Submitted:
06 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
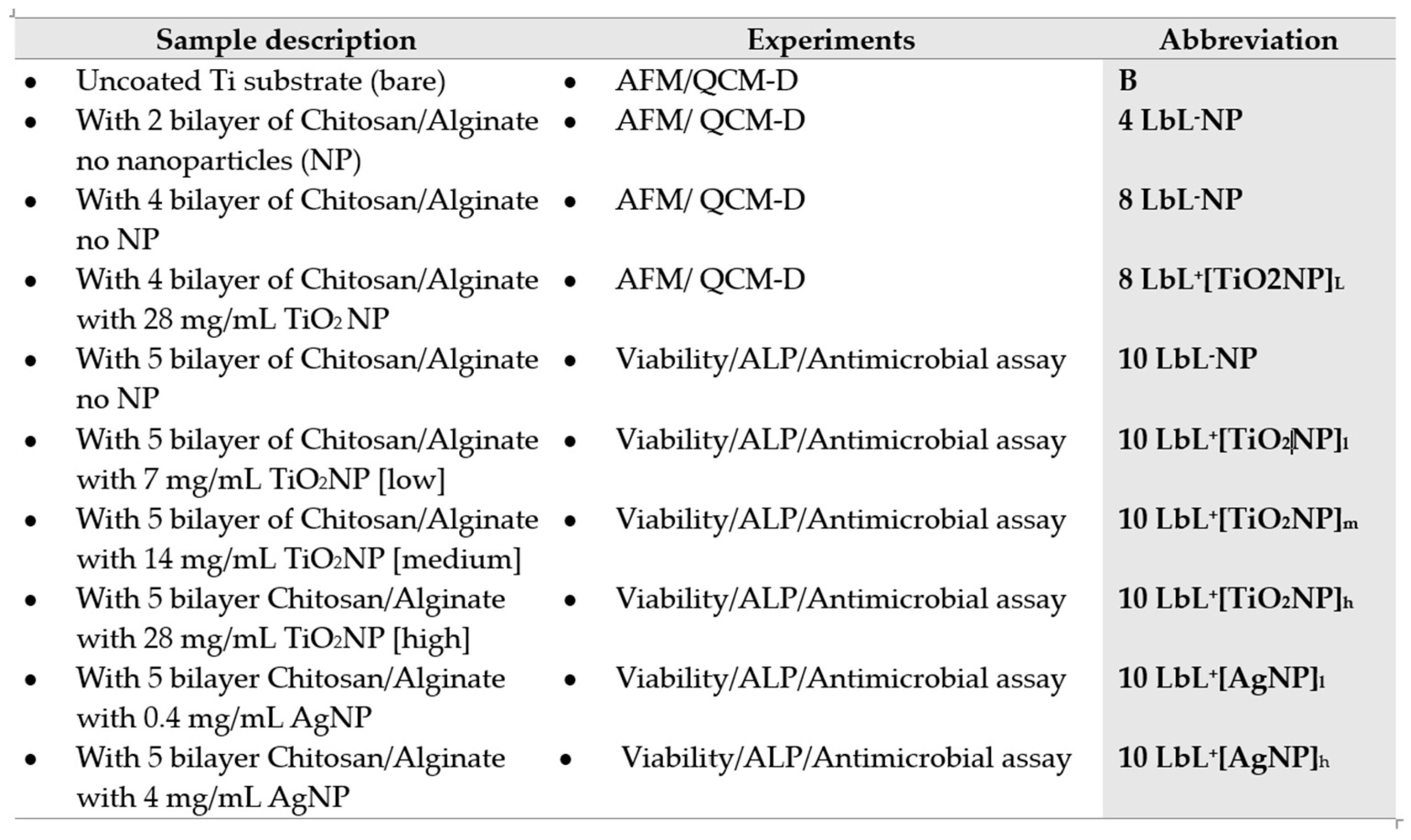
2.1. Materials
2.2. Bacterial strains
2.3. Titanium Substrate Preparation and Surface Functionalization
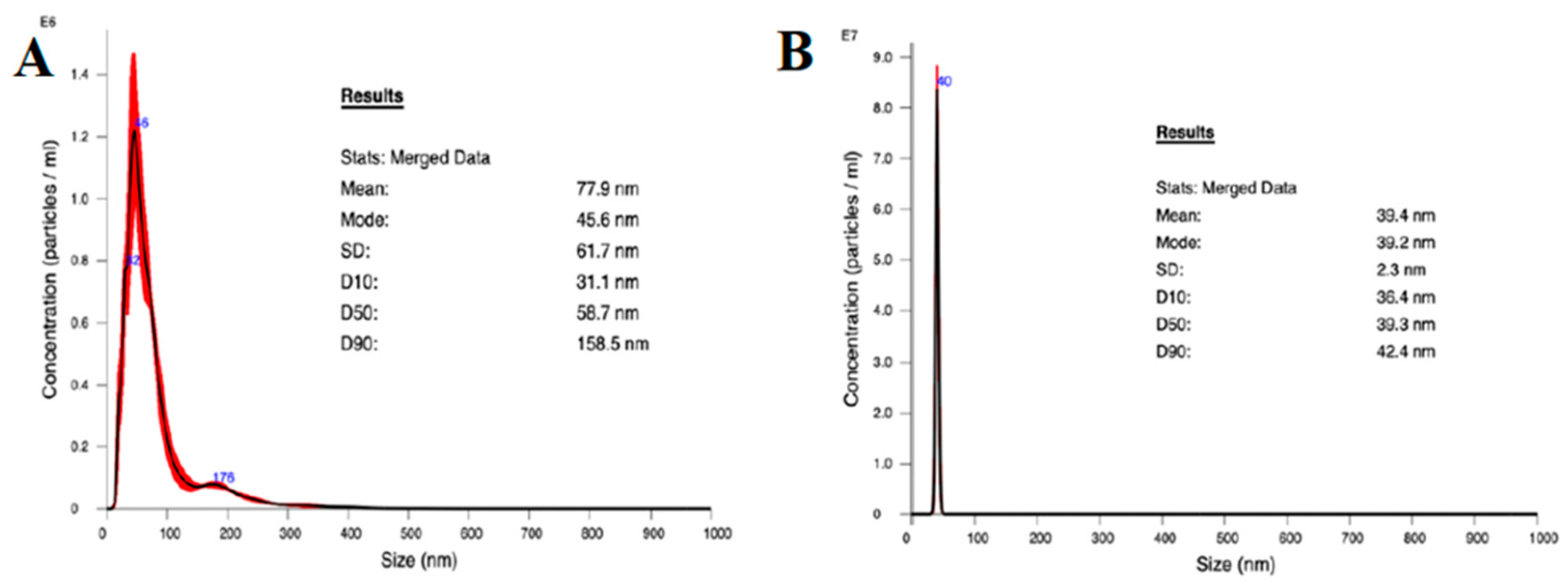
2.4. TiO2 and Ag Nanoparticles Size Measurement
2.5. Preparation of mNP Suspension Prior to Encapsulation in LbL Coating
2.6. Preparation of the Polyelectrolytes for LbL Deposition
2.7. mNP imbedding and LbL Coating Procedures
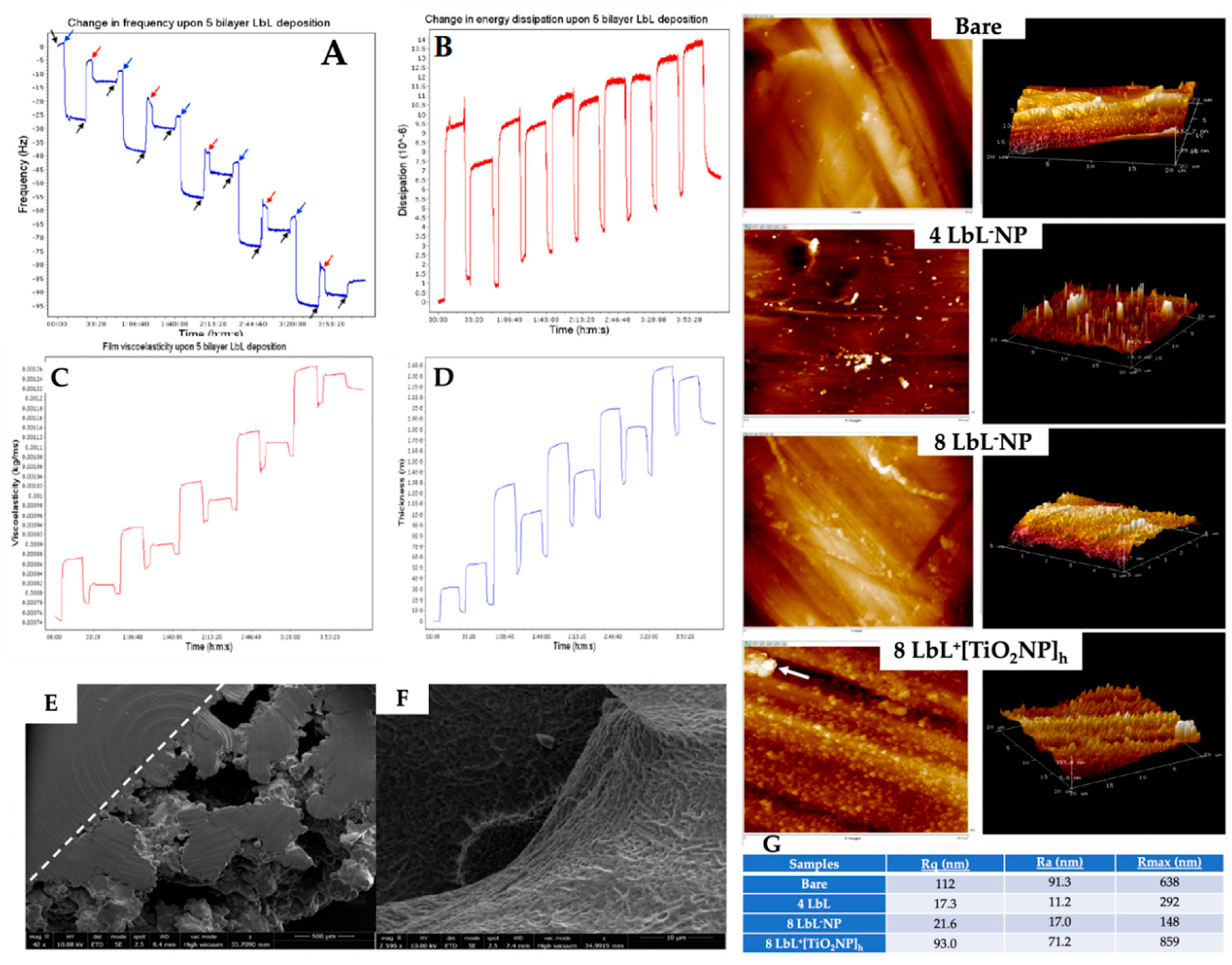
2.8. Investigation of LbL Deposition Using Quartz Crystal Microgravimetry with Dissipation (QCM-D)
2.9. Analysis of Surface Morphology and Roughness of coated Substrates by Microscopy Techniques
2.10. Cytotoxicity Assay
2.11. MC3T3-E1 Viability Assessment by AlamarBlue
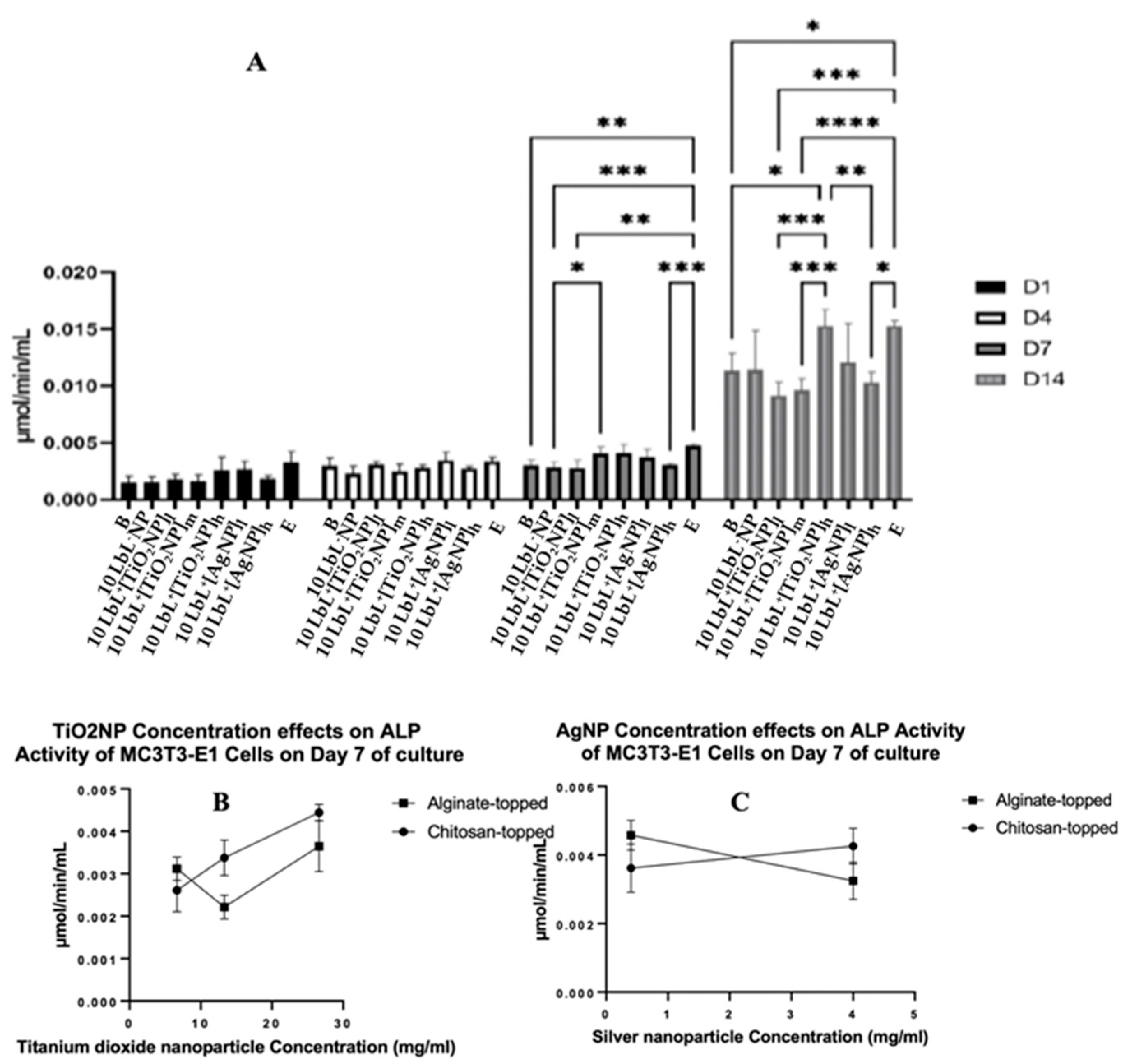
2.12. Assessment of MC3T3-E1 osteogenic differentiation by ALP Activity Analysis
2.13. Antimicrobial Assessment of Coated Ti substrates
2.14. Statistical Analysis
3. Results and discussion
3.1. Nanoparticle Characterization
3.2. Validation of LbL Deposition Using QCM-D and PEM Coating Characterization
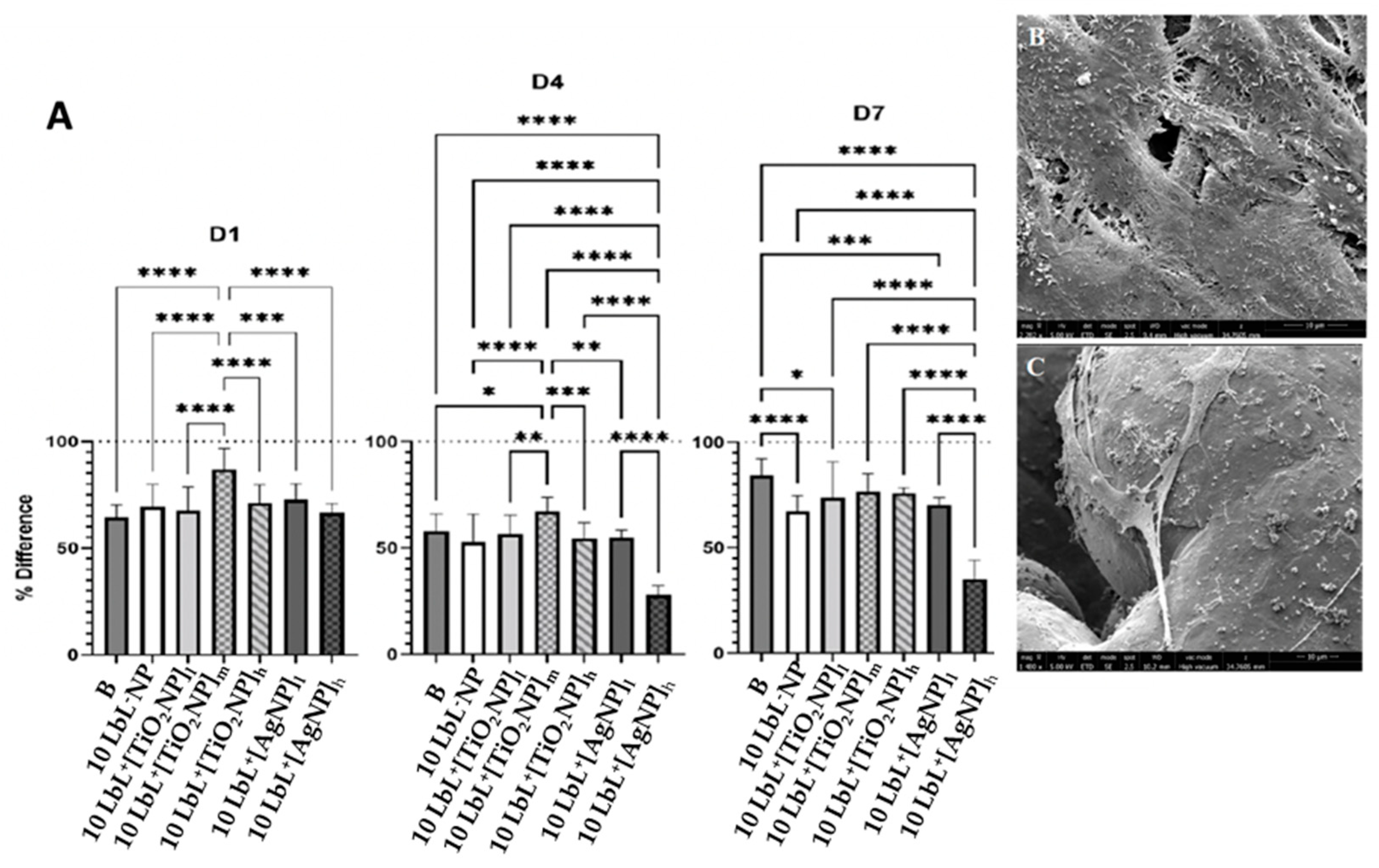
3.3. MC3T3-E1 Viability and Proliferation on PEM Coated Ti Substrates
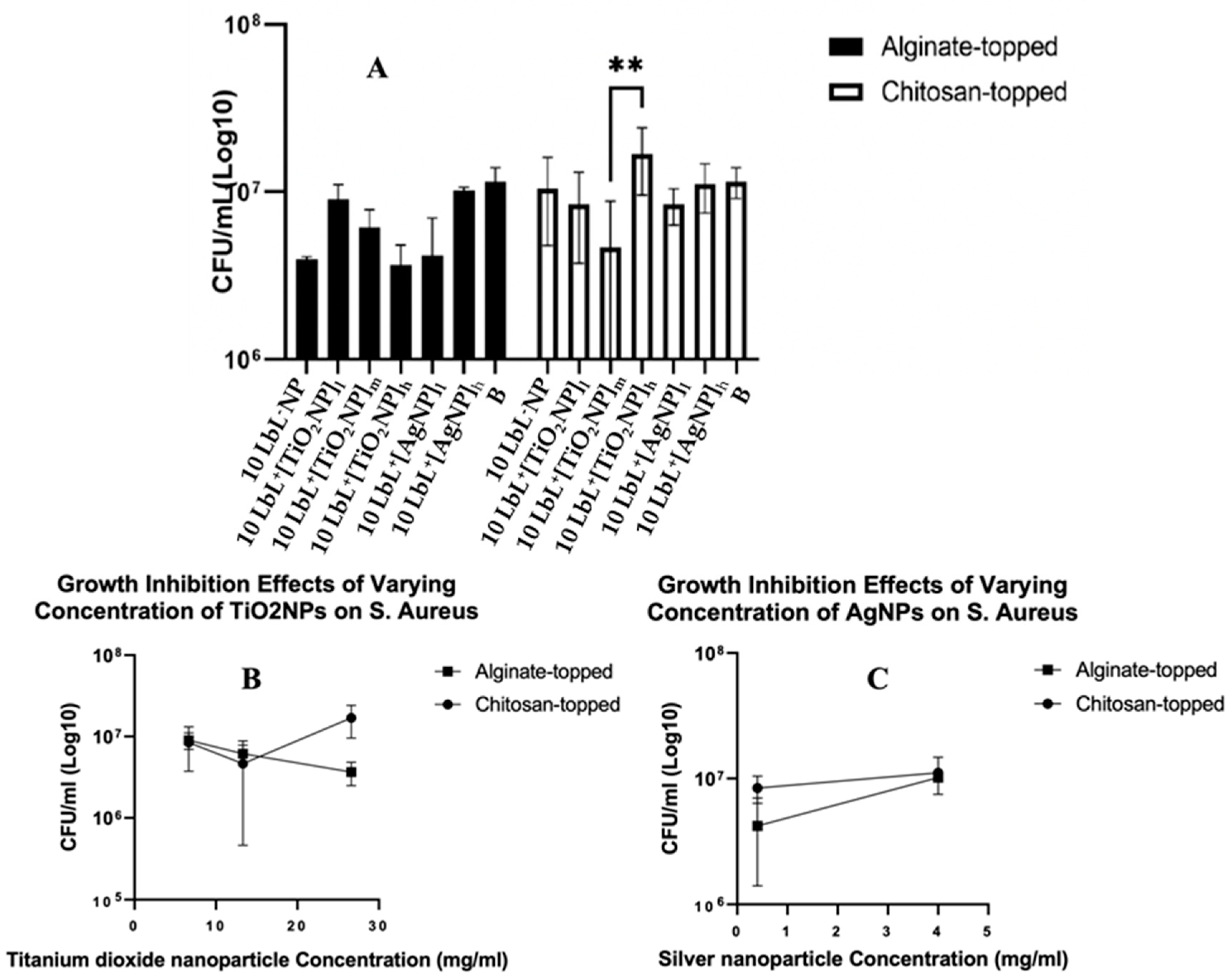
3.4. Antimicrobial activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Data Quality Documentation for Users: Canadian Joint Replacement Registry, 2020–2021 Data.
- Lazic, I.; Scheele, C.; Pohlig, F.; von Eisenhart-Rothe, R.; Suren, C. Treatment Options in PJI – Is Two-Stage Still Gold Standard? J Orthop 2021, 23, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. The Journal of Arthroplasty 2021, 36, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Luthringer, T.A.; Fillingham, Y.A.; Okroj, K.; Ward, E.J.; Della Valle, C. Periprosthetic Joint Infection After Hip and Knee Arthroplasty: A Review for Emergency Care Providers. Annals of Emergency Medicine 2016, 68, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Otto-Lambertz, C.; Yagdiran, A.; Wallscheid, F.; Eysel, P.; Jung, N. Periprosthetic Infection in Joint Replacement. Dtsch Arztebl Int 2017, 114, 347–353. [Google Scholar] [CrossRef]
- Muñoz-Gallego, I.; Meléndez-Carmona, M.Á.; Lora-Tamayo, J.; Garrido-Allepuz, C.; Chaves, F.; Sebastián, V.; Viedma, E. Microbiological and Molecular Features Associated with Persistent and Relapsing Staphylococcus Aureus Prosthetic Joint Infection. Antibiotics 2022, 11, 1119. [Google Scholar] [CrossRef]
- Patel, R. Periprosthetic Joint Infection. New England Journal of Medicine 2023, 388, 251–262. [Google Scholar] [CrossRef]
- Rajput, V.; Meek, R.M.D.; Haddad, F.S. Periprosthetic Joint Infection: What Next? The Bone & Joint Journal, 1193. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus Aureus and Staphylococcus Epidermidis Infections on Implants. Journal of Hospital Infection 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Hays, M.R.; Kildow, B.J.; Hartman, C.W.; Lyden, E.R.; Springer, B.D.; Fehring, T.K.; Garvin, K.L. Increased Incidence of Methicillin-Resistant Staphylococcus Aureus in Knee and Hip Prosthetic Joint Infection. The Journal of Arthroplasty 2023, 38, S326–S330. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Senn, L.; Bertelli, C.; Grandbastien, B.; Steinmetz, S.; Boillat-Blanco, N. Prevalence and Factors Associated with Prosthetic Joint Infections in Patients with Staphylococcus Aureus Bacteraemia: A 7-Year Retrospective Study. Antibiotics 2022, 11, 1323. [Google Scholar] [CrossRef]
- Missiakas, D.M.; Schneewind, O. Growth and Laboratory Maintenance of Staphylococcus Aureus. Current Protocols in Microbiology 2013, 28, 9C–1. [Google Scholar] [CrossRef]
- Rao, Y.; Peng, H.; Shang, W.; Hu, Z.; Yang, Y.; Tan, L.; Li, M.; Zhou, R.; Rao, X. A Vancomycin Resistance-Associated WalK(S221P) Mutation Attenuates the Virulence of Vancomycin-Intermediate Staphylococcus Aureus. Journal of Advanced Research 2022, 40, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus Aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, A.A.; Said, H.S.; Elfeky, S.M.; Shaaban, M.I. Inhibition of Erythromycin and Erythromycin-Induced Resistance among Staphylococcus Aureus Clinical Isolates. Antibiotics 2023, 12, 503. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Beswick, A.D.; Whitehouse, M.R.; Wylde, V.; Blom, A.W. Debridement, Antibiotics and Implant Retention for Periprosthetic Joint Infections: A Systematic Review and Meta-Analysis of Treatment Outcomes. Journal of Infection 2018, 77, 479–488. [Google Scholar] [CrossRef]
- Gramlich, Y.; Parvizi, J. Enough Is Enough: Salvage Procedures in Severe Periprosthetic Joint Infection. Arthroplasty 2023, 5, 36. [Google Scholar] [CrossRef]
- Liukkonen, R.; Honkanen, M.; Skyttä, E.; Eskelinen, A.; Karppelin, M.; Reito, A. Clinical Outcomes After Revision Hip Arthroplasty Due to Prosthetic Joint Infection—A Single-Center Study of 369 Hips at a High-Volume Center With a Minimum of One Year Follow-Up. The Journal of Arthroplasty 2023. [Google Scholar] [CrossRef] [PubMed]
- Olearo, F.; Zanichelli, V.; Exarchakou, A.; Both, A.; Uςkay, I.; Aepfelbacher, M.; Rohde, H. The Impact of Antimicrobial Therapy Duration in the Treatment of Prosthetic Joint Infections Depending on Surgical Strategies: A Systematic Review and Meta-Analysis. Open Forum Infectious Diseases 2023, 10, ofad246. [Google Scholar] [CrossRef]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef]
- Shabana, N.S.; Seeber, G.; Soriano, A.; Jutte, P.C.; Westermann, S.; Mithoe, G.; Pirii, L.; Siebers, T.; Have, B. ten; Zijlstra, W.; et al. The Clinical Outcome of Early Periprosthetic Joint Infections Caused by Staphylococcus Epidermidis and Managed by Surgical Debridement in an Era of Increasing Resistance. Antibiotics 2023, 12, 40. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomaterialia 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Bujdák, J.; Medvecká, V.; Pálková, H.; Barlog, M.; Bujdáková, H. Surface Characterization and Anti-Biofilm Effectiveness of Hybrid Films of Polyurethane Functionalized with Saponite and Phloxine B. Materials 2021, 14, 7583. [Google Scholar] [CrossRef] [PubMed]
- Dadi, N.C. teja; Dohál, M.; Medvecká, V.; Bujdák, J.; Koči, K.; Zahoranová, A.; Bujdáková, H. Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B. Molecules 2021, 26, 325. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.; Zia, A.W.; Devarajan, D.K.; Soni; Panayiotidis, M. I.; Joyce, T.J.; Tambuwala, M.M.; Serrano-Aroca, Á. Multi-Functional Bioactive Silver- and Copper-Doped Diamond-like Carbon Coatings for Medical Implants. Acta Biomaterialia 2023, 167, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, Y.; An, H.; Yi, J.; Li, C.; Wang, X.; Chai, W. Recent Advances in Prevention, Detection and Treatment in Prosthetic Joint Infections of Bioactive Materials. Frontiers in Bioengineering and Biotechnology 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Piñera-Avellaneda, D.; Buxadera-Palomero, J.; Ginebra, M.-P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Surface Competition between Osteoblasts and Bacteria on Silver-Doped Bioactive Titanium Implant. Biomaterials Advances 2023, 146, 213311. [Google Scholar] [CrossRef]
- Elizarova, I.S.; Luckham, P.F. Layer-by-Layer Adsorption: Factors Affecting the Choice of Substrates and Polymers. Advances in Colloid and Interface Science 2018, 262, 1–20. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Pires, R.A.; Reis, R.L.; Alves, N.M. Layer-by-Layer Films Based on Catechol-Modified Polysaccharides Produced by Dip- and Spin-Coating onto Different Substrates. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2020, 108, 1412–1427. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLOS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Marine Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate Composites for Bone Tissue Engineering: A Review. International Journal of Biological Macromolecules 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Thambirajoo, M.; Maarof, M.; Lokanathan, Y.; Katas, H.; Ghazalli, N.F.; Tabata, Y.; Fauzi, M.B. Potential of Nanoparticles Integrated with Antibacterial Properties in Preventing Biofilm and Antibiotic Resistance. Antibiotics 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. International Journal of Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. International Journal of Nanomedicine 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Abo-zeid, Y.; Williams, G.R. The Potential Anti-Infective Applications of Metal Oxide Nanoparticles: A Systematic Review. WIREs Nanomedicine and Nanobiotechnology 2020, 12, e1592. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-Coated Silver Nanoparticles in Biological and Environmental Conditions: Fate, Stability and Toxicity. Advances in Colloid and Interface Science 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Lojk, J.; Repas, J.; Veranič, P.; Bregar, V.B.; Pavlin, M. Toxicity Mechanisms of Selected Engineered Nanoparticles on Human Neural Cells in Vitro. Toxicology 2020, 432, 152364. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Kim, S.; Kim, S.-J.; Song, K.-D. Antimicrobial and Cytotoxicity Properties of Biosynthesized Gold and Silver Nanoparticles Using D. Brittonii Aqueous Extract. Arabian Journal of Chemistry 2022, 15, 104217. [Google Scholar] [CrossRef]
- Chauhan, V.; Dhiman, V.K.; Mahajan, G.; Pandey, A.; Kanwar, S.S. Synthesis and Characterization of Silver Nanoparticles Developed Using a Novel Lipopeptide(s) Biosurfactant and Evaluating Its Antimicrobial and Cytotoxic Efficacy. Process Biochemistry 2023, 124, 51–62. [Google Scholar] [CrossRef]
- Mouriya, G.K.; Mohammed, M.; Azmi, A.A.; Khairul, W.M.; Karunakaran, T.; Amirul, A.-A.A.; Ramakrishna, S.; Santhanam, R.; Vigneswari, S. Green Synthesis of Cicer Arietinum Waste Derived Silver Nanoparticle for Antimicrobial and Cytotoxicity Properties. Biocatalysis and Agricultural Biotechnology 2023, 47, 102573. [Google Scholar] [CrossRef]
- Poon, W.-L.; Alenius, H.; Ndika, J.; Fortino, V.; Kolhinen, V.; Meščeriakovas, A.; Wang, M.; Greco, D.; Lähde, A.; Jokiniemi, J.; et al. Nano-Sized Zinc Oxide and Silver, but Not Titanium Dioxide, Induce Innate and Adaptive Immunity and Antiviral Response in Differentiated THP-1 Cells. Nanotoxicology 2017, 11, 936–951. [Google Scholar] [CrossRef]
- Poon, W.-L.; Lee, J.C.-Y.; Leung, K.S.; Alenius, H.; El-Nezami, H.; Karisola, P. Nanosized Silver, but Not Titanium Dioxide or Zinc Oxide, Enhances Oxidative Stress and Inflammatory Response by Inducing 5-HETE Activation in THP-1 Cells. Nanotoxicology 2020, 14, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; He, S.; Zhou, L.; Yuan, J.; Jiang, B.; Ni, X.; Lu, K.; Zhang, P.; Zhao, Q. A Cytocompatible Microporous Sr-Doped Titanium Dioxide Coating Fabricated by Plasma Electrolytic Oxidation. Frontiers in Materials 2023, 10. [Google Scholar] [CrossRef]
- Hari Raj, K.; Gnanavel, S.; Ramalingam, S. Investigation of 3D Printed Biodegradable PLA Orthopedic Screw and Surface Modified with Nanocomposites (Ti–Zr) for Biocompatibility. Ceramics International 2023, 49, 7299–7307. [Google Scholar] [CrossRef]
- Noreen, S.; Wang, E.; Feng, H.; Li, Z. Functionalization of TiO2 for Better Performance as Orthopedic Implants. Materials 2022, 15, 6868. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaee, S.Y.; Dehnavi, S.M.; Bagheri, R.; Barjasteh, M.; Golizadeh, M.; Zamani, H.; Karimi, A. Boosting Bone Cell Growth Using Nanofibrous Carboxymethylated Cellulose and Chitosan on Titanium Dioxide Nanotube Array with Dual Surface Charges as a Novel Multifunctional Bioimplant Surface. International Journal of Biological Macromolecules 2023, 228, 570–581. [Google Scholar] [CrossRef]
- D’Agostino, A.; Bertolini, M.; Bono, N.; Pavarini, M.; Tarsini, P.; Candiani, G.; De Nardo, L.; Chiesa, R. Antibacterial Titanium Dioxide Coatings for CoCrMo Orthopaedic Implants. Applied Surface Science 2023, 609, 155300. [Google Scholar] [CrossRef]
- Popova, A.D.; Sheveyko, A.N.; Kuptsov, K.A.; Advakhova, D.Yu.; Karyagina, A.S.; Gromov, A.V.; Krivozubov, M.S.; Orlova, P.A.; Volkov, A.V.; Slukin, P.V.; et al. Osteoconductive, Osteogenic, and Antipathogenic Plasma Electrolytic Oxidation Coatings on Titanium Implants with BMP-2. ACS Appl. Mater. Interfaces 2023, 15, 37274–37289. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are Smaller Nanoparticles Always Better? Understanding the Biological Effect of Size-Dependent Silver Nanoparticle Aggregation Under Biorelevant Conditions. International Journal of Nanomedicine 2021, 16, 3021–3040. [Google Scholar] [CrossRef]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Walters, K.B. XPS Study on the Use of 3-Aminopropyltriethoxysilane to Bond Chitosan to a Titanium Surface. Langmuir 2007, 23, 6645–6651. [Google Scholar] [CrossRef]
- Holmes, C.A.; Tabrizian, M. Enhanced MC3T3 Preosteoblast Viability and Adhesion on Polyelectrolyte Multilayer Films Composed of Glycol-Modified Chitosan and Hyaluronic Acid. Journal of Biomedical Materials Research Part A 2012, 100A, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Nayef, L.; Castiello, R.; Tabrizian, M. Washless Method Enables Multilayer Coating of an Aggregation-Prone Nanoparticulate Drug Delivery System with Enhanced Yields, Colloidal Stability, and Scalability. Macromolecular Bioscience 2017, 17, 1600535. [Google Scholar] [CrossRef] [PubMed]
- Bernards, D.A.; Desai, T.A. Nanoscale porosity in polymer films: fabrication and therapeutic applications. Soft Matter. 2010, 6(8), 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yergeshov, A.A.; Al-Thaher, Y.; Avdokushina, S.; Statsenko, E.; Abdullin, T.I.; Prokopovich, P. Nanocomposite orthopaedic bone cement combining long-acting dual antimicrobial drugs. Biomaterials Advances, 2023,153, 213538. doi.org/10.1016/j.bioadv.2023.213538.
- Misra, R.D.K.; Thein-Han, W.W.; Pesacreta, T.C.; Somani, M.C.; Karjalainen, L.P. Biological Significance of Nanograined/Ultrafine-Grained Structures: Interaction with Fibroblasts. Acta Biomaterialia 2010, 6, 3339–3348. [Google Scholar] [CrossRef]
- Yazid, M.D.; Ariffin, S.H.Z.; Senafi, S.; Razak, M.A.; Wahab, R.M.A. Determination of the Differentiation Capacities of Murines’ Primary Mononucleated Cells and MC3T3-E1 Cells. Cancer Cell International 2010, 10, 42. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-Dimensional Porous Scaffold by Self-Assembly of Reduced Graphene Oxide and Nano-Hydroxyapatite Composites for Bone Tissue Engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface Potential and Charges Impact on Cell Responses on Biomaterials Interfaces for Medical Applications. Materials Science and Engineering: C 2019, 104, 109883. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Frontiers in Bioengineering and Biotechnology 2021, 9. [Google Scholar] [CrossRef]
- Shen, J.; Gao, P.; Han, S.; Kao, R.Y.T.; Wu, S.; Liu, X.; Qian, S.; Chu, P.K.; Cheung, K.M.C.; Yeung, K.W.K. A Tailored Positively-Charged Hydrophobic Surface Reduces the Risk of Implant Associated Infections. Acta Biomaterialia 2020, 114, 421–430. [Google Scholar] [CrossRef]
- Lv, H.; Chen, Z.; Yang, X.; Cen, L.; Zhang, X.; Gao, P. Layer-by-Layer Self-Assembly of Minocycline-Loaded Chitosan/Alginate Multilayer on Titanium Substrates to Inhibit Biofilm Formation. J Dent 2014, 42, 1464–1472. [Google Scholar] [CrossRef]
- Rodríguez López, A. de L.; Lee, M.-R.; Ortiz, B.J.; Gastfriend, B.D.; Whitehead, R.; Lynn, D.M.; Palecek, S.P. Preventing S. Aureus Biofilm Formation on Titanium Surfaces by the Release of Antimicrobial β-Peptides from Polyelectrolyte Multilayers. Acta Biomater 2019, 93, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of Chitosan Nano Dressing for Wound Healing: Characterization, in Vitro and in Vivo Studies. International Journal of Biological Macromolecules 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





