Submitted:
29 September 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Nanofibers Synthesis
2.2. MFC Architecture and Configuration
3. Results and Discussion
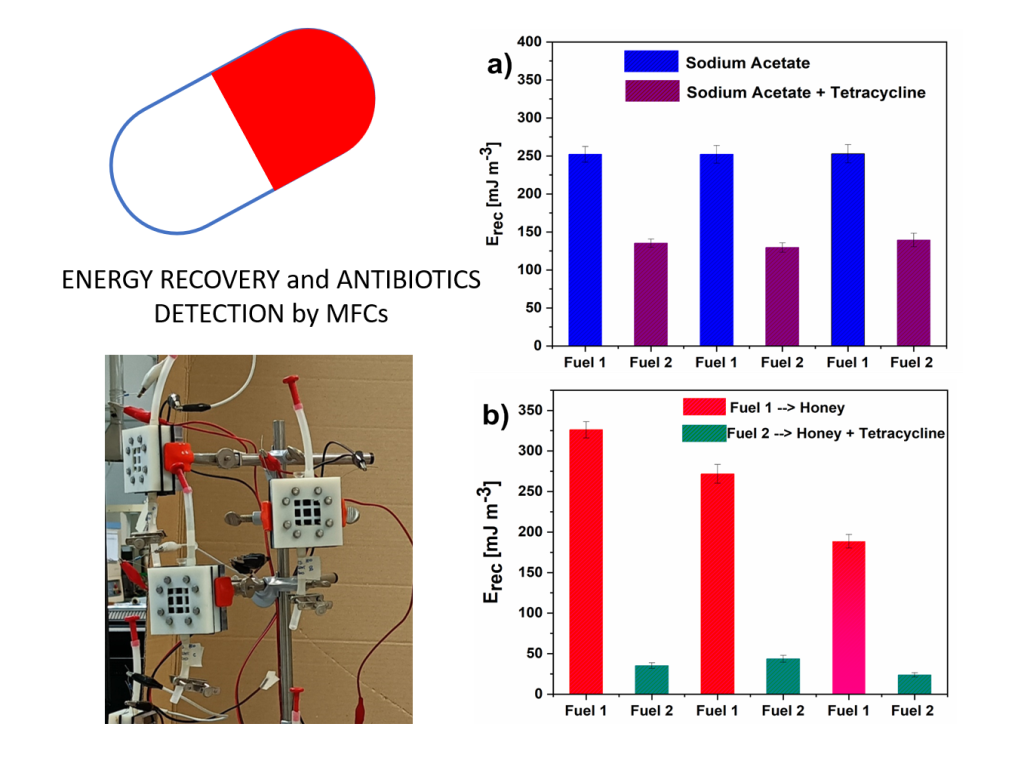
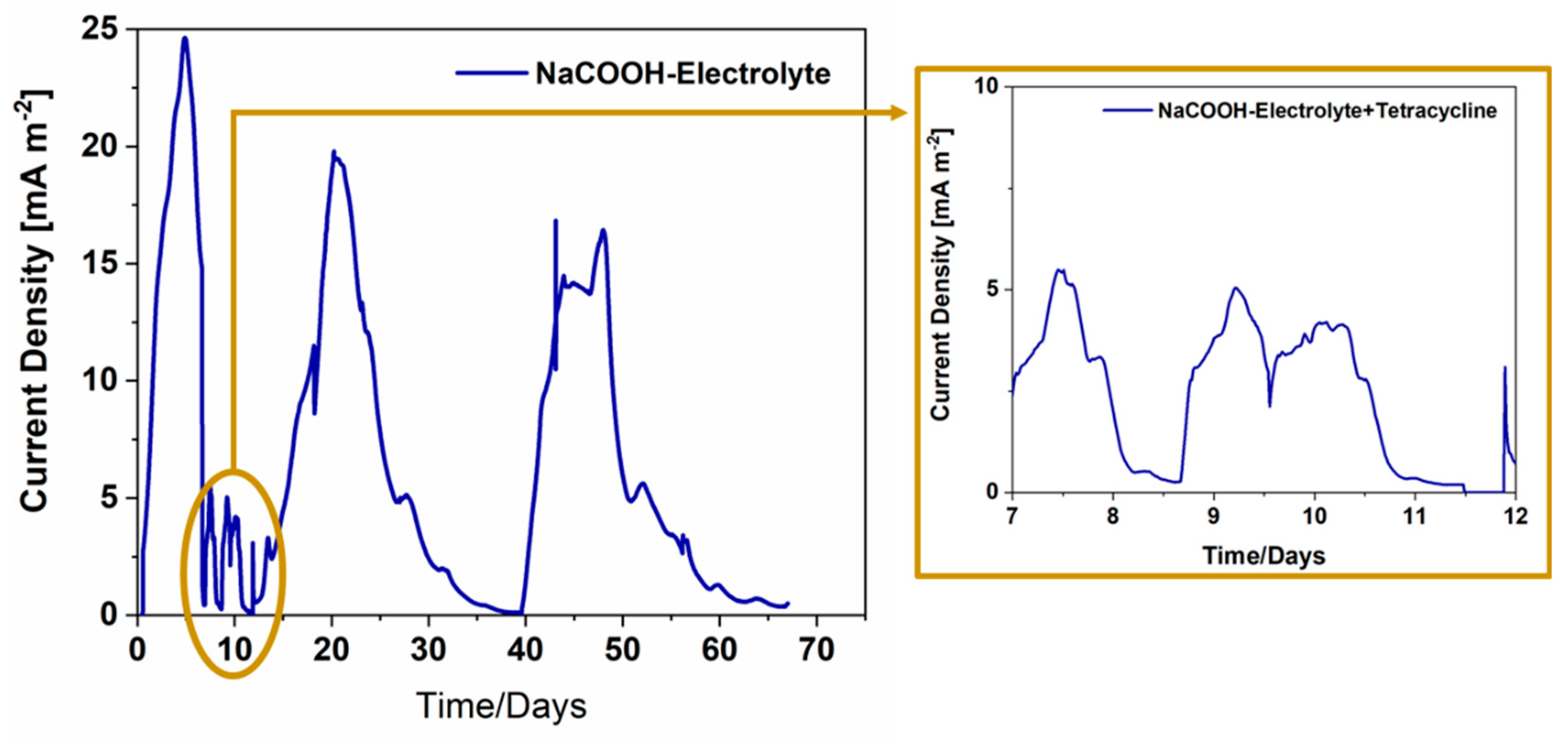
3.1. a-SCMFCs for tetracycline detection in solium acetate-based electrolyte
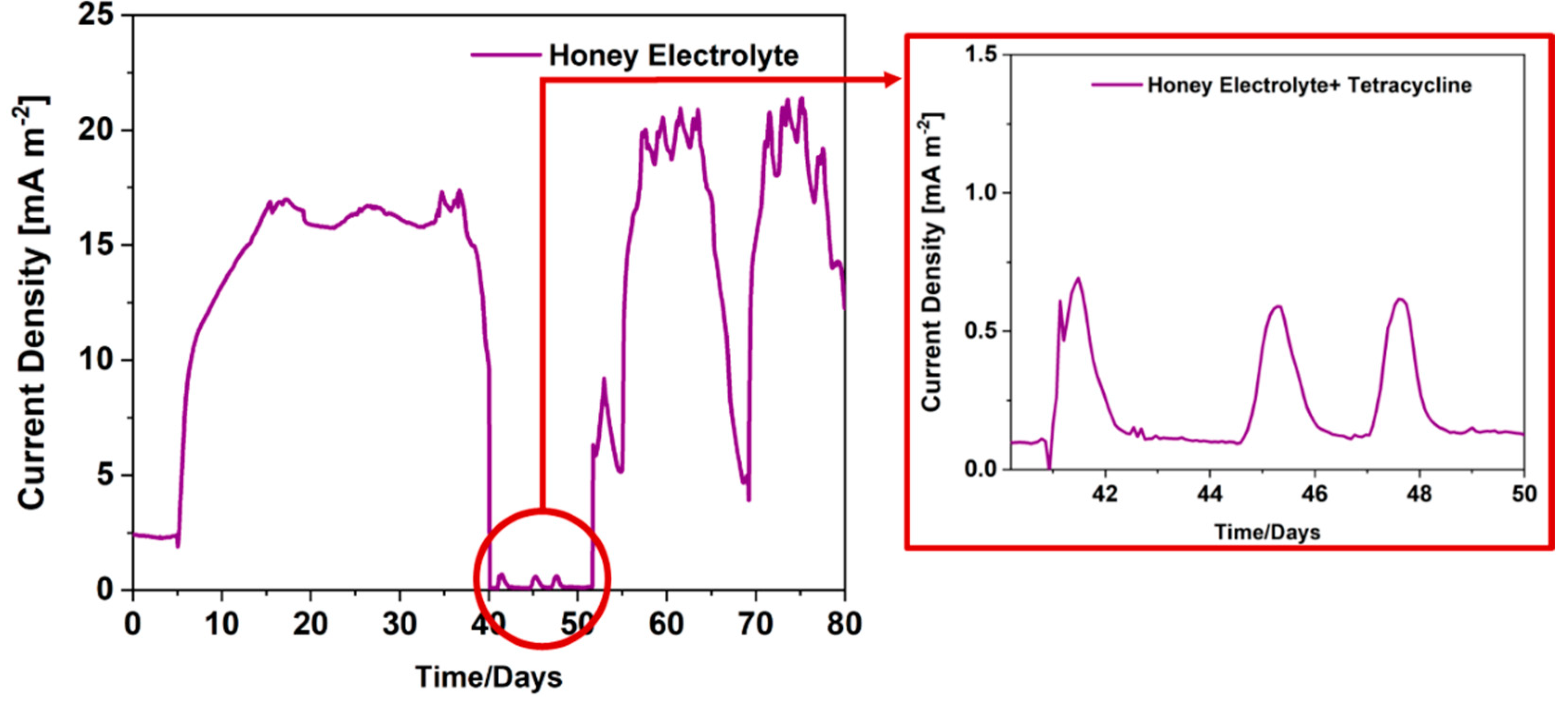
3.2. a-SCMFCs for tetracycline detection in honey-based matrix
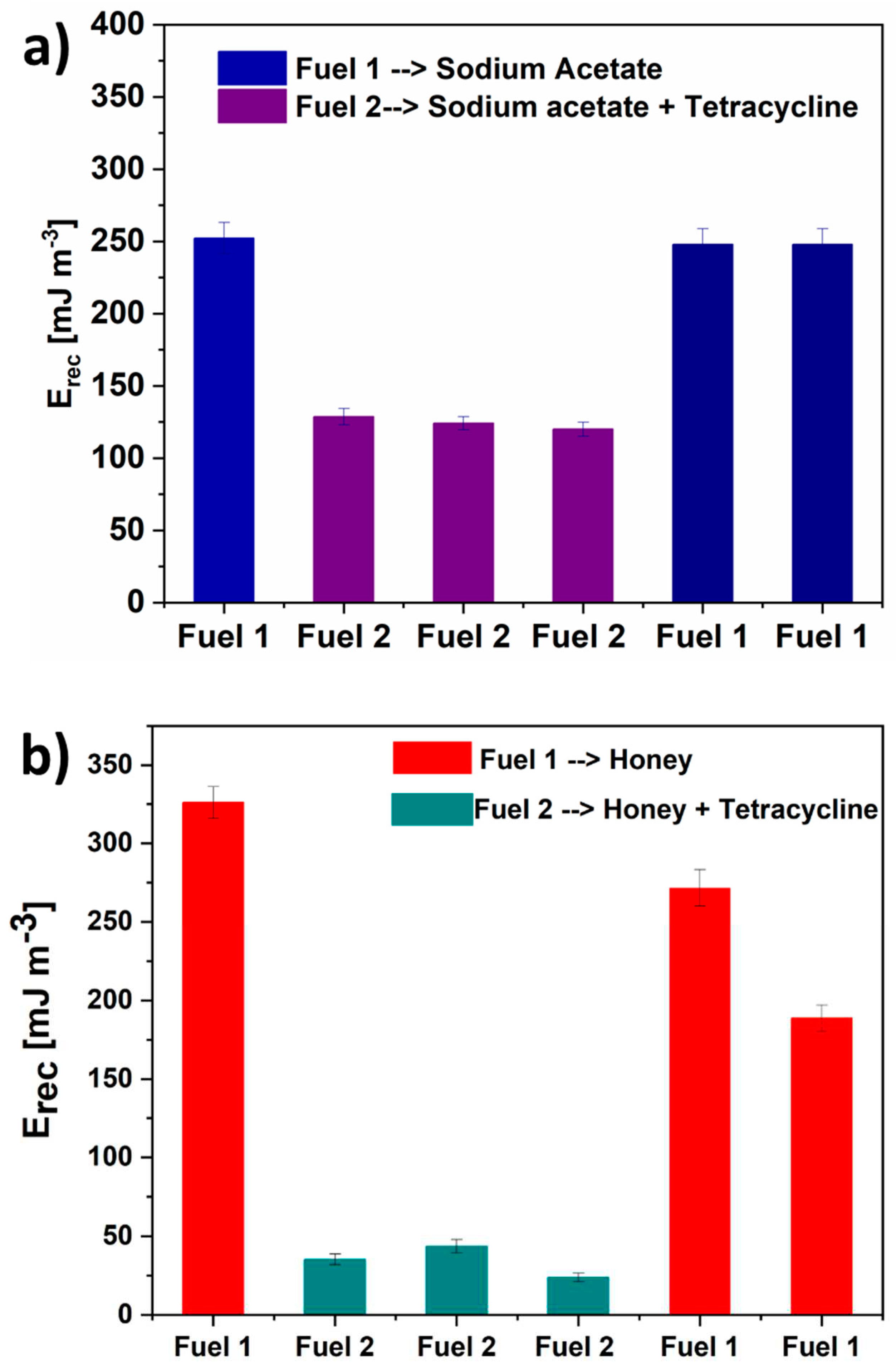
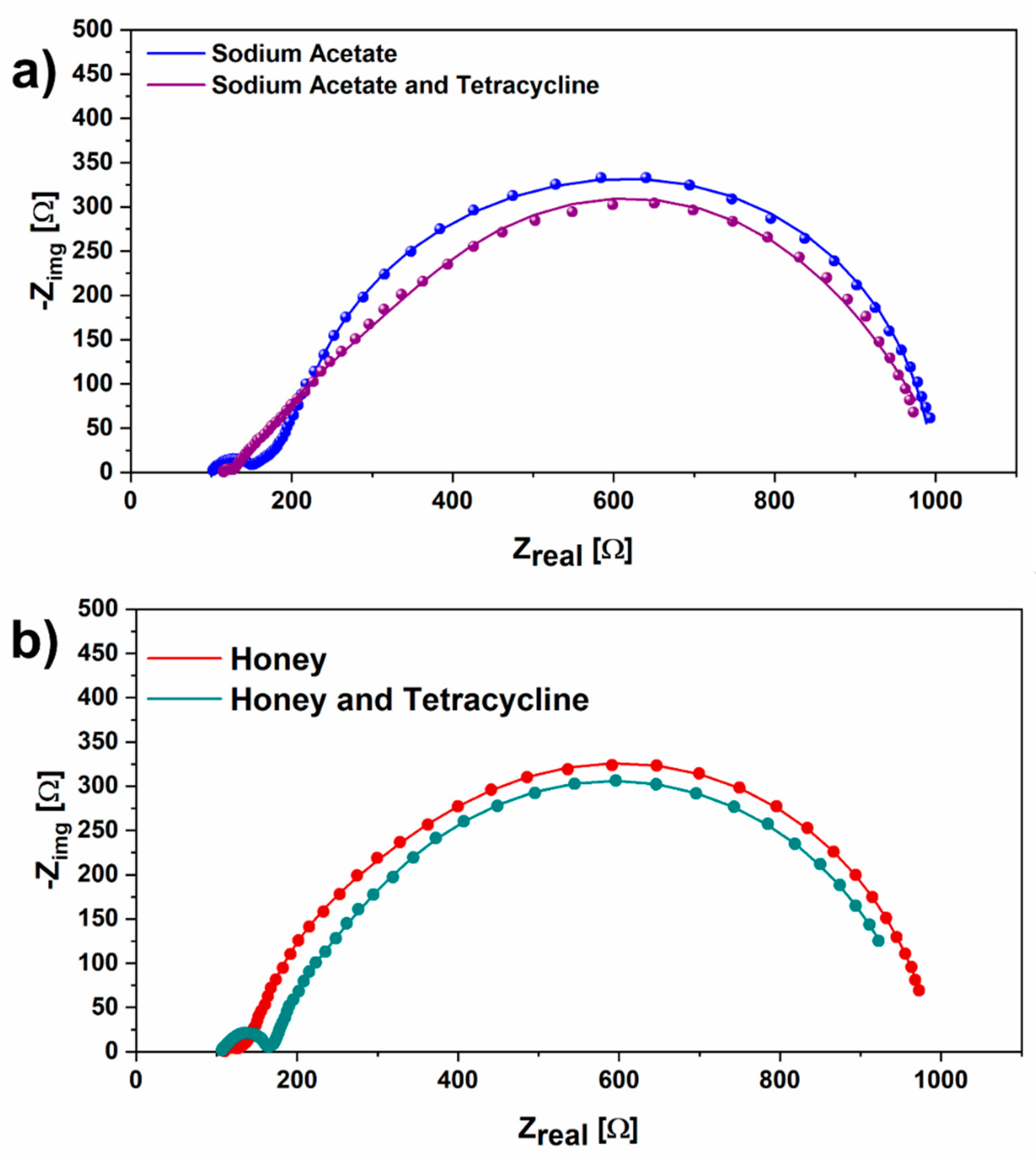
3.2. Energy Recovery analysis and Electrochemical Impedance Spectroscopy Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, D.; Du, D.; Lin, Y. Recent progress on nanomaterial-based biosensors for veterinary drug residues in animal-derived food. Trends in Analytical Chemistry 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Karp, H.; Ekholm, P.; Kemi, V.; Hivonen, T.; Lamberg-Allardt, C. Differences among total and in vitro digestible phosphorus content of meat and milk products. J. Renal Nutr. 2012, 12, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Veterinary drug residues in animal and food: compliance with safety levels still high. https://www.efsa.europa.eu/en/news/veterinary-drug-residues-animals-and-food-compliance-safety-levels-still-high (accessed on 26 September 2023).
- N. Al-Waili, K. Salom, A. Al-Ghamdi, and M. J. Ansari, “Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards,” Sci. World J. 2012, 2012, no. Table 1.
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Phillips, I. Does the Use of Antibiotics in Food Animals Pose a Risk to Human Health? A Critical Review of Published Data. J. Antimicrob. Chemother. 2003, 53, 28–52. [Google Scholar] [CrossRef]
- Carlet, J. Antibiotic resistance: Protecting antibiotics - the declaration of the world alliance against antibiotic resistance. Indian J Crit Care Med. 2014, 18, 643–645. [Google Scholar] [CrossRef]
- Chandler, C.I.R. Current Accounts of Antimicrobial Resistance: Stabilisation, Individualisation and Antibiotics as Infrastructure. Palgrave Commun. 2019, 5, 53. [Google Scholar] [CrossRef]
- Kneebone, J.; Tsang, P.C.W.; Towson, D.H. Rapid Antibiotic Screening Tests Detect Antibiotic Residues in Powdered Milk Products. J. Dairy Sci. 2010, 93, 3961–3964. [Google Scholar] [CrossRef]
- Jammoul, A.; El Darra, N. Evaluation of Antibiotics Residues in Chicken Meat Samples in Lebanon. Antibiotics 2019, 8, 69. [Google Scholar] [CrossRef]
- Dawadi, S.; Thapa, R.; Modi, B.; Bhandari, S.; Timilsina, A.P.; Yadav, R.P.; Aryal, B.; Gautam, S.; Sharma, P.; Thapa, B.B.; Aryal, N.; Aryal, S.; Regmi, B.P.; Parajuli, N. Technological Advancements for the Detection of Antibiotics in Food Products. Processes 2021, 9, 1500. [Google Scholar] [CrossRef]
- Farouk, F.; Azzazy, H.M.E.; Niessen, W.M.A. Challenges in the Determination of Aminoglycoside Antibiotics, a Review. Anal. Chim. Acta 2015, 890, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Zhou, J.; Liu, H.; Wu, H.; Zhang, Z.; Ding, Y.; Zhang, K. Simultaneous Extraction and Determination of 45 Veterinary Antibiotics in Swine Manure by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B 2020, 1154, 122286. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, A.S.; Lista, A.G.; Domini, C.E. Reverse Ultrasound-Assisted Emulsification-Microextraction of Macrolides from Chicken Fat Followed by Electrophoretic Determination. LWT 2019, 113, 108334. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Yuan, H.; Peng, Y.; Hong, Q.; Liu, M. Rapid Detection of Tetracycline Residues in Duck Meat Using Surface Enhanced Raman Spectroscopy. J. Spectrosc. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Baghani, A.; Mesdaghinia, A.; Rafieiyan, M.; Soltan Dallal, M.M.; Douraghi, M. Tetracycline and Ciprofloxacin Multiresidues in Beef and Chicken Meat Samples Using Indirect Competitive ELISA. J. Immunoass. Immunochem. 2019, 40, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Chen, J.; Rao, Z.; Guo, B.; Xu, Y. Recent Advances of Biosensors for Detection of Multiple Antibiotics. Biosensors 2023, 13, 850. [Google Scholar] [CrossRef]
- Singh, H.; Thakur, B.; Bhardwaj, S.K.; Khatri, M. ; Kim K-H.; Bhardwaj, N. Nanomaterial-based fluorescent biosensors for the detection of antibiotics in foodstuffs: A review. Food Chemistry 2023, 426, 136657. [Google Scholar] [CrossRef]
- Gaudin, V. Advances in Biosensor Development for the Screening of Antibiotic Residues in Food Products of Animal Origin-A Comprehensive Review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef]
- Mungroo, N.; Neethirajan, S. Biosensors for the Detection of Antibiotics in Poultry Industry—A Review. Biosensors 2014, 4, 472–493. [Google Scholar] [CrossRef]
- Naresh, V. , Lee N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications – A review. Journal of Oral Biology and Craniofacial Research 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Su, L. , Jia W., Hou C., Lei Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Analytica Chimica Acta 2006, 568, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Moraskie, M.; Or Roshid, M.H.; O'Connor, G.; Dikici, E.; Zingg, J-M. ; Deo, S.; Daunert, S. Microbial whole-cell biosensors: Current applications, challenges, and future perspectives. Biosens. Bioelectron. 2021, 191, 113359. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chhabra, M. The versatility of microbial fuel cells as tools for organic matter monitoring. Bioresour. Technol. 2023, 377, 128949. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Oliasab, L.; Di Lorenzo, M. Microbial fuel cells for in-field water quality monitoring. RSC Adv 2021, 11, 16307. [Google Scholar]
- Chang, I.S.; Jang, J.K.; Gil, G.C.; Kim, M.; Kim, H.J.; Cho, B.W.; Kim, B.H. Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens. Bioelectron. 2004, 19, 607–613. [Google Scholar] [CrossRef]
- Ivars-Barceló, F.; Zuliani, A.; Fallah, M.; Mashkour, M.; Rahimnejad, M.; Luque, R. Novel Applications of Microbial Fuel Cells in Sensors and Biosensors. Appl. Sci. 2018, 8, 1184. [Google Scholar] [CrossRef]
- Christwardana, M.; Yoshi, L.A.; Setyonadi, I.; Maulana, M.R.; Fudholi, A. A novel application of simple submersible yeast-based microbial fuel cells as dissolved oxygen sensors in environmental waters. Enzyme Microb. Technol. 2021, 149, 109831. [Google Scholar] [CrossRef]
- Klevinskas, A.; Kantminiene, K.; Žmuidzinaviciene, N.; Jonuškiene, I.; Griškonis, E. Microbial Fuel Cell as a Bioelectrochemical Sensor of Nitrite Ions. Processes 2021, 9, 1330. [Google Scholar] [CrossRef]
- Zhu, T-J. ; Lin, C-W.; Liu, S-H. Sensitivity and reusability of a simple microbial fuel cell-based sensor for detecting bisphenol A in wastewater. Chemosphere 2023, 320, 138082. [Google Scholar] [CrossRef]
- Chouler, J.; Di Lorenzo, M. Water Quality Monitoring in Developing Countries; Can Microbial Fuel Cells be the Answer? Biosensors 2015, 5, 450–70. [Google Scholar] [CrossRef] [PubMed]
- Agostino, v. , Massaglia G., Gerosa M., Sacco A., Saracco G., Margaria V., Quaglio M. Environmental electroactive consortia as reusable biosensing element for freshwater toxicity monitoring. New Biotechnology 2020, 55, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Massaglia, G. , Frascella F., Chiadò A., Sacco A., Marasso S.L., Cocuzza M., Pirri C.F., Quaglio M. Electrospun Nanofibers: from Food to Energy by Engineered Electrodes in Microbial Fuel Cells. Nanomaterials 2020, 10, 523. [Google Scholar] [CrossRef]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Canizares, P.; Gonzales, E.R.; Rodrigo, M.A.R. Energy recovery from winery wastewater using a dual chamber microbial fuel cell. J Chem. Technol. Biotechnol. 2016, 91, 1802–1808. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.; Zhang, H.; Jia, H.; Zhang, Y.; Cui, Z.; Gao, F. Maximizing energy recovery from homeostasis in microbial fuel cell by synergistic conversion of short-chain volatile fatty acid. Bioresour. Technol. Rep. 2019, 7, 100200; 37. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Molognoni, D.; Dallago, E.; Liberale, A.; Cella, R.; Longoni, P.; Pantaleoni, L. Microbial Fuel Cells for Direct Electrical Energy Recovery from Urban Wastewaters. Sci. World J. 2013, 2013, 634738. [Google Scholar] [CrossRef]
- Hidalgo, D.; Sacco, A.; Hernàndez, S.; Tommasi, T. Electrochemical and impedance characterization of Microbial Fuel Cells based on 2D and 3D anodic electrodes working with seawater microorganisms under continuous operation. Bioresour. Technol. 2015, 195, 139–146. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




