1. Introduction
It is widely known that physical inactivity associates with numerous chronic diseases [
1] which also has led to the recommendation from the World Health Organization that all adults should perform regular physical activity [
2]. The positive influence of systematic exercise has a multicomponent impact on human health including decreases in coronary heart diseases risk [
3] and obesity [
4], supports type 2 diabetes treatment [
5], and improves sleep quality [
3] as well as mental health [
6]. However, the efficiency of different types of physical activity could vary depending on volume and exercise intensity. Therefore, scientific verification of applied training programs seems to be an important task for researchers.
The modern lifestyle allows people to devote limited time to physical activity. Thus, since the beginning of the 21st century, the topic of low-volume, but high-intensity interval training has drawn attention and explored extensively [
7,
8]. It appears that repeated high-intensity training could be time-efficient exercise mode to induce positive adaptations skeletal muscle metabolism and to upregulate the aerobic capacity in men and women [
9,
10]. Weston et al. [
11] introduced definitions of the most popular strategies of interval training based on exercise intensity. High-intensity interval training (HIIT) includes efforts at intensity exceeding 80-85% of maximal heart rate, while sprint interval training (SIT) is defined as efforts at an intensity equal to or greater (supramaximal) than peak aerobic capacity. Moreover, to describe the continuous efforts performed at intensities lower than HIIT, moderate-intensity continuous training (MICT) is widely used [
12].
The effectiveness of mentioned above training forms has often been compared. The first such comparisons, published nearly half a decade ago [
13], reported no differences in maximal oxygen uptake (VO
2max) changes and physiological responses between continuous and interval training participants. However, more recent studies demonstrated that interval training could provide even more benefits in physiological adaptations when training volume is matched [
9]. Even when interval training volume is lower, the adaptive response has been shown to be comparable or even superior compared to endurance training [
10,
14,
15]. Ramos et al. [
16] found that HIIT enhances vascular function more effectively compared to MICT. Furthermore, Milanović et al. [
17] observed more significant improvements in VO
2max following HIIT than after traditional endurance training, which is supported by Nybo et al [
15]. Similar tendencies were noted in psychological responses. It was well established that both HIIT and MICT are beneficial in improving mental health [
18]. Previous research indicated that after HIIT, participants experienced equal or greater post-exercise enjoyment than after MICT [
19,
20]. However, repeated intense exercise results in increased reactive oxygen species (ROS) generation, which is associated with cellular function disruption [
21]. Nevertheless, this negative effect could be reduced by antioxidant defence systems, including Catalase (CAT), Superoxide Dismutase (SOD) and Reduced (GSH) and Oxidized Glutathione (GSSG) [
22]. Although the acute effect of HIIT efforts on the antioxidant status has been accurately described [
23], the number of studies investigating the influence of training programs in humans is still sparse. Complete evaluation of the training effectiveness usually covers three main areas: changes in physical performance, a physiological response and psychological reaction. All these elements should be considered when assessing the training plan's efficiency. Therefore, the purpose of this research was twofold: (1) to evaluate the effects of self-paced high-intensity interval training (Sp-HIIT) vs self-paced moderate-intensity continuous training (Sp-MICT) on aerobic fitness level, psychophysiological responses and antioxidant status in young adults; (2) to identify potential correlations between the aerobic fitness level and oxidative stress and antioxidant markers.
2. Materials and Methods
Experimental approach to the problem
The effects of Sp-HIIT vs Sp-MICT on young adults' aerobic fitness level, psychophysiological reactions, and antioxidant status were compared using a two-group, parallel research design. Pre and post-intervention measurements included a skinfold measurement, 30-15 Intermittent Fitness Test (30–15 IFT), serum oxidative stress marker [Malondialdehyde (MDA)] and antioxidant markers [Catalase (CAT), Superoxide Dismutase (SOD), Reduced (GSH) and Oxidized Glutathione (GSSG)]. Twenty-four randomized recreationally male young adults performed either the Sp-HIIT or the Sp-MICT group. They were randomly assigned to one of the groups. Two 12–24 x 30-second high-intensity runs ≥85 %HRmax were completed by the Sp-HIIT group, followed by 30-second rest periods. The participants in Sp-MICT performed 24-48 minutes of continuous running at 60-75 %HRmax. These eight-week training interventions were performed three times a week, and two-day intervals separated each training session. All measurements were performed on standard outdoor athletics track with a tartan surface between 3 and 6 o’clock in the afternoon for similar chronobiological responses. They were told to keep a regular nutritional intake both before and during the trial, and they were familiar with testing and training techniques.
Design
Before the study began, we estimated the sample size using the G*Power software program version 3.1 (Düsseldorf, Germany). After adding a partial effect size of 0.2, a power of 0.8, a p-value of 0.5 (2 groups and 2 number of measurements), and a correlation of 0.5, considering previous study findings of imposed vs self-selected training [
24]. We found a recommended total sample size of 12 for the study.
Participants
Twenty-four young male adults were randomly assigned to a Sp-HIIT (n = 12; age: 21.8 ± 1.3 years, height: 176.6 ± 5.8 cm, weight: 76.9 ± 10.6 kg) or the Sp-MICT (n = 12; age: 22.1 ± 1.6 years, height: 179.5 ± 5.9 cm, weight: 78.2 ± 12.2 kg). All participants were involved in team sports including handball, basketball, and soccer, had at least two years of experience with the training workload of four training units per week, which included core strength training, aerobic activity, and group exercise. Before the study began, written informed permission was acquired from the participants after they had been told of the research's requirements, benefits, risks, and procedures.
Procedures
Biochemical Procedure. A minimum of 10 hours fasting before their routine breakfast time (7:00-8:30 am), blood samples from each participant were collected into lithium heparin vacutainers and centrifuged at 5000 rpm for 10 min at four °C to separate plasma. The plasma samples were collected and frozen at -80°C until biochemical analysis. The spectrophotometric method was used to determine CAT [
25], SOD [
26] activities and MDA levels [
27]. Furthermore, GSH and GSSG concentrations were also determined spectrophotometrically, as described in a previous study [
28]. After the blood collection to analyse oxidative stress with MDA and antioxidant markers, the body fat percentages of participants, using the Holtain Tanner–Whitehouse skinfold calliper (Holtain, UK) and Gulick anthropometric tape (Holtain), were estimated using the validated formula for Turkish athlete [
29]. Following the anthropometric measurements, each participant performed the 30–15 IFT. This test, consisting of 30 s of running and 15 s of passive recovery, is an acoustically and reliably progressive test according to the procedures described. After the 30–15 IFT, the maximal oxygen uptake (VO
2max) was estimated from the maximum speed (30-15 V
IFT) reached in the last stage of the test [
30].
Training Interventions. Sp-HIIT and Sp-MICT interventions were performed on standard athletics track 3 days per week for eight weeks, and two-day intervals separated each training session to maximize physical and physiological performance. A progressive training design was developed to increase final performance in both training programmes. The Sp-MICT group's total training duration was divided in half during the research in accordance with the Sp-HIIT group. Two 12–24 x 30-second high-intensity runs ≥85 %HR
max were completed by the Sp-HIIT group, followed by 30-second passive resting. The participants in Sp-MICT continuously performed 24-48 minutes of running at 60-75 %HR
max. Detailed information about training is summarized in
Table 1. A 15-minute standardized warm-up, which included 10 minutes of running and 5 minutes of static and dynamic stretching activities, was the first part of each training session. Our participants were allowed to change and maintain their pace during each training session without receiving any guidance on the intensity of their activity. The rating of perceived exertion (RPE) was determined using the category level (CR-20) Borg scale immediately after each session [
31]. Brunel Mood Scale (BRUMS) was used to determine their mood profile [
32,
33] before and after the training sessions. The validated and reliable this scale, consists of 19 items and four sub-scales (e.g., fatigue, depression, anger and vigour) scored on a five-point Likert scale [
34]. Using the responses to a question of “how do you feel right now?” volunteers indicated whether they experienced such feelings on a 5-point scale (0 = not at all; 1 = a little; 2 = moderately; 3 = quite a bit; 4 = extremely).
Statistical analysis
Data were represented as mean ± SD. A mixed ANOVA was used to test for interactions and the main effects of time and group on aerobic fitness level, psychophysiological responses and antioxidant status. Correlation between aerobic fitness level and pro/antioxidant markers was performed using the Pearson (r) or Spearman (rho) correlation coefficient. The correlations were considered trivial (<0.1), small (0.1 to <0.3), moderate (0.3 to <0.5), large (0.5 to <0.7) and very large (0.7 to <0.9), extremely large (0.9 – 1.0) [
35]. SPSS version 24.0 was used to conduct all statistical analyses (SPSS, Version 24.0 for Windows; SPSS Inc., Chicago, IL, United States). Statistical significance was set at the level of p ≤ 0.05.
3. Results
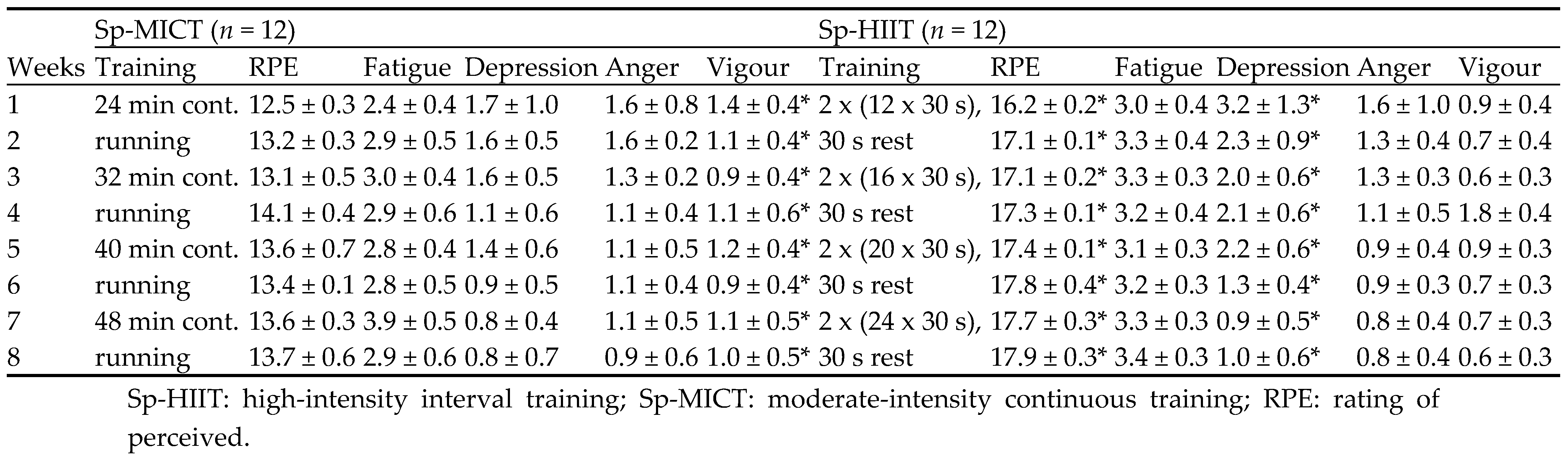
The weekly RPE and mood responses are demonstrated in
Table 1. Overall, during the 8-week self-paced training period, the Sp-HIIT showed higher RPE responses than those to Sp-MICT sessions (p ≤ 0.05, d = ranging from 7.6 to 17.4). Moreover, the Sp-HIIT also showed higher depression responses than those to Sp-MICT sessions (p ≤ 0.05, d = ranging from 0.2 to 4.1). In contrast, the vigour responses from the Sp-MICT were significantly higher than those to Sp-HIIT sessions (p ≤ 0.05, d = ranging from 0.2 to 1.3).
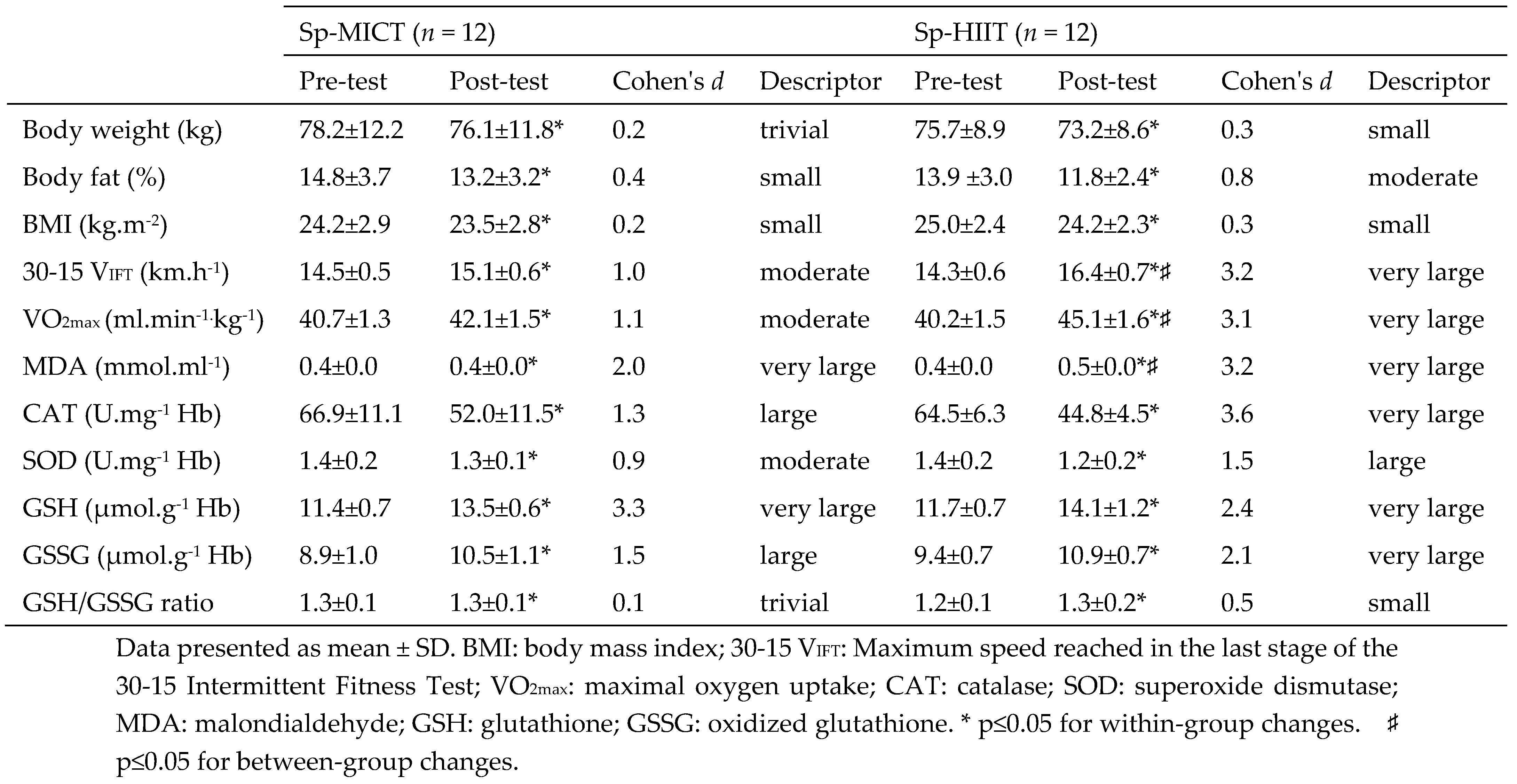
The Sp-HIIT and Sp-MICT interventions demonstrated similar anthropometric, performance responses, and antioxidant status improvements except for 30-15 VFIT, VO
2max and MDA responses in
Table 2. The Sp-HIIT showed greater improvement in the 30-15 V
IFT (14.7%, p ≤ 0.05, d = 3.20 [very large effect]), VO
2max (12.2%, p ≤ 0.05, d = 3.15 [very large effect]) and MDA (11.2%, p ≤ 0.05, d = 3.59 [very large effect]) compared with the Sp-MICT group.
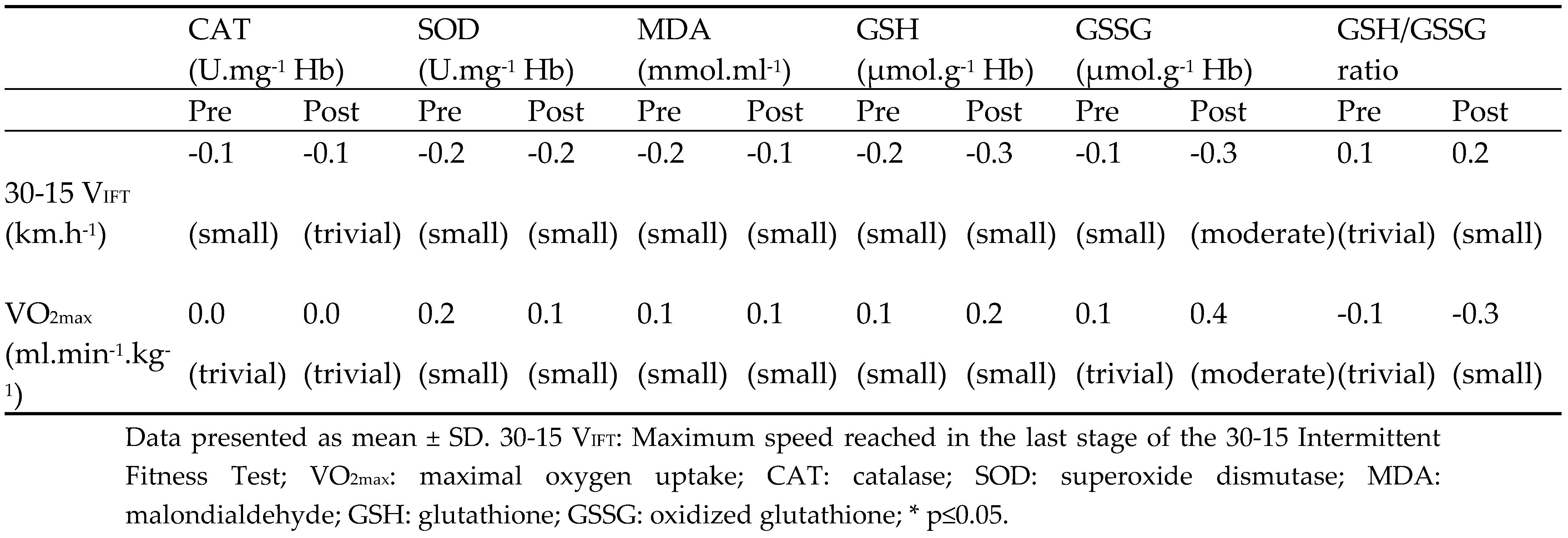
Table 3 demonstrates the correlations between aerobic fitness level, oxidative stress, and antioxidant markers during 8-week Sp-HIIT and Sp-MICT interventions. No significant differences were found in the oxidative stress and antioxidant markers with the aerobic fitness level between pre-and post-training.
4. Discussion
The main findings in our study were that both the Sp-HIIT and the Sp-MICT intervention improved body composition, aerobic fitness, and circulatory antioxidant status. However, the Sp-HIIT intervention caused greater improvement in the 30-15 VIFT, VO2max, and MDA responses, but with higher physical exertion, vigour and depression scores in comparison to Sp-MICT. Furthermore, no significant training-induced changes were found in serum oxidative stress and antioxidant markers.
The rating of psychophysiological responses, such as RPE-scores and mood profile, are validated, low-cost, and practical tools for measuring physical fatigue and psychological status in athletes. Our results are consistent with previous studies [
13,
36]. Thus, the HIIT-intervention induced higher negative changes in mood profile compared to the MICT. In addition to these supporting findings, the physical tiredness after a training session induced increased adverse feelings such as anxiety, anger, and depression [
37,
38]. The nature of HIIT and MICT might explain differences in mood state responses. While HIIT included running intervals of different durations, the MICT included prolonged exercise and monotonous nature, which may have affected the results. Furthermore, different types of training (imposed or self-paced exercise), participants' training duration, and characteristics (sex, age and training experiment) could be other influencing factors, as previously stated [
15,
39].
Regarding the effect of Sp-HIIT and Sp-MICT on body composition, the present study demonstrated significant favourable effects on body weight and fat percentage. No significant differences were observed between training interventions, which confirms some other findings in untrained hypertensive women [
40], but are in contrast to findings in sedentary males [
15]. However, none of these studies were controlled for altered nutritional habits, which is a limitation. Furthermore, the current study also demonstrated upregulated performance in the 30-15 V
IFT as well as VO
2max in Sp-HIIT compared to Sp-MICT after 8-week interventions. These findings support previous research comparing HIIT and MICT interventions with different training durations ranging from 4 to 12 weeks and in a variety of populations, such as healthy [
41], recreationally active [
13], and untrained young adults (15). In a similar study, Hottenrott et al. [
42] reported greater increases in V
IFT and VO
2max following a 12-week HIIT intervention compared to MICT in recreationally active adults. Similarly, an 8-week HIIT intervention resulted in 11% increase in VO
2max in healthy men [
43] a 11% improvement in young women [
44], which is of similar magnitude as reported by Nybo et al [
15] in healthy males and by Kristiansen et al [
45] in a frail patient with coronary heart disease after 12-wks of HIIT. Contrary to these study results supporting our findings, V̇O
2max has been shown to increase 20% after a 12-week Sp-MICT training programmes compared with Sp-HIIT in inactive women [
11]. From a practical point of view, our findings speak in favour that Sp-HIIT is a feasible, practical and efficient training method to improve VO
2max in recreationally active adults. However, there is still no consensus on which type of self-paced training intervention is optimal to improve aerobic fitness and body composition because of the lack of literature.
The present study measured circulatory oxidative stress and antioxidant markers by determining resting serum MDA, CAT, SOD, GSH and GSSG values prior and after the intervention. We demonstrated higher level of serum MDA, as well as performance in the 30-15 V
FIT and VO
2max after 8-week of Sp-HIIT compared to Sp-MICT. In contrast to our study results, some studies have found an increase in serum SOD and CAT activity and a decrease in MDA levels in untrained men after 8-week aerobic training [
46,
47], while our findings agree with Knez et al. [
48], who found that the systemic MDA concentration increases progressively during an intervention. The analysis of circulatory MDA markers of peroxidation and antioxidant enzymes in individuals with long-term exposure to metabolic oxidative stress induced by exercise training may indicate altered fatigue pattern and, consequently, the overload of adaptive stress mechanisms and depression. Although the similar training intervention protocols in these studies, some important confounding factors such as the populations’ physical activity levels, age and training intensity may explain the diverging results.
Our study demonstrated that 8-week of Sp-HIIT provoked higher increases in 30-15 V
FIT and VO
2max responses compared to Sp-MICT, but with no statistical associations between aerobic fitness level such as 30-15 V
FIT and VO
2max and circulatory antioxidant markers, which is in line with findings by others [
49]. In contrast to our study results, Peserico et al. [
50] have recently found strong correlations between GSSG and indicators of aerobic fitness level, such as peak velocity and 5-km running time in untrained men. In addition, an earlier study found that the systemic GSH values correlated with training volume and VO
2max in competitive triathletes [
51]. As a result of improved VO
2max, increasing reactive oxygen species production is associated with increased erythrocyte glutathione peroxidase activity and glutathione concentration, both of which will protect the organism against lipid peroxidation and cell membrane damage [
51]. Differences between studies in antioxidant markers and aerobic fitness level might be explained by genetic background, physical activity levels and the general participant characteristics.
The study has strengths and limitations. The applied training modalities were designed according to suggested physical activity guidelines to improve health conditions for adults, which is strength. Furthermore, the study is the first to compare the effects of self-paced training on the aerobic fitness level, psychophysiological responses and antioxidant status in young well-trained adults. However, several limitations must be addressed. The main limitation of this study is that there was a lack of direct measurement of aerobic capacity. Another important limitation is lack of control of the nutritional habits of participants, affecting physical performance in relation to body composition and blood profiles. Finally, the sample size is too small to generalize the findings and no inactive control group was included.
5. Conclusions
In conclusion, both self-paced high-intensity interval and continuous moderate-intensity training in an 8-week period demonstrated similar improvements in anthropometric performance and antioxidant status, while adaptations in 30-15 VFIT, VO2max and serum MDA were in favour of high-intensity interval training. However, the high-intensity interval training intervention induced higher negative changes in RPE and depression responses compared to moderate intensity continuous training.
Author Contributions
Conceptualization, Y.S., E.A. and B.K.; methodology, Y.S., E.A. and B.K.; formal analysis, Y.S., E.A. and B.K.; investigation, Y.S., E.A. and B.K.; resources, Y.S., E.A. and B.K.; data curation, Y.S., E.A. and B.K.; writing—original draft preparation, Y.S., P.K, M.M., E.A., B.K. and L.R.; writing—review and editing, Y.S., P.K, M.M., E.A., B.K. and L.R.; supervision, Y.S., P.K, M.M., E.A., B.K. and L.R.; project administration, Y.S.; funding acquisition, Y.S., E.A. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out in compliance with the Declaration of Helsinki and received approval from the ***** ***** University Ethics Committee (E-33490967-044-94834).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions generated for this study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012, 380, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carth, C.; Chapur, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Stanton, R.; To, Q.G.; Khalesi, S.; Williams, S.L.; Alley, S.J.; Thwaite. T.L.; Fenning, A.S.; Vandelanotte, C. Depression, Anxiety and Stress during COVID-19: Associations with Changes in Physical Activity, Sleep, Tobacco and Alcohol Use in Australian Adults. Int J Environ Res Public Health. 2020, 17, 4065. [Google Scholar] [CrossRef]
- Kim, B.Y.; Choi, D.H.; Jung, C.H.; Kang, S.K.; Mok, J.O.; Kim, C.H. Obesity and Physical Activity. J Obes Metab Syndr. 2017, 26, 15–22. [Google Scholar] [CrossRef]
- Najafipour, F.; Mobasseri, M.; Yavari, A.; Nadrian, H.; Aliasgarzadeh, A.; Mashinchi Abbasi, N.; Niafar, M.; Gharamaleki, J.H.; Sadra, V. Effect of regular exercise training on changes in HbA1c, BMI and VO 2 max among patients with type 2 diabetes mellitus: an 8-year trial. BMJ Open Diabetes Res Care. 2017, 5, e000414. [Google Scholar] [CrossRef] [PubMed]
- Paluska, S.A.; Schwenk, T.L. Physical activity and mental health: current concepts. Sports Med. 2000, 29, 167–180. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Hughes, S.C.; Heigenhauser, G.J.F.; Bradwell, S.N.; Gibala, M.J. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005, 98, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Van Essen, M.; Wilkin, G.P.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef]
- Gibala, M.J. Physiological basis of interval training for performance enhancement. Exp Physiol. 2021, 106, 2324–2327. [Google Scholar] [CrossRef]
- Iaia, F.M.; Ermanno, R.; Bangsbo, J. High-Intensity Training in Football. Int J Sports Physiol Perform. 2009, 4, 291–306. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef]
- Eddy, D.O.; Sparks, K.L.; Adelizi, D.A. The effects of continuous and interval training in women and men. Eur J Appl Physiol Occup Physiol. 1977, 37, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nordsborg, N.B.; Connolly, L.; Weihe, P.; Iuliano, E.; Krustrup, P.; Saltin, B.; Mohr, M. Oxidative capacity and glycogen content increase more in arm than leg muscle in sedentary women after intense training. J Appl Physiol. 2015, 119, 116–123. [Google Scholar] [CrossRef]
- Nybo, L.; Sundstrup, E.; Jakobsen, M.D.; Mohr, M.; Hornstrup, T.; Simonsen, L.; Bülow, J.; Randersi, M.B.; Nielsen, J.J.; Aagard, P. , et al. High-Intensity Training versus Traditional Exercise Interventions for Promoting Health. Med Sci Sport Exerc. 2010, 42, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: a Systematic Review and Meta-Analysis. Sport Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sport Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef]
- Reed, J.L.; Terada, T.; Cotie, L.M.; Tulloch, H.E.; Leenen, F.H.; Mistura, M.; Hans, H.; Wang, H.W.; Vidal-Almela, S.; et al. The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: A ra. Prog Cardiovasc Dis. 2022, 70, 73–83. [Google Scholar] [CrossRef]
- Stork, M.J.; Banfield, L.E.; Gibala, M.J.; Martin Ginis, K.A. A scoping review of the psychological responses to interval exercise: is interval exercise a viable alternative to traditional exercise? Health Psychol Rev. 2017, 11, 324–344. [Google Scholar] [CrossRef]
- Stork, M.J.; Gibala, M.J.; Martin Ginis, K.A. Psychological and Behavioral Responses to Interval and Continuous Exercise. Med Sci Sport Exerc. 2018, 10, 2110–2121. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative Stress. Sport Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Mohr, M.; Nielsen, T.S.; Weihe, P.; Thomsen, J.A.; Aquino, G.; Krustrup, P.; Nordsborg, N.B. Muscle ion transporters and antioxidative proteins have different adaptive potential in arm than in leg skeletal muscle with exercise training. Physiol Rep. 2017, 5, e13470. [Google Scholar] [CrossRef] [PubMed]
- Farney, T.M.; McCarthy, C.G.; Canale, R.E.; Schilling, B.K.; Whitehead, P.N.; Bloomer, R.J. Absence of Blood Oxidative Stress in Trained Men after Strenuous Exercise. Med Sci Sport Exerc. 2012, 44, 1855–1863. [Google Scholar] [CrossRef]
- Kellogg, E.; Cantacessi, C.; McNamer, O.; Holmes, H.; von Bargen, R.; Ramirez, R.; Gallegher, D.; Vargas, S.; Santia, B.; Rodrigez, K.; et al. Comparison of Psychological and Physiological Responses to Imposed vs. Self-selected High-Intensity Interval Training. J Strength Cond Res. 2019, 33, 2945–2952. [Google Scholar] [CrossRef]
- Adamo, A.M.; Llesuy, S.F.; Pasquini, J.M.; Boveris, A. Brain chemiluminescence and oxidative stress in hyperthyroid rats. Biochem J. 1989, 263, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Ewing, J.F.; Janero, D.R. Microplate Superoxide Dismutase Assay Employing a Nonenzymatic Superoxide Generator. Anal Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sen, C.K.; Marin, E.; Kretzschmar, M.; Hanninen, O. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol. 1992, 73, 1265–1272. [Google Scholar] [CrossRef]
- Açikada, C.; Hazir, T.; Aşçi, A.; Özkara, A. Physical and physiological profiles of a second league division soccer team during preparation period. Hacettepe J Sport Sci Technol. 1998, 9, 3–14. [Google Scholar]
- Buchheit, M. The 30–15 intermittent fitness test: 10 year review. Myorobie J. 2010, 1, 278. [Google Scholar]
- Foster, C.; Boullosa, D.; McGuigan, M.; Fusco, A.; Cortis, C.; Arney, B.E.; Orton, B.; Dodge, C.; Jaime, S.; Radtke, K.; et al. 25 Years of Session Rating of Perceived Exertion: Historical Perspective and Development. Int J Sports Physiol Perform. 2021, 16, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.C.; Lane, A.M.; Lane, H.J.; Keohane, L. Development and validation of a mood measure for adolescents. J Sports Sci. 1999, 17, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.C.; Potgieter, J.R.; Fogarty, G.J. The stellenbosch mood scale: A dual-language measure of mood. Int J Sport Exerc Psychol. 2003, 1, 231–245. [Google Scholar] [CrossRef]
- Çakiroğlu, A.A.; Demir, E.; Güclü, M. The Validity and Reliablity Study of the Brunel Mood Scale with the Adult Athletes (Turkish Adaptation). Int J Appl Exerc Physiol. 2020, 9, 126–140. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med Sci Sport Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Oliveira, B.R.R.; Slama, F.A.; Deslandes, A.C.; Furtado, E.S.; Santos, T.M. Continuous and high-intensity interval training: which promotes higher pleasure? PLoS One. 2013, 8, e79965. [Google Scholar] [CrossRef]
- Costa, K.; De Lira, C.; Penna, E.; Andrade, M.; De Suza Fonseca, F.; De Lima-Junior, D.; Gentil, P.; Sá Filho, A.S.; Texiera Costa, G.D.C. State of mood, motivation, and impulsivity of young athletes: a cross-sectional study. Hum Mov. 2023, 24, 86–92. [Google Scholar] [CrossRef]
- Morgan, W.P.; Brown, D.R.; Raglin, J.S.; O’Connor, P.J.; Ellickson, K.A. Psychological monitoring of overtraining and staleness. Br J Sports Med. 1987, 21, 107–114. [Google Scholar] [CrossRef]
- Soylu, Y.; Arslan, E.; Sogut, M.; Kilit, B.; Clemente, F. Effects of self-paced high-intensity interval training and moderateintensity continuous training on the physical performance and psychophysiological responses in recreationally active young adults. Biol Sport. 2021, 38, 555–562. [Google Scholar] [CrossRef]
- Mohr, M.; Nordsborg, N.B.; Lindenskov, A.; Steinholm, H.; Nielsen, H.P.; Mortensen, J.; Weihe, P.; Krustrup, P. High-Intensity Intermittent Swimming Improves Cardiovascular Health Status for Women with Mild Hypertension. Biomed Res Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Helgerud, J.; Hoydal, K.; Wang, E. , Karlsen, T.; Berg, P.P.; Bjerkaas, M.M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic High-Intensity Intervals Improve VO2max More Than Moderate Training. Med Sci Sport Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Hottenrott, K.; Ludyga, S.; Schulze, S. Effects of high intensity training and continuous endurance training on aerobic capacity and body composition in recreationally active runners. J Sports Sci Med. 2012, 11, 483–488. [Google Scholar] [PubMed]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int J Environ Res Public Health. 2020, 17, 3589. [Google Scholar] [CrossRef]
- Mazurek, K.; Zmijewski, P.; Krawczyk, K.; Czajkowska, A.; Kęska, A.; Kapuscinski, P.; Mazurek, T. High intensity interval and moderate continuous cycle training in a physical education programme improves health-related fitness in young females. Biol Sport. 2016, 33, 139–144. [Google Scholar] [CrossRef]
- Kristiansen, J.; Sjúrðarson, T.; Grove, E.L.; Rasmussen, J.; Kristensen, S.D.; Hvas, A.M.; Mohr, M. Feasibility and impact of whole-body high-intensity interval training in patients with stable coronary artery disease: a randomised controlled trial. Sci Rep. 2022, 12, 17295. [Google Scholar] [CrossRef] [PubMed]
- Azizbeigi, K.; Stannard, S.R.; Atashak, S.; Mosalman Haghighi, M. Antioxidant enzymes and oxidative stress adaptation to exercise training: Comparison of endurance, resistance, and concurrent training in untrained males. J Exerc Sci Fit. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; La Torre, A.; Porcelli, S. Time-Course Changes of Oxidative Stress Response to High-Intensity Discontinuous Training versus Moderate-Intensity Continuous Training in Masters Runners. Aykin-Burns N, editor. PLoS One. 2014, 9, e87506. [Google Scholar] [CrossRef] [PubMed]
- Knez, W.L.; Jenkins, D.G.; Coombes, J.S. Oxidative Stress in Half and Full Ironman Triathletes. Med Sci Sport Exerc. 2007, 39, 283–288. [Google Scholar] [CrossRef]
- Zivkovic, V.; Lazarevic, P.; Djuric, D.; Cubrilo, D.; Macura, M.; Vuletic, M.; Barudzic, N.; Nesic, M.; Jakovljevic, V. Alteration in basal redox state of young male soccer players after a six-month training programme. Acta Physiol Hung. 2013, 100, 64–76. [Google Scholar] [CrossRef]
- Peserico, C.S.; Machado, F.A. Association between endurance performance, oxidative stress, and antioxidant markers during a running training program in untrained men. Sport Sci Health. 2022, 18, 249–256. [Google Scholar] [CrossRef]
- Margaritis, I.; Tessier, F.; Richard, M.J.; Marconnet, P. No Evidence of Oxidative Stress After a Triathlon Race in Highly Trained Competitors. Int J Sports Med. 1997, 18, 186–190. [Google Scholar] [CrossRef] [PubMed]
Table 1.
8 weeks training programmes and the weekly rating of perceived exertion and mood responses.
Table 1.
8 weeks training programmes and the weekly rating of perceived exertion and mood responses.
Table 2.
Effect of 8 weeks trainings on anthropometric, aerobic fitness level and antioxidant status of the participants.
Table 2.
Effect of 8 weeks trainings on anthropometric, aerobic fitness level and antioxidant status of the participants.
Table 3.
Correlations between aerobic fitness level and oxidative stress and antioxidant markers.
Table 3.
Correlations between aerobic fitness level and oxidative stress and antioxidant markers.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).