Introduction
The locations where tendons, ligaments, fasciae, and joint capsules attach to the bone are called entheses. Pathologies of the entheses, primarily structural defects, are called enthesopathies (Sudoł-Szopińska, 2015). Enthesopathies are characterized by inflammation or degenerative changes at the entheses. Enthesopathies of the lower extremities are extremely common in adult populations regardless of athletic background. They are divided into repetitive mechanical stress and diffuse inflammatory response categories. The overall incidence is difficult to calculate given the varied nature, presentation, and etiology, but a general rise in the incidence rate has occurred over the past decade (Alvarez, 2023)(Ko, 2023). There are approximately 2.35 per 1000 adults between the ages of 21 and 60-years-old who have achilles tendinosis (Albers, 2016). Nearly 6% of the general population will experience Achilles Tendinosis in their lifetime (Maffulli, 2019). The high incidence rate of Achilles Tendinosis emphasizes the significance of the condition and the need for effective treatment options.
Achilles tendinosis is a combination of localized pain, swelling of the Achilles tendon, and loss of function. While Achilles tendonitis is rampant among athletes, it is not an exclusive pathology to athletes. Approximately 65% of Achilles injuries diagnosed in general practice are not sport-related (Silbernagel, 2020). Enthesopathy can be caused by a loss of fibrillar structure secondary to edema or mineralization, which leads to calcification and ossification, diffuse inflammatory disease, or altered structure of collagen fibers leading to a thickened enthesis (Alvarez, 2023). Factors leading to Achilles tendinosis include mechanical stress, poor blood supply from the calcaneal insertion, diminished collagen density, age, and trauma. Diagnostic measures typically include assessing the history of the symptoms, physical exam focusing on tenderness and decreased range of motion, and imaging modalities including ultrasound and MRI (Longo, 2009). This report focuses on addressing the loss and damage of structural tissues with a Wharton’s jelly tissue allograft and encouraging the body’s natural healing processes with EPAT and laser therapies.
Standard care practices for Achilles tendinosis vary from over-the-counter anti-inflammatories or corticosteroid injections with functional rehabilitation therapy to surgical intervention in severe cases. Standard non-invasive treatment options include activity modification, orthotics, heel lifts, massage, hot and cold compresses, strengthening exercises, ultrasound, and NSAIDs or oral corticosteroids. However, given that there are no prostaglandin inflammatory mediators in Achilles tendonitis, the efficacy of NSAIDs is questionable (Lopez, 2015). Despite several conservative treatment options, one in four patients will require surgery (Feeney, 2022). Between 24% to 45.5% of patients with Achilles tendinosis have failed conservative treatment, leading to the need for surgical intervention. However, poor postoperative results require a greater reoperation risk as the condition progresses. Best practices are still widely debated as the Achilles tendon’s pain mechanisms are poorly understood (Alfredson, 2007). It is common for surgeons to experience patients with significant retrocalcaneal exostosis and no pain as frequently as the reverse circumstance. Without the sensation of pain, promptly identifying Achilles tendinopathy may be difficult for some patients, resulting in further complications.
Symptom improvement with the standard of care typically occurs between 3 and 12 months but not beyond 12 months and incurs the need for research to find an optimal treatment (Vlist, 2021). Recent literature has demonstrated promising alternative interventions, including extracorporeal pulse wave therapy, laser therapy, and regenerative tissue allograft supplementation (Saxena, 2011) (Bjordal, 2006). Shock wave therapy creates microtraumas to stimulate neovascularization, producing greater blood flow and promoting tissue improvement. The benefits of laser therapy include enhanced adenosine triphosphate production, cell function, and protein synthesis (Lopez, 2015). WJ’s composition of growth factors, cytokines, and collagen matrix provides excellent potential in catalyzing the supplementation and repair of wounds and musculoskeletal injuries (Gupta, 2020). This retrospective case study aims to present novel application techniques and preliminary evidence of the efficacy and safety of EPAT, Wharton’s jelly, and lasers on a patient who has failed standard-of-care practices for over two years.
Materials and Method
All methods complied with the FDA and American Association of Tissue Banks (AATB) standards. This study was conducted under an Institute of Regenerative and Cellular Medicine IRB-approved protocol (RL-UCT-001), and informed consent was obtained from the study participants. Wharton’s jelly tissue allograft was processed and distributed by Regenative Labs. Patient recruitment, allograft application, and patient tracking were performed at Parker Foot and Ankle.
Human umbilical cords were obtained from consenting mothers following full-term Caesarian section deliveries. Prior to delivery, birth mothers underwent comprehensive medical, social, and blood testing. An independent certified laboratory tested all of the donations for infectious disease in accordance with Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 CFR part 493, and FDA regulations. Each birth mother was tested for hepatitis B core antibody (HBcAb), hepatitis B surface antigen (HBsAg), hepatitis C antibody (HCV), human immunodeficiency virus antibody (HIV-1/HIV-2 Plus O), human T-lymphotropic virus antibody (HLTV-I/11), syphilis (RPR), HIV-1/HCV/HBV, NAT, and West Nile virus (WNV). Each test was performed with an FDA-approved testing kit (see
Appendix A). All test results were required to be negative or non-reactive before processing the umbilical cord tissue.
Wharton’s jelly was aseptically dissociated from the rinsed umbilical cord. After dissociation, 150 mg of Wharton’s Jelly was suspended in approximately 2 mL of sterile sodium chloride 0.9% solution (normal saline). Dimethyl sulfoxide (DMSO) was added to the suspension as a cryoprotectant. The volume of DMSO was calculated as 5% of the total suspension volume. The cryoprotectant functions to preserve the integrity of the fibroblasts, pericytes, immune cells, and growth factors of the allograft while being stored in −40 °C freezers. [
20] The sample was not combined with cells, tissues, or articles other than the exceptions outlined in 21 CFR Part 1271.10 (a) (3) (Human Cells, Tissues, and Cellular and Tissue-Based Product Regulation).
Case Presentation
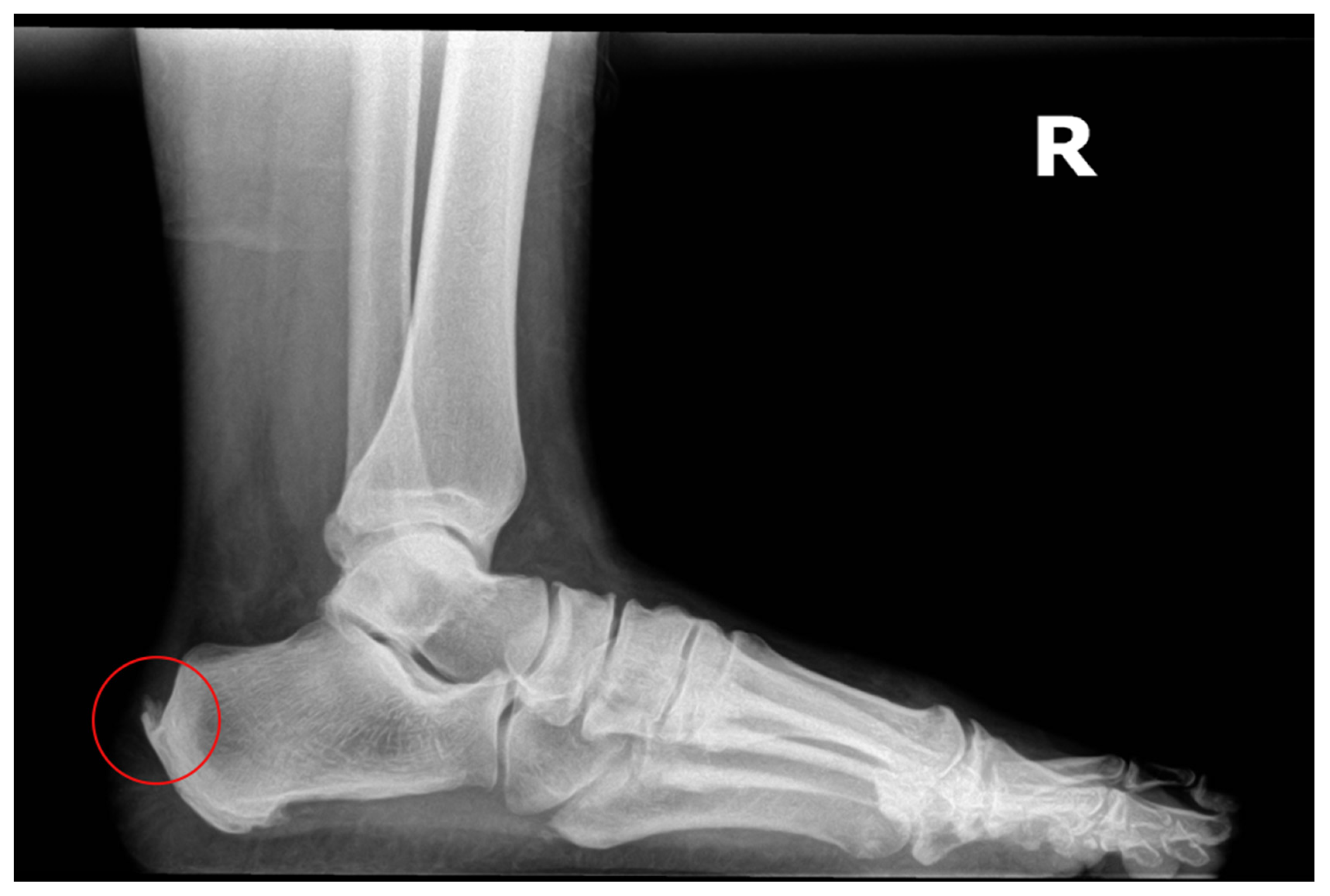
The patient in this study is a 54-year-old female with slow-onset chronic Achilles tendinosis, enthesopathy at the insertion, and retrocalcaneal exostosis. She has been suffering from her condition for three years, which has been progressively worsening. She has failed several standard care treatment modalities, including rest and two procedures. She presented with a retrocalcaneal exostosis of the right leg, severe pain, and declining surgical intervention (
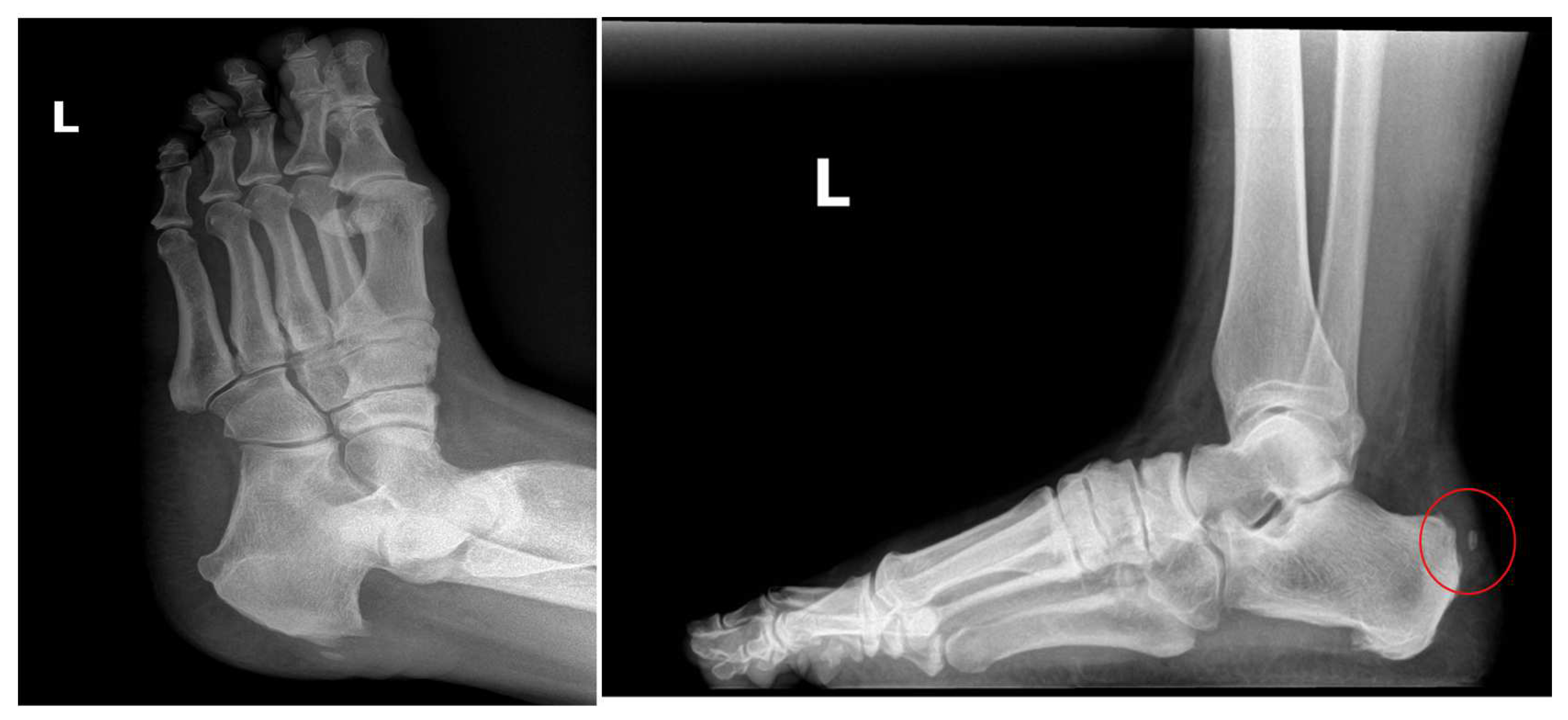
Figure 1). Previously, the patient underwent two procedures performed by previous surgeons: one successful inferior calcaneal exostectomy and one minimally successful retro calcaneal resection of the contra-lateral foot (
Figure 2). Her work requires her to be on her feet 75% of the time, further aggravating the disorder and causing sharp burning pain. Exacerbating factors include ambulating and standing. To avoid surgery and loss of productive time at work, she sought treatment from Dr. Robert Parker at his clinic, Parker Foot and Ankle, in Houston, Texas. There, she was offered an alternative conservative Wharton’s Jelly structural connective tissue to supplement the structural defects in conjunction with EPAT and class IV laser therapy to promote her body’s own repair processes. This study aims to report pain alleviation secondary to supplementing damaged tendon tissue with Wharton’s jelly and priming the local tendon with EPAT and lasers.
Patient Care Procedures
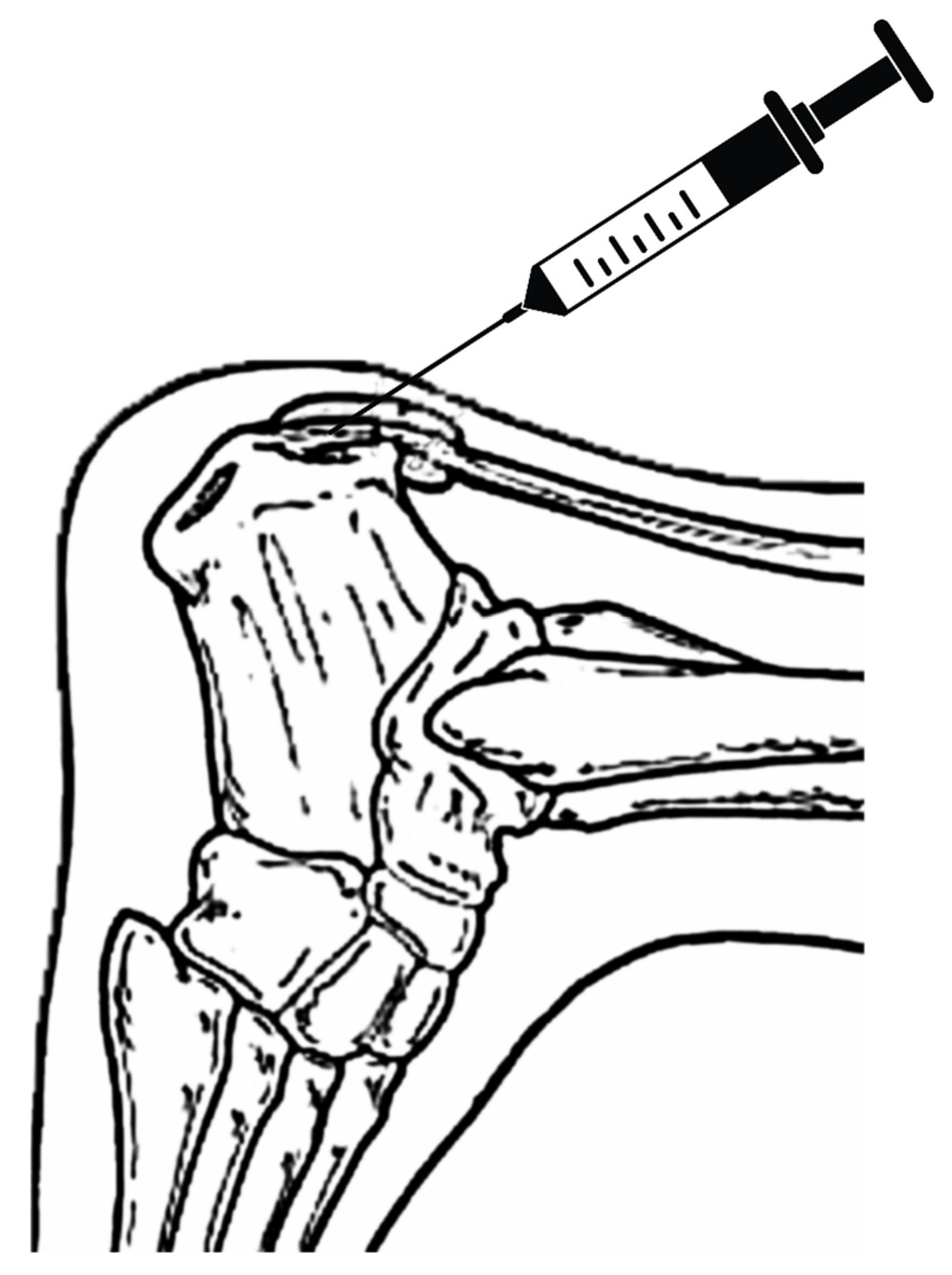
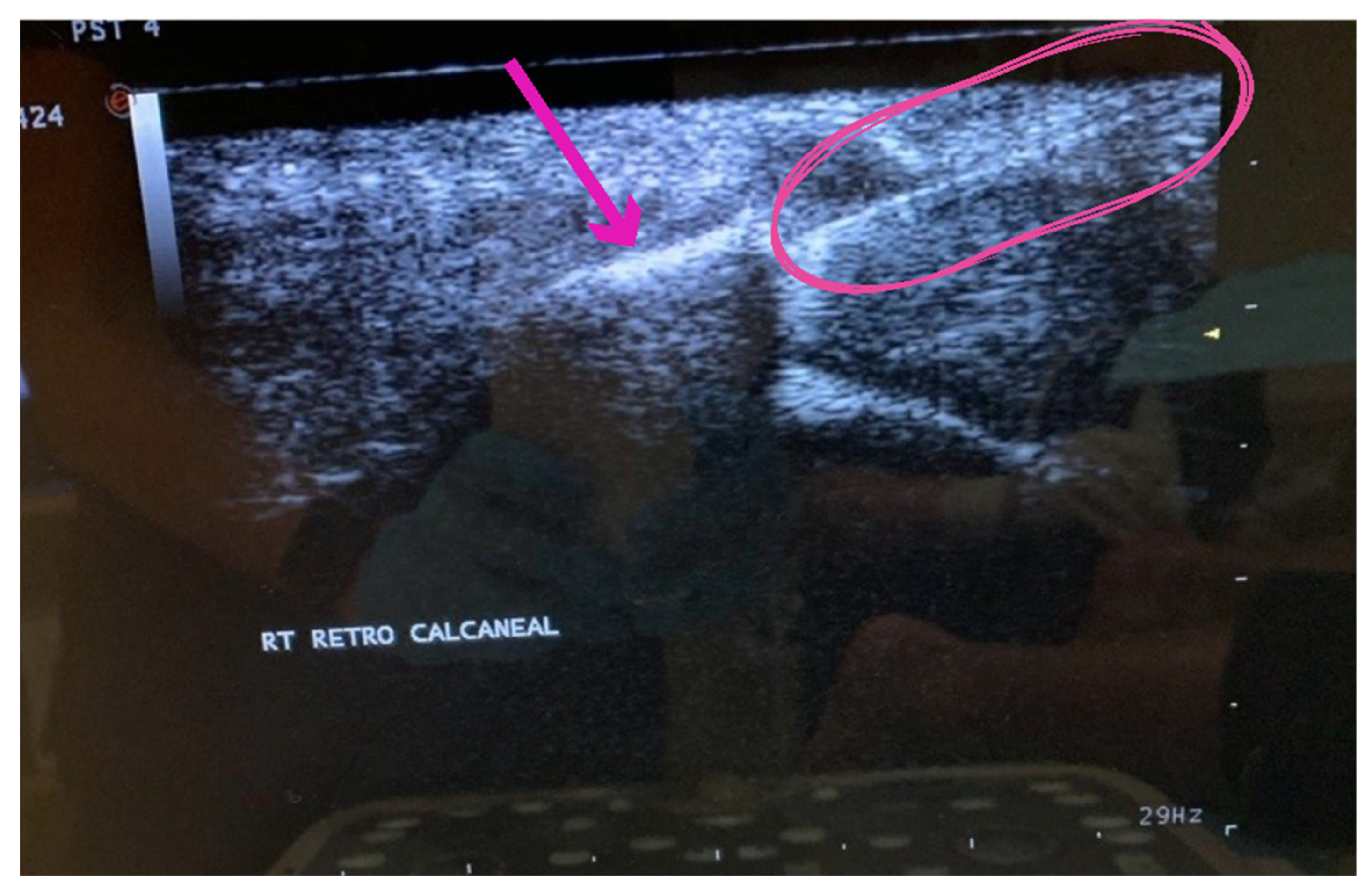
The procedure included the application of an umbilical cord tissue matrix, also known as Wharton’s Jelly, extracorporeal pulsed-activated therapy (EPAT), and class IV laser therapy. The patient was placed in the prone position without a tourniquet. It is essential to place the local anesthetic of choice more proximal away from the area to be treated. Lidocaine, 1% plain, was utilized to block the retrocalcaneal nerves, including the posterior tibial and sural nerves. The lower extremity was prepped and draped in the standard sterile technique. Prior to the application of the tissue allografts, the patient received EPAT at 11Hrtz, 1.4 bars, for 3521 pulses to the affected tissue. While the above EPAT was performed, 2ml of cryotext, a minimally manipulated tissue allograft, was thawed slowly per laboratory guidelines in a 35-degree bath. Under real-time ultrasound guidance using Esoate My Labs 15.0 MHz with a 4cm transducer head (
Figure 3,
Figure 4 and
Figure 5), the Wharton’s jelly allograft was transplanted along the insertion (enthesis) in the frontal plane in a fanning-like technique as well as fanning in the sagittal plane. This application was accomplished just proximal within the intratendinous area beneath the paratenon around the tendon proper by strategic supplementation, as well as between the paratenon and circumferentially around the tendon proper. Further “needling” with a 22 gauge needle was performed to encourage neovascularization. The patient was instructed not to take NSAIDs or steroids and to refrain from high-impact activities. The patient was given acetaminophen to take as needed. The patient was scheduled for biweekly class IV laser treatments for the next three weeks to provide photobiomodulation. A pneumatic boot was fitted for two weeks.
Results
The patient began treatment on 3/21/2023, reporting a visual analog scale (VAS) pain involvement of 10/10. Upon follow-up examination on week 5 (4/25/2023), the patient reported an 80% improvement in functionality with VAS pain involvement of 2/10, noting most residual pain presented in the mornings. At the final exam on week 13 (6/20/2023), the patient reported VAS pain involvement of 0/10, with her only complaint being that the two weeks of wearing the pneumatic boot caused acute hip pain. The use of an equivalent height heel or “level up” device was advised on the contralateral foot to relieve the stress on her hip. The patient will be tracked for any recurrence of pain over the next six months.
Discussion
These preliminary observations on using umbilical cord structural tissue allografts as a tissue supplementation in combination with EPAT and class IV laser therapy show promising improvement in patient-reported pain and functionality. EPAT prepares the tissue for allograft incorporation by sending acoustic waves through the tissue to promote the regenerative growth factors secreted by the body to begin repairing the tissues. The WJ allograft, applied in a fanning technique to agitate the surrounding tissue and distribute the allograft evenly, supplements the damaged tissues of the tendon. In addition, the contact the needle makes in the tissue stimulates new blood vessels in the tissue, allowing for accelerated blood flow to deliver nutrients and oxygen to catalyze the process of tissue regrowth. The follow-up with Laser therapy has been shown to increase microcirculation and ATP production and lower inflammatory markers. With each modality providing independent improvement, combining the three modalities has excellent potential for success.
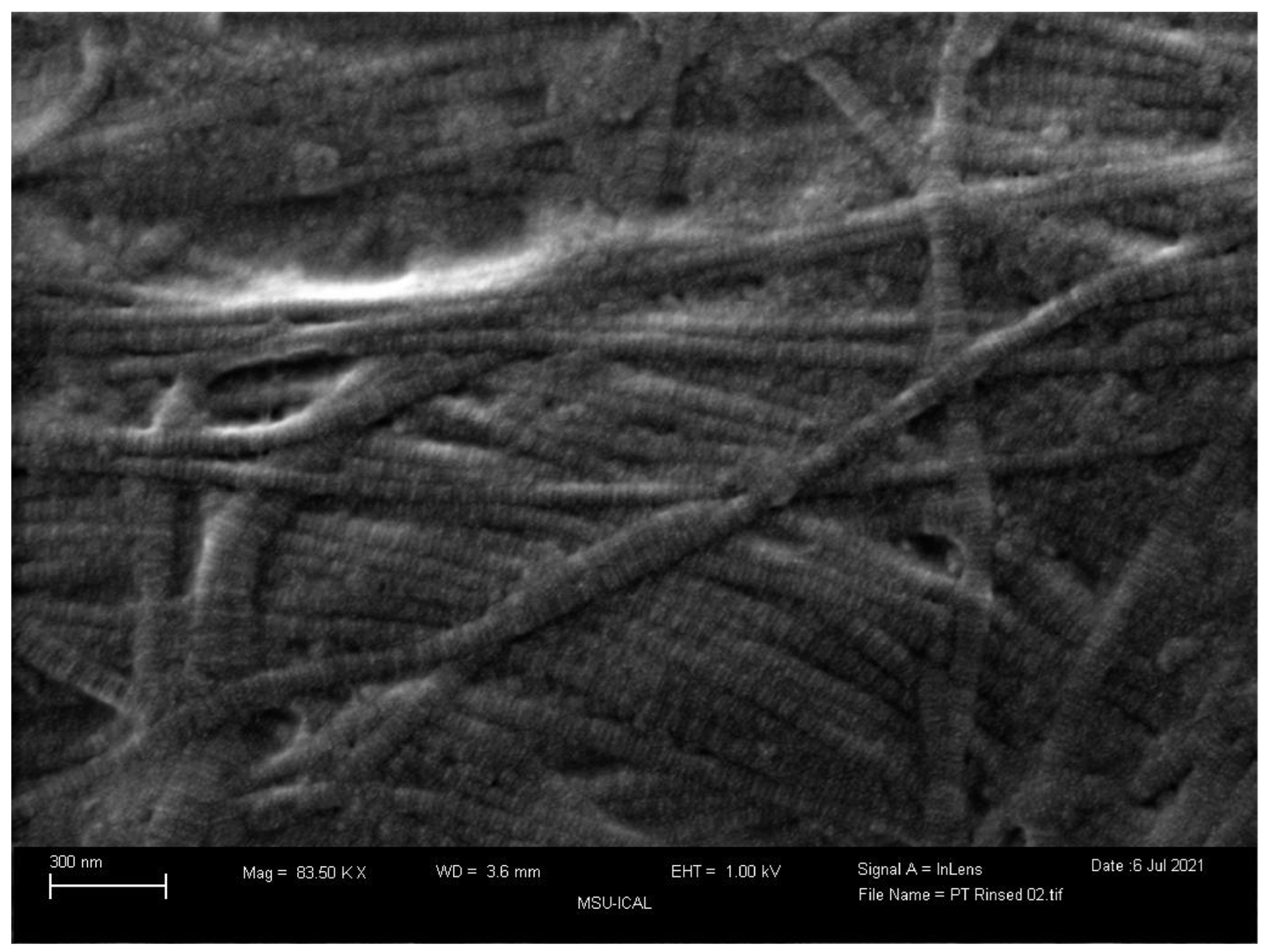
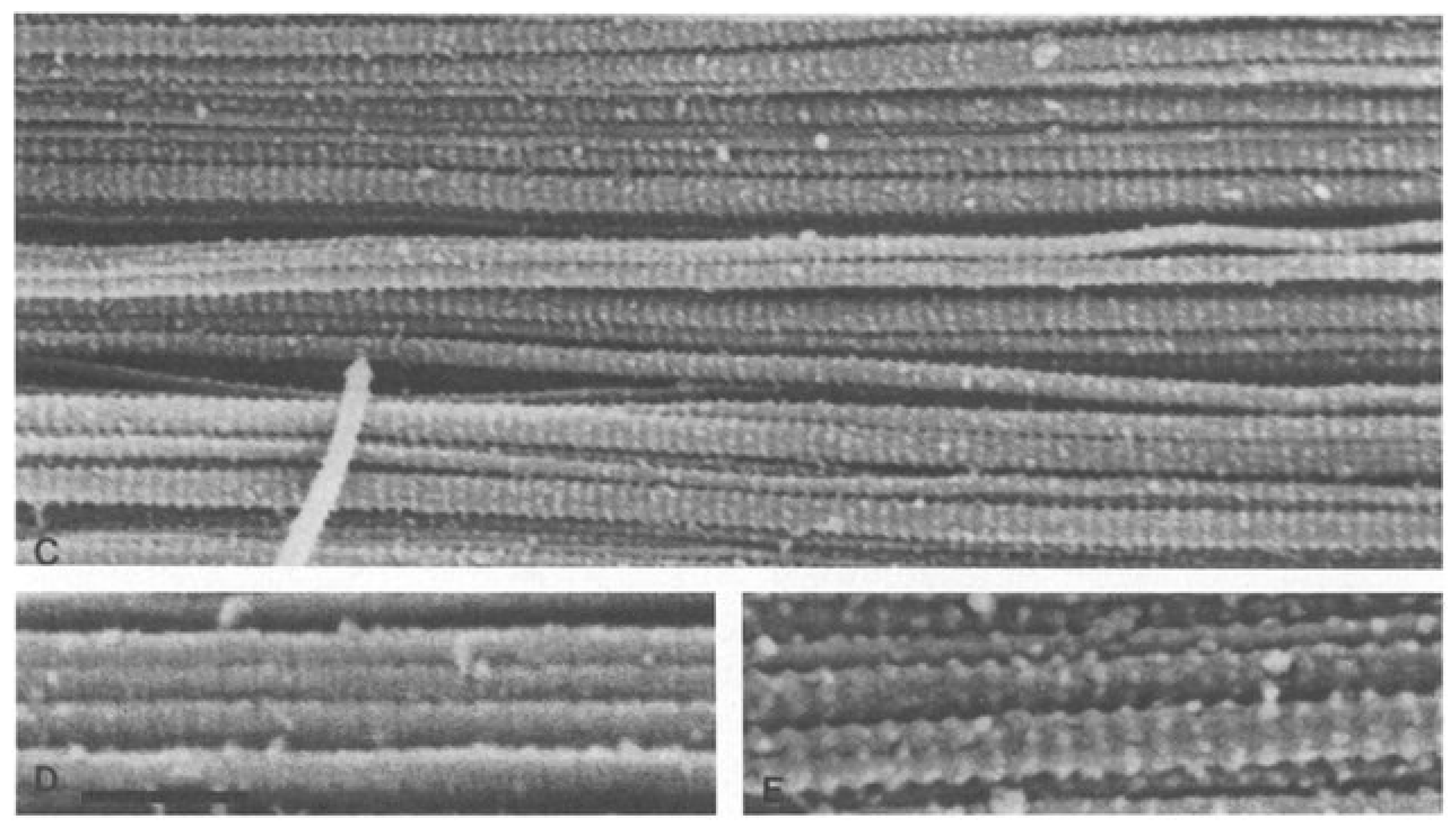
Closely evaluating the tissue composition,
Figure 6 and
Figure 7 compare a scanning electron microscope (SEM) image of WJ tissue product’s preserved cross-linked collagen structure to an SEM image of an Achilles Tendon specimen from 77 to 80-year-old individuals (Keene, 1987). The images show the structural similarity of the collagen matrix in WJ compared to the Achilles tendon. The similarity in the images demonstrates the effectiveness of WJ allografts. With such structural similarity, the homologous implementation of WJ provides significant results in supplementing degenerated tissue associated with Achilles Tendinosis and supports the regeneration process. With these images, WJ showcases the ability to function as an architectural scaffold for ECM supplementation in the Achilles Tendon. The act of comparing SEM images of various tissue types to WJ helps to confirm consistent structural characteristics. The structural similarities between different tissue types and WJ help support the homologous use of WJ in various anatomical locations.
The outcomes for this patient, 100% pain reduction after 90 days, align with and exceed the positive results presented in other literature regarding each separate element used in the care procedure as stand-alone applications. After utilizing Wharton’s Jelly alone, positive results have been found for applications in the sacroiliac joint and osteoarthritis. A WJ product was applied to the structural defect in these studies and produced statistically significant improvements. The sacroiliac joint study reported an 84% reduction in Numeric Pain Rating Scale (NPRS) scores and a 76% reduction in Western Ontario and McMaster University Arthritis Index (WOMAC) scores from the initial application to the 90-day follow-up (Lai, 2023). In comparison, the degenerated knee study reported statistically significant patient-reported outcome improvements with an overall 20.52% improvement in pain, joint stiffness, and physical function (Davis, 2022). Throughout current literature, WJ has been proven effective in supplementing structural defects in multiple anatomical locations.
Laser therapy is another minimally invasive and affordable option for patients with persistent pain who want an alternative to surgery. High-intensity laser therapy (HILT) of class 4 laser diodes delivering at least 500 mW of power has shown promising results. HILT functions to decrease erythrocyte deformability and platelet aggregation, resulting in membrane revitalization, viscosity reduction, and erythrocyte stress adaptation (Brandl, 2023). After utilizing infrared thermography, skin thermal changes were recorded to increase significantly by 9.45 degrees Celsius (Brandl, 2023). The increase in more effective blood flow allows for the body’s natural healing factors to be able to be delivered to the defective area at a quicker rate.
Additionally, EPAT has been tested as an independent treatment option for patients suffering from Achilles tendinosis. A study by Saxena (2011) found that 78.38% of tendons showed clinically significant improvement by at least one-year post-treatment. Given that no adverse reactions were recorded, EPAT exists as a safe, viable, and practical option for the treatment of Achilles tendinosis. This study has shown that by combining WJ allografts, EPAT, and class IV laser therapy, they work in conjunction to significantly relieve chronic pain from structural defects of the Achilles tendon. The patient in this study showed 100% improvement in just three months after receiving a unique combination of WJ, class IV laser therapy, and EPAT. Future research with a larger cohort will further evaluate the efficacy and safety of Wharton’s jelly alongside EPAT and class IV laser therapies and assist in defining dosage protocols.
Conclusion
In conclusion, the application of Wharton’s Jelly in combination with EPAT and class IV laser therapy showed improvement in pain and functionality in a patient suffering from structural defects of the tissue leading to chronic Achilles Tendinosis, immediately proximal enthesopathy, and significant retrocalcaneal exostosis. After failing three years of conservative treatment, she achieved total relief in just 13 weeks. This study demonstrates WJ allografts as a prospective treatment option for patients suffering from Achilles Tendinosis who have failed standard care treatments and wish to avoid surgical intervention. Additional randomized controlled trials are necessary to warrant WJ allograft application to degenerative tissue associated with Achilles Tendinosis as a standardized treatment and an alternative option to surgery. Continued research involving larger sample sizes will further evaluate the efficacy and safety of Wharton’s Jelly alongside EPAT and class IV laser therapies and assist in defining dosage protocols.
Appendix A
Test Kits
HBcAb: Catalog number: 06P06, Abbott Laboratories, Abbott Park, IL, USA 223
HbsAg: Catalog number: 06P02, Abbott Laboratories, Abbott Park, IL, USA 224
HCV: Catalog number: 06P04, Abbott Laboratories, Abbott Park, IL, USA 225
HIV1, HIV2, plus O: Catalog number: 06P01, Abbott Laboratories, Abbott Park, IL, 226 USA 227
HTLV-I/II: Catalog number: 06P07, Abbott Laboratories, Abbott Park, IL, USA 228
RPR: Catalog number: 900025, Arlington Scientific, Springville, UT, USA 229
HIV1, HCV, HBV, NAT: Catalog number: 303330, 303331, 303719, 303334, 303344 230
WNV: Catalog number: 07001061190, Roche Diagnostics, Indianapolis, IN, USA
References
- Aicale, R., Oliviero, A., & Maffulli, N. (2020). Management of Achilles and patellar tendinopathy: what we know, what we can do. Journal of foot and ankle research, 13(1), 59. [CrossRef]
- Albers, I. S., Zwerver, J., Diercks, R. L., Dekker, J. H., & Van den Akker-Scheek, I. (2016). Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC musculoskeletal disorders, 17, 16. [CrossRef]
- Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br J Sports Med. 2007 Apr;41(4):211-6. Epub 2007 Feb 20. PMID: 17311806; PMCID: PMC2658946. [CrossRef]
- Alvarez A, Tiu TK. Enthesopathies. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559030/.
- Bjordal JM, Lopes-Martins RA, Iversen VV. A randomised, placebo controlled trial of low level laser therapy for activated Achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br J Sports Med. 2006 Jan;40(1):76-80; discussion 76-80. PMID: 16371497; PMCID: PMC2491942. [CrossRef]
- Brandl, A., Egner, C., Reisser, U., Lingenfelder, C., & Schleip, R. (2023). Influence of high-energy laser therapy to the patellar tendon on its ligamentous microcirculation: An experimental intervention study. PloS one, 18(3), e0275883. [CrossRef]
- Davis JM, Sheinkop MB, Barrett TC. Evaluation of the Efficacy of Cryopreserved Human Umbilical Cord Tissue Allografts to Augment Functional and Pain Outcome Measures in Patients with Knee Osteoarthritis: An Observational Data Collection Study. Physiologia. 2022; 2(3):109-120. [CrossRef]
- Feeney K. M. (2022). The Effectiveness of Extracorporeal Shockwave Therapy for Midportion Achilles Tendinopathy: A Systematic Review. Cureus, 14(7), e26960. [CrossRef]
- Genah S, Cialdai F, Ciccone V, Sereni E, Morbidelli L, Monici M. Effect of NIR Laser Therapy by MLS-MiS Source on Fibroblast Activation by Inflammatory Cytokines in Relation to Wound Healing. Biomedicines. 2021 Mar 16;9(3):307. PMID: 33809724; PMCID: PMC8002295. [CrossRef]
- Gupta, A., El-Amin, S.F., Levy, H.J. et al. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J Orthop Surg Res 15, 49 (2020). [CrossRef]
- Keene, D. R., Sakai, L. Y., Bächinger, H. P., & Burgeson, R. E. (1987). Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. The Journal of cell biology, 105(5), 2393–2402. [CrossRef]
- Ko, V. M., Cao, M., Qiu, J., Fong, I. C., Fu, S. C., Yung, P. S., & Ling, S. K. (2023). Comparative short-term effectiveness of non-surgical treatments for insertional Achilles tendinopathy: a systematic review and network meta-analysis. BMC musculoskeletal disorders, 24(1), 102. [CrossRef]
- Lai A, Shou J, Traina SA, Barrett T. The Durability and Efficacy of Cryopreserved Human Umbilical Cord Tissue Allograft for the Supplementation of Cartilage Defects Associated with the Sacroiliac Joint: A Case Series. Reports. 2023; 6(1):12. [CrossRef]
- Longo, U. G., Ronga, M., & Maffulli, N. (2009). Achilles tendinopathy. Sports medicine and arthroscopy review, 17(2), 112–126.
- Lopez, R. G., & Jung, H. G. (2015). Achilles tendinosis: treatment options. Clinics in orthopedic surgery, 7(1), 1–7. [CrossRef]
- Maffulli, Nicola, et al. “Achilles insertional tendinopathy: state of the art.” Journal of ISAKOS 4.1 (2019): 48-57.
- Saxena A, Ramdath S Jr, O’Halloran P, Gerdesmeyer L, Gollwitzer H. Extra-corporeal pulsed-activated therapy (“EPAT” sound wave) for Achilles tendinopathy: a prospective study. J Foot Ankle Surg. 2011 May-Jun;50(3):315-9. Epub 2011 Mar 15. PMID: 21406328. [CrossRef]
- Silbernagel, K. G., Hanlon, S., & Sprague, A. (2020). Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. Journal of athletic training, 55(5), 438–447. [CrossRef]
- Sudoł-Szopińska, I., Kwiatkowska, B., Prochorec-Sobieszek, M., & Maśliński, W. (2015). Enthesopathies and enthesitis. Part 1. Etiopathogenesis. Journal of ultrasonography, 15(60), 72–84. [CrossRef]
- Van der Vlist, A. C., Winters, M., Weir, A., Ardern, C. L., Welton, N. J., Caldwell, D. M., Verhaar, J. A. N., & de Vos, R. J. (2021). Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. British journal of sports medicine, 55(5), 249–256. [CrossRef]
- Damaged connective tissues between the bone and tendons or ligaments are common among adults regardless of activity level. Achilles tendinosis is one of the most common tissue defects and enthesopathies. The patient in this study is a 54-year-old female with chronic Achilles tendinosis from chronic enthesopathy at the Achilles tendon insertion with a retrocalcaneal exostosis for three years who has failed standard-of-care practices for over two years. The patient received extracorporeal pulsed-activated therapy (EPAT) before applying 2ml of CryoText, a Wharton’s jelly tissue allograft. The patient then received class IV laser therapy treatments twice weekly for three weeks. The patient started with a 10/10 VAS at the initial visit. At five weeks post-application, the patient’s score had improved by 80% at 2/10 VAS. By week 13, the patient rated her pain as 0/10 VAS. The improvement in patient-reported pain and functionality reported in this study after the application of Wharton’s Jelly, EPAT, and class IV laser therapy warrants future research studying the safety and efficacy of these patient care modalities together.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).