Submitted:
29 September 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell lines
2.2. Hybridomas
2.3. Flow cytometric analysis
2.4. Western blotting
2.5. Immunohistochemical analyses
3. Results
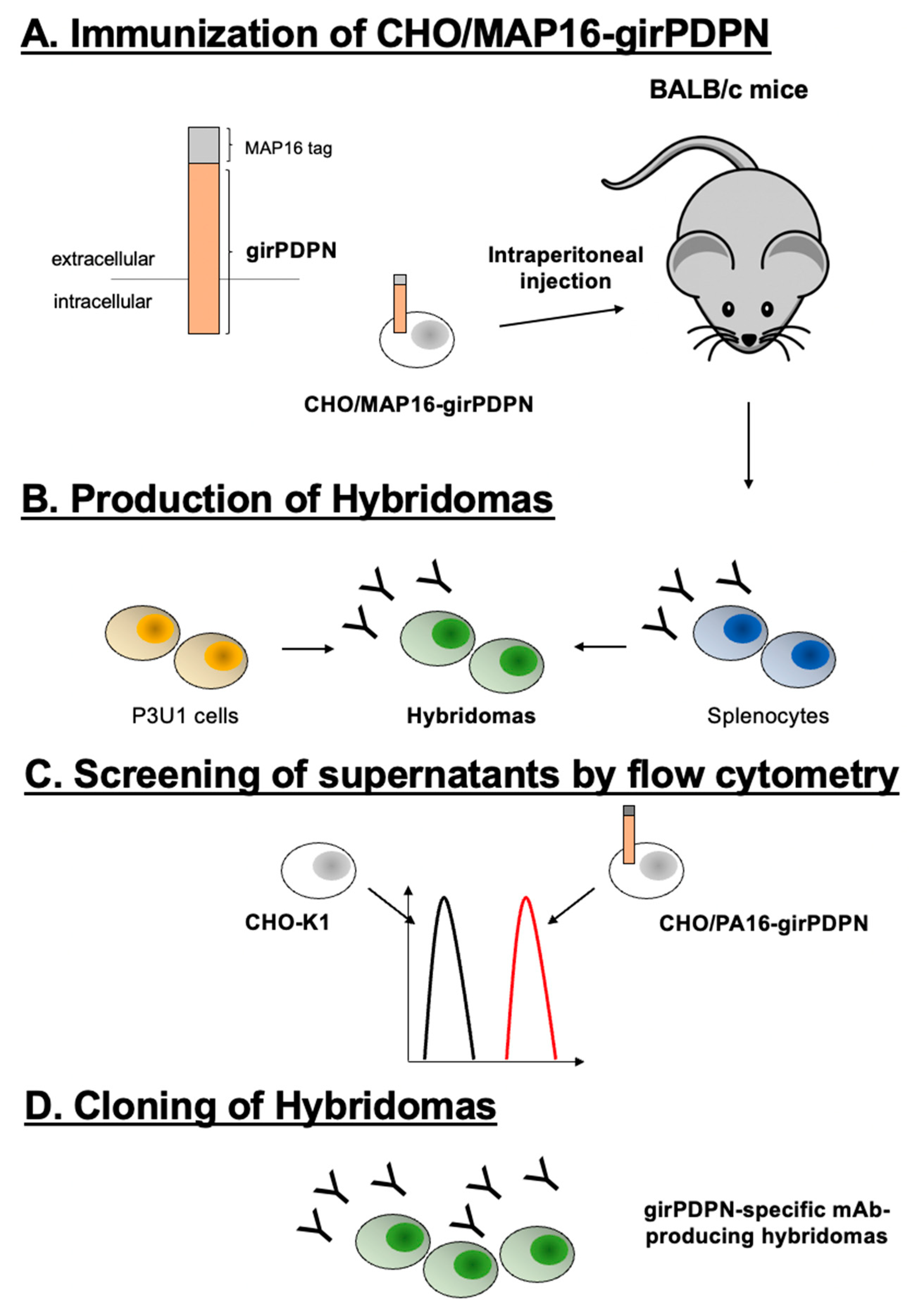
3.1. Establishment of a novel anti-girPDPN antibody
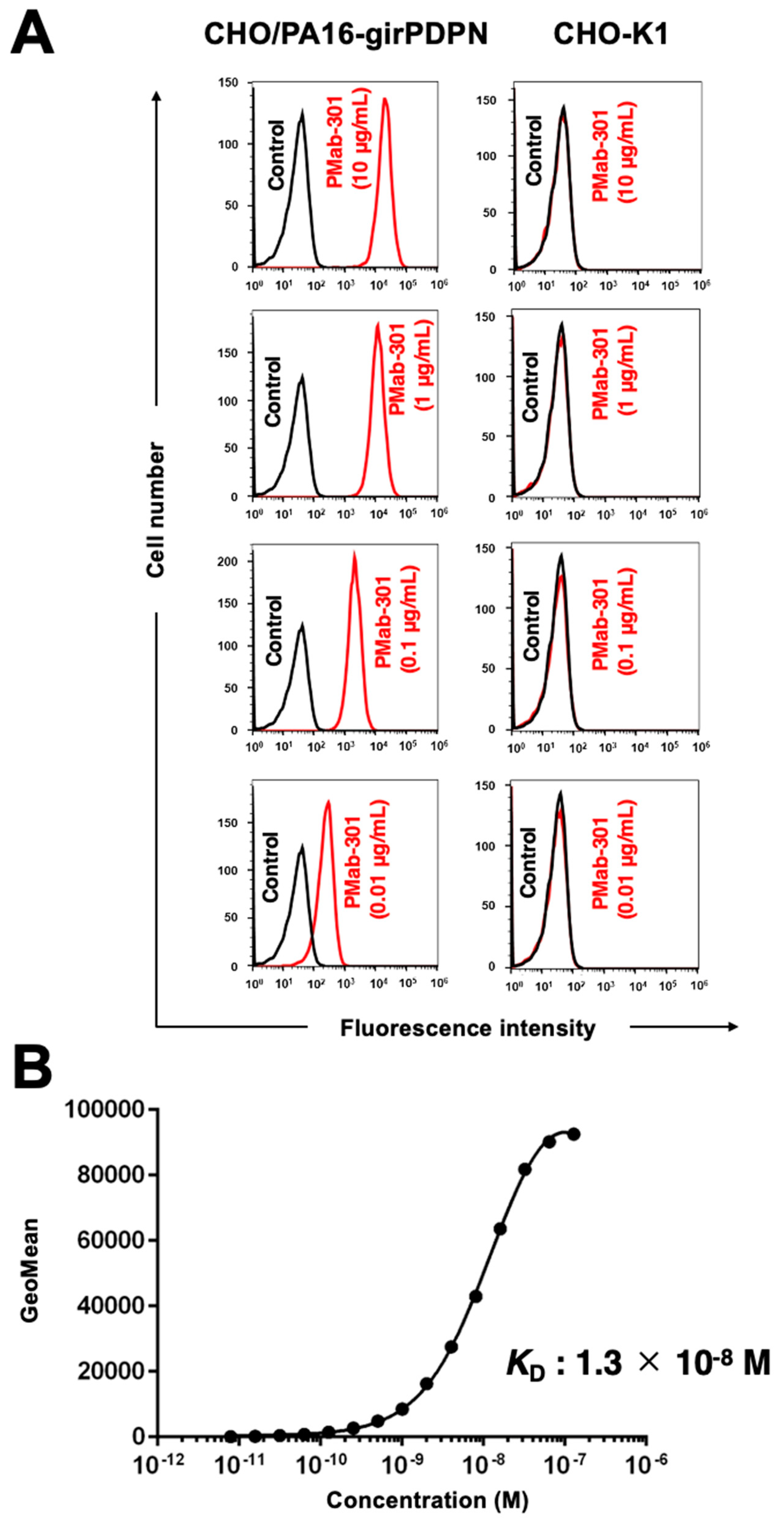
3.2. PMab-301 reacted girPDPN-overexpressing CHO-K1 in flow cytometry
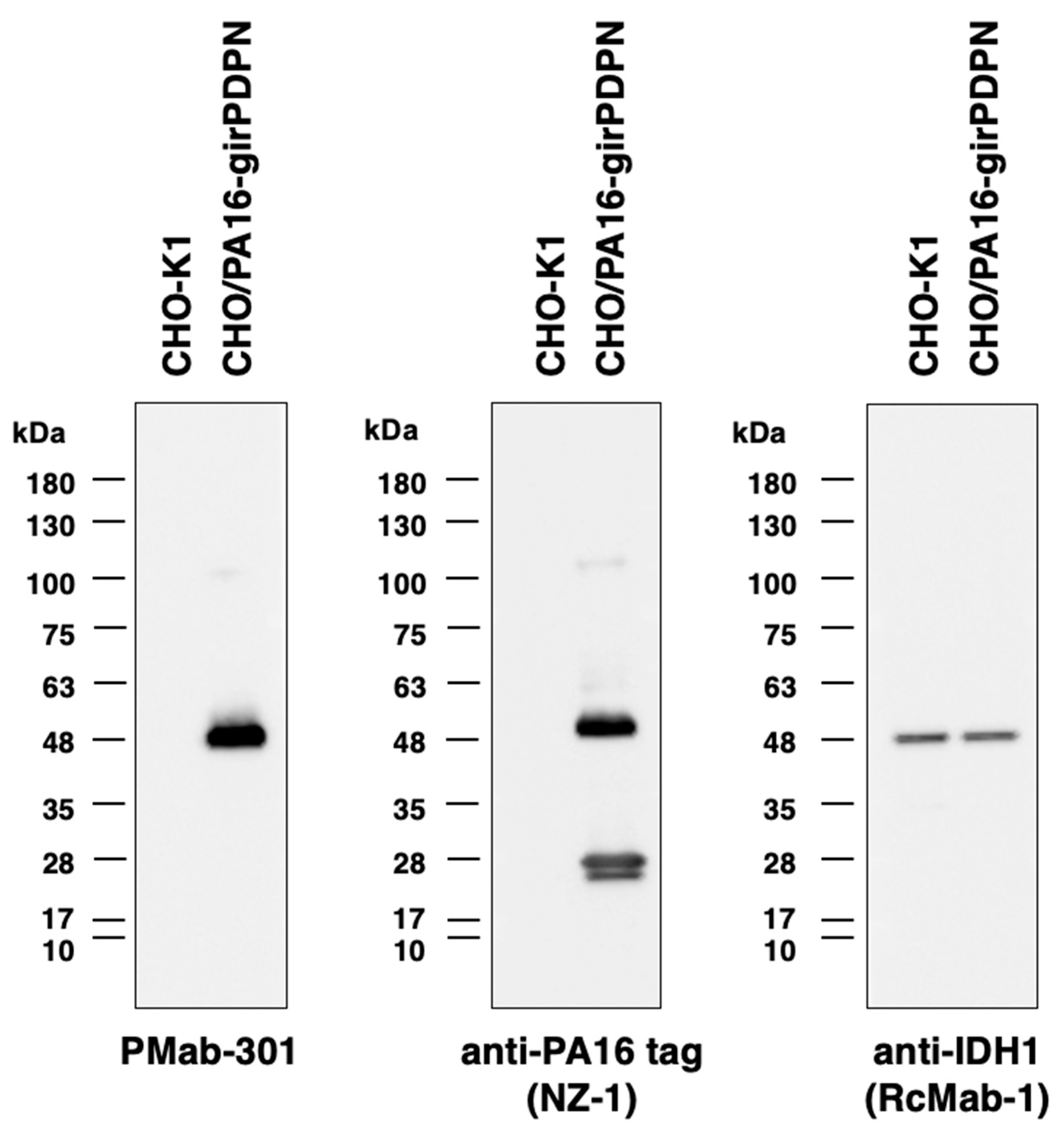
3.3. girPDPN was detected by western blot by PMab-301
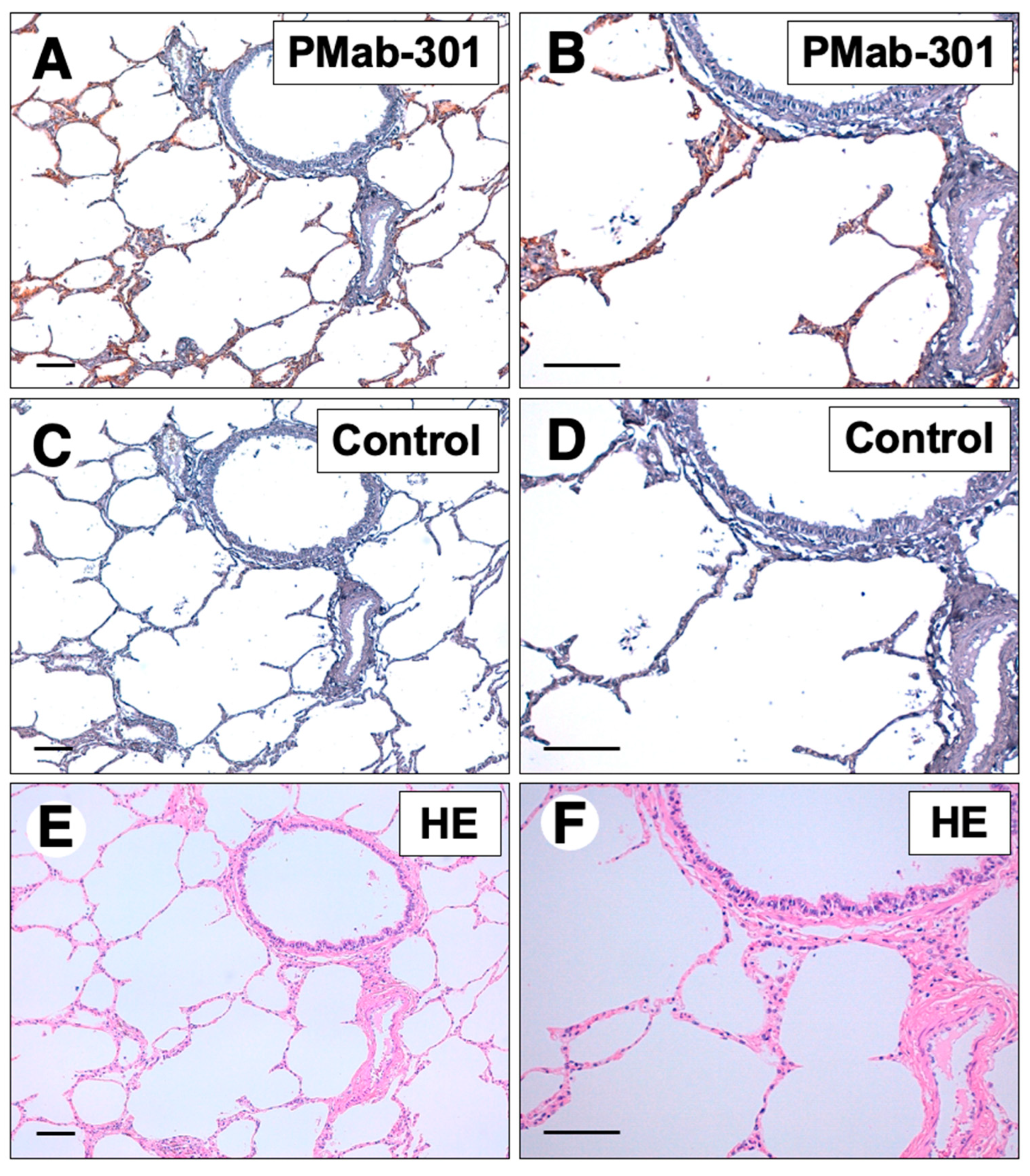
3.4. PMab-301 recognized girPDPN in immunohistochemistry
4. Discussion
References
- Breiteneder-Geleff, S.; Matsui, K.; Soleiman, A.; Meraner, P.; Poczewski, H.; Kalt, R.; Schaffner, G.; Kerjaschki, D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 1997, 151, 1141–1152. [Google Scholar]
- Schacht, V.; Ramirez, M.I.; Hong, Y.K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J 2003, 22, 3546–3556. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Kato, Y.; Inoue, O.; Kaneko, M.K.; Mishima, K.; Yatomi, Y.; Yamazaki, Y.; Narimatsu, H.; Ozaki, Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem 2007, 282, 25993–26001. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kunita, A.; Ito, H.; Kameyama, A.; Ogasawara, S.; Matsuura, N.; Hasegawa, Y.; Suzuki-Inoue, K.; Inoue, O.; et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci 2008, 99, 54–61. [Google Scholar] [CrossRef]
- Bertozzi, C.C.; Schmaier, A.A.; Mericko, P.; Hess, P.R.; Zou, Z.; Chen, M.; Chen, C.Y.; Xu, B.; Lu, M.M.; Zhou, D.; et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 2010, 116, 661–670. [Google Scholar] [CrossRef]
- Ochoa-Alvarez, J.A.; Krishnan, H.; Pastorino, J.G.; Nevel, E.; Kephart, D.; Lee, J.J.; Retzbach, E.P.; Shen, Y.; Fatahzadeh, M.; Baredes, S.; et al. Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms. Oncotarget 2015, 6, 9045–9060. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.; Sata, M.; Fujita, N.; Tsuruo, T.; Osawa, M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol 2005, 26, 195–200. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kuno, A.; Uchiyama, N.; Amano, K.; Chiba, Y.; Hasegawa, Y.; Hirabayashi, J.; Narimatsu, H.; Mishima, K.; et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem Biophys Res Commun 2006, 349, 1301–1307. [Google Scholar] [CrossRef]
- Suzuki, H.; Kaneko, M.K.; Kato, Y. Roles of Podoplanin in Malignant Progression of Tumor. Cells 2022, 11. [Google Scholar] [CrossRef]
- Dobbs, L.G.; Williams, M.C.; Gonzalez, R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta 1988, 970, 146–156. [Google Scholar] [CrossRef]
- Hirakawa, S.; Hong, Y.K.; Harvey, N.; Schacht, V.; Matsuda, K.; Libermann, T.; Detmar, M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol 2003, 162, 575–586. [Google Scholar] [CrossRef]
- Furusawa, Y.; Kaneko, M.K.; Nakamura, T.; Itai, S.; Fukui, M.; Harada, H.; Yamada, S.; Kato, Y. Establishment of a Monoclonal Antibody PMab-231 for Tiger Podoplanin. Monoclon Antib Immunodiagn Immunother 2019, 38, 89–95. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Saidoh, N.; Chang, Y.W.; Handa, S.; Harada, H.; Kagawa, Y.; Ichii, O.; et al. PMab-52: Specific and Sensitive Monoclonal Antibody Against Cat Podoplanin for Immunohistochemistry. Monoclon Antib Immunodiagn Immunother 2017, 36, 224–230. [Google Scholar] [CrossRef]
- Kato, Y.; Yamada, S.; Furusawa, Y.; Itai, S.; Nakamura, T.; Yanaka, M.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K. PMab-213: A Monoclonal Antibody for Immunohistochemical Analysis Against Pig Podoplanin. Monoclon Antib Immunodiagn Immunother 2019, 38, 18–24. [Google Scholar] [CrossRef]
- Kato, Y.; Yamada, S.; Itai, S.; Kobayashi, A.; Konnai, S.; Kaneko, M.K. Anti-Horse Podoplanin Monoclonal Antibody PMab-219 is Useful for Detecting Lymphatic Endothelial Cells by Immunohistochemical Analysis. Monoclon Antib Immunodiagn Immunother 2018, 37, 272–274. [Google Scholar] [CrossRef]
- Honma, R.; Ogasawara, S.; Kaneko, M.K.; Fujii, Y.; Oki, H.; Nakamura, T.; Takagi, M.; Konnai, S.; Kato, Y. PMab-44 Detects Bovine Podoplanin in Immunohistochemistry. Monoclon Antib Immunodiagn Immunother 2016, 35, 186–190. [Google Scholar] [CrossRef]
- Furusawa, Y.; Yamada, S.; Nakamura, T.; Sano, M.; Sayama, Y.; Itai, S.; Takei, J.; Harada, H.; Fukui, M.; Kaneko, M.K.; et al. PMab-235: A monoclonal antibody for immunohistochemical analysis against goat podoplanin. Heliyon 2019, 5, e02063. [Google Scholar] [CrossRef]
- Kato, Y.; Furusawa, Y.; Sano, M.; Takei, J.; Nakamura, T.; Yanaka, M.; Okamoto, S.; Handa, S.; Komatsu, Y.; Asano, T.; et al. Development of an Anti-Sheep Podoplanin Monoclonal Antibody PMab-256 for Immunohistochemical Analysis of Lymphatic Endothelial Cells. Monoclon Antib Immunodiagn Immunother 2020, 39, 82–90. [Google Scholar] [CrossRef]
- Kato, Y.; Furusawa, Y.; Yamada, S.; Itai, S.; Takei, J.; Sano, M.; Kaneko, M.K. Establishment of a monoclonal antibody PMab-225 against alpaca podoplanin for immunohistochemical analyses. Biochem Biophys Rep 2019, 18, 100633. [Google Scholar] [CrossRef]
- Furusawa, Y.; Yamada, S.; Itai, S.; Nakamura, T.; Takei, J.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K.; Kato, Y. Establishment of a monoclonal antibody PMab-233 for immunohistochemical analysis against Tasmanian devil podoplanin. Biochem Biophys Rep 2019, 18, 100631. [Google Scholar] [CrossRef]
- Takei, J.; Furusawa, Y.; Yamada, S.; Nakamura, T.; Sayama, Y.; Sano, M.; Konnai, S.; Kobayashi, A.; Harada, H.; Kaneko, M.K.; et al. PMab-247 Detects Bear Podoplanin in Immunohistochemical Analysis. Monoclon Antib Immunodiagn Immunother 2019, 38, 171–174. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Takei, J.; Sano, M.; Konnai, S.; Kobayashi, A.; Nakagun, S.; Kobayashi, Y.; Kaneko, M.K.; et al. Immunohistochemical Analysis of the Harbor Porpoise Using Antipodoplanin Antibody PMab-237. Monoclon Antib Immunodiagn Immunother 2019, 38, 104–107. [Google Scholar] [CrossRef]
- Tanaka, T.; Asano, T.; Sano, M.; Takei, J.; Hosono, H.; Nanamiya, R.; Nakamura, T.; Yanaka, M.; Harada, H.; Fukui, M.; et al. Development of Monoclonal Antibody PMab-269 Against California Sea Lion Podoplanin. Monoclon Antib Immunodiagn Immunother 2021, 40, 124–133. [Google Scholar] [CrossRef]
- Nanamiya, R.; Suzuki, H.; Takei, J.; Li, G.; Goto, N.; Harada, H.; Saito, M.; Tanaka, T.; Asano, T.; Kaneko, M.K.; et al. Development of Monoclonal Antibody 281-mG(2a)-f Against Golden Hamster Podoplanin. Monoclon Antib Immunodiagn Immunother 2022, 41, 311–319. [Google Scholar] [CrossRef]
- Goto, N.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Monoclonal Antibody PMab-292 Against Ferret Podoplanin. Monoclon Antib Immunodiagn Immunother 2022, 41, 101–109. [Google Scholar] [CrossRef]
- Asano, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Nanamiya, R.; Takei, J.; Asano, T.; Tanaka, T.; Sano, M.; Nakamura, T.; Yanaka, M.; Hosono, H.; Kaneko, M.K.; Kato, Y. Development of Anti-Human CC Chemokine Receptor 9 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 101–106. [Google Scholar] [CrossRef]
- Sayama, Y.; Kaneko, M.K.; Kato, Y. Development and characterization of TrMab-6, a novel anti-TROP2 monoclonal antibody for antigen detection in breast cancer. Mol Med Rep 2021, 23. [Google Scholar] [CrossRef]
- Sayama, Y.; Kaneko, M.K.; Takei, J.; Hosono, H.; Sano, M.; Asano, T.; Kato, Y. Establishment of a novel anti-TROP2 monoclonal antibody TrMab-29 for immunohistochemical analysis. Biochem Biophys Rep 2021, 25, 100902. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Kaneko, M.K.; Kato, Y. Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C(44)Mab-5. Biochem Biophys Rep 2018, 14, 64–68. [Google Scholar] [CrossRef]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; Nogi, T.; Kato, Y.; Takagi, J. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014, 95, 240–247. [Google Scholar] [CrossRef]
- Fujii, Y.; Kaneko, M.K.; Kato, Y. MAP Tag: A Novel Tagging System for Protein Purification and Detection. Monoclon Antib Immunodiagn Immunother 2016, 35, 293–299. [Google Scholar] [CrossRef]
- Yamada, S.; Honma, R.; Kaneko, M.K.; Nakamura, T.; Yanaka, M.; Saidoh, N.; Takagi, M.; Konnai, S.; Kato, Y. Characterization of the Anti-Bovine Podoplanin Monoclonal Antibody PMab-44. Monoclon Antib Immunodiagn Immunother 2017, 36, 129–134. [Google Scholar] [CrossRef]
- Potter, J.S.; Clauss, M. Mortality of captive giraffe (Giraffa camelopardalis) associated with serous fat atrophy: a review of five cases at Auckland Zoo. J Zoo Wildl Med 2005, 36, 301–307. [Google Scholar] [CrossRef]
- Sullivan, K.; van Heugten, E.; Ange-van Heugten, K.; Poore, M.H.; Dierenfeld, E.S.; Wolfe, B. Analysis of nutrient concentrations in the diet, serum, and urine of giraffe from surveyed North American zoological institutions. Zoo Biol 2010, 29, 457–469. [Google Scholar] [CrossRef]
- Garijo, M.M.; Ortiz, J.M.; Ruiz de Ibanez, M.R. Helminths in a giraffe (Giraffa camelopardalis xgiraffa) from a zoo in Spain. Onderstepoort J Vet Res 2004, 71, 153–156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




