1. Introduction
Active packaging development has become essential in the plastics industry, particularly in food packaging, because this packaging is necessary for maintaining the quality, freshness, and safety of food products. Active packaging can increase product shelf life by controlling the interaction between the development and its environment, reducing food waste and the risk of product damage (Dirpan et al., 2023; Martinez et al., 2023). The use of petrochemical plastics in food packaging has had several drawbacks, particularly in terms of damaging the environment and causing a larger plastic waste problem. The most important aspect, however, is that petrochemical plastics can leach dangerous chemical compounds into food, such as bisphenol A (BPA), which can disrupt the human hormonal system. Aside from that, plastic can harm the appearance and texture of food products, as well as inhibit oxygen exchange, causing food ingredients to spoil faster (Glicerina et al., 2023; tomic et al., 2023).
The development of PLA-based active packaging is a significant innovation in the food packaging industry. PLA is a biodegradable polymer derived from renewable sources such as corn or potato starch. One of the most significant advantages of PLA is that it is biodegradable, which means that it can be naturally broken down by microorganisms in certain environments (Pandey et al., 2022). Aside from that, PLA provides sufficient strength and resistance to water penetration to be used in a variety of food packaging applications (Arias et al., 2022; Radhalakshmi et al., 2023). Since PLA is brittle and has limited resistance to water vapor permeability, it is commonly employed in industrial packaging applications alongside other polymers such as PCL. PCL as a more flexible polymer with a lower level of crystallinity, blends with PLA to improve the mechanical, strength, and barrier properties of packaging (Opálková et al., 2022). This mixture provides PLA-PCL copolymer, which improves flexibility and resistance to water vapor permeation (Staffa et al., 2022). PCL was selected as the best polymer to combine with PLA in packaging because it has a lower melting point and crystallinity than PLA, allowing PCL to provide the elasticity required to overcome PLA's brittleness in active packaging production.
Active packaging is produced by incorporating active agents such as antioxidants and antimicrobials. Plant extracts, specifically extracts containing polyphenols such as Syzygium cumini seed waste, are among the most frequently employed natural antioxidant sources in the development of active packaging. Polyphenols are natural compounds with high antioxidant activity that can protect food from oxidative damage (Ahmed et al., 2022; Zhang et al., 2022; Ullah et al., 2022). When compounds in a material are exposed to oxygen, they undergo oxidative damage, also known as oxidation (Wu et al., 2022). This reaction can cause color, taste, and texture changes, as well as nutritional value loss in food ingredients (nawaz et al., 2022). Apart from that, Syzygium cumini seed waste contains phenolics, such as gallic acid, ellagic acid, and catechin (Qamar et al., 2022; Kumar et al.,2022). This phenolic has the ability to capture free radicals which contribute to oxidative damage in food ingredients (Periyasamy et al., 2022). Its flavonoid content protects food products by inhibiting lipid and protein oxidation.
Antibacterial properties in active packaging, alongside antioxidants, are highly crucial and may offer further advantages in protecting food products. Bacteria, mold, and other pathogenic microorganisms are common causes of food spoilage and food poisoning (Manaa et al., 2022). The antibacterial properties of active packaging can inhibit the growth and development of these microorganisms, thereby preserving product freshness and safety. Furthermore, in the food industry, consumer safety is a top priority (Kumari et al., 2022). Antibacterials in active packaging can help reduce the risk of cross-contamination or contamination by pathogenic microorganisms. Nanochitosan, or chitosan in nanoparticle form, has characteristics that make it suitable for the production of active packaging, especially because it is a source of antibacterial, the amino group (NH2), which plays an important role in antibacterial properties (Khairy et al., 2022; Abdullazadeh et al., 2023). Chitosan has a large surface area in the nanostructure, facilitating to interact effectively with microorganisms such as bacteria (Sen et al., 2022).
This study develops environmentally friendly active packaging by utilizing Syzygium cumini seed waste as an antioxidant. This aids in attempts to reduce organic waste and replace synthetic chemicals in food packaging systems. This active packaging may improve food safety and quality by preventing oxidative damage and the growth of harmful microorganisms. Therefore, this research assists in reducing food waste and the risk of food poisoning while also supporting sustainability principles in the food industry. Moreover, the addition of nano chitosan to the packaging provides antibacterial properties, causing it to be more effective in preserving product freshness.
2. Material and method
2.1. The extraction of syzygium cumini seed as sources of antioxidant
The dried seeds of Syzygium cumini are ground into a fine powder as much as 30 g. The seed powder is then placed in the extraction container. Water 5:1 is used as the solvent, and the extraction process is carried out by heating the solvent to around 100°c using a controlled heater (Kant et al., 2022). The seeds are soaked in boiling water for 45 minutes during the extraction process, allowing water-soluble compounds such as polyphenols to be extracted into the solvent. Right after extraction, the extract can be separated from the seeds by filtration and centrifuged (320R Benchtop centrifuge).
2.2. Preparation of active packaging
The solvent casting method was used to produce neat films according to Sutil et al., (2022). The films were made with PLA (IngeoTM Biopolymer 3052D, Nature Works LLC), PCL (Aldrich, Mn = 80,000), Nanochitosan (Sigma Aldrich, Jakarta), and chloroform (98%, Sigma Aldrich, Jakarta). Polymers blend utilizing using PLA which dissolved in 1250 mL of chloroform and PCL (1.5 g) in 750 mL of chloroform in separate glasses, then mixed together using a magnetic stirrer at 300 rpm for 8 hours at 35
0C. After that, the mixture was poured into a Petri dish and dried in a 50°C oven.
Table 1 summarises the various mixture compositions studied and the processing conditions used in the active agent mixing process.
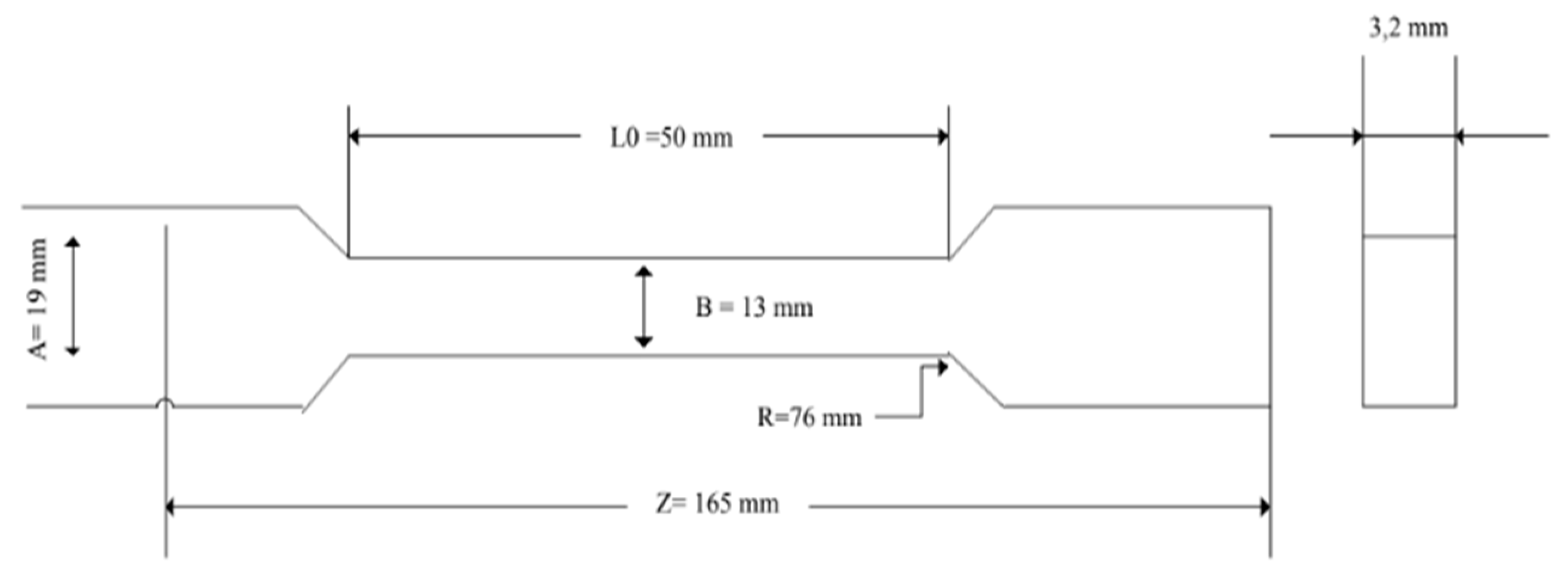
PLA/PCL blends are extruded using a single screw extruder (Chello0023 jigsaw) at a temperature of 160
0C for 50 minutes with the aaddition of active agent. The sample printed using a UTM printing machine to produce a tensile test sample. A specimen of a tensile rod is shown in
Figure 1.
2.3. Characterization techniques
The Universal Tensile Machine (UTM) tensile test is a method for testing the strength of a material by applying an axial force load at a speed of 2 mm/minute. The FT-IR instrument operates on the spectroscopy principle. Because of its complex spectrum of many peaks, infrared spectroscopy/FTIR is used for organic compound identification (qualitative analysis). FTIR is also used to determine the types of functional groups that can indicate a material's general composition at 500 cm-1- 4000 cm-1. The detailed morphological features of the active packaging films were explored using a scanning electron microscope (SEM-JEOL model).
2.4. Aplication of active packaging on chicken breast
2.4.1. Colony reduction
Minced chicken breasts were analyzed for S. aureus on day 0 to determine the presence or absence of this pathogen, while experimentally contaminated samples were analyzed for S. aureus on day 0 and on days 3, 6, and 9 of storage. Minced chicken breast that had not been inoculated with S. aureus was analyzed for total survival at 0 and days 3, 6, and 9 of storage. After opening from active packaging, 10 g of minced meat was aseptically weighed, and transferred into a sterile bag, and 180 ml of distilled water was added to each sample for bacterial counting. The chopped samples were homogenized in the blender for 2 minutes. For the enumeration of different bacteria, serial decimal dilutions are prepared, and 1 ml or 0.1 ml of appropriately diluted suspension is inoculated directly on the surface of the appropriate medium. S. aureus was counted on PDA agar media and incubated at 33 °C for 24 hours. Bacterial colonies were incubated for 24 hours at 37 °C in accordance with ISO 21528-2 (2004). According to ISO 15214 (1998), colony reduction was calculated using a Scan 300 colony counter after 72 hours of incubation at 33 °C. The results were measured in colony-forming units per gram (CFU/g).
2.4.2. pH
Before measuring pH, minced chicken breast samples were left at room temperature for 10 minutes. A pH meter Testo 205 (Testo AG, Lenzkirch, Germany) was used to measure the pH.
2.4.3. Total volatile basic nitrogen
Total volatile nitrogen (TVBN) was determined as described by Shao et al., (2022).
3. Result and discussion
3.1. Tensile strength of active packaging films
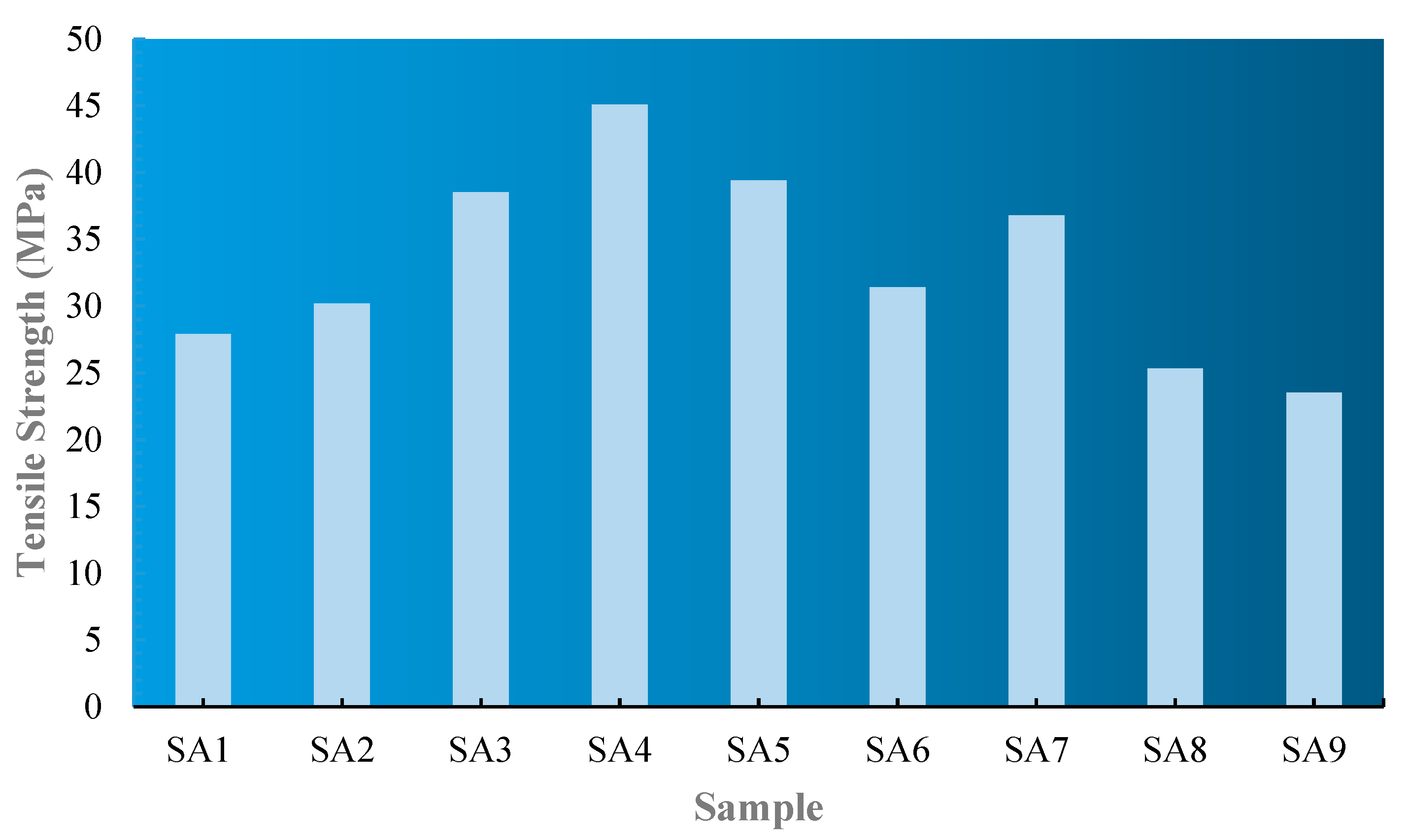
The tensile strength analysis aims to determine the influence of PLA/PCL/nanochitosan/Syzygium cumini seed extract variations on the tensile strength and percentage elongation of the produced plastic films. One key finding of this research is that the addition of Syzygium cumini seed extract and nanochitosan as reinforcements results in a significant increase in the tensile strength of the produced nanocomposite plastic.
The addition of
Syzygium cumini seed extract as a natural antioxidant source acts as a strengthening agent, enhancing the intermolecular interactions within the polymer matrix. This strengthening effect leads to increased load-bearing capacity and resistance to deformation under pressure. The combination of nanochitosan with its amino and hydroxyl functional groups exhibits excellent chemical compatibility with the polymer matrix. This compatibility facilitates a strong interfacial bond between chitosan and polymer chains, contributing to the overall tensile strength of the produced films. In
Figure 2, the combination of
Syzygium cumini seed extract and nanochitosan also creates a synergistic effect further enhancing the tensile strength, as observed in sample SA4 (45.1 MPa).
Syzygium cumini's seed extract ability to neutralize free radicals and inhibit the oxidation process complements the strengthening properties of chitosan, resulting in a stronger polymer network.
Nanochitosan is chitosan that has been transformed into nanoparticles. This provides a larger surface area and good dispersion properties within polymer matrices such as PLA/PCL. Nanochitosan also has nanometer-sized particles, giving it a greater ability to reinforce the polymer matrix. The uniform distribution of nanochitosan within the matrix can enhance tensile strength, elastic modulus, and impact resistance more effectively than regular chitosan. Nanochitosan is becoming increasingly popular globally as a potential food preservative due to its biocompatible, bioactive, biodegradable, polycationic, and non-toxic properties. Generally, nanochitosan is produced from chitin (the second most abundant biopolymer after cellulose) through a deacetylation process, typically sourced from crustaceans such as shrimp. Through advancements in science and technology, nanochitosan has been modified to exhibit superior properties, including antibacterial, antifungal, antioxidant activities, and other remarkable characteristics, such as film-forming capabilities.
3.2. FT-IR of active packaging films
The functional group analysis was conducted to identify the functional groups present in the plastic film samples using Fourier Transform Infrared (FT-IR) spectroscopy. This analysis is based on the characteristic peak wavelengths of a sample. These peak wavelengths indicate the presence of specific functional groups in the sample because each functional group has its own characteristic peak. The FT-IR analysis results can be seen in the figure below.
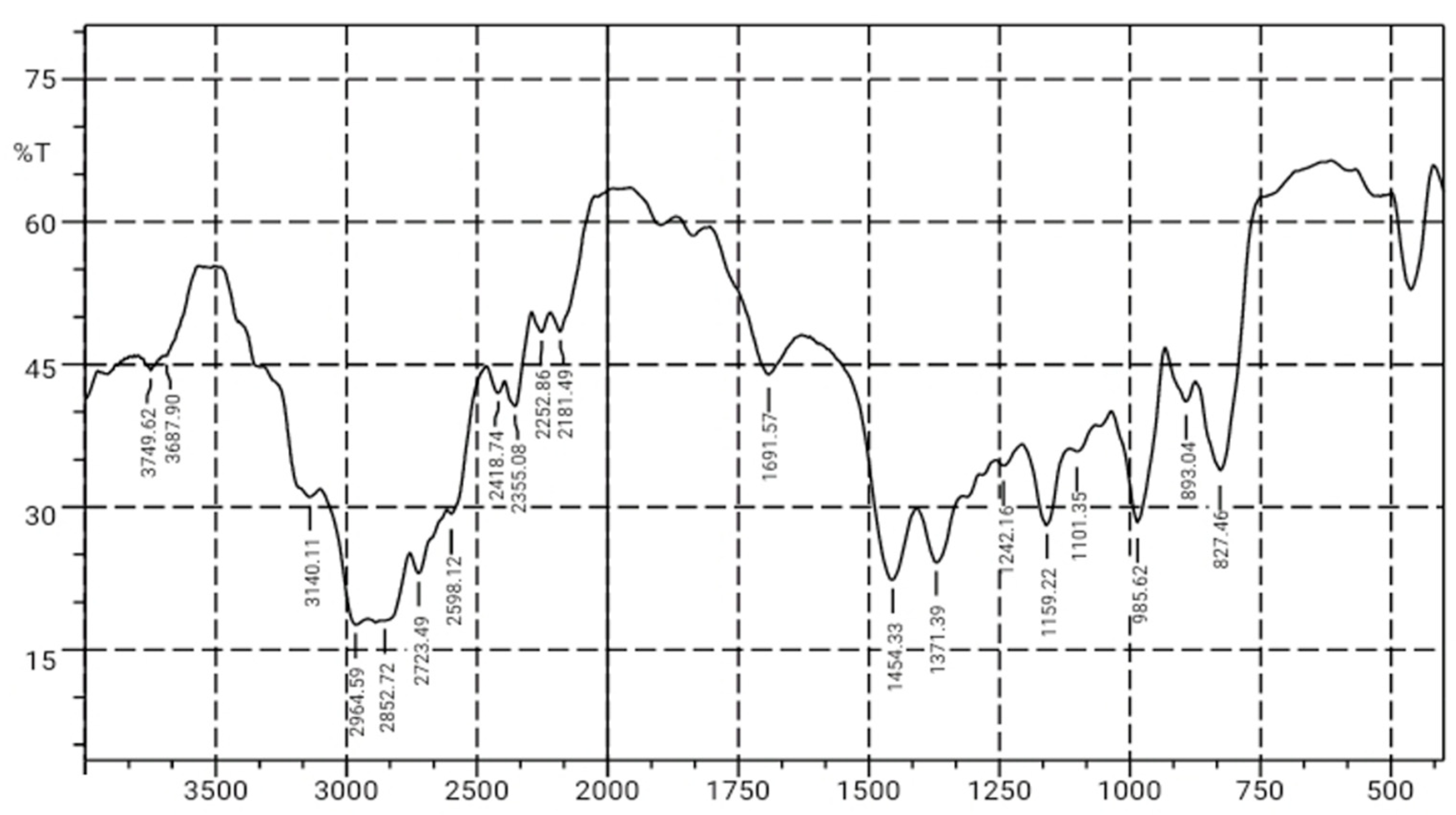
Figure 3. shows the analysis results of the best-blended sample (SA4) from the research, which indicates absorption at 3140.11 cm
-1, confirming the presence of N-H groups. This is consistent with the literature, which states that the broad absorption appearing in the 3300-3500 cm
-1 region is attributed to N-H groups, indicating the presence of nanochitosan.
In the 3000-2850 cm-1 region, there is an absorption at 2964.59 cm-1 indicating the presence of C-H groups, while in the 2500-2000 region, there is absorption at 2355.08 cm-1 indicating the presence of C=O groups, and in the 1500-1250 cm-1 region, there is absorption at 1454.33 cm-1 indicating the presence of C-O groups, which suggests the presence of Syzygium cumini seed extract groups. These results reveal the functional groups that make up the plastic polymer. The functional group identification results in Table 3. show that all the functional groups present are consistent with the base materials used, namely PLA, PCL, nanochitosan, and Syzygium cumini seed extract, without the formation of new functional groups. It can be concluded that the plastic film manufacturing process is perfectly executed as its constituent components have been successfully identified.
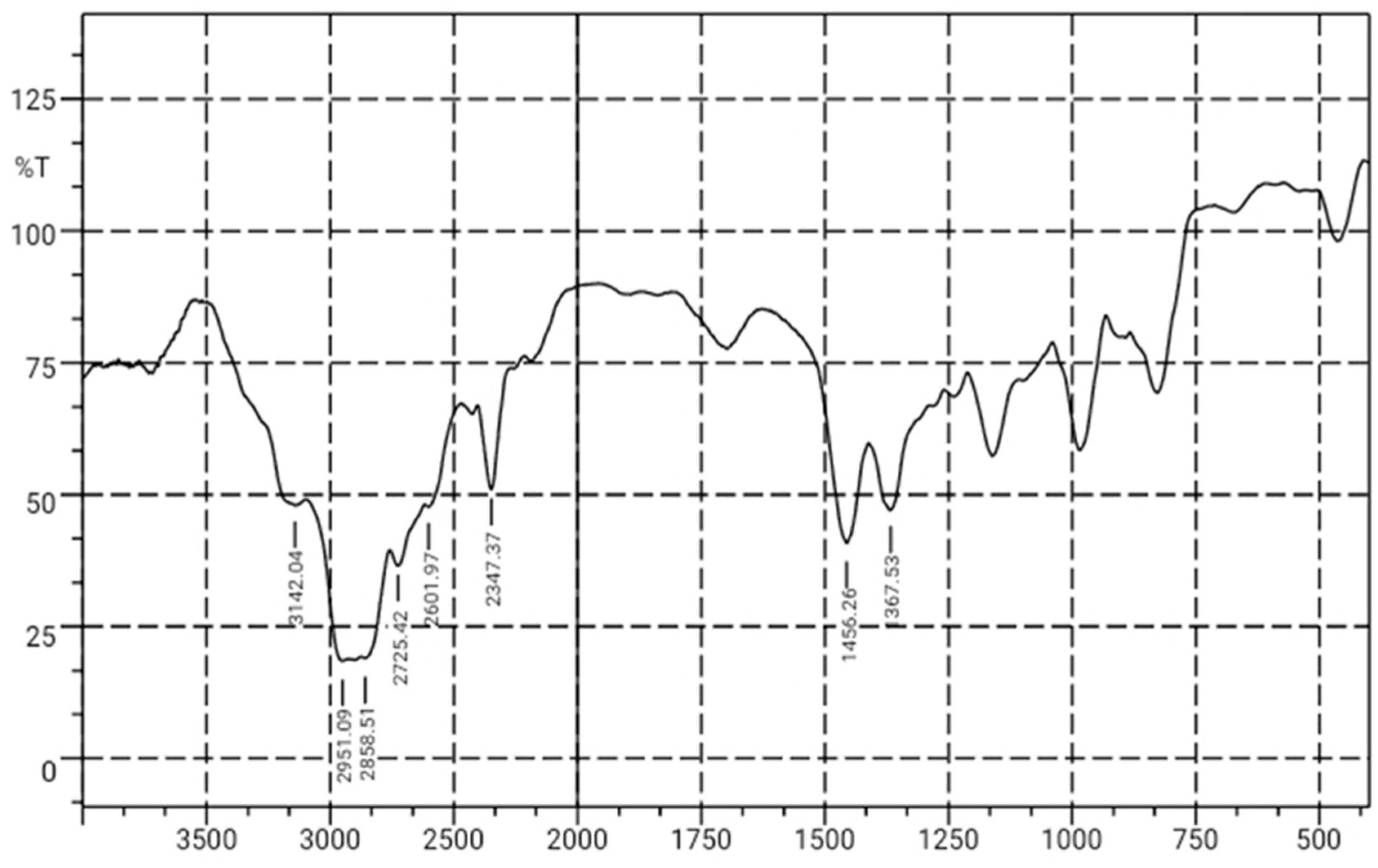
Figure 4. shows the results of sample SA5 as a reference. It is known that there is absorption at 3142.04 cm
-1, indicating the presence of N-H groups, consistent with the literature that the broad absorption appearing in the 3300-3500 cm
-1 region is attributed to N-H groups. In the 3000-2850 cm
-1 region, there is an absorption at 2951.09 cm
-1 indicating the presence of C-H groups, in the 2500-2000 cm
-1 region, there is absorption at 2347.37 cm
-1 indicating the presence of C=O groups, and in the 1500-1250 cm
-1 region, there is absorption at 1456.26 cm
-1 indicating the presence of C-O groups. These results show that all the samples have the same functional groups but with different peak absorbance levels. The differences in absorbance peaks occur because each type of molecule has its own unique molar absorptivity coefficient, also known as the molar absorptivity coefficient. This value represents how strongly a specific functional group or chemical bond in a molecule interacts with infrared radiation. When there is a change in the concentration of a substance, the number of molecules present in the sample also changes. This means that with higher concentrations, there are more molecules available to absorb infrared light, resulting in higher absorbance values. In complex samples or mixtures, various molecules or functional groups can interact with infrared radiation in various ways. These interactions can lead to overlapping absorbance peaks, making it difficult to separate the contributions of individual components. Changes in concentration can affect the intensity and position of these peaks. It is important to note that absorbance is not a linear response to concentration over a wide range. At high concentrations, absorbance can reach a saturation point where further increases in concentration may not significantly increase absorbance. This occurs because all available molecules have absorbed as much as they can at a specific wavelength.
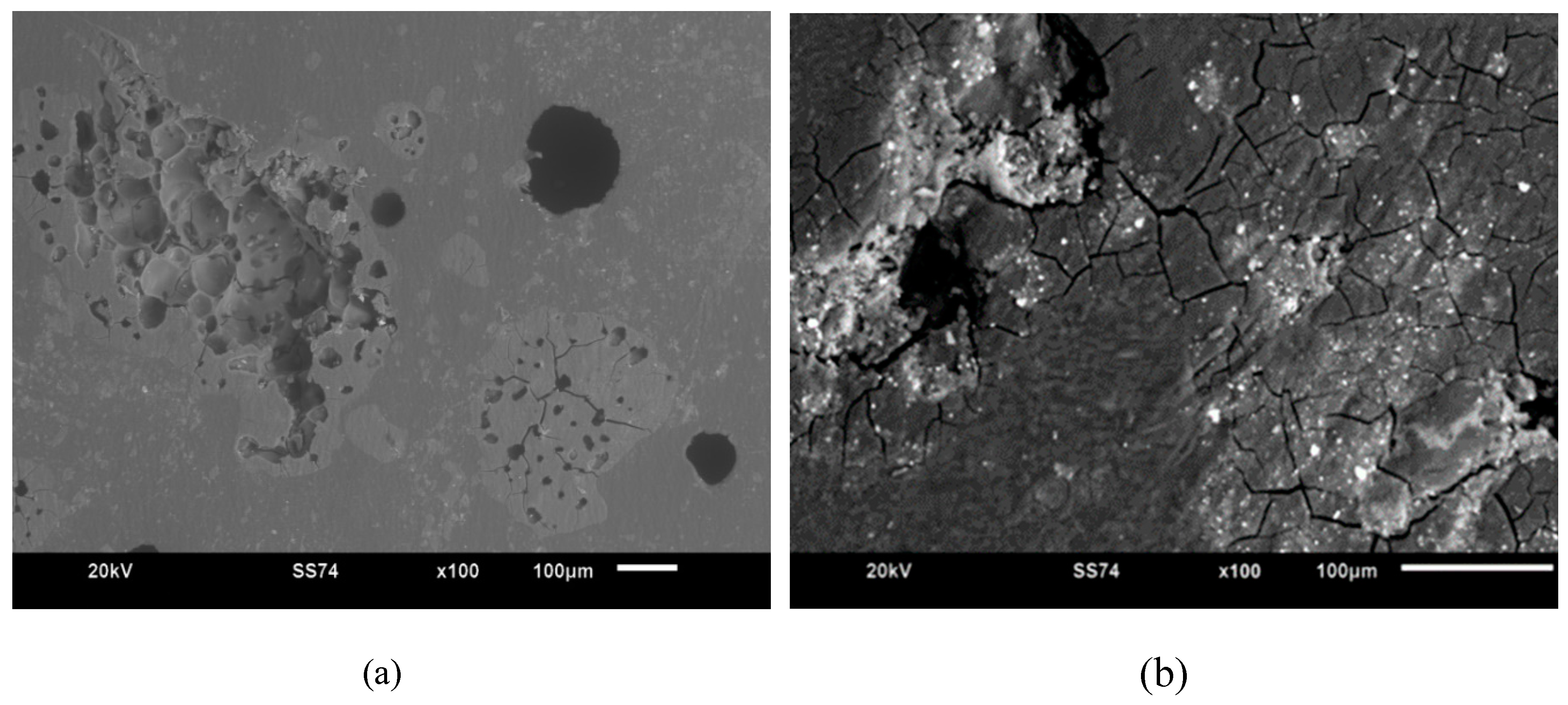
3.3. SEM of active packaging film
Morphological characterization is an additional test in this research aimed at supporting the results of the best samples from the biodegradation test. This test aims to observe the morphological structure of the eco-friendly biocomposite blending process of PLA/PCL/nanochitosan/
Syzygium cumini seed extract using a microscope that relies on electron beams to depict the surface morphology of the analyzed material. The following is the image of the analysis results using Scanning Electron Microscopy (SEM).
Figure 5. (a) indicate a surface structure that exhibits excessive agglomeration of nanochitosan particles (25% by weight). Nanochitosan particles exhibit strong Van der Waals forces between each other.
When too many nanochitosan particles are in close proximity, these forces can cause the particles to agglomerate. This is also influenced by the nanochitosan molecules attracting each other, leading these particles to cluster together. When the concentration of nanochitosan in the solution is very high, the likelihood of agglomeration increases. When too many nanochitosan particles are present in a limited space, they have more opportunities to come into contact and interact with each other, which can result in agglomeration. Additionally, the choice of solvent and compatibility between the solvent and polymer matrix can affect the nanoparticle distribution. If the solvent used to disperse nanoparticles is not compatible with the polymer, it can lead to phase separation, causing the nanoparticles to cluster in certain areas. Furthermore, factors such as particle size, shape, and surface chemistry can also influence dispersion. Smaller nanoparticles and surface-modified particles tend to disperse more evenly, while larger or unmodified particles are more prone to aggregation. Achieving uniform nanoparticle distribution in active packaging materials involves addressing various factors through proper formulation, mixing techniques, and the use of suitable dispersants to enhance overall performance and effectiveness of the packaging material.
Figure 5. (b) indicate a smoother surface structure of the sample. This suggests that a smaller quantity of nanochitosan (20% by weight) in the matrix minimizes high-energy distribution. When the amount of nanochitosan is limited, the energy required to move the particles within the matrix becomes lower, making it easier to achieve even dispersion. Nanochitosan can also interact with the matrix through surface adhesion. Based on
Figure 6, it implies that nanochitosan has the ability to adhere evenly to the matrix due to compatibility as well as improved mixing and dispersion.
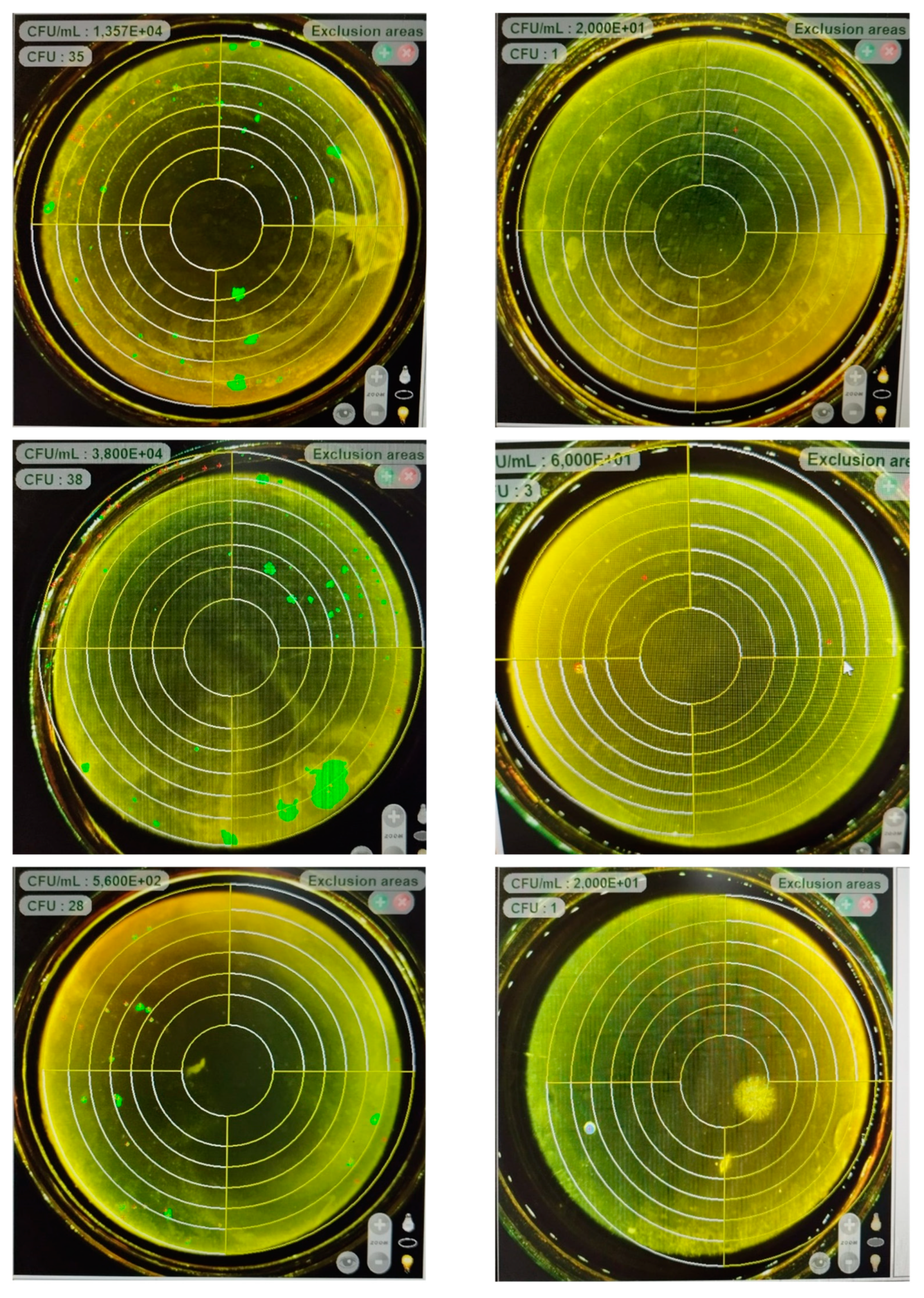
3.4. Colony reduction
Nanochitosan has the scientific ability to reduce Staphylococcus aureus (S. aureus) colonies due to various factors, including its physicochemical properties and antimicrobial mechanisms. S. aureus is a pathogenic bacterium commonly responsible for human infections, including skin infections, respiratory tract infections, and food poisoning. Nanochitosan is a derivative of chitosan, a natural polysaccharide obtained from the exoskeleton of shrimp and crabs. Chitosan possesses strong antimicrobial properties due to its positive charge. Nanochitosan, with its very small particle size, has a large surface area, allowing it to interact with more S. aureus bacteria. The amine groups in the chitosan structure interact with the phosphate groups on the cell walls of S. aureus bacteria, disrupting cellular membrane integrity and membrane permeability, thereby inhibiting growth and causing cell lysis. Moreover, nanochitosan can penetrate bacterial cell membranes, leading to greater internal damage.
Nano-sized chitosan at 20% by weight makes it easier for it to penetrate bacterial aggregates and biofilms that may form from S. aureus. When nanochitosan successfully penetrates bacterial biofilms, they can interact directly with the protected bacterial cells within. This is important because S. aureus often forms biofilms that reduce the effectiveness of conventional antibiotics.
Good dispersion of nanochitosan in solution also allows them to evenly distribute in the environment surrounding the bacteria, enhancing their ability to interact with a large number of S. aureus bacterial cells. Syzygium cumini seed extract can also penetrate the double lipid membrane of bacteria, disrupting membrane integrity. This can lead to osmotic imbalance and leakage of essential substances from bacterial cells. As a result, bacteria experience cellular stress that can inhibit their growth and division. Syzygium cumini seed extract can also inhibit the activity of vital enzymes in bacterial cells. These enzymes are essential for bacterial metabolism and reproduction processes. By inhibiting the activity of these enzymes, Syzygium cumini seed extract can disrupt various metabolic pathways within bacterial cells, thereby reducing bacterial colonies.
3.5. pH
In this research, the pH of minced chicken breast with the addition of nanochitosan/
Syzygium cumini seed extract additives (SA4) was evaluated under cold conditions (20°C) and compared with samples containing only PLA/PCL polymer (SA8). The optimal pH of chicken breast typically ranges from 5.8 to 6.07, depending on the chicken type used and the slaughtering process. In this study, the pH of minced chicken breast on the first day was 5.82 and did not show significant differences between samples. Subsequently, the pH of chicken breast in SA8 packaging decreased after the first day, as indicated in
Figure 7. The pH decrease that occurred after a few days may be due to the reaction of dissolved CO2 in water and the lipid fraction in chicken breast, resulting in the formation of carbonic acid, which subsequently lowered the pH. Furthermore, there was a further decrease in pH at specific periods, likely related to the growth of lactic acid bacteria.
One of the factors influencing the pH decrease is the population of lactic acid bacteria. During this study, it was observed that the lowest pH occurred on the 18th day, especially in chicken breast wrapped with the SA8 sample. In samples with SA4, the addition of nanochitosan and Syzygium cumini seed extract could affect pH by reducing the population of lactic acid bacteria, which ultimately could result in a lower level of acidity. Another factor contributing to the pH increase may be related to the presence of alkaline and nitrogen compounds formed during protein changes in chicken breast due to bacterial growth during storage. The possibly low storage temperature in this study might have only slowed down food degradation but could not prevent the deterioration of food quality, especially in food items with high water content. This research revealed that the SA4 sample was able to maintain the quality of chicken breast better than the polymer sample.
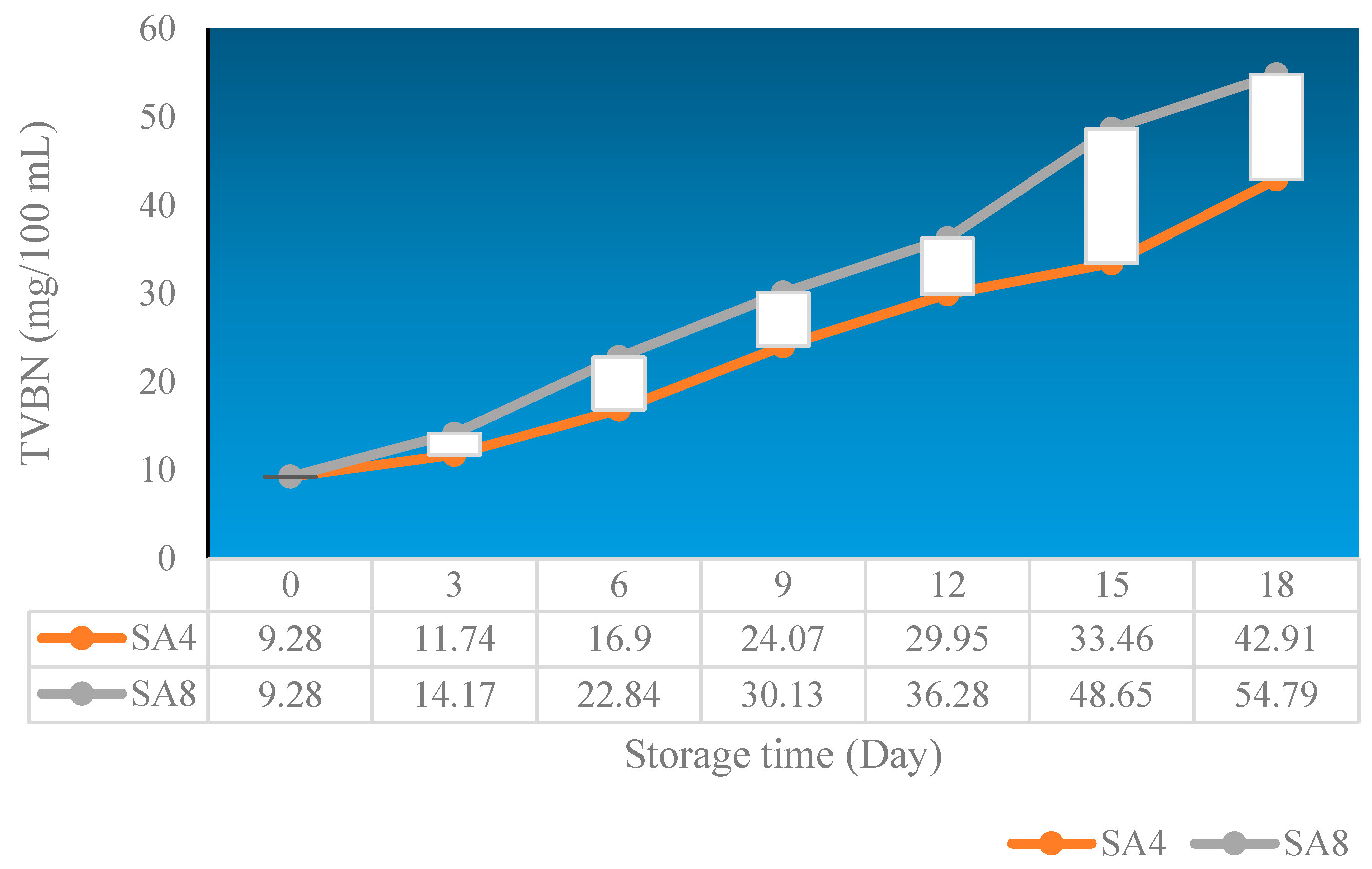
3.6. TVBN of chicken breast
TVBN is an indicator used to evaluate the freshness of chicken breast. When the meat is fresh and in good condition, the level of volatile basic nitrogen (TVBN) is usually low. However, over time and due to changes in storage conditions, the concentration of TVBN can increase. An increase in TVBN typically indicates changes and damage to proteins in chicken breast, which can be caused by bacterial activity, enzymes, and chemical reactions during storage. TVBN testing is an important tool in monitoring the quality of chicken breast products during the storage and distribution cycle. It allows manufacturers and suppliers to assess how long a product can be stored while maintaining the freshness levels acceptable to consumers.
A high TVBN level can be an indicator that the product is no longer fit for consumption or has low quality. From
Figure 8. it can be observed that sample SA4 can better maintain the quality of chicken breast compared to sample SA8. This is because
Syzygium cumini, as an antioxidant source, can protect the proteins in chicken breast from oxidation, which can lead to changes and degradation. Protein oxidation is one of the main factors in meat quality changes.
Syzygium cumini, with its antioxidant properties, can help slow down the protein oxidation process during storage. The SA4 film used as packaging, enriched with nanochitosan, can have better barrier properties compared to polymer plastic (SA8) without additives. The SA4 packaging film can help reduce the entry of oxygen and water into the chicken breast packaging. Oxygen and water are factors that can accelerate protein changes. By reducing permeability to oxygen and water, plastic with nanochitosan can slow down protein changes. Nanochitosan also has the potential to interact with proteins in chicken breast. This can occur through hydrogen bonding or ionic bonding. These interactions can help maintain the structure and stability of proteins, inhibiting conformational changes that can occur during storage.
Hydrogen bonding is an interaction between a hydrogen atom from one molecule and oxygen, nitrogen, or fluorine atoms from another molecule. These bonds form due to differences in electronegativity between these atoms, resulting in the hydrogen atom having a partial positive charge that can interact with the partial negative charge of other atoms. Nanochitosan and Syzygium cumini seed extract, with their structures rich in hydroxyl groups (-OH), have the potential to form hydrogen bonds with the functional groups on the side chains of amino acids in chicken breast proteins. These hydrogen bonds can occur between the hydrogen atom on the hydroxyl group of nanochitosan and the oxygen or nitrogen atom in the protein. Hydrogen bonding can help maintain the three-dimensional structure of proteins. When hydrogen bonds form, they can help maintain the relative positions of atoms in the protein, which in turn preserves the correct protein conformation. During storage, this can inhibit conformational changes that can occur due to environmental pressures or interactions with other molecules.
4. Conclusion
In summary, the SA4 sample exhibited the highest tensile strength due to enhanced intermolecular interactions within the polymer matrix facilitated by the addition of Syzygium cumini seed extract and uniform distribution of nanochitosan. FT-IR analysis confirmed successful interactions between the PLA/PCL matrix and nanochitosan and Syzygium cumini additives. However, excess nanochitosan in the SA5 sample led to particle agglomeration. SA4-coated samples showed a significant reduction in S. aureus bacterial colonies, up to 98%, and improved shelf life based on TVBN and pH values.
Author Contributions
Conceptualization, S.S.; methodology, T.R and S.S; investigation, A.S. and T.R.; resources, F.; data curation, S.S.; T.R and A.S.; writing—original draft preparation, S.S., T.R.; and A.S.; writing—review and editing, T.R.; project administration, T.R.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The author expresses his gratitude and highest appreciation to Sincere appreciation to the Ministry of Research, Technology and Higher Education of the Republic of Indonesia and Lhokseumawe State Polytechnic which has funded through grants number: 461/BAP/SPK/04/PPK.01.APTV/VI/PNL/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author wishes to express her gratitude and appreciation to the Republic of Indonesia's Ministry of Research, Technology, and Higher Education, as well as Politeknik Negeri Lhokseumawe.
Conflicts of Interest
Not applicable.
References
- Dirpan, A., Ainani, A. F., & Djalal, M. (2023). A bibliometrics visualization analysis of active packaging system for food packaging. Heliyon. [CrossRef]
- Martínez-Aguilar, V., Peña-Juárez, M. G., Carrillo-Sanchez, P. C., López-Zamora, L., Delgado-Alvarado, E., Gutierrez-Castañeda, E. J., ... & Gonzalez-Calderon, J. A. (2023). Evaluation of the Antioxidant and Antimicrobial Potential of SiO2 Modified with Cinnamon Essential Oil (Cinnamomum Verum) for Its Use as a Nanofiller in Active Packaging PLA Films. Antioxidants, 12(5), 1090. [CrossRef]
- Glicerina, V., Siroli, L., Gottardi, D., Ticchi, N., Capelli, F., Accorsi, R., ... & Romani, S. (2023). Influence of an innovative, biodegradable active packaging on the quality of sunflower oil and “pesto” sauce during storage. Applied Food Research, 3(2), 100313.
- Tomić, A., Šovljanski, O., & Erceg, T. (2023). Insight on Incorporation of Essential Oils as Antimicrobial Substances in Biopolymer-Based Active Packaging. Antibiotics, 12(9), 1473. [CrossRef]
- Pandey, S., Sharma, K., & Gundabala, V. (2022). Antimicrobial bio-inspired active packaging materials for shelf life and safety development: A review. Food Bioscience, 48, 101730. [CrossRef]
- Arias, A., Feijoo, G., & Moreira, M. T. (2022). Technological feasibility and environmental assessment of polylactic acid-nisin-based active packaging. Sustainable Materials and Technologies, 33, e00460. [CrossRef]
- Radhalakshmi, V., Raman, M., & Joy, M. R. (2023). Development of active packaging film based on poly (lactic acid) incorporated with Piper betel leaf ethanolic extract and its application in the shelf-life extension of tuna meat. International Journal of Biological Macromolecules, 246, 125751. [CrossRef]
- Opálková Šišková, A., Mosnáčková, K., Musioł, M., Opálek, A., Bučková, M., Rychter, P., & Eckstein Andicsová, A. (2022). Electrospun Nisin-Loaded Poly (ε-caprolactone)-Based Active Food Packaging. Materials, 15(13), 4540.
- Staffa, L. H., Huysecom, A. S., Bettini, S. H. P., Moldenaers, P., & Chinelatto, M. A. (2022). Effect of molecular structure of PEG/PCL multiblock copolymers on the morphology and interfacial properties of PLA/PCL blends. Journal of Polymer Research, 29(9), 388. [CrossRef]
- Ahmed, M., Khan, K. U. R., Ahmad, S., Aati, H. Y., Ovatlarnporn, C., Rehman, M. S. U., ... & Anwar, M. (2022). Comprehensive phytochemical profiling, biological activities, and molecular docking studies of Pleurospermum candollei: An insight into potential for natural products development. Molecules, 27(13), 4113.
- Zhang, Y., Cai, P., Cheng, G., & Zhang, Y. (2022). A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Natural Product Communications, 17(1), 1934578X211069721.
- Ullah, H., Hussain, Y., Santarcangelo, C., Baldi, A., Di Minno, A., Khan, H., ... & Daglia, M. (2022). Natural polyphenols for the preservation of meat and dairy products. Molecules, 27(6), 1906. [CrossRef]
- Wu, H., Richards, M. P., & Undeland, I. (2022). Lipid oxidation and antioxidant delivery systems in muscle food. Comprehensive Reviews in Food Science and Food Safety, 21(2), 1275-1299. [CrossRef]
- Nawaz, A., Irshad, S., Khan, I. A., Khalifa, I., Walayat, N., Aadil, R. M., ... & Lorenzo, J. M. (2022). Protein oxidation in muscle-based products: Effects on physicochemical properties, quality concerns, and challenges to food industry. Food Research International, 157, 111322. [CrossRef]
- Qamar, M., Akhtar, S., Ismail, T., Wahid, M., Abbas, M. W., Mubarak, M. S., ... & Esatbeyoglu, T. (2022). Phytochemical profile, biological properties, and food applications of the medicinal plant Syzygium cumini. Foods, 11(3), 378.
- Kumar, M., Hasan, M., Lorenzo, J. M., Dhumal, S., Nishad, J., Rais, N., ... & Zhang, B. (2022). Jamun (Syzygium cumini (L.) Skeels) seed bioactives and its biological activities: A review. Food Bioscience, 102109. [CrossRef]
- Periyasamy, A. P. (2022). Natural dyeing of cellulose fibers using syzygium cumini fruit extracts and a bio-mordant: A step toward sustainable dyeing. Sustainable Materials and Technologies, 33, e00472. [CrossRef]
- Manaa, O., Mahran, A., Ahmed, A., Helal, I., & Shaheen, H. (2022). Rapid Assessment of Spoilage and Food Poisoning Microbes in Common Meat Products. Suez Canal Veterinary Medical Journal. SCVMJ, 27(2), 243-258.
- Kumari, R., Jayachandran, L. E., & Kumar, S. (2022). Role of Microbes in the Food Industry. Role of Microbes in Industrial Products and Processes, 59-79.
- Khairy, A. M., Tohamy, M. R., Zayed, M. A., Mahmoud, S. F., El-Tahan, A. M., El-Saadony, M. T., & Mesiha, P. K. (2022). Eco-friendly application of nano-chitosan for controlling potato and tomato bacterial wilt. Saudi Journal of Biological Sciences, 29(4), 2199-2209. [CrossRef]
- Abdollahzadeh, M., Elhamirad, A. H., Shariatifar, N., Saeidiasl, M., & Armin, M. (2023). Effects of nano-chitosan coatings incorporating with free/nano-encapsulated essential oil of Golpar (Heracleum persicum L.) on quality characteristics and safety of rainbow trout (Oncorhynchus mykiss). International Journal of Food Microbiology, 385, 109996. [CrossRef]
- Sen, S. K., Tripathi, R., Mandal, P., & Choudhuri, C. (2022). Assessment of antifungal activity of nano-chitosan against mung bean seed borne pathogen Aspergillus flavus through solid matrix priming. South African Journal of Botany, 150, 372-386. [CrossRef]
- Kant, R., & Kumar, A. (2022). Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustainable Chemistry and Pharmacy, 30, 100829. [CrossRef]
- Sutil, G. A., Andrade, K. S., Rebelatto, E. A., & Lanza, M. (2022). Effects of incorporation of pure or multicomponent active agents in biopolymers for food packaging using supercritical CO2. Trends in Food Science & Technology, 120, 349-362. [CrossRef]
- Shao, L., Tian, Y., Chen, S., Xu, X., & Wang, H. (2022). Characterization of the spoilage heterogeneity of Aeromonas isolated from chilled chicken meat: in vitro and in situ. Lwt, 162, 113470. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).