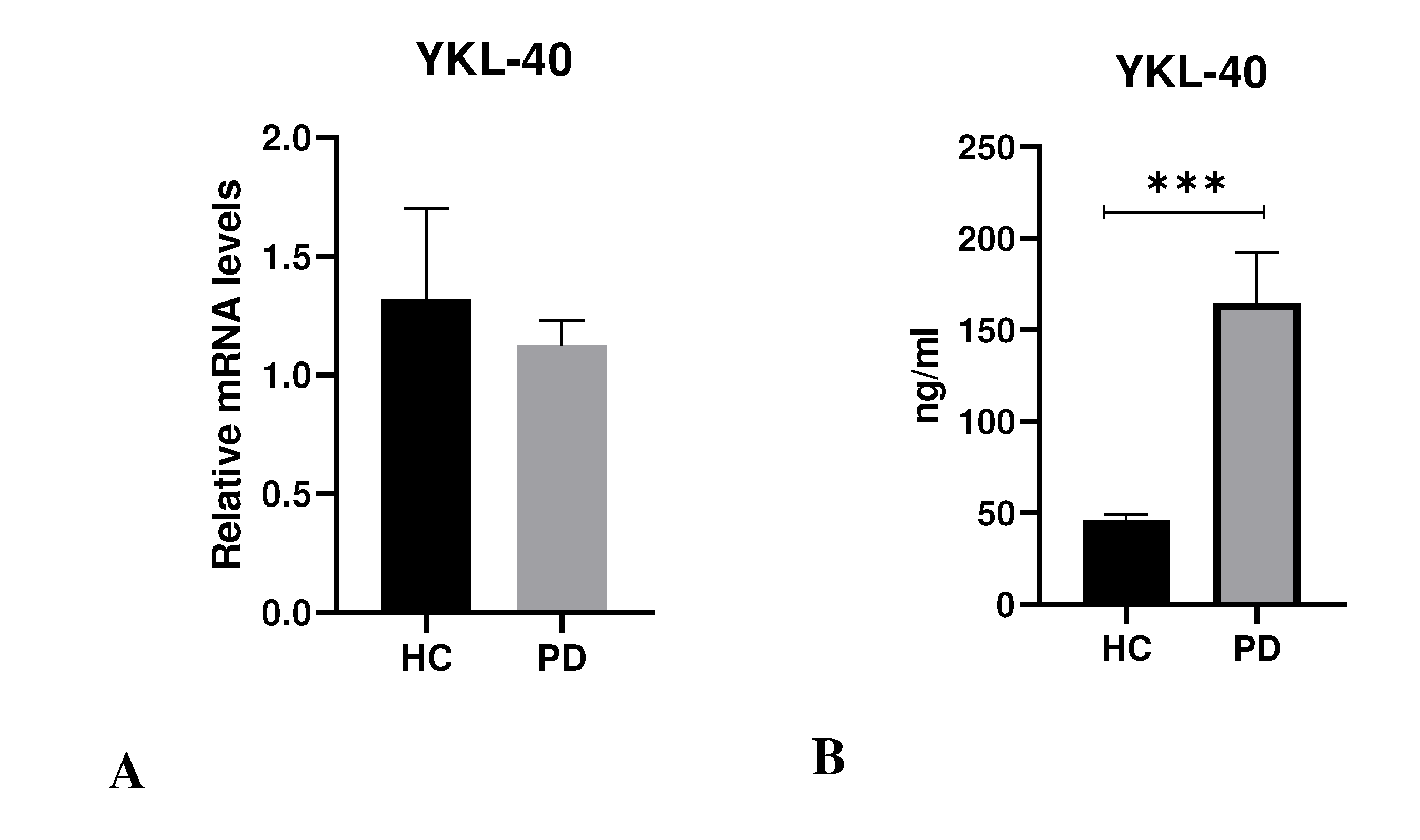
The expression levels of YKL-40 showed only a trend for decreased mRNA levels in patients compared to controls without any statistical significance (p=0.651) in contrast to protein levels (p=0.0001) (
Figure 2). Mean YKL-40 mRNA values in PD were 1.125, while in HC they were 1.317. Interestingly, YKL-40 plasma levels in patients with PD were three times higher than these of the HC. The reported mean values for protein levels in PD were 164.70 ng/ml, compared to the control group - 46.06 ng/ml.
2.4. Correlation analysis
A possible relationship between YKL-40 protein levels and bioenergetic parameters was serched. The glycoprotein was shown to be directly related to ATP production (R = 0.9, p = 0.012) and to basal respiration (R = 0.92, p = 0.008).
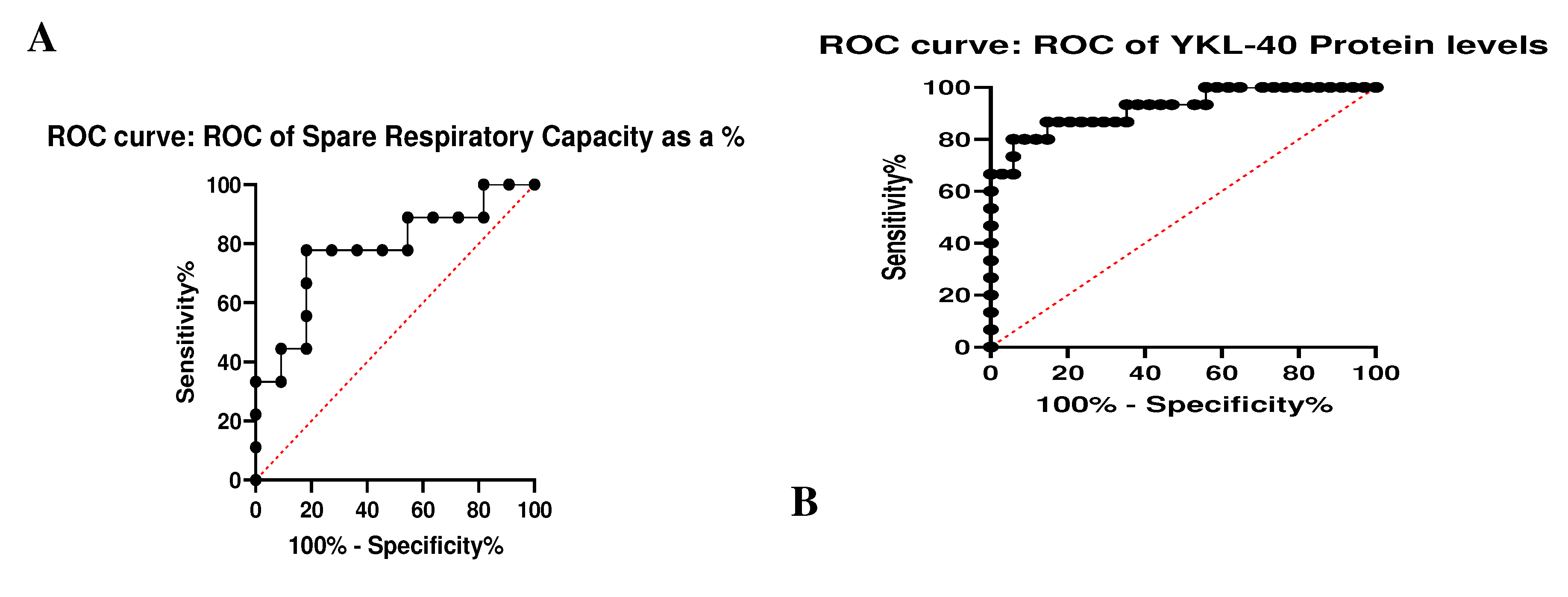
Receiver Operating Characteristic (ROC) analysis
To evaluate the predictive value of the two statistically significant indicators – the spare respiratory capacity and YKL-40 protein levels, ROC analysis was performed (
Figure 3). Our results revealed Sensitivity%- 66.08 and Specificity%-63.15 for the reserve respiratory capacity and Sensitivity%-- 79.13 and Specificity%-- 92.78 for p. Our data show that YKL-40 plasma levels appear to be an excellent marker for the differentiation of PD from the HC. The spare respiratory capacity also differentiates the studied groups but is less important than the plasma levels of the glycoprotein.
3. Discussion
A better knowledge of mitochondrial function is key for maintaining cellular homeostasis as these organelles serve as the hub of many metabolic pathways crucial for the progression of PD. A deeper understanding of clinical-pathological mechanisms underlying the disease is fun-damental for designing new strategies for therapeutic intervention in PD.
The data presented in our study show a characteristic PD bioenergetic profile [
15,
16] with an increased almost two-fold reserve respiratory capacity compared to HC. The results obtained are consistent with other studies reporting changes in mitochondrial activity in PD [
15]. The dif-ference between the examined groups is statistically significant (P=0.038) and shows that the reserve capacity is very sensitive to oxidative stress and is therefore a suitable marker for the de-tection of physiological abnormalities in neurodegenerative diseases [
17]. Schirinzi et al (2022) also reported increased reserve capacity and maximal respiration in PD that correlated with the severity of motor impairment and with CSF Aβ42 levels [
15]. The team hypothesizes that mito-chondrial respiration capacity may increase with PD progression and is interpreted as a compensa-tory bioenergetic activation or as a consequence of abnormal mitochondrial function [
15]. How-ever, we detected that the other bioenergetic parameters reduced in the patient group - ATP-coupled respiration, coupling efficiency, maximal respiration (p=0.680) and proton leak were not statistically significant. This suggests that mitochondrial function is suppressed as observed by Walter J et al. as well [
17]. The possible explanation is that the observed abnormalities in the investigated parameters are intrinsic to PD mitochondria or that their activity is related to other pathogenic processes leading to PD.
It is known that mitochondrial dysfunction can provoke deposition of α-synuclein and formation of Lewy bodies, as well as a neuronal loss [
18]. Altered astrocytic function also leads to α-synuclein accumulation, neuroinflammation, impaired mitochondrial metabolism and oxida-tive stress [
19]. Metabolic reprogramming resulted in less efficient production of ATP, and ROS generation [
20]. Neurons and differentiated neuronal cells are largely dependent upon oxidative phosphorylation and usually exhibit a substantial mitochondrial spare respiratory capacity, to rap-idly meet the requirements of increased functional demands and stress [
21]. Furthermore, respira-tory capacity features the grade of toxicity, ischemia, and oxidative stress [
21,
22]. As we expected, mitochondrial function was altered in our PD patients’. Our findings are in accordance with previous studies describing fluctuation in the OCR parameters in different neurological pa-thologies. It was found that the spare respiratory capacity in fibroblasts correlated with early neu-ropsychiatric symptoms in Alzheimer’s disease [
23]. This finding together with our data suggest that moderate changes in the activity of the electron transport chain in mitochondria can signifi-cantly affect the disease phenotype. Reduced mitochondrial ATP production was also found to correlate with the downregulation of the PD-related genes in human neurons [
24]. Another factor involved in PD development, α-synuclein, was proven to modulate the activity of ATP synthase through interaction with its α-subunit [
25].
In addition to mitochondrial changes, we report a correlation of bioenergetic indices and YKL-40 protein levels. We assume that YKL-40 might reflect different sides of the response to brain injury, such as neuroinflammation and brain damage. Many studies have reported that the levels of YKL-40 were associated with the number of cells involved in neurodegeneration and glial activation [
26]. It was found that the concentration of YKL-40 was lower in patients with PD compared to those with atypical Parkinson’s syndrome, but still higher than the levels in the control group. No correlations with disease stages or severity were observed in this study [
27].
Interestingly, the data regarding YKL-40 in PD remain controversial. Our results showed significantly increased plasma YKl-40 levels compared to HC without any important change at the mRNA level. We could propose that YKL-40 expression might be regulated post transcrip-tionally. In previous studies, a possible regulatory axis lncRNAs/miR-30e/YKL-40 was proven in sysyemic sclerosis [
28]. It is quite possible that this glycoprotein is post-transcriptionally regulated in this pathology too.
Plasma levels of YKL-40 were detected in neurodegenerative dementias. Significantly high-er plasma YKL-40 levels in Creutzfeldt-Jakob disease (CJD) with a moderate potential to dis-criminate CJD cases from controls was determined. Additionally, YKL-40 concentrations appear significantly higher at late disease stages [
29]. Furthermore, higher YKL-40 levels were related to the deterioration of cognitive abilities [
26]. On the contrary, some authors revealed lower levels of YKL-40 in patients with PD than in healthy controls or in other neurodegenerative conditions [
30].
4. Materials and Methods
4.1. Patients and controls
After signing a written informed consent in accordance with the instructions of the Ethics Committee at the Medical University of Plovdiv (Protocol № 6/2021) and following the principles of the Declaration of Helsinki, patients and healthy controls were recruited in the present study. It included 18 naïve PD patients and a control group (n=7) of age-matched healthy individuals from a Bulgarian cohort. Venous blood was collected in EDTA-Vacutainer monovette. The diagnosis was verified by a team of certified professionals from the Department of Neurology according to the internationally accepted criteria MDS-PD (Movement Disorder Society – Parkinson’s Disease, 2015) (Postuma et al; 2015) and the severity of the symptoms was assessed by UPDRS (Unified Parkinson's Disease Rating Scale)] and Hoen – Yahr scale. The full revised version of UPDRS contains 50 questions, divided into four parts: Nonmotor aspects of experiences of daily living, Motor experiences of daily living, Motor examination, Motor Complications. Each question is anchored with five responses. The total UPDRS-score ranges between 0 (normal neurological examination) and 199 (the highest severity of the symptoms). The Hoen–Yahr scale is a short five-point scale that emphasizes the lateralization of symptoms and the patients' need for assistance in performing activities of daily living.
The control group included volunteers without a history or clinical data of neurological diseases. According to the exclusion criteria, subjects with acute/chronic infectious/inflammatory and autoimmune diseases were not involved in the study. Demographic and medical history information was also collected.
4.2. Isolation of peripheral blood mononuclear cells (PBMC)
The peripheral blood was processed immediately and PBMCs were isolated using Pancoll (Pan Biotech Cat # P04-60500) according to the protocol established by the manufacturer. Cells were cultured overnight in RPMI-1640 medium (Pan Biotech Cat # P04-22100) with 10% FBS, 1% penicillin/streptomycin in a cell culture incubator at 37°C, 5% CO2, and high humidity. The number of cells and their viability were determined using an automatic counter “LUNA” (Logos Biosystems, Anyang, South Korea). Cells were diluted with RPMI-1640 to a final concentration of 2.105 cells per well and then plated in Seahorse microplates for analysis. Separated plasma was frozen and stored at -800 for subsequent analysis.
4.3. Metabolic analysis in real time
Mitochondrial activity was measured using a Seahorse XFp analyzer (Agilent, Santa Clara, CA, USA). The advantage of our study is that the cells are processed immediately after their isolation, which limits the negative effect of freezing. PBMCs were cultured on poly-D-lysine wells at a density of 2 × 105 cells/well. To verify that the cells were evenly distributed in a monolayer, the wells were visualized using an inverted microscope (Nikon eclipse TS 100, NIKON, Amstelveen, Netherlands).
4.4. Mito stress test
A mitochondrial stress test was performed according to recommendations of Agilent (Agilent, Santa Clara, CA, USA) and following the protocol established by the manufacturer. Before analysis, the growth medium was replaced with Seahorse XF medium (Agilent Seahorse, pH = 7.4), and PBMCs were incubated in a 37 °C incubator without CO2. Each sample was tested in triplicate, and the results were averaged. The analyzer measures oxygen consumption rate (OCR) as an indicator of mitochondrial respiration. The first measurement determines the baseline OCR followed by OCR after injection of the following inhibitors: oligomycin (1.5 µM), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (1 µM) (FCCP) and rotenone (0.5 µM), according to the manufacturer's instructions.
The use of the indicated inhibitors allows measurement of ATP-coupled respiration (oligomycin inhibits ATP synthase), maximal respiration (FCCP, reveals maximal ETC capacity), reserve respiratory capacity (difference between maximal respiration and basal respiration) and non-mitochondrial respiration (rotenone inhibits complex I).
4.5. YKL-40 gene expression by qPCR
Total RNA from white blood cells was extracted using Trizol (Invitrogen, No. 15596026) according to the manufacturer's instructions. The concentration of RNA was measured on a NanoDrop Nucleic Acid Quantification (Thermo Fisher Scientific, Walthan, MA, United Sates). cDNA was transcribed from 2 μg RNA using Genaxon GreenMasterMix (2x) (Genaxxon bioscienceGmbH, Ulm, Germany, Lot. No. M3023.0500) according to the kit‘s instructions. The primer combinations used for the longest RNA transcripts of CHI3L1 (YKL-40) were synthesized by Integrated DNA Technologies, (Leuven, Belgium). The sequences were as follows: Fw 5’-CTGCTCCAGTGCTGCTCT-3’, Rev 5’-TACAGAGGAAGCGGTCAAGG-3’. RNA normalization was performed using several internal controls: GAPDH (Fw 5'-AGG TCCACCACTGACACGTTG-3', Rw5'-AGCTGAACGGGATGCTCACT-3'), ACTINβ (Fw 5'-AGTGTGACG TGGACATCCGGA-3', Rev 5'- GCC AGGGCAGTGATCTCCTCCT-3') and hUBC (Fw 5'TCCTCAGGCAGAG-GTT GATCTT-3', Rev 5'-GGACCAAGTGCAGAGGTGGACTCTT-3') (Integrated DNA Technologies, Leuven, Belgium). The qPCR reactions were run on Rotor-Gene Q 600 (Qiagen, Germany) and quantitative evaluations were performed by the 2−△△Ct method. Each sample was analyzed 2 times.
4.6. Detection of YKL-40 in plasma
Plasma was isolated from peripheral blood after centrifugation and then supernatant was frozen at -80 oC. It was then used to measure YKL-40 levels with an ELISA kit, specific for the protein encoded by the longer isoform (383 amino-acids) (MicroVue YKL-40, Quidel, 9975 Summers Ridge Road, San Diego, CA92121, USA, Lot. No. 088337), following the manufacturer's instructions. The assay employs the sandwich-based ELISA method with optical density measured at 405 nm.
4.7. Statistical analysis
Preprocessing of OCR values was performed using Wave Software (Seahorse Bioscience, Agilent, (available at
https://www.agilent.com/). Statistical analyzes of data were performed with Graphpad Prism. Differences between normally distributed variables were evaluated for significance using Welch's t-test for independent samples or paired t-test for dependent samples and Wilcoxon-Mann-Whitney test. Significance threshold was set at p-value < 0.05.
5. Conclusions
Diagnosis of PD relies almost entirely on evaluating the clinical presentations of the disease. The efforts in clarifying the complex interplay between neuroinflammation and mitochondrial metabolism in PD development still do not provide convincing evidence. In the present study we report high concentrations of plasma YKL-40 related to bioenergetic parameters. In addition, we found an increased respiratory reserve capacity, which defines a characteristic PD profile. We suggest that YKL-40 levels and respiratory reserve capacity may serve as markers to distinguish PD from healthy controls. Larger cohorts and additional metabolic analysis are needed to definitively confirm a possible relationship between peripheral mitochondrial function and plasma levels of YKL-40. In conclusion, our study reveals new aspects of a possible link between the YKL-40 glycoprotein and mitochondrial function of PD PBMC. The development of clinically relevant biomarkers to aid diagnosis represents a huge challenge in PD due to its heterogeneous phenotype and multifactorial etiology. We consider that YKL-40 protein levels in combination with the dynamics in mitochondrial metabolism might serve as an additional tool to monitor in-flammatory activity and the clinical course of PD.
Author Contributions
Conceptualization, V.S. and M.K.; methodology, M.G.; software, M.G.; formal analysis, M.G.; investigation, M.K.; M.G.; writing—original draft preparation, M.G; M.K..; writing—review and editing, V.S.; supervision, V.S.; A.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The study was financed by European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0007-C01
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the of the Ethics Committee at the Medical University of Plovdiv (Protocol № 6/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalia, V.; Lang, E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Váradi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson's disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.; Kanaan, M.; Kordower, H. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat. Rev. Neurosci. 2011 12, 359–366. [CrossRef]
- Park, S.; Davis, L.; Sue, M. ; Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. 2018, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Rango, M.; Bresolin, N. Brain mitochondria, aging, and Parkinson's disease. Genes. 2018, 9, 250. [Google Scholar] [CrossRef]
- Yadava, N.; Nicholls, G. ; Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J. Neurosci. 2007, 27, 7310–7317. [Google Scholar] [CrossRef]
- Renkema, G.H.; Boot, R.G.; Au, F.L.; Strijland, A.; Muijsers, A.O.; Hrebicek, M.; Aerts, J.M.F.G.; Donker-Koopman, W.E. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. JBIC J. Biol. Inorg. Chem. 1998, 251, 504–509. [Google Scholar] [CrossRef]
- Jensen B., V.; Johansen J., S.; Price P., A. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin. Cancer Res. 2003, 9, 4423–4434. [Google Scholar]
- E Hakala, B.; White, C.; Recklies, A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar] [CrossRef]
- Brasso, K. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate 2006, 66, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Shao, R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front. Physiol. 2013, 4, 122. [Google Scholar] [CrossRef]
- Prakash, M.; Bodas, M.; Prakash, D.; Nawani, N.; Khetmalas, M.; Mandal, A. Diverse pathological implications of YKL-40: answers may lie in ‘outside-in’ signaling. Cell Signal 2013, 25, 1567–73. [Google Scholar] [CrossRef] [PubMed]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Salvatori, I.; Zenuni, H.; Grillo, P.; Valle, C.; Martella, G.; Mercuri, N.B.; Ferri, A. Pattern of Mitochondrial Respiration in Peripheral Blood Cells of Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10863. [Google Scholar] [CrossRef]
- Minchev D, Kazakova M, Sarafian V. Neuroinflammation and Autophagy in Parkinson’s Disease—Novel Perspectives. Int. J. Mol. Sciences. 2022, 23, 14997. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Bolognin, S.; Antony, P.; Nickels, S.; Poovathingal, S.; Salamanca, L.; Magni, S.; Perfeito, R.; Hoel, F.; Qing, X.; Jarazo, J. Neural stem cells of Parkinson's disease patients exhibit aberrant mitochondrial morphology and functionality. Stem cell reports, 2019, 14, 878–89. [Google Scholar] [CrossRef]
- Valdinocci, D.; Simões, R.F.; Kovarova, J.; Cunha-Oliveira, T.; Neuzil, J.; Pountney, D.L. Intracellular and Intercellular Mitochondrial Dynamics in Parkinson’s Disease. Front. Neurosci. 2019, 13, 930. [Google Scholar] [CrossRef]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Booth, H.D.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Yadava, N.; Nicholls, G. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J. Neurosci. 2007, 4, 7310–7. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.; Choi, S.; Day, N.; Gerencser, A.; Hubbard, A.; Melov, S. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free Radic. Biol. Med. 2011, 1, 866–73. [Google Scholar] [CrossRef]
- Bell, S.M.; De Marco, M.; Barnes, K.; Shaw, P.J.; Ferraiuolo, L.; Blackburn, D.J.; Mortiboys, H.; Venneri, A. Deficits in Mitochondrial Spare Respiratory Capacity Contribute to the Neuropsychological Changes of Alzheimer’s Disease. J. Pers. Med. 2020, 10, 32. [Google Scholar] [CrossRef]
- Gandhi, P.; Chen, S.; Wilson-Delfosse, A. Leucine-rich repeat kinase 2 (LRRK2): a key player in the pathogenesis of Parkinson's disease. J. Neurosci. Res. 2009, 1, 1283–95. [Google Scholar] [CrossRef]
- Ludtmann, M.H.; Angelova, P.R.; Ninkina, N.N.; Gandhi, S.; Buchman, V.L.; Abramov, A.Y. Monomeric Alpha-Synuclein Exerts a Physiological Role on Brain ATP Synthase. J. Neurosci. 2016, 36, 10510–10521. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Janelidze, S.; Surova, Y.; Hansson, O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci. Rep. 2018, 5, 13276. [Google Scholar] [CrossRef]
- Magdalinou, N.K.; Paterson, R.W.; Schott, J.M.; Fox, N.C.; Mummery, C.; Blennow, K.; Bhatia, K.; Morris, H.R.; Giunti, P.; Warner, T.T.; et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Dichev, V.; Mehterov, N.; Kazakova, M.; Karalilova, R.; Batalov, A.; Sarafian, V. The lncRNAs/miR-30e/CHI3L1 Axis Is Dysregulated in Systemic Sclerosis. Biomedicines 2022, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Villar-Piqué, A.; Schmitz, M.; Hermann, P.; Goebel, S.; Bunck, T.; Varges, D.; Ferrer, I.; Riggert, J.; Llorens, F.; Zerr, I. Plasma YKL-40 in the spectrum of neurodegenerative dementia. J. Neuroinflamm. 2019, 16, 1–5. [Google Scholar] [CrossRef]
- Olsson, B.; Hertze, J.; Lautner, R.; Zetterberg, H.; Nägga, K.; Höglund, K.; Basun, H.; Annas, P.; Lannfelt, L.; Andreasen, N.; Minthon, L. Microglial markers are elevated in the prodromal phase of Alzheimer's disease and vascular dementia. J. Alzheimer's Dis. 2013, 1, 45–53. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).