Submitted:
04 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
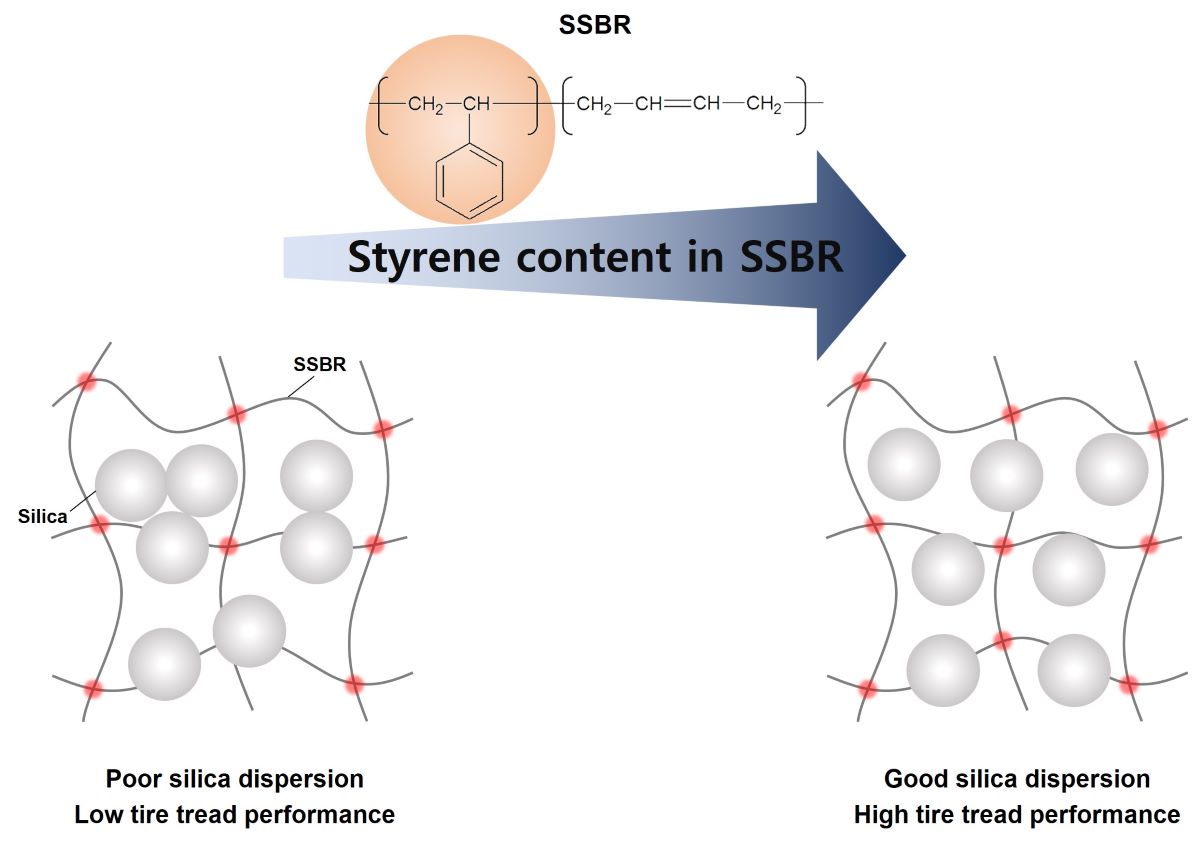
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample preparation
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.-Y.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.-J.; Kim, W. The effect of accelerator contents on the vulcanizate structures of SSBR/silica vulcanizates. Compos. Interfaces 2017, 24, 563–577. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.S.; Lim, S.H.; Jang, S.H.; Kim, D.H.; Park, N.-H.; Jung, J.W.; Choi, J. The Investigation of the Silica-Reinforced Rubber Polymers with the Methoxy Type Silane Coupling Agents. Polymers 2020, 12, 3058. [Google Scholar] [CrossRef]
- Iz, M.; Kim, D.; Hwang, K.; Kim, W.; Ryu, G.; Song, S.; Kim, W. The effects of liquid butadiene rubber and resins as processing aids on the physical properties of SSBR/silica compounds. Elastomers Compos. 2020, 55, 289–299. [Google Scholar]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of natural rubber with silica/carbon black hybrid filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Santana, M.H.; Verdejo, R.; López-Manchado, M.A. Giving a Second Opportunity to Tire Waste: An Alternative Path for the Development of Sustainable Self-Healing Styrene–Butadiene Rubber Compounds Overcoming the Magic Triangle of Tires. Polymers 2019, 11, 2122. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.; Tang, Z.; Guo, B. Solving “magic triangle” of tread rubber composites with phosphonium-modified petroleum resin. Polymer 2020, 190, 122244. [Google Scholar] [CrossRef]
- Jung, J.; Sodano, H.A. Synergetic effect of aramid nanofiber-graphene oxide hybrid filler on the properties of rubber compounds for tire tread application. J. Appl. Polym. Sci. 2021, 139, 51856. [Google Scholar] [CrossRef]
- Qu, L.; Yu, G.; Xie, X.; Wang, L.; Li, J.; Zhao, Q. Effect of silane coupling agent on filler and rubber interaction of silica reinforced solution styrene butadiene rubber. Polym. Compos. 2013, 34, 1575–1582. [Google Scholar] [CrossRef]
- Lee, D.-H.; Li, X.X.; Cho, U.-R. Comparison on mechanical properties of SSBR composites reinforced by modified carbon black, silica, and starch. Elastomers Compos. 2018, 53, 175–180. [Google Scholar]
- Liu, S.; Liu, L.; Wu, Q.; Zhang, L. Silica reinforced epoxidized solution-polymerized styrene butadiene rubber and epoxidized polybutadiene rubber nanocomposite as green tire tread. Polymer 2023, 281. [Google Scholar] [CrossRef]
- Lim, S.-H.; Lee, S.; Lee, N.; Ahn, B.K.; Park, N.; Kim, W. Effect of 1,3-Diphenyl-guanidine (DPG) Mixing Step on the Properties of SSBR-silica Compounds. Elastomers Compos. 2016, 51, 81–92. [Google Scholar] [CrossRef]
- Hassan, A.A.; Formela, K.; Wang, S. Enhanced interfacial and mechanical performance of styrene-butadiene rubber/silica composites compatibilized by soybean oil derived silanized plasticization. Compos. Sci. Technol. 2020, 197, 108271. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, B.; Kim, K.; Lee, J.; Kim, I.J.; Kim, W. Effects of Molecular Weight of Functionalized Liquid Butadiene Rubber as a Processing Aid on the Properties of SSBR/Silica Compounds. Polymers 2021, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Park, N.; Kim, D.; Kim, W. Influence of end-functionalized solution styrene–butadiene rubber on silica-filled vulcanizates with various silica–silane systems. Rubber Chem. Technol. 2019, 92, 364–377. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, N.; Lim, S.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.-J.; Kim, W. Influence of the silanes on the crosslink density and crosslink structure of silica-filled solution styrene butadiene rubber compounds. Compos. Interfaces 2016, 24, 711–727. [Google Scholar] [CrossRef]
- Kim, W.; Ryu, G.; Hwang, K.; Song, S.; Kim, W. Effect of coagulant type on the silica dispersion and properties of functionalized RAFT ESBR silica wet masterbatch. Elastomers Compos. 2020, 55, 167–175. [Google Scholar]
- Shin, W.S.; Kwon, Y.R.; Kim, J.S.; Hong, S.J.; Kim, Y.J.; Lim, S.H.; Chang, Y.W.; Kim, D.H. Improved Silica Dispersibility in Silica-rubber Compounds for a Tire Tread by Using an Itaconic Acid-based Polymeric Dispersant. Fibers Polym. 2021, 22, 196–204. [Google Scholar] [CrossRef]
- Choi, S.-S. Improvement of the filler dispersion in silica-filled SBR compounds using low molecular weight polybutadiene treated with maleic anhydride. Elastomers Compos. 2006, 41, 10–18. [Google Scholar]
- Manoharan, P.; Naskar, K. Exploring a highly dispersible silica–elastomer composite for tire applications. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Sun, C.; Wen, S.; Ma, H.; Li, Y.; Chen, L.; Wang, Z.; Yuan, B.; Liu, L. Improvement of Silica Dispersion in Solution Polymerized Styrene–Butadiene Rubber via Introducing Amino Functional Groups. Ind. Eng. Chem. Res. 2018, 58, 1454–1461. [Google Scholar] [CrossRef]
- Hassanabadi, M.; Najafi, M.; Motlagh, G.H.; Garakani, S.S. Synthesis and characterization of end-functionalized solution polymerized styrene-butadiene rubber and study the impact of silica dispersion improvement on the wear behavior of the composite. Polym. Test. 2020, 85, 106431. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Han, S.; Kim, W.-S.; Mun, D.-Y.; Ahn, B.; Kim, W. Effect of coupling agents on the vulcanizate structure of carbon black filled natural rubber. Compos. Interfaces 2020, 27, 355–370. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black loaded natural rubber vulcanizates. Part II. J. Appl. Polym. Sci. 1962, 6, 368–372. [Google Scholar] [CrossRef]
- Robertson, C.G.; Lin, C.J.; Bogoslovov, R.B.; Rackaitis, M.; Sadhukhan, P.; Quinn, J.D.; Roland, C.M. Flocculation, reinforcement, and glass transition effects in silica-filled styrene-butadiene rubber. Rubber Chem. Technol. 2011, 84, 507–519. [Google Scholar] [CrossRef]
- Seo, M.; Lee, C.; Kim, D.; Ahn, B.; Lee, G.-R.; Kim, W.; Li, S. Saccharide-containing conjugates as eco-friendly coupling agents for silica reinforced rubber compounds. Polym. Test. 2021, 104, 107379. [Google Scholar] [CrossRef]
- Parks, C.R.; Brown, R.J. Crosslink Density of Elastomers. A New Gas-Chromatographic Method. Rubber Chem. Technol. 1976, 49, 233–236. [Google Scholar] [CrossRef]
- Choi, S. Improvement of properties of silica-filled natural rubber compounds using polychloroprene. J. Appl. Polym. Sci. 2002, 83, 2609–2616. [Google Scholar] [CrossRef]
- Ansarifar, A.; Nijhawan, R.; Nanapoolsin, T.; Song, M.; Tanabe, Y.; Miyoshi, K.; Kirino, Y.; Liang, J.; Feng, N.; Chang, S.; et al. Reinforcing Effect of Silica and Silane Fillers on the Properties of Some Natural Rubber Vulcanizates. Rubber Chem. Technol. 2003, 76, 1290–1310. [Google Scholar] [CrossRef]
- Ansarifar, A.; Wang, L.; Ellis, R.J.; Kirtley, S.P.; Riyazuddin, N. Enhancing the mechanical properties of styrene–butadiene rubber by optimizing the chemical bonding between silanized silica nanofiller and the rubber. J. Appl. Polym. Sci. 2007, 105, 322–332. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Yang, L.; Wang, H.; Zhong, J. Effect of styrene content on mechanical and rheological behavior of styrene butadiene rubber. Mater. Res. Express 2020, 8, 015302. [Google Scholar] [CrossRef]
- Choi, S.-S.; Chung, K.-H.; Nah, C. Improvement of properties of silica-filled styrene-butadiene rubber (SBR) compounds using acrylonitrile-styrene-butadiene rubber (NSBR). Polym. Adv. Technol. 2003, 14, 557–564. [Google Scholar] [CrossRef]
- Halasa, A.F.; Prentis, J.; Hsu, B.; Jasiunas, C. High vinyl high styrene solution SBR. Polymer 2005, 46, 4166–4174. [Google Scholar] [CrossRef]
- Padenko, E.; Berki, P.; Wetzel, B.; Karger-Kocsis, J. Mechanical and abrasion wear properties of hydrogenated nitrile butadiene rubber of identical hardness filled with carbon black and silica. J. Reinf. Plast. Compos. 2015, 35, 81–91. [Google Scholar] [CrossRef]
- Lee, S.; Son, C.E.; Hong, U.; Lee, K.; Choi, S.-S. Influence of Cure Condition on Crosslink Types and Densities and Physical Properties of NR/BR Blend Rubber Vulcanizate. Polym. Korea 2018, 42, 895–900. [Google Scholar] [CrossRef]
- Kim, D.-W.; Seo, B.-H.; Kim, H.-J.; Paik, H.-J.; Kang, J.-W.; Kim, W.-H. Mechanical Properties of Acrylonitrile Functionalized Emulsion SBR/silica Compounds. Elastomers Compos. 2012, 47, 54–64. [Google Scholar] [CrossRef]
- Lolage, M.; Parida, P.; Chaskar, M.; Gupta, A.; Rautaray, D. Green Silica: Industrially scalable & sustainable approach towards achieving improved “nano filler – Elastomer” interaction and reinforcement in tire tread compounds. Sustain. Mater. Technol. 2020, 26, e00232. [Google Scholar] [CrossRef]
- Wang, M.-J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Kim, W.-S.; Lee, D.-H.; Kim, I.-J.; Son, M.-J.; Kim, W.; Cho, S.-G. SBR/organoclay nanocomposites for the application on tire tread compounds. Macromol. Res. 2009, 17, 776–784. [Google Scholar] [CrossRef]
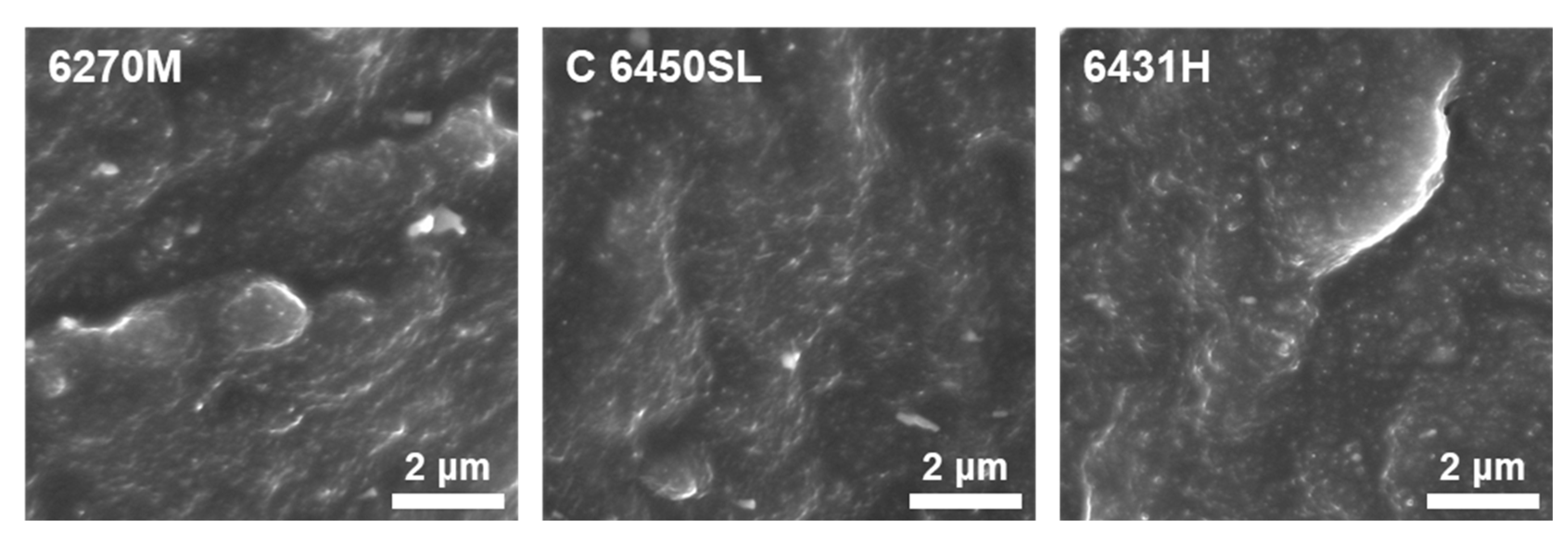
| Styrene content$(%) | Vinyl content$(%, in butadiene) | Mooney viscosity | Tg (°C) | |
|---|---|---|---|---|
| 6270M | 25 | 63 | 51 | -30 |
| C 6450 SL | 35 | 40 | 53 | -36 |
| 6431H | 40 | 35 | 62 | -30 |
| 6270M | C 6450SL | 6431H | ||
|---|---|---|---|---|
| Stage 1$(SMB Mixing) | SSBR(6270M) | 100 | - | - |
| SSBR(C 6450SL) | - | 100 | - | |
| SSBR(6431H) | - | - | 100 | |
| Silica | 60 | |||
| TESPT | 6 | |||
| ZnO | 5 | |||
| StA | 1 | |||
| PEG 4000 | 3 | |||
| Stage 2$(FMB Mixing) | Sulfur | 0.5 | ||
| CBS | 1.5 | |||
| DPG | 1.5 | |||
| Step | Time (min) | Action |
|---|---|---|
| 1st step$(mixing in a kneader) | 0:00 | Add rubber (80 °C) |
| 1:00 | Add ZnO + StA + PEG 4000 | |
| 2:00 | Add silica + TESPT (1/4) | |
| 5:00 | Add silica + TESPT (2/4) | |
| 8:00 | Add silica + TESPT (3/4) | |
| 11:00 | Add silica + TESPT (4/4) | |
| 15:00 | Dump (120–125 ℃) | |
| 2nd step$(mixing in a two-roll mill) | 0:00 | Add SMB compound |
| 1:00 | Add sulfur + CBS + DPG | |
| 5:00 | Dump |
| 6270M | C 6450SL | 6431H | |
|---|---|---|---|
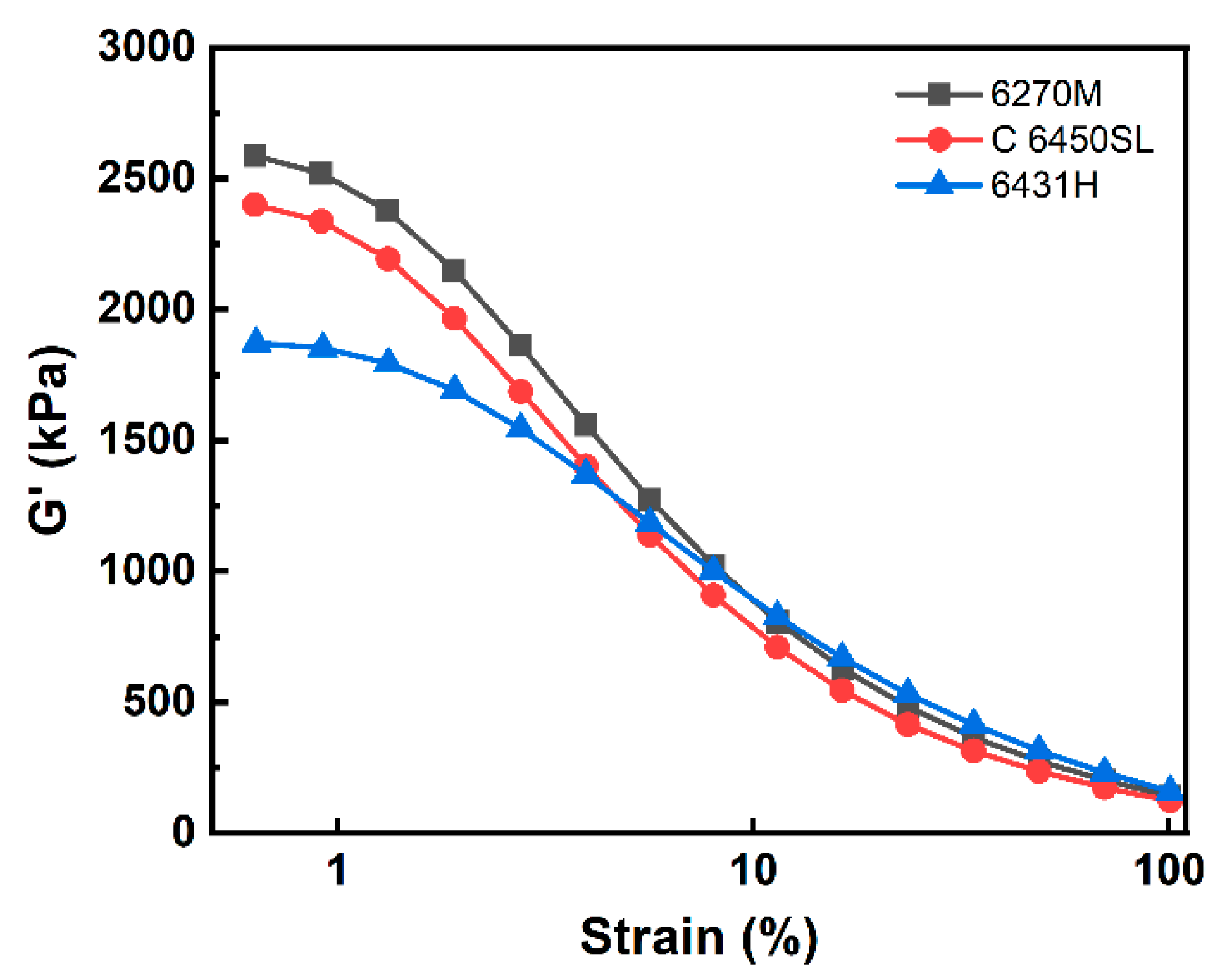
| G′ at initial point (kPa) | 2585 | 2400 | 1871 |
| G′ at final point (kPa) | 143.0 | 123.7 | 159.3 |
| ∆G′ (kPa) | 2442 | 2276 | 1712 |
| 6270M | C 6450SL | 6431H | |
|---|---|---|---|
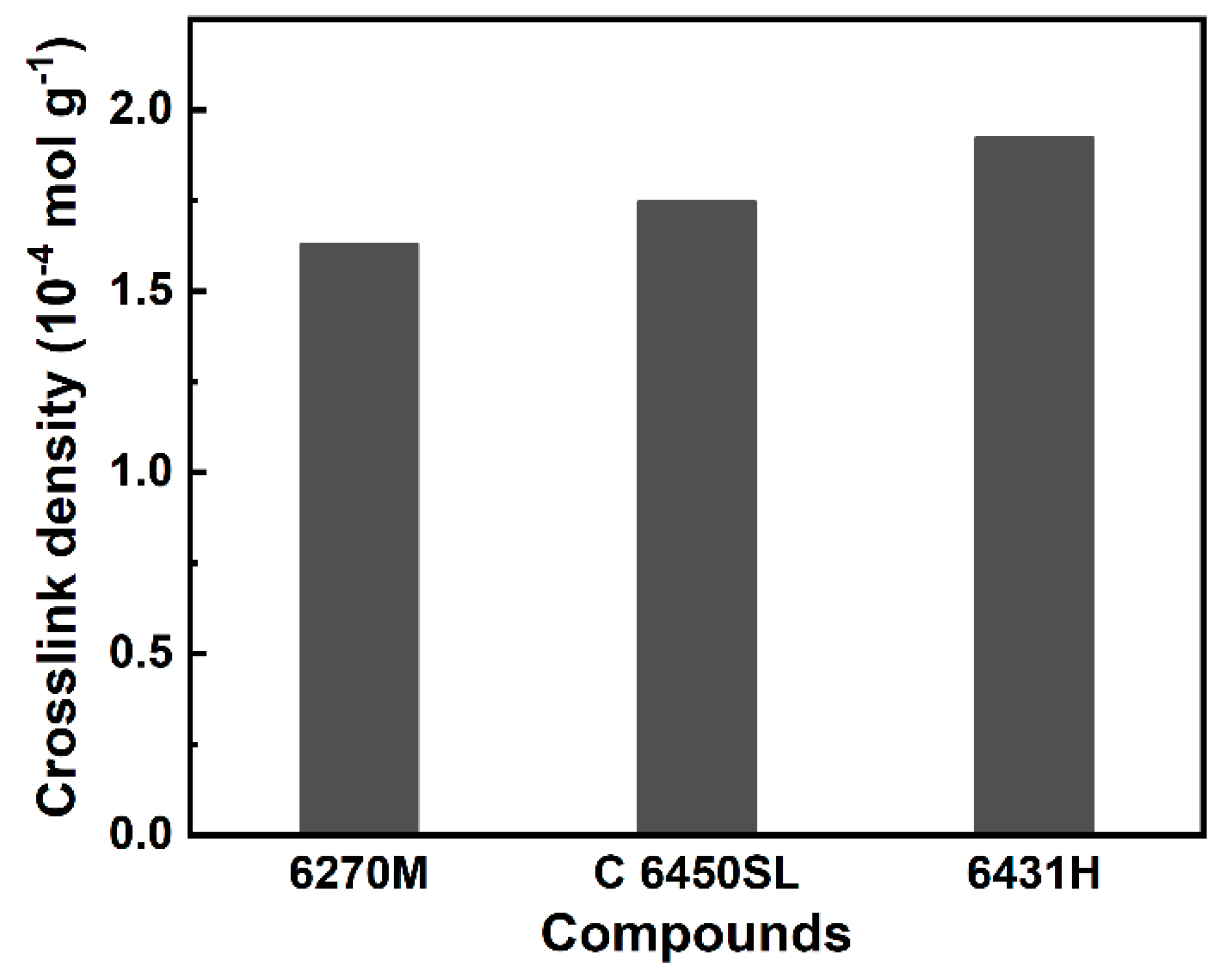
| Crosslink density (10-4 mol g-1) | 1.629 | 1.747 | 1.923 |
| 6270M | C 6450SL | 6431H | |
|---|---|---|---|
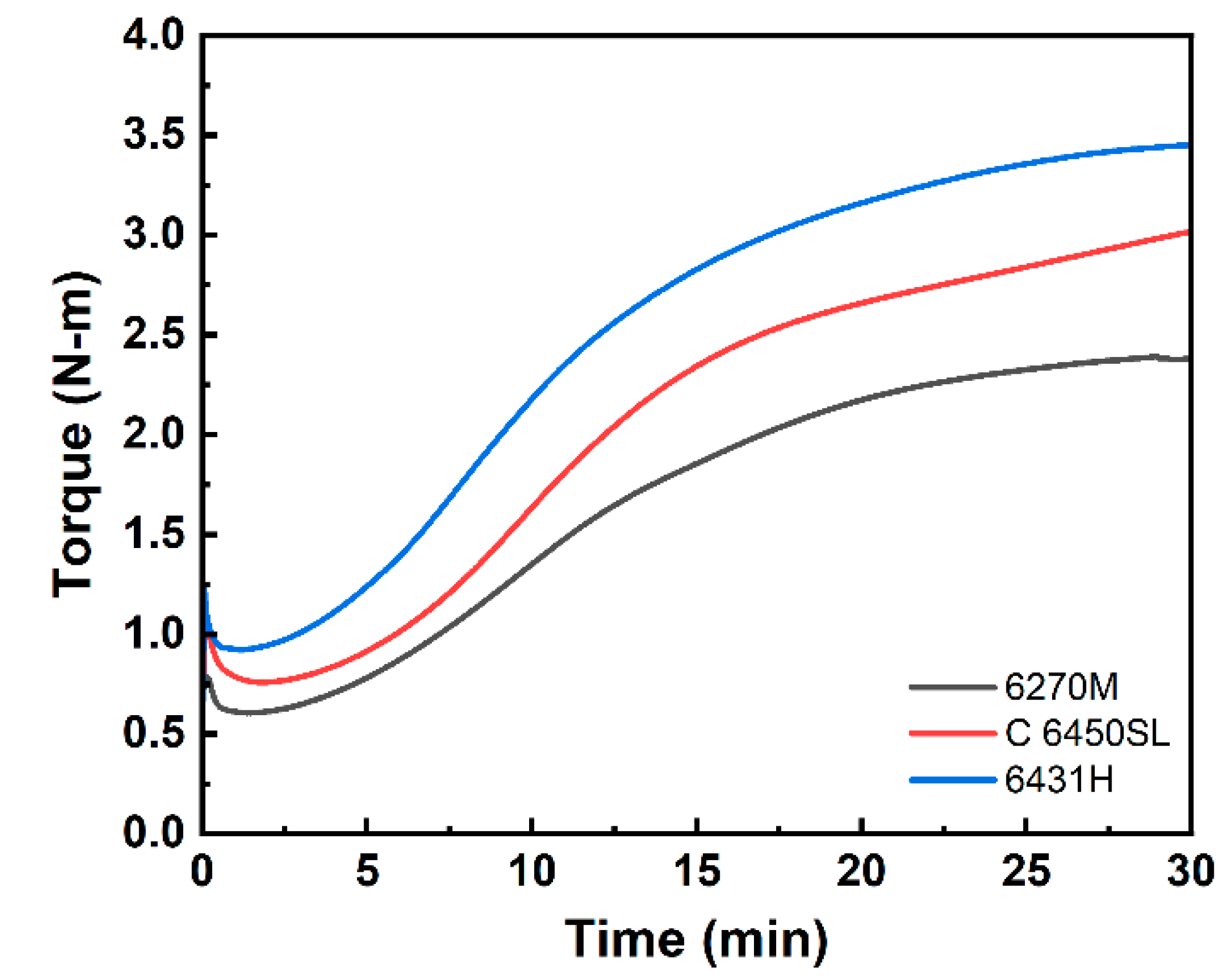
| t10 (min) | 5.03 | 5.72 | 4.52 |
| t90 (min) | 20.94 | 23.60 | 20.79 |
| Tmin (N-m) | 0.61 | 0.76 | 0.92 |
| Tmax (N-m) | 2.39 | 3.02 | 3.45 |
| ∆T (N-m) | 1.78 | 2.26 | 2.53 |
| 6270M | C 6450SL | 6431H | |
|---|---|---|---|
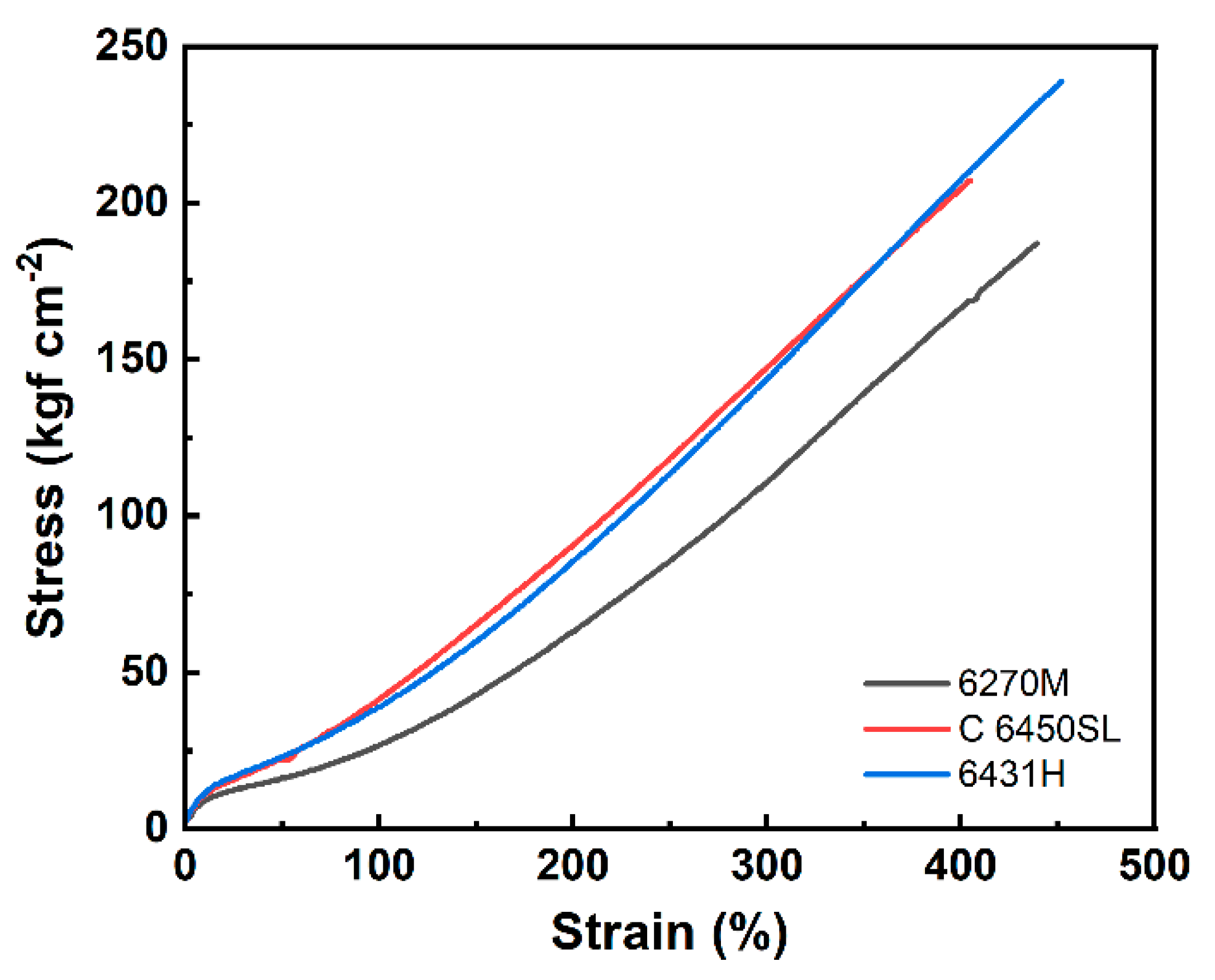
| Hardness (Shore A) | 74 | 75 | 76 |
| 300% modulus (kgf cm-2) | 111 | 148 | 144 |
| Elongation at the break (%) | 440 | 410 | 450 |
| Tensile strength (kgf cm-2) | 187 | 208 | 239 |
| Tear strength (kgf cm-1) | 62.0 | 80.7 | 81.5 |
| 6270M | C 6460SL | 6431H | |
|---|---|---|---|
| DIN abrasion loss (mg) | 203 | 178 | 164 |
| DIN abrasion loss (mm3) | 168 | 147 | 134 |
| 6270M | C 6450SL | 6431H | |
|---|---|---|---|
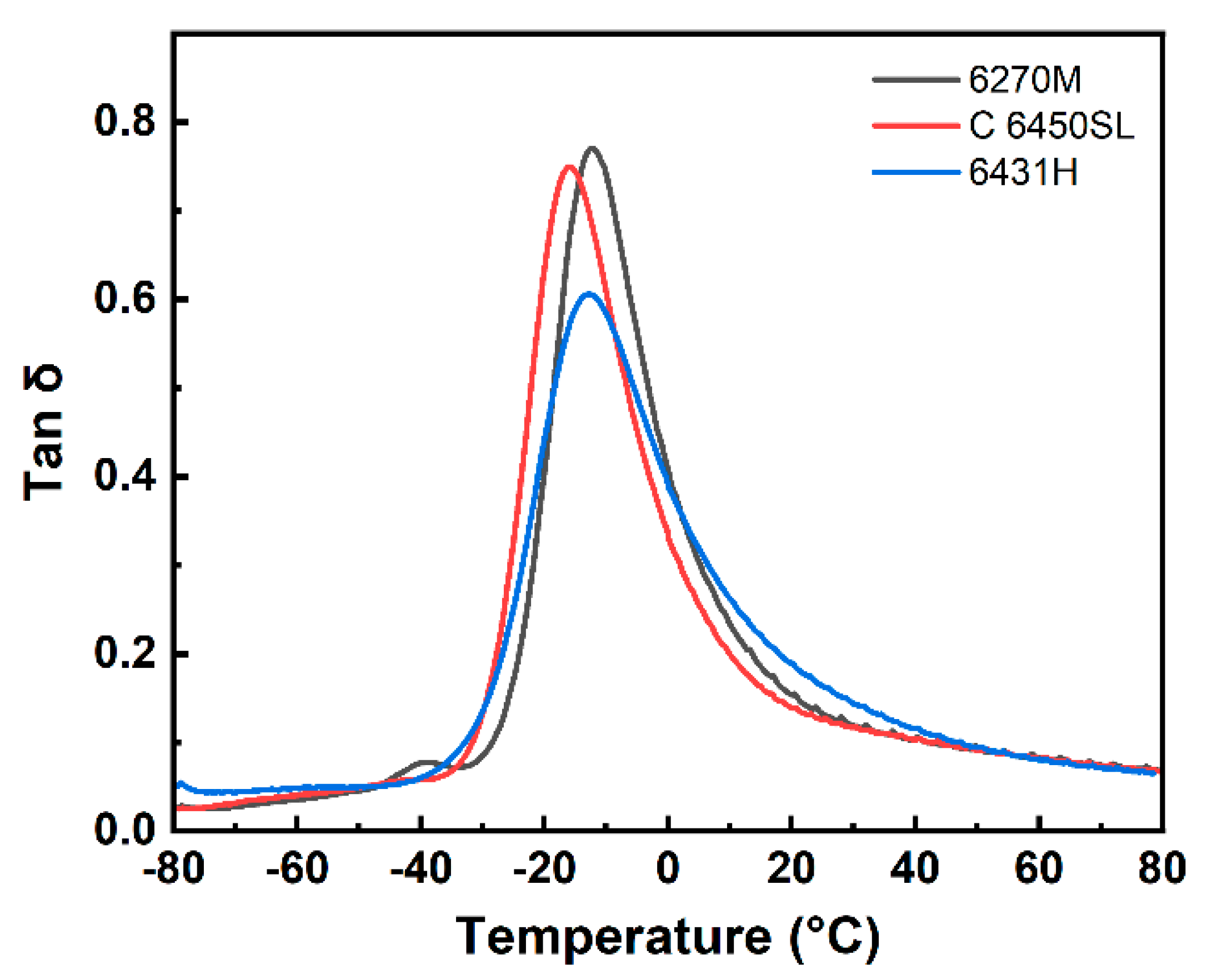
| Tg (°C) | -12.08 | -16.20 | -12.91 |
| Peak tan δ | 0.7704 | 0.7494 | 0.6057 |
| Tan δ at 0 °C | 0.4068 | 0.3300 | 0.3883 |
| Tan δ at 60 °C | 0.0823 | 0.0818 | 0.0805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





