Submitted:
08 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Interaction under static conditions
2.2.2. Physical integrity under dynamic conditions
2.2.3. Linear swelling
3. Results and Discussion
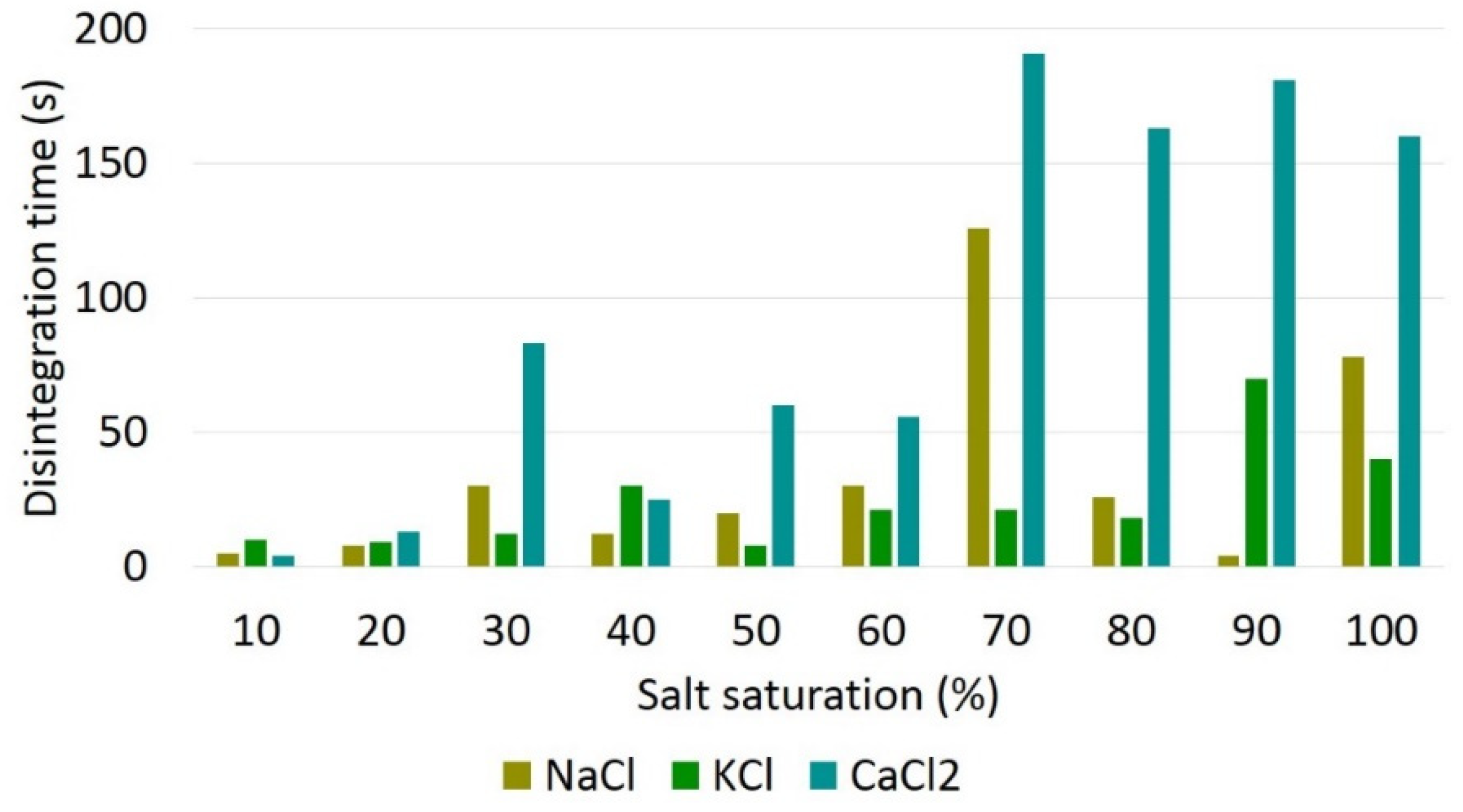
3.1. Interaction under static conditions
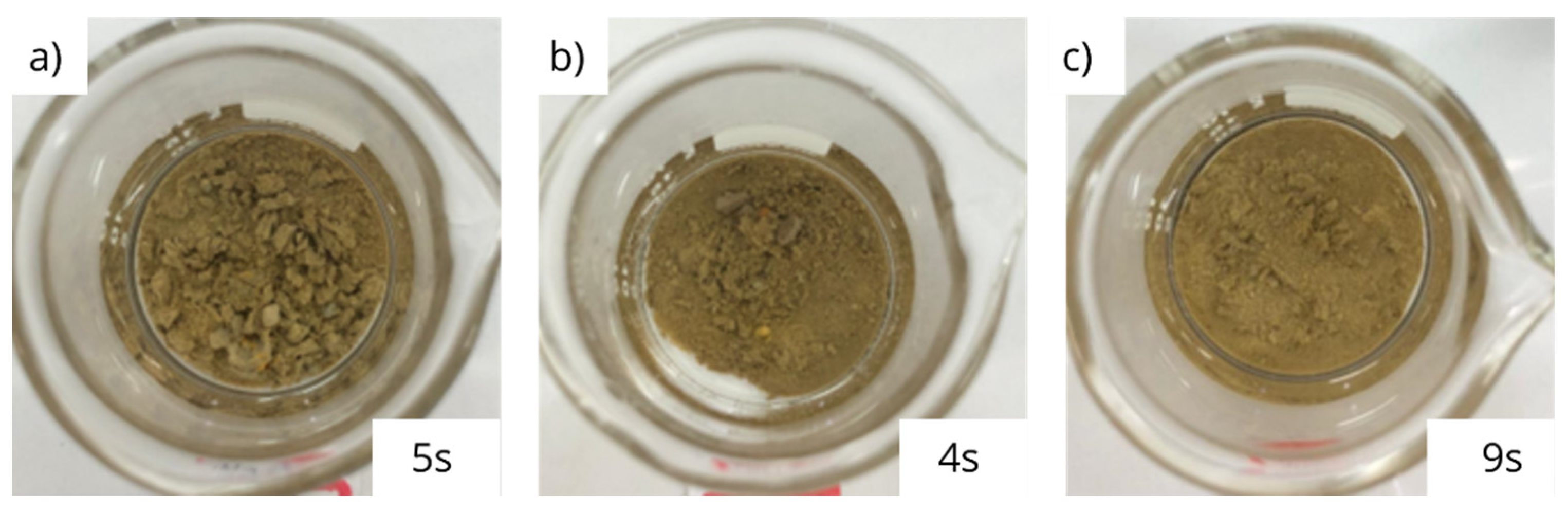
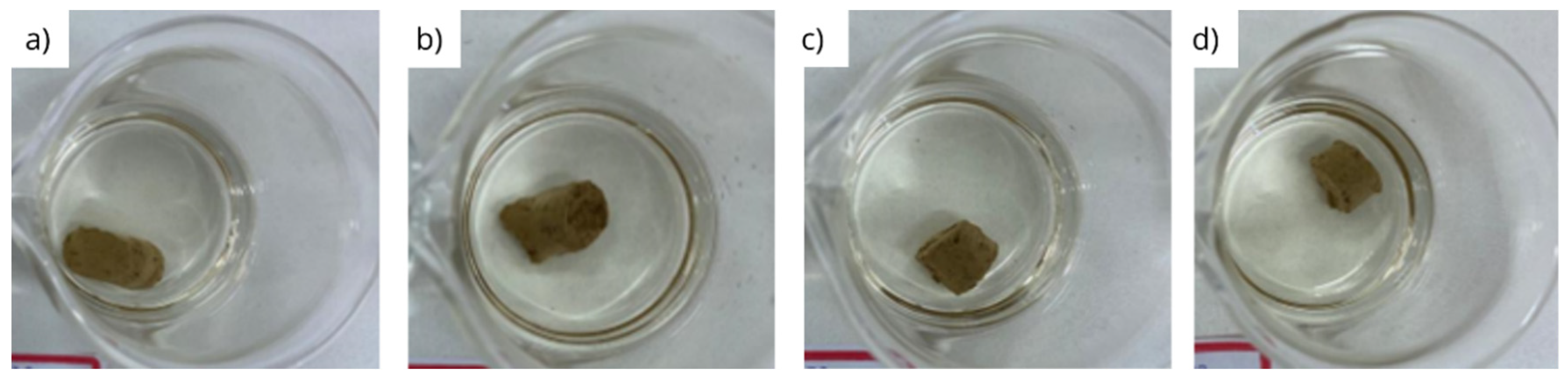
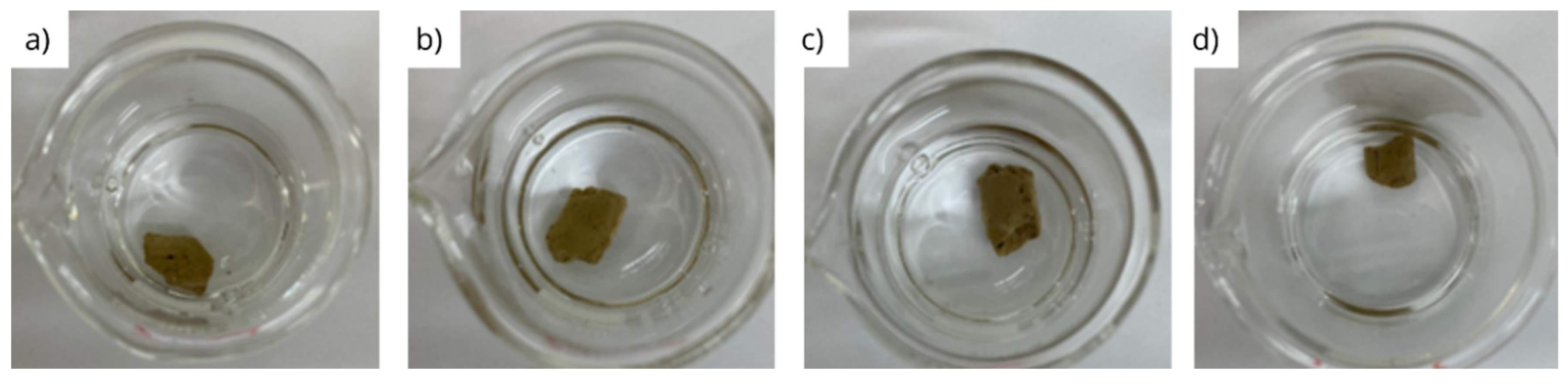
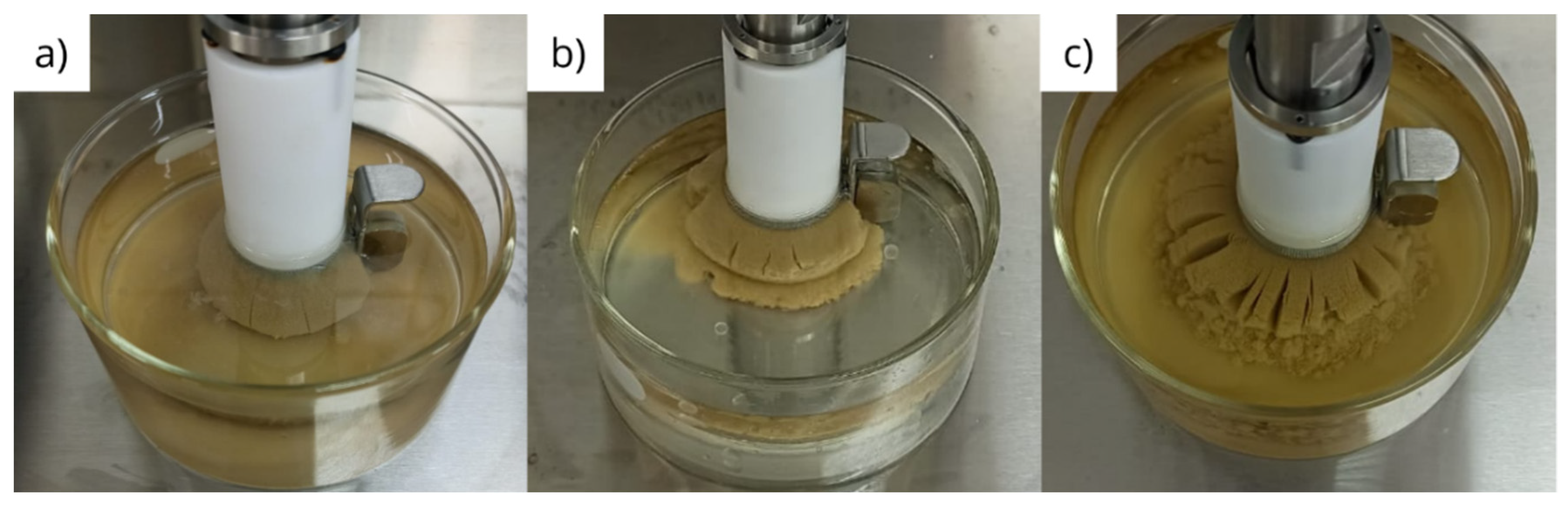
3.2. Physical integrity under dynamic conditions
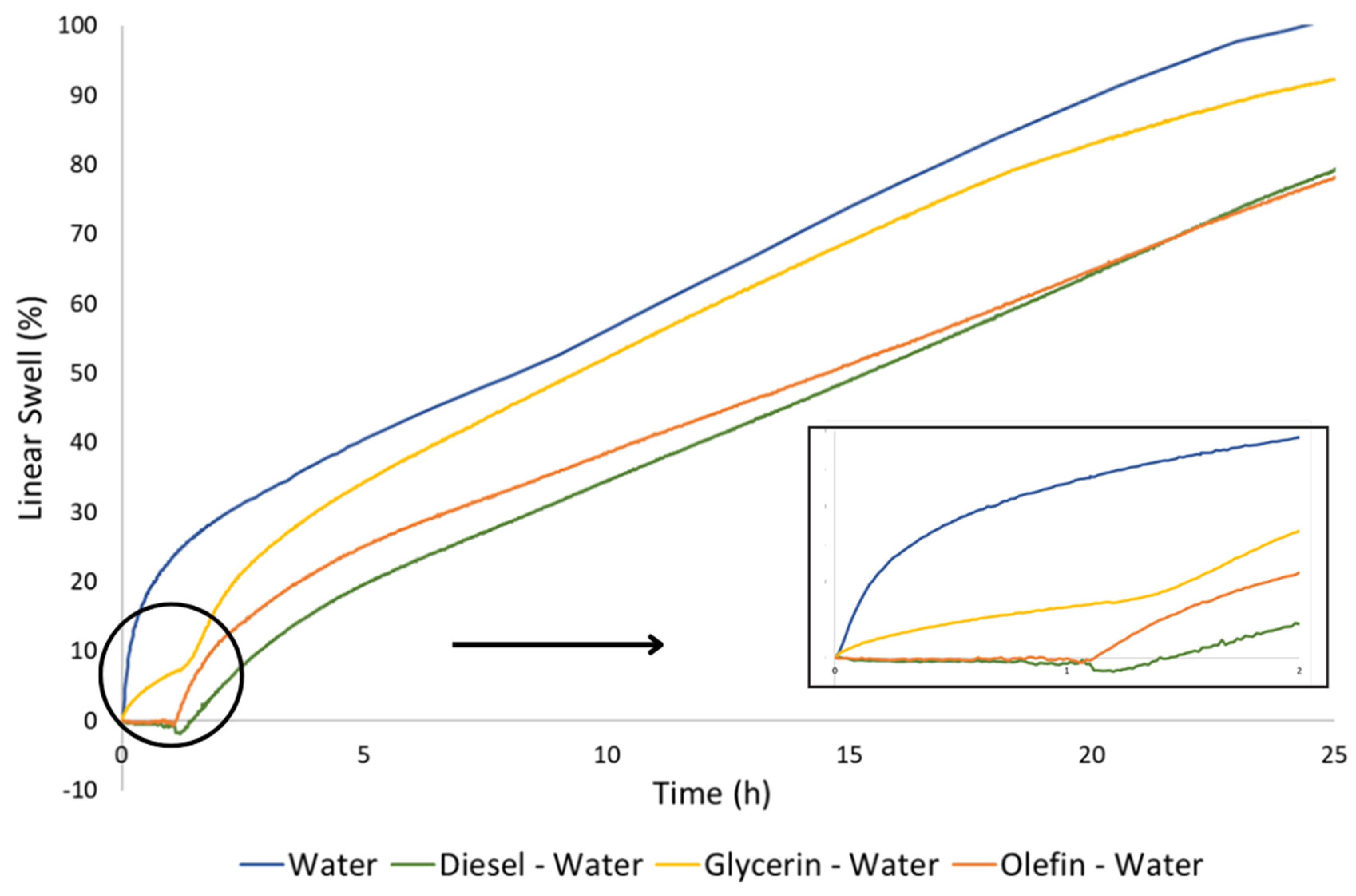
3.3. Linear swelling
4. Conclusions
- Saline solutions consisting of sodium chloride (NaCl), calcium chloride (CaCl2) and potassium chloride (KCl) brines, in different saturations, compromise the physical integrity of the bentonite pellets and are unsuitable for use as displacement fluids;
- Saline solutions with lower saturation, around 10 to 20%, promote faster and more significant disintegration of bentonite pellets;
- The interaction between glycerin and bentonite pellets results in partial physical disintegration of the pellets, which is significantly enhanced by shear under dynamic conditions;
- Bentonite pellets maintain their physical integrity when exposed to diesel and olefin, even under dynamic conditions;
- Diesel and olefin seems to present minor effects on pellets hydration and swelling, since it was observed a linear swelling in deionized water of 78% after the previous contact with these fluids, which represents a decrease of only 20% on its total swelling capacity in water. It does not impair the formation of a hydraulically solid plug of bentonite in the well, neither the use of diesel and olefin as displacement fluids;
- Among the fluids considered for displacement of pellets in offshore wells, diesel and olefin proved to be a highly promising alternative.
Author Contributions
Funding
Conflicts of Interest
References
- Towler, B. F.; Firouzi, M.; Mortezapour, A.; Hywel-Evans, P. D. Plugging CSG wells with bentonite: Review and preliminary lab results. In SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9-11 November 2015. [CrossRef]
- B.F. Towler; M. Firouzi; H.G. Holl; R. Gandhi; A. Thomas. Field trials of plugging oil and gas wells with hydrated bentonite. SPE Asia Pacific Oil & Gas Conference and Exhibition, Perth, Australia, 25 – 27 October 2016. [CrossRef]
- Achang, M.; Yanyao, L.; Radonjic, M. A review of past, present, and future technologies for permanent plugging and abandonment of wellbores and restoration of subsurface geologic barriers. Environ. Eng. Sci. 2020, 37, 395–408. [Google Scholar] [CrossRef]
- Ogden, F. L.; Ruff, J. F. Strength of bentonite water-well annulus seals in confined aquifers. J. Irrig. Drain. Eng. 1993, 119, 242–250. [Google Scholar] [CrossRef]
- James, M. C. Using coarse ground bentonite to plug abandoned holes. Water Well J. 1996, 50, 44–47. [Google Scholar]
- Kale, R. C.; Ravi, K. A review on the impact of thermal history on compacted bentonite in the context of nuclear waste management. Environ. Technol. Innov 2021, 23, 101728. [Google Scholar] [CrossRef]
- Clark, J.; Salsbury, B. Well Abandonment Using Highly Compressed Sodium Bentonite-An Australian Case Study. In Exploration and Production Environmental Conference, San Antonio, USA, 10-12 March 2003. [CrossRef]
- Towler, B. F.; Ehlers, G. C. Friction factors for hydrated bentonite plugs. In SPE Rocky Mountain Regional Meeting, Casper, USA, 18-21 May 1997. [CrossRef]
- Towler, B. F.; Victorov, H.; Zamfir, G.; Ignat, P. Plugging Wells with Hydrated Bentonite, Part 2: Bentonite Bars. In SPE Annual Technical Conference and Exhibition, Colorado, USA, 21-24 September 2008. [CrossRef]
- Bell, S.T.; Opitz, L.A.; Sundstrom, E.; Taylor, J.F. Plugging Wells with Hydrated Bentonite, Part 3: Further Lab Results. University of Wyoming, Department of Chemical Engineering. 2009.
- Englehardt, J.; Wilson, M. J.; Woody, F. New abandonment technology new materials and placement techniques. In SPE Exploration and Production Environmental Conference, San Antonio, USA, 26-28 February 2001. [CrossRef]
- Holl, H. G.; Scheuermann, A. Characterisation of geomechanical properties of bentonite clay used for plug and abandonment operations of coal seam gas wells. J. Miner. Mater. Characteriz. Eng. 2018, 6, 218–234. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhang, B. Evaluation and prediction of the swelling pressures of GMZ bentonites saturated with saline solution. Appl. Clay. Sci. 2015, 105, 207–216. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, W. M.; Chen, Y. G.; Chen, B.; Cui, Y. J. Influences of salt solution concentration and vertical stress during saturation on the volume change behavior of compacted GMZ01 bentonite. Eng. Geol. 2016, 207, 48–55. [Google Scholar] [CrossRef]
- Chen, Y. G.; Dong, X. X.; Zhang, X. D.; Ye, W. M.; Cui, Y. J. Combined thermal and saline effects on the swelling pressure of densely compacted GMZ bentonite. Appl. Clay. Sci. 2018, 166, 318–326. [Google Scholar] [CrossRef]
- Bian, X.; Cui, Y. J.; Li, X. Z. Voids effect on the swelling behaviour of compacted bentonite. Geotech. 2019, 69, 593–605. [Google Scholar] [CrossRef]
- AMERICAN PETROLEUM INSTITUTE SPECIFICATIONS, API 10B-5. Recommended Practice on Determination of Shrinkage and Expansion of Well Cement Formulations at Atmospheric Pressure, 2004.
- Tan, L.; Zheng, P.; Liu, Q. Effects of saline solutions on the desiccation cracking and shrinkage behavior of gaomiaozi bentonite. Adv. Civ. Eng. 2020, 1–13. [Google Scholar] [CrossRef]
- Rao, S. M.; Shivananda, P. Role of osmotic suction in swelling of salt-amended clays. Can. Geotech. J. 2005, 42, 307–315. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Niu, Z.; Xu, Y.; Wei, X.; Chen, X.; Pan, D.; Wu, W. Kinetic determination of sedimentation for GMZ bentonite colloids in aqueous solution: Effect of pH, temperature and electrolyte concentration. Appl. Clay. Sci. 2020, 184, 105393. [Google Scholar] [CrossRef]
- Patel, A.; Stamatakis, S.; Young, S.; Friedheim, J. Advances in Inhibitive Water-Based Drilling Fluids—Can They Replace Oil-Based Muds? In International Symposium on Oilfield Chemistry, Houston, USA, 28 February – 02 March 2007. [CrossRef]
- Sanchez-Martin, M. J.; Rodriguez-Cruz, M. S.; Andrades, M. S.; Sanchez-Camazano, M. Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: influence of clay type and pesticide hydrophobicity. Appl. Clay Sci. 2006, 31, 216–228. [Google Scholar] [CrossRef]
- Lara, S. C.; Salcedo, F. Gelatinization and retrogradation phenomena in starch/montmorillonite nanocomposites plasticized with different glycerol/water ratios. Carbohydr. Polym. 2016, 151, 206–212. [Google Scholar] [CrossRef]
- Corrêa, C. C.; Cruz, G. F. D.; Vaz, A. S.; Araújo, B. D. S.; Silva, A. A. D.; Rodrigues, R. A.; Lomba, R. F. T.; Waldmann, A. T. D. A. . Avaliação do potencial uso de bioglicerina como base para formulação de fluidos de perfuração aquosos para poços de petróleo e gás. Quím. Nova. 2017, 40, 378–387. [Google Scholar] [CrossRef]
- Montes-h, G.; Duplay, J.; Martinez, L.; Mendoza, C. Swelling–shrinkage kinetics of MX80 bentonite. Appl. Clay. Sci. 2003, 22, 279–293. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Pan, Wei. P.; Hunter, D.; Singh, A.; Vaia, R. Thermal Degradation of alkyl quaternary ammonium montmorillonite. Chem. Mater. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]
- Bertuoli, P. T.; Piazza, D.; Scienza, L. C.; Zattera, A. J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay. Sci. 2014, 87, 46–51. [Google Scholar] [CrossRef]
- Goyal, S.; Hernández, N. B.; Cochran, E. W. An update on the future prospects of glycerol polymers. Polym. Int. 2021, 70, 911–917. [Google Scholar] [CrossRef]
- Perez, F. M.; Legarto, C.; Lombardi, M. B.; Santori, G. F.; Pompeo, F.; Nichio, N. N. 2022. Activated Bentonite Nanocomposite for the Synthesis of Solketal from Glycerol in the Liquid Phase. Catal. 2002, 12, 673. [Google Scholar] [CrossRef]
- Hewelke, E.; Szatyłowicz, J.; Hewelke, P.; Gnatowski, T.; Aghalarov, R. The impact of diesel oil pollution on the hydrophobicity and CO 2 efflux of forest soils. Water, Air, Soil Pollut. 2018, 229, 1–11. [Google Scholar] [CrossRef]
- Alzamel, M.; Fall, M.; Haruna, S. Swelling ability and behaviour of bentonite-based materials for deep repository engineered barrier systems: Influence of physical, chemical and thermal factors. J. Rock Mech. Geotech. Eng. 2022, 14, 689–702. [Google Scholar] [CrossRef]


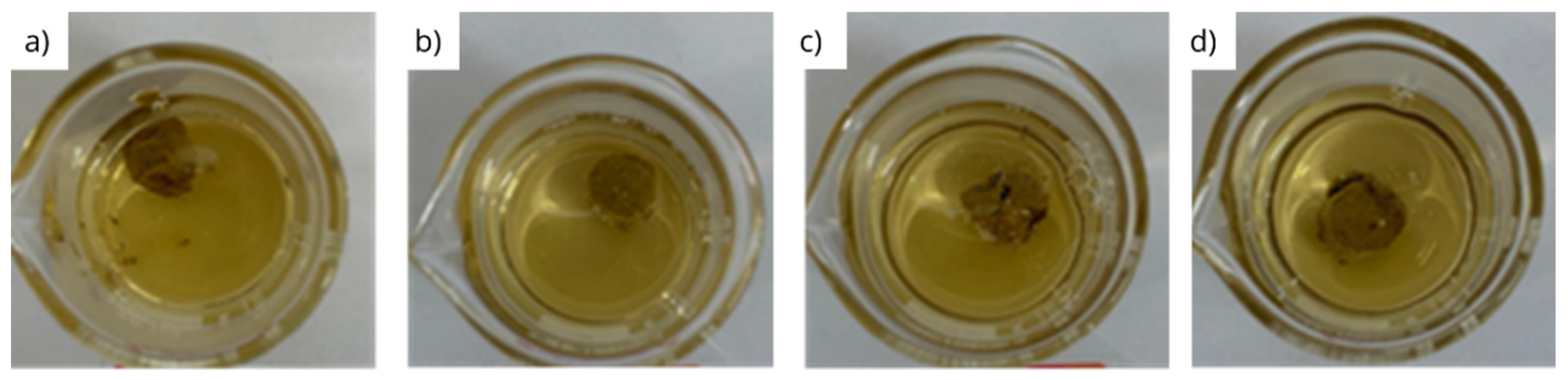
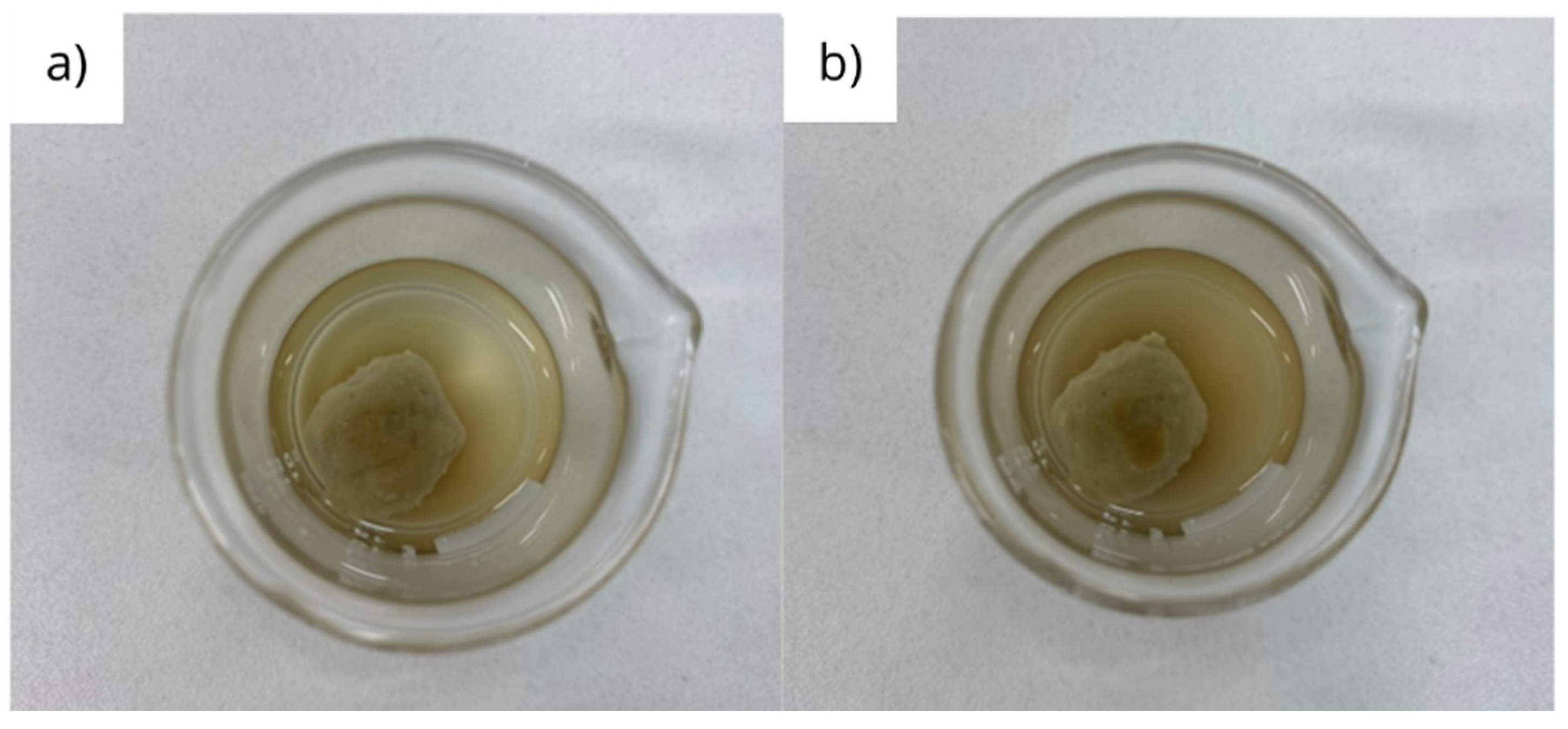
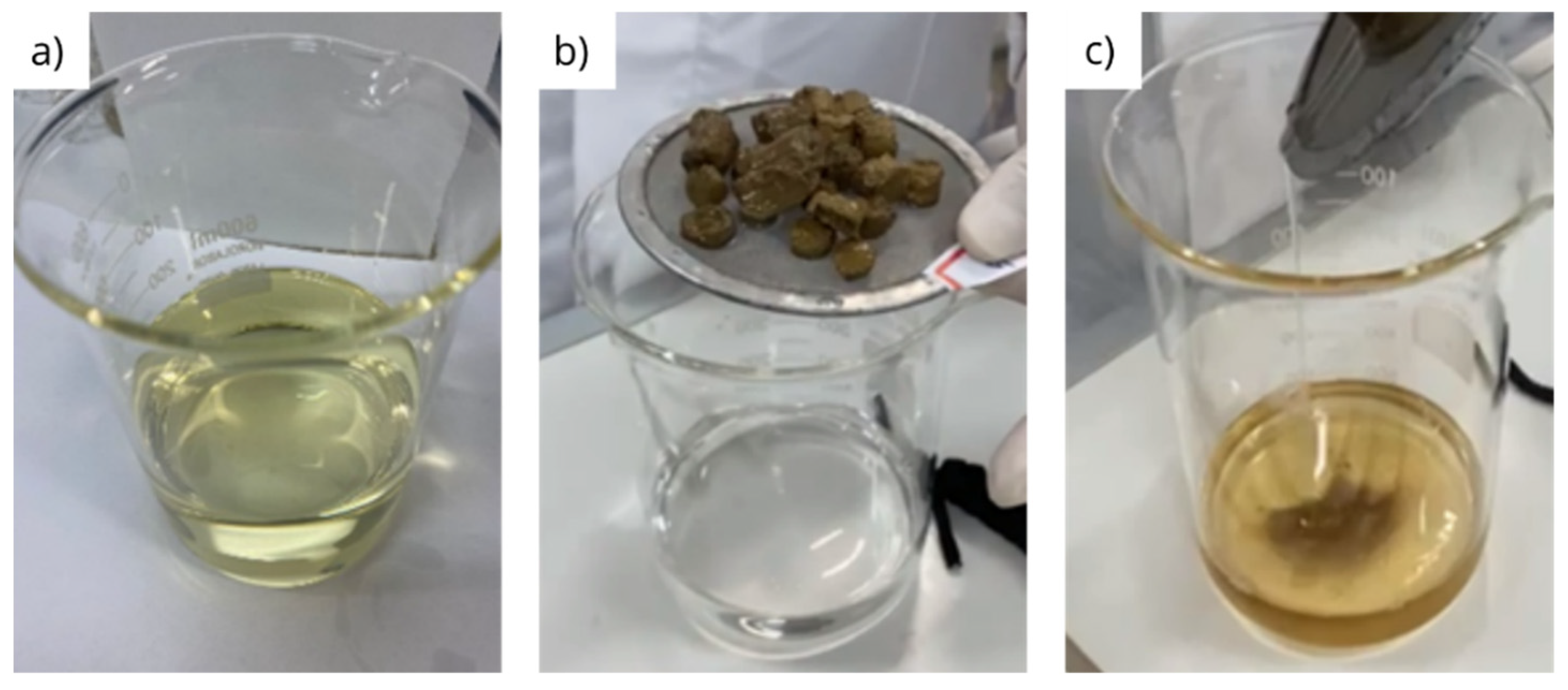
| Fluid | Initial mass (g) | Final mass (g) | Disintegration rate (%) |
|---|---|---|---|
| Diesel | 20.00 | 17.98 | 10.10 |
| Olefin | 20.00 | 17.49 | 12.55 |
| Glycerin | 20.00 | 17.13 | 14.35 |
| Initial mass (g) | Final mass (g) | Moisture loss (%) |
|---|---|---|
| 20.00 | 17.22 | 13.90% |
| Fluid | Initial mass (g) | Mass after immersion in fluid (g) | Final mass (g) | Mass variation (%) |
|---|---|---|---|---|
| Diesel | 20.00 | 20.71 | 17.86 | - 10.70 |
| Olefin | 20.00 | 20.45 | 17.55 | -12.25 |
| Glycerin | 20.00 | 26.06 | 23.57 | +17.85 |
| Fluids | Corrected disintegration rate (%) |
| Diesel | - 0.60 |
| Olefin | 0.24 |
| Glycerin | 31.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





