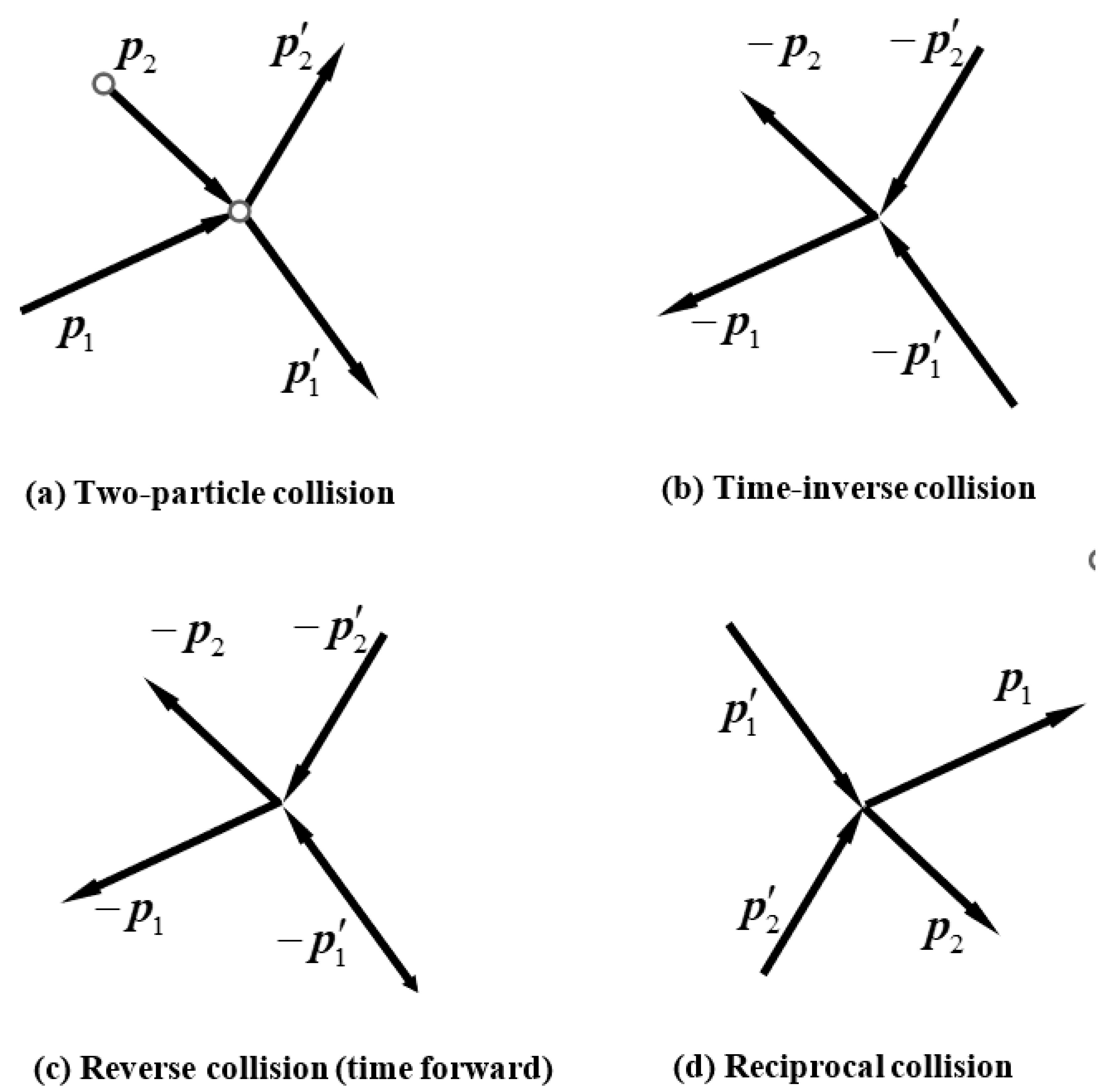
We consider an ideal gas composed of a large number of molecules which collide with each other frequently. This is a collision system. We will only concern two-particle elastic collisions. Because of the collisions, energy and momentum transfer between molecules, which causes the rapid change of the microscopic state of the gas and moreover the change of the macroscopic state. So, examining whether the two-particle collision process is reversible is important. If it is reversible, the motion of the gas is either, otherwise the motion of the gas is not guaranteed to be reversible.
Equations (59) are not differential equations, but they directly describe the motion of the two-particle collision. They are actually the solutions of equation of motion with given initial condition. The initial conditions are that the momenta and before the collision are known.
A. Two-particle collision in classical mechanics
We first propose a definition of the reversibility of the collision process in
Figure 1a. If the collision
Figure 1a occurs, the reverse collision
Figure 1c necessarily also occurs, that is, the probability of the occurrence of the reverse process
Figure 1c is 1, then we say that the original collision
Figure 1a is reversible; If the probability is less than 1, then the original collision is said to be irreversible. Let us analyze whether the probability of the reverse process occurring is equal to 1.
In one-dimensional space, Equation (59) contains two equations. The
and
of the incident particles are known. From (59), the two momenta of the final state can be uniquely solved. Therefore, the final state is uniquely determined. In particular, since the particles are identical, the solution is
, i.e., the two particles simply exchange their momenta. This case is as if no collision occurs, which was treated in [
26,
45]. That is why we classified one-dimensional identical particle systems as collision-free systems in subsection I.C.
In two-dimensional space, each momentum has two components, so Equation (59) contains three equations. There are four momentum components to be solved. Therefore, there is an uncertain quantity in the final state, e.g., the angle θ between and can vary in the range of 0~2π.
In three-dimensional space, each momentum has three components, so Equation (59) contains four equations. There are six momentum components
to be solved. Thus, there are two uncertain quantities of the two outgoing particles, e.g., the angles
between
and
can vary in the range of 4π solid angles. Therefore, the momenta of the outgoing particles have a distribution [
73].
We consider the case of three-dimensional space. In
Figure 1a, only a pair of specific outgoing momenta
and
are plotted. The probability of the occurrence of this pair
and
is denoted as
. It must be that
, because there are other outgoing
and
pairs, as long as they meet Equation (59).
The reciprocal collision of
Figure 1a is the exchange of
and
as in
Figure 1d, that is to say, the initial state is
and the final state
, the occurrence probability of
Figure 1d is denoted as
. By classical mechanics, it is demonstrated that
[
52]. One may believe [
50] that the
reflects “principle of dynamic reversibility”. This recognition is not right, because the reverse collision is not involved in proving
.
Now, assume that the collision process
Figure 1a takes place. We examine if its reverse collision necessarily occurs, or the occurrence probability of
Figure 1c is 1. In
Figure 1c, the momentum of the incident particles are
and
, and only a specific pair of outgoing momenta
and
is plotted. In fact, there can be whatever pairs of outgoing
and
as long as they meet Equation (59). Therefore, the occurrence probability of the specific collision in
Figure 1c is certainly less than 1. By definition, the original collision in
Figure 1a is irreversible. Similarly, the collision process in
Figure 1c is also irreversible. It is concluded that in two-dimensional and three-dimensional space, the two-particle collision is irreversible.
Since individual microscopic collision events are irreversible, why can a frictionless quasi-static process of a macroscopic system composed of a large number of microscopic particles colliding with each other be reversible?
At every moment of a frictionless quasi-static process, the gas is in equilibrium state. Therefore, let us first review the concept of equilibrium. The definition of equilibrium is: “A macroscopic state which does not tend to change in time, except for random fluctuation.” [
32] When a system is in equilibrium, its macro parameters, such as volume, temperature, pressure, ect., do not change with time. If a macroscopic parameter such as pressure can be measured with an instrument, the measured value does not change with time. An equilibrium state is a macro state. There can be a huge number of micro states corresponding to this one macro state. In an equilibrium state, micro state changes vary rapidly. The change is caused by the collisions between molecules. The molecules’ momenta follow Maxwell-Boltzmann distribution. The reason that the micro states changes while the macro state does not is that the distribution remains unchanged.
That the frequent collisions between molecules do not change the distribution is determined by the principle of detailed balance.
B. Detailed balance
From Boltzmann’s
H theorem [
51], it can be shown that detailed balance is the sufficient and necessary conditions for a gas to reach equilibrium [
15,
52,
72,
74]. Here, we review the principle of detailed balance.
For the two-molecule collision in
Figure 1a, the mathematical expression of the detailed balance is
The is the momentum distribution function of the gas. In equilibrium, it is the Maxwell-Boltzmann distribution.
We inspect the physical meaning of Equation (60). It reveals that for the two particles participating the collision, the product of the distribution of the initial state is equal to that of the final state. In Equation (60), the left hand side equals to the right hand side. On the other hand, the right hand side also equals to the left hand side. That is, not only the collision
in
Figure 1a, but also its reciprocal collision
shown by
Figure 1d, satisfies Equation (60).
The detailed balance tells us that a pair of collisions reciprocal to each other retains the distribution function unchanged. Suppose that in an equilibrium gas there are four molecules. The momenta of molecules 1 and 2 are
and
, and those of 3 and 4 are
and
, respectively. Let molecules 1 and 2 collide as in
Figure 1a,
. After the collision, two extra
and
molecules are generated and two
and
molecules are reduced. As a result, the distribution function of the gas may deviate from the equilibrium one. If, meanwhile, another collision between molecules 3 and 4 takes place as in
Figure 1d,
, then, the extra
and
are reduced and the reduced
and
are restored. This retrieves the equilibrium distribution.
It is seen that the occurrence of a pair of reciprocal collisions can keep the distribution function unchanged and this unchanged distribution is an equilibrium one. Conversely, if a gas is in equilibrium, when a collision of
Figure 1a takes place, there must also a reciprocal collision of
Figure 1d, although this pair of reciprocal collisions changes the microscopic state of the gas. Someone [
50] thought that the detailed balance was a sufficient but not necessary condition for equilibrium, but it has been proved that the detailed balance was a sufficient and necessary condition [
7,
52].
As a comparison, we consider a pair of reverse collisions
Figure 1a,c. Assume that there are four molecules. The momenta of molecules 1 and 2 are
and
, and those of 3 and 4 are
and
, respectively. Let molecules 1 and 2 collide as in
Figure 1(a),
, and 1 and 2 collide as in
Figure 1c,
. After this pair of collisions, four molecules,
,
,
, and
are produced, and other four,
,
,
, and
are reduced. Apparently, the momentum distribution function changes. Therefore, a pair of reverse collisions cannot retain the distribution unchanged.
For the detailed balance, we address the following points. Before doing so, we remind the distinguish between macro and micro infinitesimals in time and space [
46]. The macro (micro) infinitesimal in time is called macro-instant (micro-instant). Macroscopic instruments can do measurements during macro-instant. Spatially, a macro subregion is a very small region but it still belongs to a macro one. A micro region can contain merely a small number of molecules.
(i) Detailed balance is achieved within a macro-instant.
When a gas is in equilibrium, a pair of reciprocal collisions
Figure 1a,d occur within the same macro-instant but not the same micro-instant. Macroscopically, the pair of
and
occur simultaneously, but microscopically, they occur sequentially. The system is in equilibrium at every macro-instant.
(ii) Detailed balance is achieved in a macro region.
A pair of reciprocal collisions occurs within a macro subsystem but not in a micro region. This is also what equilibrium requires. “If a closed macroscopic system is in a state such that in any macroscopic subsystem the macroscopic physical quantities are to a high degree of accuracy equal to their mean values, the system is said to be in a state of
statistical equilibrium (or
thermodynamic or
thermal equilibrium).” [
14] Equilibrium is reached in any macro subsystem, while in a micros region, detailed balance cannot be reached.
Based on the discussion of (i) and (ii) above, detailed balance is achieved statistically in macro subsystems. The equilibrium refers to that in macro subregions and at macro-instant. Microscopically, the gas is nonequilibrium everywhere and every time. Micro states cannot be distinguished by instruments that measure macro parameters.
(iii) Detailed balance is independent of reverse collisions.
Detailed balance (60) ensures that in equilibrium, the pair of reciprocal collisions occurs, with no requirement of the reverse collision
Figure 1c. The pair of reciprocal collisions takes place clockwise, irrespective to any anticlockwise process [
72]. On the other hand, as mentioned above, a pair of reverse collisions does not guarantee the invariance of the momentum distribution function. Therefore, the discussing whether a macro process is reversible is not based on whether two-particle collisions are reversible.
Detailed balance itself also contains
That is to say, the collision process
Figure 1c and its reciprocal process also keep the momentum distribution unchanged in the case of equilibrium.
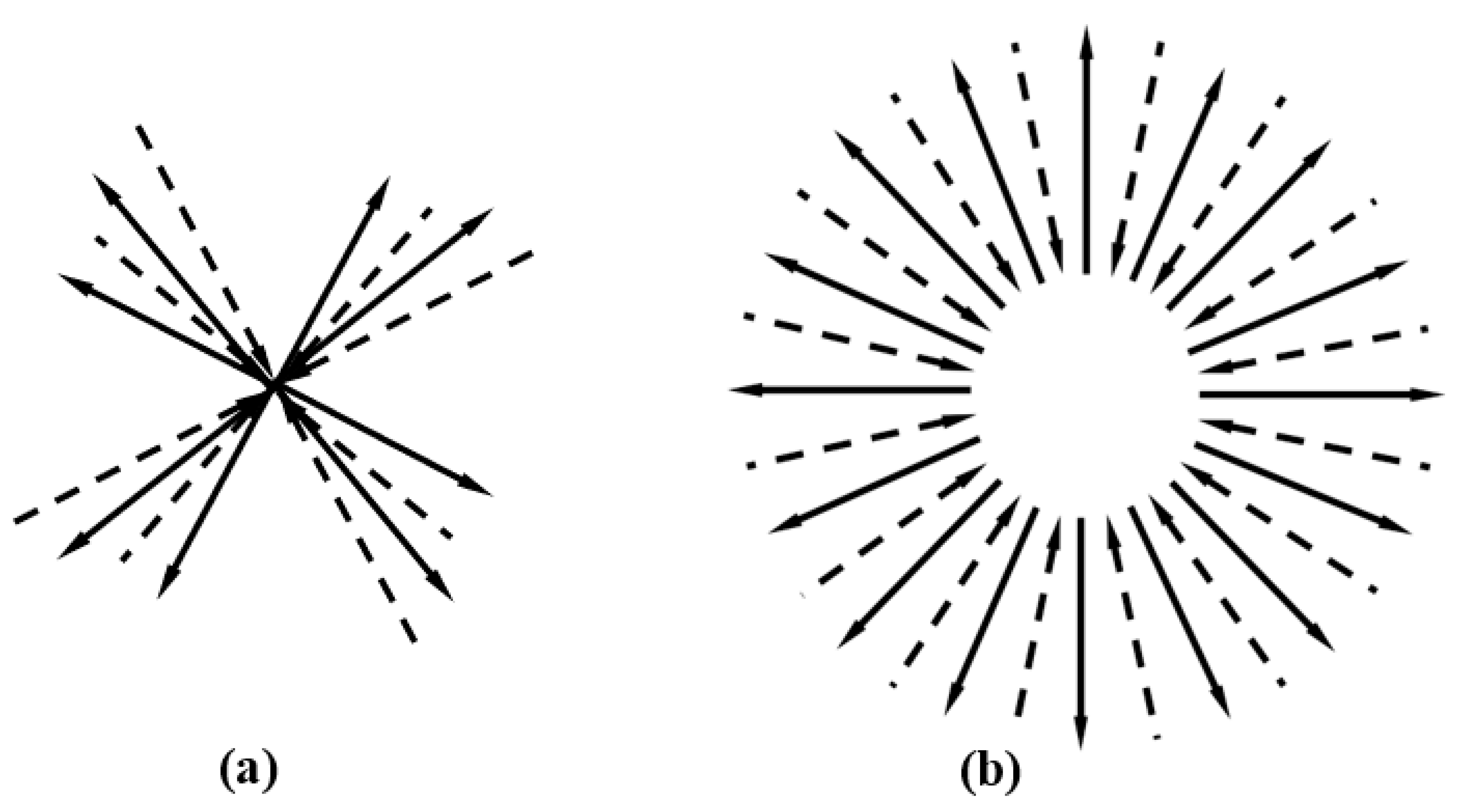
We imagine a picture of the angular distribution of molecular momentum in a gas in equilibrium. Since the number of molecules in the gas is sufficiently large, by detailed balanced, there must be many reciprocal collisions, all of which have the same values of the incident and outgoing momenta, but in different directions. We superimpose events in one figure.
Figure 2a is a superposition of four events of
Figure 1d, where the incident and outgoing particles are represented by dashed and solid lines, respectively.
Figure 2b roughly illustrates the superposition of a large number of collision events. The angle between the momenta of the two incident particles evenly distributes within solid angle 0~4π, and that of the outgoing particles also does so. Since the number of particles is large enough, both are dense enough and the angular distribution is uniform.
Reversing the directions of the arrows for both the solid and dashed lines in
Figure 2b leads to the schematic diagram of Equation (61). This is a superposition of schematic diagram of
Figure 1c in all directions. This shows that for reciprocal collisions, as well as for reverse collisions, the momentum of the incoming and outgoing particles are uniformly distributed in all directions. This means that if there is a collision of
Figure 1a, then there will be a reverse collision
Figure 1c.
Therefore, although the detailed balance itself does not require the reverse process of a collision to appear, we believe that once the detailed balance is reached, then, in the equilibrium state, the reverse collision process of any collision also inevitably occur. That a pair of reverse collisions, Figures 1a,c occur in a macro-instant should be a by-product when an equilibrium state is reached.
Figure 2.
(a) The superposition of four
Figure 1d in different directions. Incident and outgoing particles are represented by dashed and solid lines, respectively. (b) The superposition of a large number of similar collisions, which shows that in equilibrium, the momentum distribution is isotropic.
Figure 2.
(a) The superposition of four
Figure 1d in different directions. Incident and outgoing particles are represented by dashed and solid lines, respectively. (b) The superposition of a large number of similar collisions, which shows that in equilibrium, the momentum distribution is isotropic.
We conceive a situation that slightly deviates from detailed balance.
When Boltzmann derived the
H theorem, he obtained the following inequality [
13].
The equal sign holds only when the detailed balance (60) is reached. Suppose that the gas is very close to equilibrium, such that the momentum distribution of the two particles before the collision belongs to the equilibrium distribution with temperature
T.
After the collision, the distribution belongs to that with temperature
.
For an elastic collision (59), the total energy before and after the collision is denoted by
ε,
. We derive from Equation (62) that
No matter whether the two molecules become a hotter or cooler distribution after the collision, this expression is not less than zero. It is equal to zero only if . This means that areas with slightly lower (higher) temperatures will increase (decrease) temperatures. Heat always flows from the hotter to the cooler area, making the temperature approach the same everywhere.
The isotropic distribution shown in
Figure 2b is ideal only when the number of molecules in the gas approached infinity. In this equilibrium state the momentum distribution remains unchanged.
Therefore, a necessary condition for achieving detailed balance is that the number of molecules in the gas is sufficiently large. Although the molecule number in the actual gas is as high as the order of magnitude of Avogadro constant, it is not really infinity after all. So, even in every macro-instant, it is not guaranteed that for every two-particle collision, there always occur a corresponding reciprocal process. As a result, the momentum distribution of the molecule may deviate from the equilibrium distribution. This deviation is the fluctuation. Macro physical quantities are averages calculated by means of the distribution function. Therefore, they fluctuate around their average values. Obviously, the larger the molecule number, the smaller the fluctuation.
Now, consider a region in the gas which is macroscopically small enough, say, a submillimeter-scale region, the particle number in this region is much smaller than that of the whole gas. If in 1 m3 there are particles, then in m3 there are particles, such that . In such a small region fluctuation will be comparatively large. It is possible that the number of particles with momentum in a certain direction in a macro-instant is much greater than that with opposite momentum. If a pollen granule is positioned in this region, the molecular impact it receives is not isotropic, so that it suffers a net force. The pollen is subject to this force to move. This is the reason of Brownian motion, which reflects that in such a small area, detailed balance cannot be achieved.
Brownian motion also indicates that at a macro-instant, the number of particles entering and leaving the region is not always equal. That is to say, the density of particle numbers in this region is variable with times. The molecular number density in different small regions at a micro-instant are not the same. The density varies around its average value. The fluctuation in a gas actually embodies the non-equilibrium at micro-instant.
When the detailed balance is not achieved, the left and right side of Equation (60) are not equal. This mean that at least a collision of
Figure 1a does not have its reciprocal collision at a macro-instant. Consequently, the momentum distribution function will change. The gas cannot tend to equilibrium. Rather, it is in a non-equilibrium state. As a result, the macroscopic state may change.
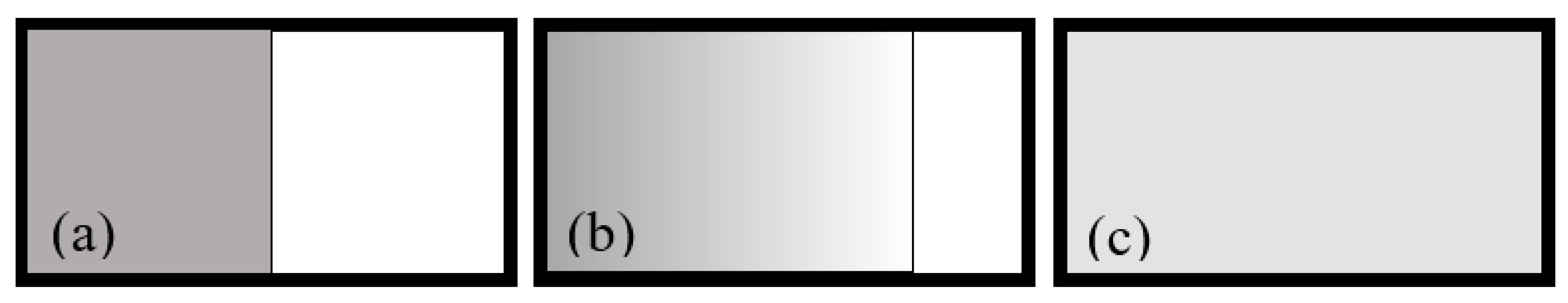
Now consider a case that an ideal gas expands into a vacuum, shown in
Figure 3.
Figure 3a shows that the gas is confined in the left half of a box by a partition. At the initial moment, the partition is removed and the gas begins to expand. The border line between the gas and vacuum is called frontline.
Figure 3.
Schematic diagram of a gas expansion into vacuum. The dotted line indicates the border line between gas and vacuum, called frontline. (a) The gas is confine inside the left half box by a partition. At the initial moment, the partition is drawn and the gas starts expansion. (b) the gas expands to 3/4 box. (c) The gas is evenly distributed in the whole box, i.e., the equilibrium state is reached.
Figure 3.
Schematic diagram of a gas expansion into vacuum. The dotted line indicates the border line between gas and vacuum, called frontline. (a) The gas is confine inside the left half box by a partition. At the initial moment, the partition is drawn and the gas starts expansion. (b) the gas expands to 3/4 box. (c) The gas is evenly distributed in the whole box, i.e., the equilibrium state is reached.
At the moment that the partition is just drawn, there is no particles on the right side of the frontline. Suppose that on the left side of the frontline, two particles moving rightward collide, as in
Figure 1a, and after the collision, they enter the vacuum on the right side of the frontline. If a corresponding reciprocal collision of another two particle occurs as in
Figure 1d, they also enter the vacuum. At this moment, since there are no particles on the right side of the frontline, there is no reverse collision in
Figure 1c. Therefore, the molecules always enter the vacuum from the left side of the frontline. There is no mechanism for particles to immediately return to the left side of the frontline. The frontline moves rightward.
Assuming that at the moment in
Figure 3b, the momenta of all molecules in the gas are reversed, will the frontline move leftward? The analysis is actually the same as that for
Figure 3a. Since the right side of the frontline is a vacuum, the molecules on the left side of the frontline still enter the vacuum, and there is no a mechanism for particles return to the left of the frontline. The frontline continues moving rightward. This shows that Loschmidt’s reasoning does not apply.
We next see the equilibrium state in
Figure 3c. At every micro-instant, it is in a micro state. Every micro state in a macro state is equiprobable to appear. For a micro state in the macro state, when all the molecules’ momentum directions are reversed, the resultant is still a micro state in this macro state. When a gas evolves from a non-equilibrium state to the equilibrium state of
Figure 3c, it can be any of these micro states. Conversely, any micro state in
Figure 3c can be the one, denoted as A, that is achieved by reversing all the molecule momentum directions of a micro state, denoted as B, the latter being achieved from a non-equilibrium state. Following Loschmidt’s reasoning, we assume reversing all the molecular momentum in A to obtain state B, and with time going, the B will undergo a reverse process to go back to the original non-equilibrium state, such as
Figure 1b. However, we know that such a process has never happened and we do not expect so. This again shows that Loschmidt's reasoning does not again.
On account of that the molecule number in the gas considered is not infinite, when there is a collision
Figure 1a, the probability that its reverse collision
Figure 1c is not zero. It seems that there is a nonzero probability for the reverse process of the gas to happen, say, from
Figure 3c to
Figure 3b. Nevertheless, this probability is so small that it is equivalent to impossibility. In Boltzmann’s language, although the evolution from
Figure 3b to
Figure 3a or from
Figure 3c to Figure3d “has a definite calculable (though inconceivably small) probability, which approaches zero only in the limiting case when the number of molecules is infinite.” The time needed is so remote that “One may recognize that this is practically equivalent to
never. …… If a much smaller probability than this is not practically equivalent to impossibility, then no one can be sure that today will be followed by a night and then a day.” [
58]
A frictionless quasi-static process is the exhibition of a series of equilibrium states. Therefore, such a process is reversible. However, this reversibility does not come from the reversibility of the motion of individual particles, nor from the reversibility of the two-particle collision. Rather, it is the statistical average effect of a large number of molecules colliding each other when the detailed balance is reached.
It is seen that macroscopic reversibility requires a large number of molecules in the gas. Usually, a gas contains molecules in the order of magnitude of the Avogadro constant, is very close to meeting this requirement.
In summary, the motion of microscopic particles is irreversible. Nevertheless, when the number of molecules that make up the gas is very large, the macro state of the gas can be in equilibrium when detailed balance is reached. The equilibrium state is the statistical average effect of the motion of a large number of microscopic molecules. Frictionless quasi-static processes are reversible.
C. Two-particle scattering in quantum mechanics
We have demonstrated in subsection II.E that single-particle motion in QM is irreversible.
We [
65] rigorously derived the generalized scattering formula in QM. The state of particles before (after) the scattering is called the initial (final) state. For single-particle scattering, if the initial state is a plane waves of a free particle,
, after scattered by a scattering center, the final state is a complicated spherical wave function
. This spherical wave has projected amplitudes on plane waves
in all directions.
We first consider the case that the two particles are both free before scattering, and then transit to two new plane waves after the scattering.
Suppose that two free particles with momenta and collide. The initial state is the product of two plane waves, . The final state is complex, which can spread in all directions. If we intend to implement the reverse process of the scattering, we must prepare the as the initial state for the reverse process, which is apparently impossible. So, this scattering process of two free particles in QM is irreversible.
Nevertheless, the
can be expanded by two-particle plane waves, i.e., it has project amplitude on every pair of plane wave
. That is to say, there is a probability for the two particles with momenta
and
to transit to two plane waves with specific momenta
and
. This probability is denoted as
. In
Figure 1, we use a piece of arrowed line to represent a plane wave with a momentum.
Figure 1a is the sketch that the two
plane waves transit to two new plane waves
, denoted as
.
Figure 1b–d are respectively the time-inverse transition, reverse transition, and reciprocal transition of
Figure 1a. All of the transitions follow the total energy conservation and total momentum conservation (59). The transition probability of the reciprocal process is denoted as
.
We have rigorously proved that [
65]
The transition probability of the process
Figure 1a,
is the same as that of
Figure 1d
. This conclusion is derived from the scattering theory in QM, so that it is always valid, irrespective to whether a system reaches detailed balance, as well as irrelevant to the properties of the whole gas [
75].
The previous discussion about the two-particle collisions and detailed balance in classical mechanics is entirely applicable to the case here, as long as the two-particle collision in classical mechanics is replaced by the scattering transition of two free particles,
, in QM. In particular, the detailed balance only concerns a pair of reciprocal scattering transition
Figure 1a,d, and is irrelative to the reverse transition
Figure 1c.
If a scattering transition
Figure 1a occurs, the probability of the occurrence of its reverse process
Figure 1c is equal to 1, then, the original process
Figure 1a is said reversible. However, it is actually that
. Similarly, the probability that the process
Figure 1c happen is necessarily less than 1. Therefore, the process
Figure 1a is irreversible.
It was believed that [
51,
52]
This relationship means that the transition probabilities of the processes
Figure 1a,b are the same. This was called detailed balance and seemed obtained from QM [
76], the authors of which equated the time-inverse and reverse processes. We stress that in deriving scattering formulas [
65], we always considered clockwise processes. We do not know how to include the clockwise and anticlockwise processes in one formulas. In Section II, it is seen that the retarded and advanced processes have to be studied separately. Therefore, we can prove Equation (67) but are unable to verify (68) by the scattering theory in QM.
We address three points here. First, we make it clear that detailed balance (60), but not (67), is a content in statistical mechanics. Equation (67) is proved by the scattering theory in QM. Second, Equation (60) is expressed by momentum distribution function, which involves all molecules in the gas, while (67) only concerns two molecules, irrespective to other molecules. Third, Equation (60) only applies to equilibrium states, while (67) is always correct for two-particle scattering, regardless of whether the whole gas reaches equilibrium.
In Ref. [
51], Equation (68) is put down first. Then, with known Maxwell-Boltzmann distribution for equilibrium state, it is postulated that the numbers of the collision and reverse collision in unit time and unit phase space volume are the same so as to achieve (60). This procedure is different from that used by Boltzmann, who first obtained (60) for equilibrium state, and then derive Maxwell-Boltzmann distribution from Equation (60).
We turn to discuss the collision between two non-free particles. That is to say, before and after the collision, particles may be in bound states. A non-free particle is also called a bound particle. On account of the identity of particles, detailed balance should be written in the following form [
11,
13,
15,
74].
In Equation (69), the
is the average number of particles in the energy state
. From the left to right hand sides, Equation (69) represents that two particles in the energy levels
and
collide, and then transit to energy levels
and
, respectively, denoted as
. From the right to left hand side, Equation (69) refers to its reciprocal process,
. The transition probabilities of the two processes are denoted by
and
, respectively. It can be verified [
65] that
In the reciprocal courses, the total energy is conserved.
Please note the difference of the two bound particles’ collision and two free-particles’ collision. A free particle has a momentum. Its state has an index momentum. In its revers motion, its momentum direction is opposite to that in its original motion. This direction reversion helps us distinguish the reverse and reciprocal motions, e.g.,
Figure 1c,d. In both original and reverse motions, its total energy and total momentum are conserved, see Equation (59).
A bound particle is in one of its discrete energy levels, and there is no momentum such a state index. Thus, a collision requires the conservation of total energy, see Equation (70), without the relationship of momentum conservation. In this case, there is no difference between reverse and reciprocal collisions.
It may be due to the identity of the reverse and reciprocal collisions of bound particles, some ones believed that the collisions are reversible. We reiterate the definition of reversibility of a process: if after an original process occurs, the occurrence probability of the reverse process is less than 1, i.e., , then the original process is irreversible. This is because the particles in the state can also transit to states other than states by collision, as long as Equation (71) is meet. The conclusion is that in any case, two-particle collisions are irreversible.
Messiah [
77] thought that Equation (67) was a microreversibility relation. The author of Ref. [
78] thought that Equation (67) meant the detailed balance. “This notion is based solely on the reversibility of the microscopic equation of motion. (Or, more technically, on the Hermitian nature of the scattering Hamiltonian.)” The detailed balance (69) only involves the distribution function, and has nothing to do with whether the equation of motion is reversible. Moreover, the two-particle scattering transition is irreversible. We emphasize that when deriving equation (67), we do not use the time inversion invariance of the microscopic equations of motion [
65].
For the cases of two free particle collisions, that equilibrium is reached is not due to the reversibility of the collision, but form detailed balance (60). Similarly, in the case of bound particle collisions, equilibrium is neither due to the reversibility of the collisions, but due to (69). In the case of quantum statistics, detailed balance can be obtained by master equation, although in a form superficially different from (69) [
9,
11,
53]. By the form from master equation, it is possible to define a “distance” measuring the violation of detailed balance [
19].