Submitted:
07 October 2023
Posted:
08 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Chemical Recycling via Depolymerization: Polymers to Small Valuable Molecules
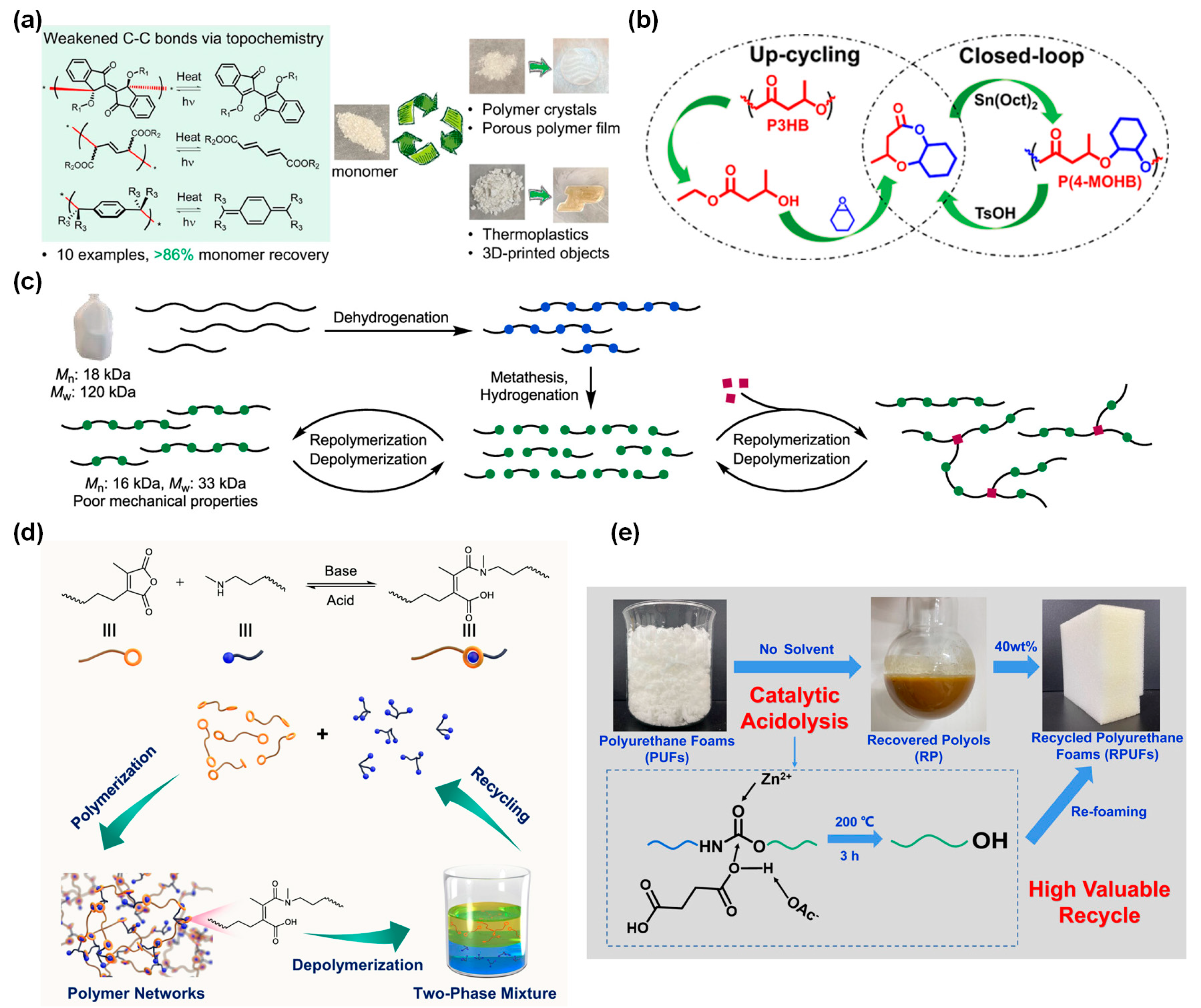
3. Chemical Recycling for Closed-loop Cycles: Polymers to Monomers, and/or to Polymers
4. Conclusion & Perspectives
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gibb, B.C. Plastics are forever. Nat. Chem. 2019, 11, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef]
- Antonopoulos, I.; Faraca, G.; Tonini, D. Recycling of post-consumer plastic packaging waste in the EU: Recovery rates, material flows, and barriers. Waste Manage. 2021, 126, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manage. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Haque, F.M.; Ishibashi, J.S.A.; Lidston, C.A.L.; Shao, H.; Bates, F.S.; Chang, A.B.; Coates, G.W.; Cramer, C.J.; Dauenhauer, P.J.; Dichtel, W.R.; Ellison, C.J.; Gormong, E.A.; Hamachi, L.S.; Hoye, T.R.; Jin, M.; Kalow, J.A.; Kim, H.J.; Kumar, G.; LaSalle, C.J.; Liffland, S.; Lipinski, B.M.; Pang, Y.; Parveen, R.; Peng, X.; Popowski, Y.; Prebihalo, E.A.; Reddi, Y.; Reineke, T.M.; Sheppard, D.T.; Swartz, J.L.; Tolman, W.B.; Vlaisavljevich, B.; Wissinger, J.; Xu, S.; Hillmyer, M.A. Defining the Macromolecules of Tomorrow through Synergistic Sustainable Polymer Research. Chem. Rev. 2022, 122, 6322–6373. [Google Scholar] [CrossRef]
- Korley, L.T.J.; Epps, T.H.; Helms, B.A.; Ryan, A.J. Toward polymer upcycling—adding value and tackling circularity. Science 2021, 373, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.-S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Uchida, N.; Tuyen, L.H.; Tanaka, K.; Matsukami, H.; Kunisue, T.; Takahashi, S.; Viet, P.H.; Kuramochi, H.; Osako, M. Mechanical recycling of plastic waste as a point source of microplastic pollution. Environ. Pollut. 2022, 303, 119114. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.B.; Arora, J.S.; Chew, J.W.; Dauenhauer, P.J.; Mushrif, S.H. Effect of Temperature and Transport on the Yield and Composition of Pyrolysis-Derived Bio-Oil from Glucose. Energy Fuels 2018, 32, 6008–6021. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Giridharan, K.; Stalin, B.; Kumaran, S.; Kavimani, V.; Nagaprasad, N.; Tesfaye Jule, L.; Krishnaraj, R. Energy recovery of waste plastics into diesel fuel with ethanol and ethoxy ethyl acetate additives on circular economy strategy. Sci.Rep. 2022, 12, 5330. [Google Scholar] [CrossRef]
- Zolghadr, A.; Sidhu, N.; Mastalski, I.; Facas, G.; Maduskar, S.; Uppili, S.; Go, T.; Neurock, M.; Dauenhauer, P.J. On the Method of Pulse-Heated Analysis of Solid Reactions (PHASR) for Polyolefin Pyrolysis. ChemSusChem 2021, 14, 4214–4227. [Google Scholar] [CrossRef]
- Antelava, A.; Jablonska, N.; Constantinou, A.; Manos, G.; Salaudeen, S.A.; Dutta, A.; Al-Salem, S.M. Energy Potential of Plastic Waste Valorization: A Short Comparative Assessment of Pyrolysis versus Gasification. Energy Fuels 2021, 35, 3558–3571. [Google Scholar] [CrossRef]
- Wulandari, Y.R.; Chen, S.S.; Hermosa, G.C.; Hossain, M.S.A.; Yamauchi, Y.; Ahamad, T.; Alshehri, S.M.; Wu, K.C.W.; Wu, H.-S. Effect of N2 flow rate on kinetic investigation of lignin pyrolysis. Environ. Res. 2020, 190, 109976. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.R.; Avasarala, S.; Murali, D.; Rajagopalan, N.; Sharma, B.K. Materials and Energy Recovery from E-Waste Plastics. ACS Sustainable. Chem. Eng. 2018, 6, 4594–4602. [Google Scholar] [CrossRef]
- Briassoulis, D.; Hiskakis, M.; Babou, E.; Antiohos, S.K.; Papadi, C. Experimental investigation of the quality characteristics of agricultural plastic wastes regarding their recycling and energy recovery potential. Waste Manage. 2012, 32, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Tullo, A.H. Plastic has a problem; is chemical recycling the solution. Chem. Eng. News 2019, 97, 39. [Google Scholar]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Pant, D. Recycling of Polyethylene Terephthalate Bottles. 4 – Chemical Depolymerization of PET Bottles via Glycolysis, William Andrew Publishing, United States, 2019; pp. 61–84.
- Damayanti; Wu, H. -S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Ghosal, K.; Nayak, C. Recent advances in chemical recycling of polyethylene terephthalate waste into value added products for sustainable coating solutions – hope vs. hype. Mat. Adv. 2022, 3, 1974–1992. [Google Scholar] [CrossRef]
- Štrukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. ChemSusChem 2021, 14, 330–338. [Google Scholar] [CrossRef]
- Hofmann, M.; Sundermeier, J.; Alberti, C.; Enthaler, S. Zinc(II) acetate Catalyzed Depolymerization of Poly(ethylene terephthalate). ChemistrySelect 2020, 5, 10010–10014. [Google Scholar] [CrossRef]
- Mohammadi, S.; Enayati, M. Dual catalytic activity of antimony (III) oxide: The polymerization catalyst for synthesis of polyethylene terephthalate also catalyze depolymerization. Polym. Degrad. Stab. 2022, 206, 110180. [Google Scholar] [CrossRef]
- Chandra, A.; Siddiqua, S. Sustainable utilization of chemically depolymerized polyethylene terephthalate (PET) waste to enhance sand-bentonite clay liners. Waste Manage. 2023, 166, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.H.; Bhatty, J.I.; Gamlen, G.A.; Dollimore, D. Aspects of the chemistry of poly(ethylene terephthalate): 5. Polymerization of bis(hydroxyethyl)terephthalate by various metallic catalysts. Polymer 1984, 25, 1333–1336. [Google Scholar] [CrossRef]
- Imran, M.; Kim, B.-K.; Han, M.; Cho, B.G.; Kim, D.H. Sub- and supercritical glycolysis of polyethylene terephthalate (PET) into the monomer bis(2-hydroxyethyl) terephthalate (BHET). Polym. Degrad. Stab. 2010, 95, 1686–1693. [Google Scholar] [CrossRef]
- Le, N.H.; Ngoc Van, T.T.; Shong, B.; Cho, J. Low-Temperature Glycolysis of Polyethylene Terephthalate. ACS Sustainable. Chem. Eng. 2022, 10, 17261–17273. [Google Scholar] [CrossRef]
- Luo, Y.; Selvam, E.; Vlachos, D.G.; Ierapetritou, M. Economic and Environmental Benefits of Modular Microwave-Assisted Polyethylene Terephthalate Depolymerization. ACS Sustainable. Chem. Eng. 2023, 11, 4209–4218. [Google Scholar] [CrossRef]
- Selvam, E.; Luo, Y.; Ierapetritou, M.; Lobo, R.F.; Vlachos, D.G. Microwave-assisted depolymerization of PET over heterogeneous catalysts. Cat. Today 2023, 418, 114124. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manage. 2021, 296, 113267. [Google Scholar] [CrossRef]
- Motonobu, G.; Hiroshi, K.; Akio, K.; Tsutomu, H.; Shoji, N. Depolymerization of polyethylene terephthalate in supercritical methanol. J. Phys.: Condens. Matter 2002, 14, 11427. [Google Scholar]
- Attallah, O.A.; Janssens, A.; Azeem, M.; Fournet, M.B. Fast, High Monomer Yield from Post-consumer Polyethylene Terephthalate via Combined Microwave and Deep Eutectic Solvent Hydrolytic Depolymerization. ACS Sustainable. Chem. Eng. 2021, 9, 17174–17185. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M. Aminolysis of polyethylene terephthalate waste. Polym. Degrad. Stab. 2006, 91, 1850–1854. [Google Scholar] [CrossRef]
- Fukushima, K.; Lecuyer, J.M.; Wei, D.S.; Horn, H.W.; Jones, G.O.; Al-Megren, H.A.; Alabdulrahman, A.M.; Alsewailem, F.D.; McNeil, M.A.; Rice, J.E.; Hedrick, J.L. Advanced chemical recycling of poly(ethylene terephthalate) through organocatalytic aminolysis. Polym. Chem. 2013, 4, 1610–1616. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Conversion of waste bottles’ PET to a hydrogel adsorbent via PET aminolysis. J. Environ. Chem. Eng. 2021, 9, 106129. [Google Scholar] [CrossRef]
- Behera, S.; Dinda, S.; Saha, R.; Mondal, B. Quantitative Electrocatalytic Upcycling of Polyethylene Terephthalate Plastic and Its Oligomer with a Cobalt-Based One-Dimensional Coordination Polymer Having Open Metal Sites along with Coproduction of Hydrogen. ACS Cat. 2023, 13, 469–474. [Google Scholar] [CrossRef]
- Delle Chiaie, K.R.; McMahon, F.R.; Williams, E.J.; Price, M.J.; Dove, A.P. Dual-catalytic depolymerization of polyethylene terephthalate (PET). Polym. Chem. 2020, 11, 1450–1453. [Google Scholar] [CrossRef]
- Yang, B.; Li, W.; Zhang, M.; Wang, L.; Ding, X. Recycling of High-Value-Added Aramid Nanofibers from Waste Aramid Resources via a Feasible and Cost-Effective Approach. ACS Nano 2021, 15, 7195–7207. [Google Scholar] [CrossRef] [PubMed]
- Navarre, N.; Mogollón, J.M.; Tukker, A.; Barbarossa, V. Recycled plastic packaging from the Dutch food sector pollutes Asian oceans. Resour. Conserv. Recycl. 2022, 185, 106508. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Cho, J.; Kim, B.; Kwon, T.; Lee, K.; Choi, S.-I. Electrocatalytic upcycling of plastic waste. Green Chem. 2023. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, X.; Deng, K.; Yu, H.; Xu, Y.; Wang, H.; Wang, Z.; Wang, L. Electrocatalytic upcycling of polyethylene terephthalate plastic to formic acid coupled with energy-saving hydrogen production over hierarchical Pd-doped NiTe nanoarrays. Appl. Catal., B 2024, 340, 123236. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Kong, X.; Zheng, L.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 4679. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Ramasamy, K.K. Electrochemical Oxidation of Lignin and Waste Plastic. ACS Omega 2020, 5, 27735–27740. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, J.E.; Troyano-Valls, C.; Beckham, G.T.; Román-Leshkov, Y. Hydrogenolysis of Polypropylene and Mixed Polyolefin Plastic Waste over Ru/C to Produce Liquid Alkanes. ACS Sustainable. Chem. Eng. 2021, 9, 11661–11666. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, L.; Conley, M.P. Cationic Tantalum Hydrides Catalyze Hydrogenolysis and Alkane Metathesis Reactions of Paraffins and Polyethylene. J. Am. Chem. Soc. 2023, 145, 4964–4968. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gorbea, G.D.; Danielson, E.; Cui, S.; Ellison, C.J.; Bates, F.-S. Threading-the-Needle: Compatibilization of HDPE/i-PP blends with butadiene-derived polyolefin block copolymers. PNAS 2023, 120, e2301352120. [Google Scholar] [CrossRef] [PubMed]
- Eagan, J.M.; Xu, J.; Di Girolamo, R.; Thurber, C.M.; Macosko, C.W.; LaPointe, A.M.; Bates, F.-S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/i-PP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef]
- Duan, J.; Chen, W.; Wang, C.; Wang, L.; Liu, Z.; Yi, X.; Fang, W.; Wang, H.; Wei, H.; Xu, S.; Yang, Y.; Yang, Q.; Bao, Z.; Zhang, Z.; Ren, Q.; Zhou, H.; Qin, X.; Zheng, A.; Xiao, F.-S. Coking-Resistant Polyethylene Upcycling Modulated by Zeolite Micropore Diffusion. J. Am. Chem. Soc. 2022, 144, 14269–14277. [Google Scholar] [CrossRef]
- Huang, Z.; Shanmugam, M.; Liu, Z.; Brookfield, A.; Bennett, E.L.; Guan, R.; Vega Herrera, D.E.; Lopez-Sanchez, J.A.; Slater, A.G.; McInnes, E.J.L.; Qi, X.; Xiao, J. Chemical Recycling of Polystyrene to Valuable Chemicals via Selective Acid-Catalyzed Aerobic Oxidation under Visible Light. J. Am. Chem. Soc. 2022, 144, 6532–6542. [Google Scholar] [CrossRef]
- Coeck, R.; De Bruyne, A.; Borremans, T.; Stuyck, W.; De Vos, D.E. Ammonolytic Hydrogenation of Secondary Amides: An Efficient Method for the Recycling of Long-Chain Polyamides. ACS Sustainable. Chem..Eng. 2022, 10, 3048–3056. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, L.; Conley, M.P. Polypropylene Degradation Catalyzed by Tantalum Hydrides Supported on Sulfated Alumina. ACS Catal. 2023, 13, 10765–10769. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Liang, X.; Shen, J.; Wang, J.; Sun, W.; Wang, D.; Jiang, J.; Li, Y. Polystyrene Waste Thermochemical Hydrogenation to Ethylbenzene by a N-Bridged Co, Ni Dual-Atom Catalyst. J. Am. Chem. Soc. 2023, 145, 16218–16227. [Google Scholar] [CrossRef] [PubMed]
- Kiel, G.R.; Lundberg, D.J.; Prince, E.; Husted, K.E.L.; Johnson, A.M.; Lensch, V.; Li, S.; Shieh, P.; Johnson, J.A. Cleavable Comonomers for Chemically Recyclable Polystyrene: A General Approach to Vinyl Polymer Circularity. J. Am. Chem. Soc. 2022, 144, 12979–12988. [Google Scholar] [CrossRef] [PubMed]
- Ukei, H.; Hirose, T.; Horikawa, S.; Takai, Y.; Taka, M.; Azuma, N.; Ueno, A. Catalytic degradation of polystyrene into styrene and a design of recyclable polystyrene with dispersed catalysts. Cat. Today 2000, 62, 67–75. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Aguado, R.; Olazar, M.; x; Gaisán, B. ; Prieto, R.; Bilbao, J. Kinetics of polystyrene pyrolysis in a conical spouted bed reactor. Chem. Eng. J. 2003, 92, 91–99. [Google Scholar] [CrossRef]
- Rabot, C.; Chen, Y.; Lin, S.-Y.; Miller, B.; Chiang, Y.-M.; Oakley, C.E.; Oakley, B.R.; Wang, C.C.C.; Williams, T.J. Polystyrene Upcycling into Fungal Natural Products and a Biocontrol Agent. J. Am. Chem. Soc. 2023, 145, 5222–5230. [Google Scholar] [CrossRef] [PubMed]
- Alberti, C.; Figueira, R.; Hofmann, M.; Koschke, S.; Enthaler, S. Chemical Recycling of End-of-Life Polyamide 6 via Ring Closing Depolymerization. ChemistrySelect 2019, 4, 12638–12642. [Google Scholar] [CrossRef]
- Kamimura, A.; Yamamoto, S. An Efficient Method To Depolymerize Polyamide Plastics: A New Use of Ionic Liquids. Org. Lett. 2007, 9, 2533–2535. [Google Scholar] [CrossRef]
- Češarek, U.; Pahovnik, D.; Žagar, E. Chemical Recycling of Aliphatic Polyamides by Microwave-Assisted Hydrolysis for Efficient Monomer Recovery. ACS Sustainable Chem. Eng. 2020, 8, 16274–16282. [Google Scholar] [CrossRef]
- Jehanno, C.; Demarteau, J.; Mantione, D.; Arno, M.C.; Ruipérez, F.; Hedrick, J.L.; Dove, A.P.; Sardon, H. Synthesis of Functionalized Cyclic Carbonates through Commodity Polymer Upcycling. ACS Macro Lett. 2020, 9, 443–447. [Google Scholar] [CrossRef]
- Kim, J.G. Chemical recycling of poly(bisphenol A carbonate). Polym. Chem. 2020, 11, 4830–4849. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.-B. Chemical recycling to monomers: Industrial Bisphenol-A-Polycarbonates to novel aliphatic polycarbonate materials. J. Polym Sci. 2022, 60, 3256–3268. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hillmyer, M.A.; Ellison, C.J. Enhanced Polyester Degradation through Transesterification with Salicylates. J. Am. Chem. Soc. 2021, 143, 15784–15790. [Google Scholar] [CrossRef]
- Kim, H.J.; Reddi, Y.; Cramer, C.J.; Hillmyer, M.A.; Ellison, C.J. Readily Degradable Aromatic Polyesters from Salicylic Acid. ACS Macro Lett. 2020, 9, 96–102. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, J.; Wang, M.; Yu, S.; Xu, Y.; Tian, S.; Gao, Z.; Xiao, D.; Liu, G.; Zhou, W.; Wang, M.; Ma, D. Valorization of waste biodegradable polyester for methyl methacrylate production. Nat. Sustainability 2023, 6, 712–719. [Google Scholar] [CrossRef]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Vora, N.; Christensen, P.R.; Demarteau, J.; Baral, N.R.; Keasling, J.D.; Helms, B.A.; Scown, C.D. Leveling the cost and carbon footprint of circular polymers that are chemically recycled to monomer. Sci. Adv. 2021, 7, eabf0187. [Google Scholar] [CrossRef]
- Shi, C.; Reilly, L.T.; Phani Kumar, V.S.; Coile, M.W.; Nicholson, S.R.; Broadbelt, L.J.; Beckham, G.T.; Chen, E.Y.-X. Design principles for intrinsically circular polymers with tunable properties. Chem 2021, 7, 2896–2912. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Peterson, G.I.; Kulik, H.J.; Kaitz, J.A.; Mar, B.D.; May, P.A.; White, S.R.; Martínez, T.J.; Boydston, A.J.; Moore, J.S. Mechanically triggered heterolytic unzipping of a low-ceiling-temperature polymer. Nat. Chem. 2014, 6, 623–628. [Google Scholar] [CrossRef]
- Cederholm, L.; Wohlert, J.; Olsén, P.; Hakkarainen, M.; Odelius, K. “Like Recycles Like”: Selective Ring-Closing Depolymerization of Poly (L-Lactic Acid) to L-Lactide. Angew. Chem. 2022, 134, e202204531. [Google Scholar] [CrossRef]
- Stevens, M.P. , Polymer chemistry, Oxford university press New York, 1990.
- Schneiderman, D.K.; Vanderlaan, M.E.; Mannion, A.M.; Panthani, T.R.; Batiste, D.C.; Wang, J.Z.; Bates, F.S.; Macosko, C.W.; Hillmyer, M.A. Chemically Recyclable Biobased Polyurethanes. ACS Macro Lett. 2016, 5, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, Z.-C.; Caporaso, L.; Cavallo, L.; Falivene, L.; Chen, E.Y.-X. Hybrid monomer design for unifying conflicting polymerizability, recyclability, and performance properties. Chem 2021, 7, 670–685. [Google Scholar] [CrossRef]
- Shi, C.; Clarke, R.W.; McGraw, M.L.; Chen, E.Y.-X. Closing the “One Monomer–Two Polymers–One Monomer” Loop via Orthogonal (De)polymerization of a Lactone/Olefin Hybrid. J. Am. Chem. Soc. 2022, 144, 2264–2275. [Google Scholar] [CrossRef]
- Abel, B.A.; Snyder, R.L.; Coates, G.W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 2021, 373, 783–789. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Yan, Q.; Liu, F.; Shen, Y.; Li, Z. Rapid and Controlled Polymerization of Bio-sourced δ-Caprolactone toward Fully Recyclable Polyesters and Thermoplastic Elastomers. Angew. Chem. Int. Ed. 2022, 61, e202201407. [Google Scholar] [CrossRef]
- Shen, Y.; Xiong, W.; Li, Y.; Zhao, Z.; Lu, H.; Li, Z. Chemoselective ring-opening polymerization of bio-renewable α-methylene-γ-butyrolactone via an organophosphazene/urea binary synergistic catalytic system toward a sustainable polyester. CCS Chem. 2020, 3, 620–630. [Google Scholar] [CrossRef]
- Li, X.-L.; Clarke, R.W.; An, H.-Y.; Gowda, R.R.; Jiang, J.-Y.; Xu, T.-Q.; Chen, E.Y.-X. Dual Recycling of Depolymerization Catalyst and Biodegradable Polyester that Markedly Outperforms Polyolefins. Angew. Chem. Int. Ed. 2023, 62, e202303791. [Google Scholar] [CrossRef]
- Gallin, C.F.; Lee, W.-W.; Byers, J.A. A Simple, Selective, and General Catalyst for Ring Closing Depolymerization of Polyesters and Polycarbonates for Chemical Recycling. Angew. Chem. 2023, 135, e202303762. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. Aliphatic Polyester Block Polymer Design. Macromolecules 2016, 49, 2419–2428. [Google Scholar] [CrossRef]
- Shi, C.; McGraw, M.L.; Li, Z.-C.; Cavallo, L.; Falivene, L.; Chen, E.Y.-X. High-performance pan-tactic polythioesters with intrinsic crystallinity and chemical recyclability. Sci. Adv. 2020, 6, eabc0495. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Westlie, A.H.; Watson, E.M.; Chen, E.Y.-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 2019, 366, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Westlie, A.H.; Caporaso, L.; Cavallo, L.; Falivene, L.; Chen, E.Y.-X. Biodegradable polyhydroxyalkanoates by stereoselective copolymerization of racemic diolides: stereocontrol and polyolefin-like properties. Angew. Chem. 2020, 132, 7955–7964. [Google Scholar] [CrossRef]
- Zhu, J.-B.; Watson, E.M.; Tang, J.; Chen, E.Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 2018, 360, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, L.; Olsén, P.; Hakkarainen, M.; Odelius, K. Chemical recycling to monomer: thermodynamic and kinetic control of the ring-closing depolymerization of aliphatic polyesters and polycarbonates. Polym. Chem. 2023, 14, 3270–3276. [Google Scholar] [CrossRef]
- Bruckmoser, J.; Remke, S.; Rieger, B. Ring-Opening Polymerization of a Bicyclic Lactone: Polyesters Derived from Norcamphor with Complete Chemical Recyclability. ACS Macro Lett. 2022, 11, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Clarke, R.W.; Jiang, J.-Y.; Xu, T.-Q.; Chen, E.Y.-X. A circular polyester platform based on simple gem-disubstituted valerolactones. Nat. Chem. 2023, 15, 278–285. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Shi, C.; Scoti, M.; Barange, D.K.; Gowda, R.R.; Chen, E.Y.-X. Chemically circular, mechanically tough, and melt-processable polyhydroxyalkanoates. Science 2023, 380, 64–69. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 2016, 8, 42–49. [Google Scholar] [CrossRef]
- Falconnet, A.; Nicolas, M.; Vollgraff, T.; Konradi, R.; Bruchmann, B.; Rodewald, D.; Hashmi, A.S.K.; Schaub, T. Facile preparation of biodegradable poly(γ-butyrolactone) via base-assisted ring-opening polymerization. Green Chem. 2023, 25, 3624–3632. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, X.; Wu, J.; Hu, X.; Zhu, N.; Guo, K. Access to high-molecular-weight poly(γ-butyrolactone) by using simple commercial catalysts. Polym. Chem. 2022, 13, 439–445. [Google Scholar] [CrossRef]
- Liu, X.; Hong, M.; Falivene, L.; Cavallo, L.; Chen, E.Y.-X. Closed-Loop Polymer Upcycling by Installing Property-Enhancing Comonomer Sequences and Recyclability. Macromolecules 2019, 52, 4570–4578. [Google Scholar] [CrossRef]
- Shen, T.; Chen, K.; Chen, Y.; Ling, J. Ring-Opening Polymerization of Cyclic Acetals: Strategy for both Recyclable and Degradable Materials. Macromol. Rapid Commun. 2023, 44, 2300099. [Google Scholar] [CrossRef] [PubMed]
- Kaya, K.; Debsharma, T.; Schlaad, H.; Yagci, Y. Cellulose-based polyacetals by direct and sensitized photocationic ring-opening polymerization of levoglucosenyl methyl ether. Polym. Chem. 2020, 11, 6884–6889. [Google Scholar] [CrossRef]
- Hester, H.G.; Abel, B.A.; Coates, G.W. Ultra-High-Molecular-Weight Poly(Dioxolane): Enhancing the Mechanical Performance of a Chemically Recyclable Polymer. J. Am. Chem. Soc. 2023, 145, 8800–8804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications. Meerwein’s salt 5th Ed. Springer International Publishing, Switzerland, 2014; pp. 384–385.
- Westlie, A.H.; Chen, E.Y.-X.; Holland, C.M.; Stahl, S.S.; Doyle, M.; Trenor, S.R.; Knauer, K.M. Polyolefin Innovations toward Circularity and Sustainable Alternatives. Macromol. Rapid Commun. 2022, 43, 2200492. [Google Scholar] [CrossRef] [PubMed]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Y.; Jia, X.; Huang, Z. Selective Catalytic Transfer Dehydrogenation of Alkanes and Heterocycles by an Iridium Pincer Complex. Angew. Chem. Int. Ed. 2014, 53, 1390–1394. [Google Scholar] [CrossRef]
- Bresciani, G.; Zacchini, S.; Pampaloni, G.; Marchetti, F. Carbon–Carbon Bond Coupling of Vinyl Molecules with an Allenyl Ligand at a Diruthenium Complex. Organometallics 2022, 41, 1006–1014. [Google Scholar] [CrossRef]
- Luo, X.; Wei, Z.; Seo, B.; Hu, Q.; Wang, X.; Romo, J.A.; Jain, M.; Cakmak, M.; Boudouris, B.W.; Zhao, K.; Mei, J.; Savoie, B.M.; Dou, L. Circularly Recyclable Polymers Featuring Topochemically Weakened Carbon–Carbon Bonds. J. Am. Chem. Soc. 2022, 144, 16588–16597. [Google Scholar] [CrossRef]
- Li, Z.; Shen, Y.; Li, Z. Chemical Upcycling of Poly(3-hydroxybutyrate) into Bicyclic Ether–Ester Monomers toward Value-Added, Degradable, and Recyclable Poly(ether ester). ACS Sustainable Chem. Eng. 2022, 10, 8228–8238. [Google Scholar] [CrossRef]
- Arroyave, A.; Cui, S.; Lopez, J.C.; Kocen, A.L.; LaPointe, A.M.; Delferro, M.; Coates, G.W. Catalytic Chemical Recycling of Post-Consumer Polyethylene. J. Am. Chem. Soc. 2022, 144, 23280–23285. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Liu, S.; Huang, Z.; Zeng, L.; Xu, J.-F.; Zhang, X. Closed-loop chemical recycling of cross-linked polymeric materials based on reversible amidation chemistry. Nat. Commun. 2022, 13, 7595. [Google Scholar] [CrossRef]
- He, H.; Su, H.; Yu, H.; Du, K.; Yang, F.; Zhu, Y.; Ma, M.; Shi, Y.; Zhang, X.; Chen, S.; Wang, X. Chemical Recycling of Waste Polyurethane Foams: Efficient Acidolysis under the Catalysis of Zinc Acetate. ACS Sustainable Chem. Eng. 2023, 11, 5515–5523. [Google Scholar] [CrossRef]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Z.; Shen, Y.; Li, Z. Crystalline Stereoregular Poly(ether-ester) via MeAl[Salen]-Catalyzed Well-Controlled Ring-Opening Polymerization of Enantiopure Cyclic Ether-Ester Monomer. Macromolecules 2023, 56, 6019–6026. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Heo, Y.; Sodano, H.A. Self-Healing Polyurethanes with Shape Recovery. Adv. Funct. Mater. 2014, 24, 5261–5268. [Google Scholar] [CrossRef]
- Jin, K.; Banerji, A.; Kitto, D.; Bates, F.S.; Ellison, C.J. Mechanically Robust and Recyclable Cross-Linked Fibers from Melt Blown Anthracene-Functionalized Commodity Polymers. ACS Appl. Mater. Interfaces 2019, 11, 12863–12870. [Google Scholar] [CrossRef]
- Sheppard, D.T.; Jin, K.; Hamachi, L.S.; Dean, W.; Fortman, D.J.; Ellison, C.J.; Dichtel, W.R. Reprocessing Postconsumer Polyurethane Foam Using Carbamate Exchange Catalysis and Twin-Screw Extrusion. ACS Cent. Sci. 2020, 6, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Alfarhan, S.; Brown, J.; Liu, B.; Long, T.; Jin, K. Chemically recyclable crosslinked thiol-ene photopolymers via thiol-disulfide exchange reactions. J. Polym. Sci. 2022, 60, 3379–3390. [Google Scholar] [CrossRef]
- Sternberg, J.; Pilla, S. Chemical recycling of a lignin-based non-isocyanate polyurethane foam. Nat. Sustainability 2023, 6, 316–324. [Google Scholar] [CrossRef]
- Fortman, D.J.; Sheppard, D.T.; Dichtel, W.R. Reprocessing Cross-Linked Polyurethanes by Catalyzing Carbamate Exchange. Macromolecules 2019, 52, 6330–6335. [Google Scholar] [CrossRef]
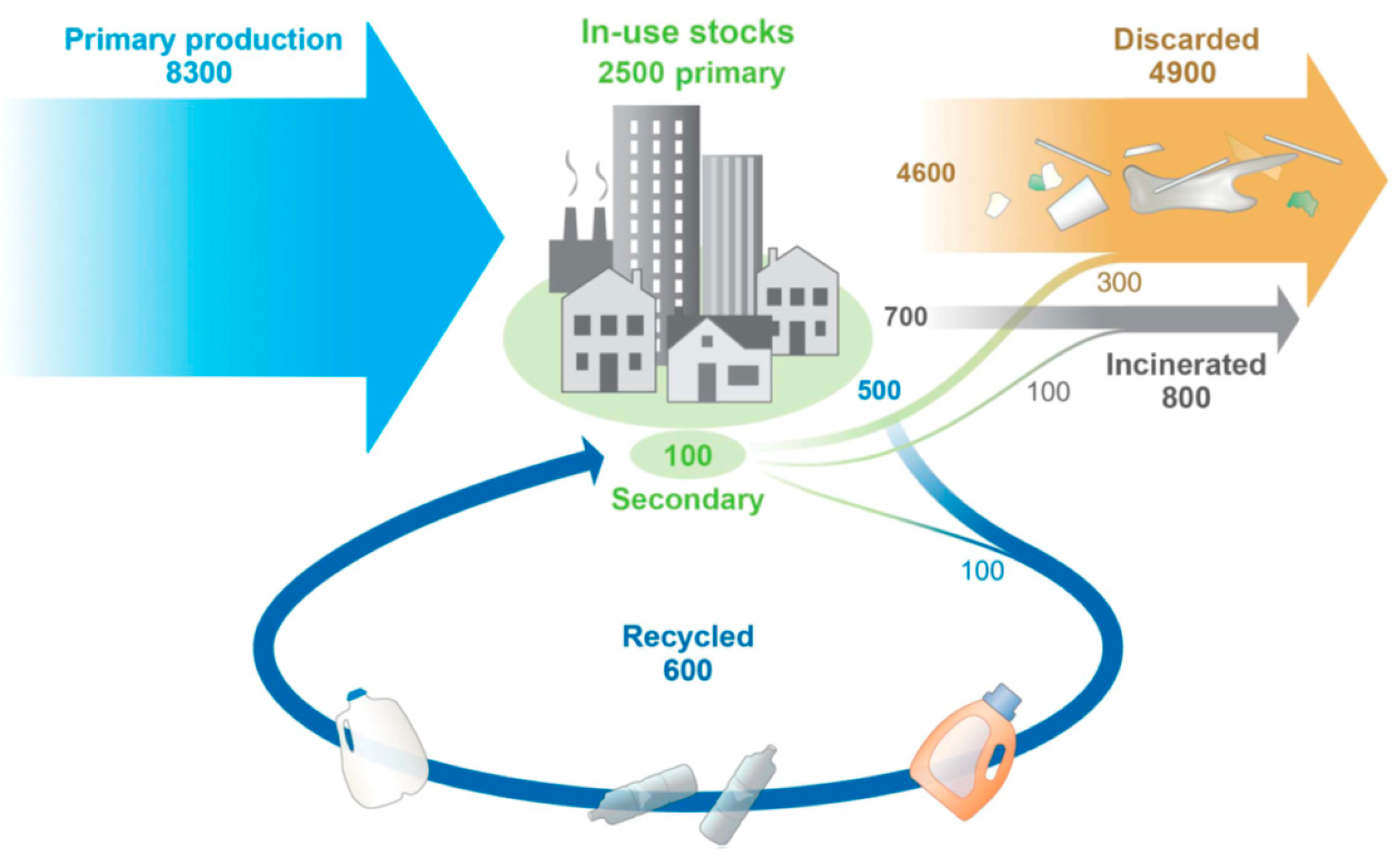
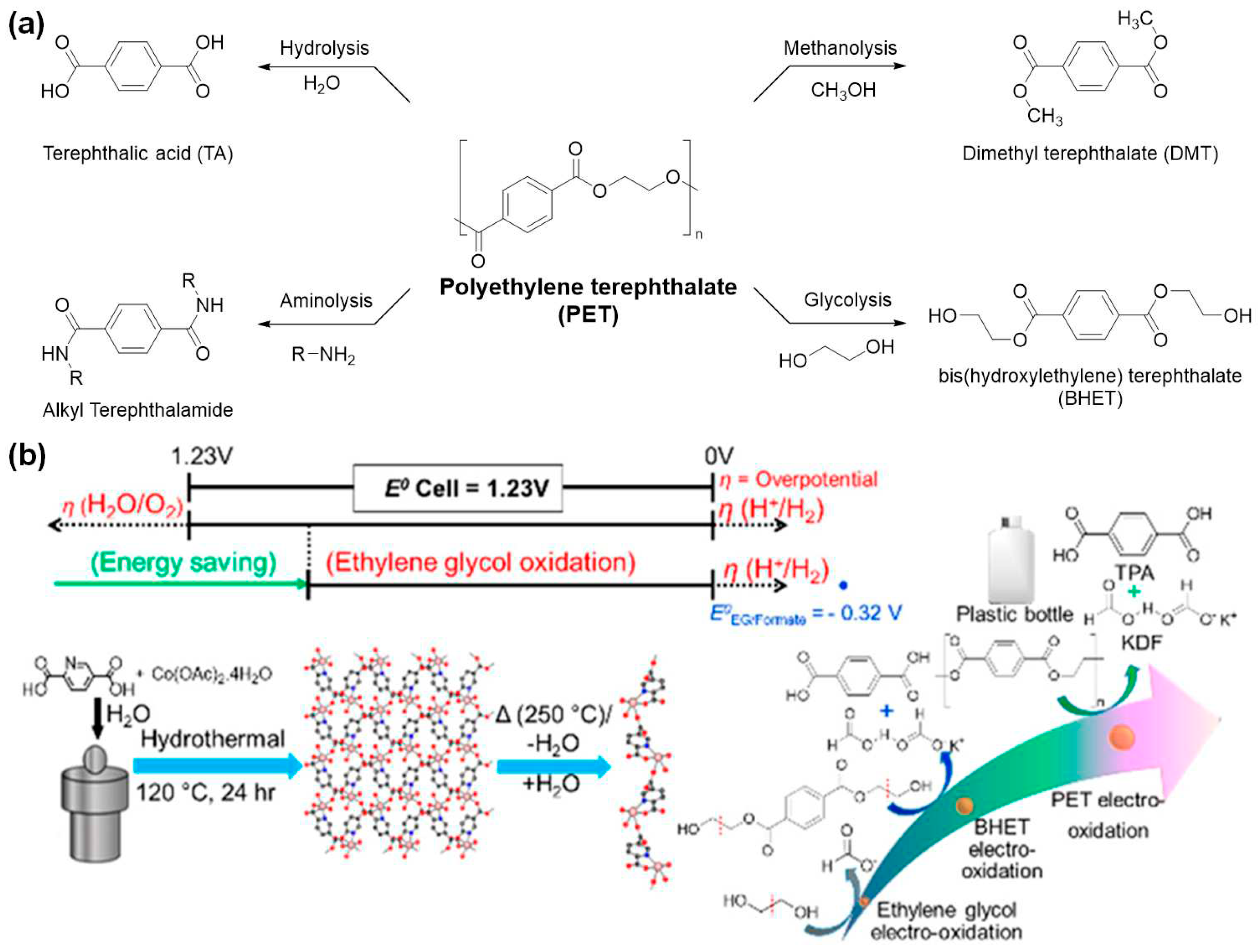
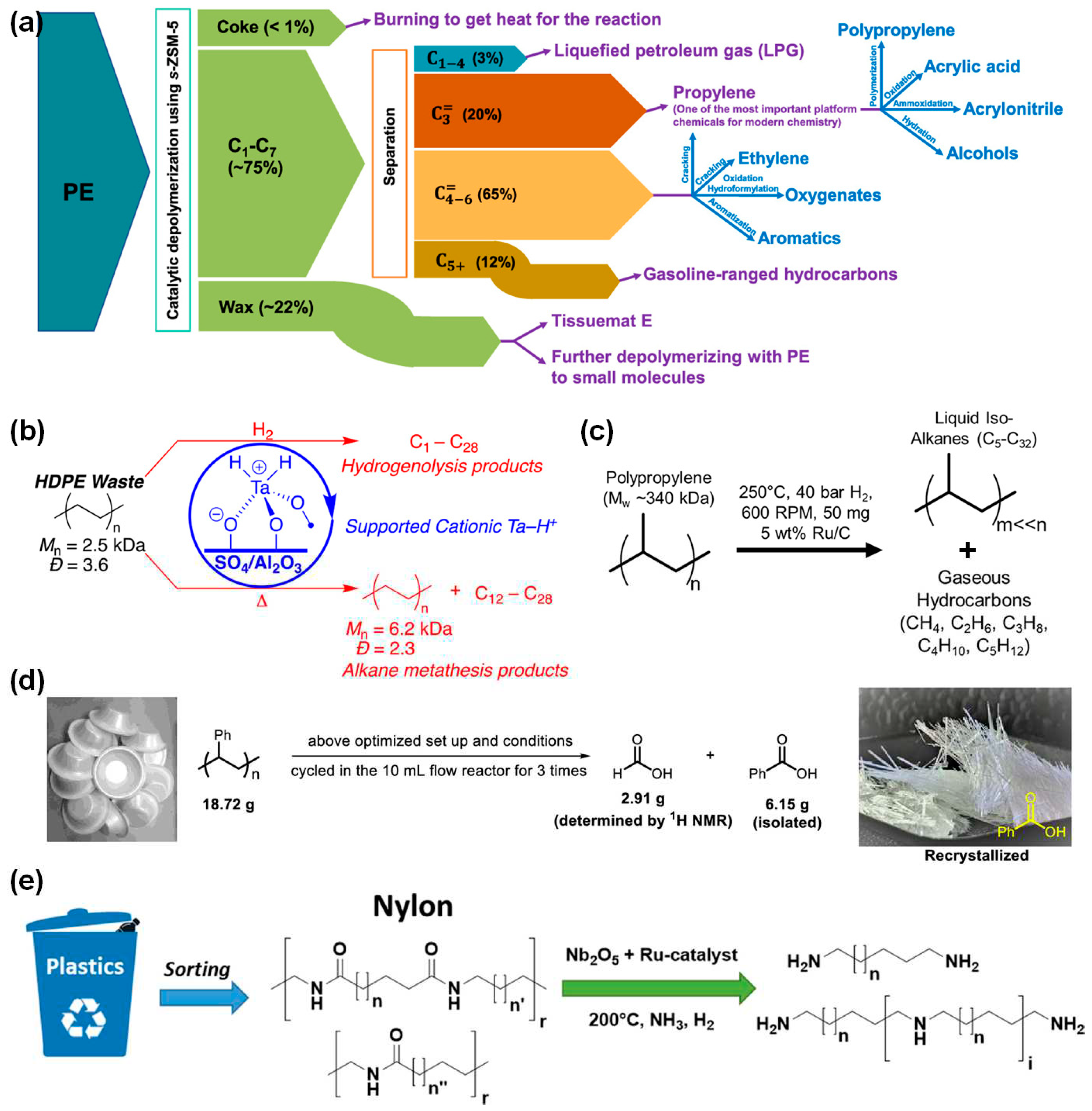
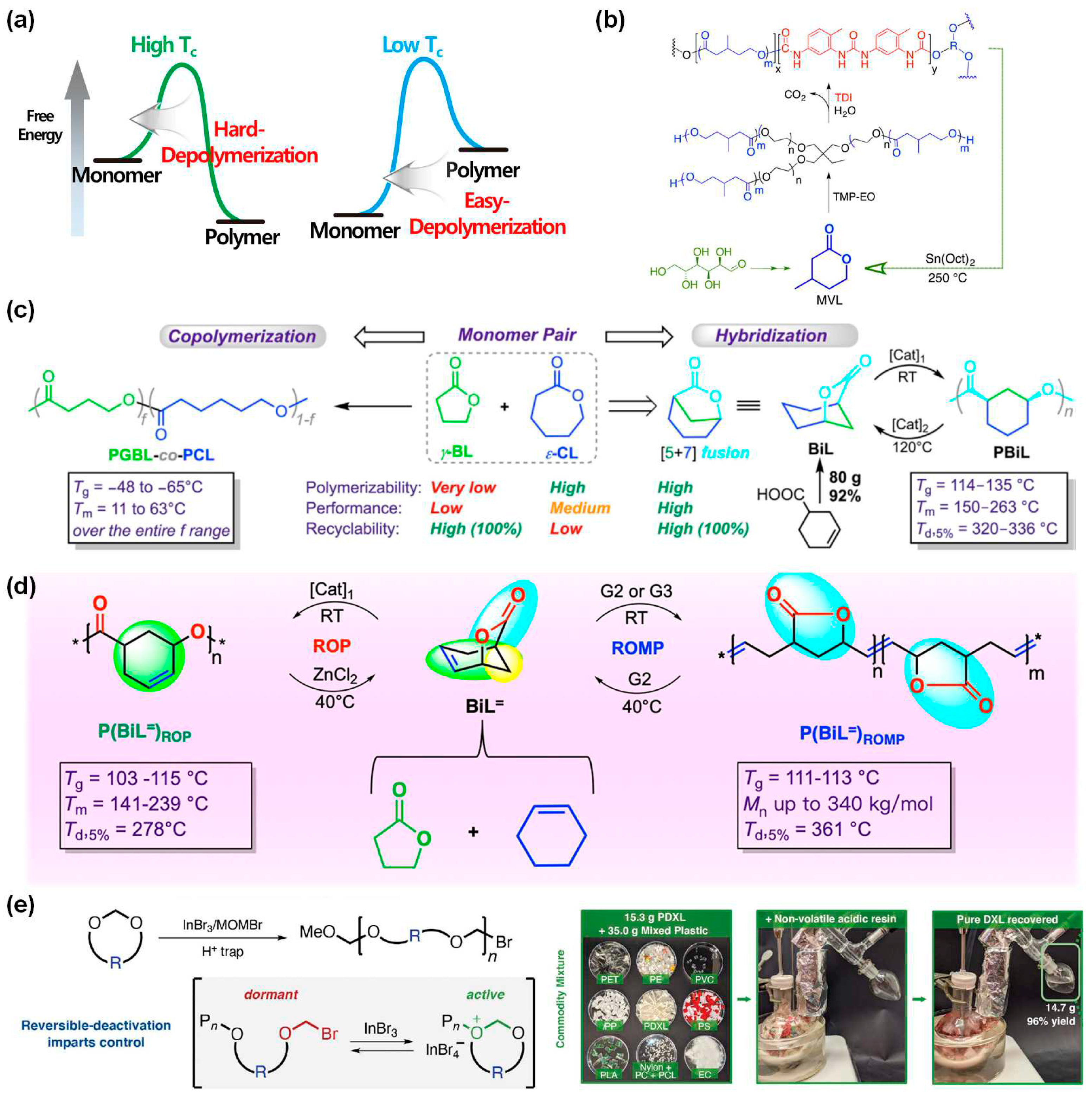
| ASTM D7209 | ISO 15270:2008 | Example |
| Primary (1st) recycling | Mechanical recycling | PET bottle to PET bottle |
| Secondary (2nd) recycling | Mechanical recycling | PET bottle to PET fiber |
| Tertiary (3rd) recycling | Chemical recycling | Glycolysis of PET |
| Quaternary (4th) recycling | Energy recovery | Pyrolysis of PET |
| Monomer | Polymer | Tc (°C) |
| Ethylene | Polyethylene | 610 |
| Isoprene | Polyisoprene | 466 |
| 1,3-butadiene | Polybutadiene | 585 |
| Methyl methacrylate | Polymethyl methacrylate | 198 |
| Styrene | Polystyrene | 395 |
| Tetrafluoroethylene | Polytetrafluoroethylene (Teflon™) |
1100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




