1. Introduction
Hypertension, indicated by systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, is a common risk factor for cardiovascular disease and can be both a cause and a consequence of chronic kidney disease (CKD) [
1,
2]. Similarly, environmental exposure to the nephrotoxic metal pollutant cadmium (Cd) is a common problem worldwide [
3]. This metal is found in cigarette smoke, contaminated air, and nearly all food types, staples included, and as such dietary exposure is inevitable for most people [
3]. Women of reproductive age and children are particularly susceptible to Cd-induced nephrotoxicity due to enhanced intestinal absorption of Cd [
3]. A prospective cohort study of residents in a Cd-polluted region of Japan reported a 49% increase in mortality from kidney failure among women who had lifetime Cd intake ≥ 1 g [
4]. Of further note, a rice Cd concentration of 0.27 mg/kg was associated with signs of kidney and bone damage [
5].
The effects of environmental exposure to Cd on blood pressure have been well documented in studies of various populations, including in the U.S. [
6,
7,
8], Canada [
9], China [
10,
11,
12], Korea [
13,
14], and Japan [
15]. A cross-sectional study of U.S. population found there was an increased risk of hypertension due to Cd in non-smoking women [
6], while a prospective cohort study of American Indians suggested a causal role of Cd in hypertension [
8].
The kidney is regarded as the key player in blood pressure control and the principal site of Cd toxicity due to a preferential accumulation of Cd in the proximal tubular epithelium [
16,
17,
18,
19]. As these cells die, Cd complexed with metallothionein (CdMT) is released into tubular lumen and excreted [
17]. Thus, urinary excretion of Cd (E
Cd) signifying cellular cytotoxicity is used widely as a measure of long-term Cd exposure or Cd body burden [
3,
16,
17,
18,
19]. There are only a few studies which have explored the mechanism by which Cd accumulation in the kidney raises blood pressure.
The present study aimed to test the hypothesis increases in blood pressure are the result of a declining glomerular filtration rate (GFR) induced by kidney damage from Cd accumulation. Thus, we quantified changes in SBP and DBP according to Cd exposure levels, indicated by E
Cd and [Cd]
b in those diagnosed with and without hypertension. To obtain a wide range of Cd exposure levels amenable to this dose-response evaluation, we used archived data from 447 participants in two Thai population-based studies conducted in a Cd-contaminated and non-contaminated regions of Thailand. Although reductions in GFR due to Cd nephropathy may also be attributable to glomerular injury, Cd causes damage primarily to tubular cells, which disables glomerular filtration, and ultimately leads to nephron atrophy, glomerulosclerosis, and interstitial inflammation and fibrosis [
3,
17,
18,
19,
20,
21].
2. Materials and Methods
2.1. Study subjects
A total of 447 persons were selected from two population-based studies conducted in a Cd-contaminated area of the Mae Sot District, Tak Province, and a non-contaminated location in the Nakhon-Si-Thammarat Province of Thailand [
22,
23]. The Institutional Ethical Committees of Chiang Mai University and the Mae Sot Hospital approved the study protocol for the Mae Sot group [
23]. The Office of the Human Research Ethics Committee, Walailak University of Thailand, approved the study protocol for the Nakhon Si Thammarat group [
23]. All subjects were provided with details of study objectives, study procedures, benefits, and potential risks, and they all provided written informed consent.
The Cd levels in soils and food crops recorded in a nationwide survey indicated that environmental exposure to Cd in Nakhon Si Thammarat was low [
24]. In comparison, the Cd concentration of the paddy soil samples from the Mae Sot district exceeded the standard of 0.15 mg/kg and the rice samples collected from household storage contained four times the amount of the permissible Cd level of 0.1 mg/kg [
25].
2.2. Blood Pressure and Cadmium Exposure Assessment
The diagnosis of hypertension was based on questionnaires, physician diagnosis or use of antihypertensive medication, and ascertained by a single measurement of SBP and DBP. Assessment of Cd exposure and blood pressure was based on a single measurement of the urinary Cd excretion (ECd) and blood Cd concentration ([Cd]b). Samples of urine and whole blood were collected after overnight fast. Blood samples were collected within 3 h of urine collection. Aliquots of blood and urine samples were stored at −80 °C for later analysis.
Urinary and blood levels of Cd ([Cd]u and [Cd]b) were quantified by atomic absorption spectrophotometry. Multielement standards (Merck KGaA, Darmstadt, Germany) were used for instrument calibration. The quality control and quality assurance of Cd quantitation were accomplished by simultaneous analysis of blood control samples (ClinChek, Munich, Germany) and the reference urine metal controls (Lyphocheck, Bio-Rad, Hercules, CA, USA).
The limit of detection (LOD) for Cd in blood or urine, defined as 3 times the standard deviation of at least 10 sample blank measurements, was 0.3 µg/L for [Cd]
b and 0.1 µg/L for [Cd]
u. The sample blanks and reference standards for urine and blood were included in the assay together with study urine or blood samples. Deionized water was used to zero the instrument. The coefficient of variation for Cd in the reference urine and blood were within acceptable clinical chemistry standards. The Cd concentration assigned to a sample that contained Cd below its LOD was assigned a value of the LOD divided by the square root of 2 [
26].
2.3. Normalization of Cadmium Excretion Rate
As the excreted Cd (E
Cd) derives from the damages and dying tubular epithelial cells, as consequence of Cd accumulation [
17], it is logical to normalize E
Cd to creatinine clearance (C
cr) rather than creatinine excretion (E
cr). C
cr is a surrogate marker of GFR, which is a measurable analogue of nephron number. This normalization to C
cr corrects for differences in urine dilution and the number of functioning nephrons among cohort of participants; they are unaffected by creatinine excretion (E
cr) [
27].
Excretion of Cd (E
Cd) was normalized to C
cr as E
Cd/C
cr = [Cd]
u[cr]
p/[cr]
u, where [Cd]
u = urine concentration of Cd (mass/volume); [cr]
p = plasma creatinine concentration (mg/dL) and [cr]
u = urine creatinine concentration (mg/dL). E
Cd/C
cr was expressed as an amount of Cd excreted per volume of the glomerular filtrate [
27].
ECd was normalized to Ecr as [Cd]u/[cr]u, where [Cd]u = urine concentration of Cd (mass/volume) and [cr]u = urine creatinine concentration (mg/dL). ECd/Ecr was expressed in μg/g creatinine. Ecr-normalization corrects for urine dilution, but it is influenced by differences in the skeletal muscle mass, which has no biologic link to kidney function. The effect of Cd exposure on GFR was obscure, when ECd was normalized to Ecr. The severity of Cd-induced kidney damage was markedly underestimated by this Ecr normalization.
2.4. Estimated Glomerular Filtration Rate (eGFR)
We used the GFR estimating equations, known as the chronic kidney disease epidemiology collaboration (CKD-EPI) equations to compute the estimated GFR (eGFR) [
28]. The CKD-EPI equations have been validated with inulin clearance [
29].
Male eGFR = 141 × [cr]p/0.9Y × 0.993age, where Y = −0.411 if [cr]p ≤ 0.9 mg/dL and Y = −1.209 if [cr]p > 0.9 mg/dL.
Female eGFR = 144 × [cr]p/0.7Y × 0.993age, where Y = −0.329 if [cr]p ≤ 0.7 mg/dL and Y = −1.209 if [cr]p > 0.7 mg/dL.
2.5. Statistical Analysis
Data were analyzed with IBM SPSS Statistics 21 (IBM Inc., New York, NY, USA). The Kruskal–Wallis test was used to assess differences in means across ECd/Ccr tertiles, and the Pearson chi-squared test was used to assess differences in percentages. The one-sample Kolmogorov–Smirnov test was used to identify departures of continuous variables from a normal distribution, and a logarithmic transformation was applied to variables that showed rightward skewing before they were subjected to parametric statistical analysis.
Logistic regression was used to determine the Prevalence Odds Ratio (POR) for hypertension, which was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg [
2]. Multiple linear regression was used to identify variables affecting eGFR, SBP, and DBP. Univariate/covariance analysis with Bonferroni correction in multiple comparisons was used to obtain mean eGFR, mean SBP and mean DBP, adjusted for covariates and interactions. For all tests,
p-values ≤ 0.05 were considered as statistical significance.
3. Results
3.1. Characteristics of study subjects
A total of 447 persons (333 women and 114 men) with a mean age of 51.1 years, were included in this study (
Table 1).
Overall mean [Cd]u and [Cd]b were 4.23 and 2.75 µg/L, respectively. Subjects were grouped according to tertile of the Cd excretion rate [(ECd/Ccr) ×100]. Corresponding mean (ECd/Ccr) ×100 in the low, middle and high tertile groups were 0.38, 2.28 and 6.89 µg/L filtrate, equivalent to ECd/Ecr of 0.48, 3.07 and 8.48 µg/g creatinine. Mean blood Cd concentration [Cd]b in the low, middle and high tertiles of Cd burden were 0.72, 2.37 and 5.14 µg/L, respectively.
The percentages (%) of smoking in the middle (34.9%) and high (42.7%) tertiles were higher than the low (16.2%) ECd/Ccr tertile group. The % hypertension across ECd/Ccr tertiles were similar, but diabetes was more prevalent in the low tertile (39.2%) compared with the middle (3.4%) and high (4.0%) tertile groups. In parallel, low eGFR was more prevalent in the low tertile (10.3%) than in the middle (1.3%) and high (8.7%) tertiles.
Mean age (56.6 years), mean BMI (25.5 kg/m2), mean SBP (134 mmHg) and mean DBP (83 mmHg) in the low tertile group were all statistically higher than the middle and high ECd/Ccr tertile groups. The mean eGFR of 84 mL/min/1.73m2 in the low tertile was 7-12 mL/min/1.73m2 below the mean eGFR values in the middle and high tertiles.
3.2. Hypertension prevalence in relation to Cd burden
We used a logistic regression to identify risk factors for hypertensin (
Table 2).
Six independent variables incorporated in a model were age, BMI, gender, smoking, diabetes, and Cd burden described as, mild, moderate, and heavy. The prevalence odds ratio (POR) for hypertension was associated with two of these six variables; BMI (POR 1.082, 95% CI:1.027-1.140) and a medium Cd burden (POR 2.114, 95% CI: 1.049-4.260). An increase in POR for hypertension was statistically insignificantly in those who had heavy Cd burden (p = 0.092).
Table 3 provides results from an analogous logistic regression analysis, where [Cd]
b was used as a Cd exposure indicator.
POR for hypertension was again associated with the increment of BMI and Cd exposure levels. POR for hypertension rose to 1.083 (95% CI: 1.029-1.140) per every 1 kg/m2 increase in BMI. Compared with [Cd]b < 0.60 µg/L, the POR for hypertension were increased to 2.113 (95% CI: 1.191-3.749) and 1.833 (95% CI: 1.000-3.360) in those with [Cd]b of 0.61-1.69, and 1.70-3.38 µg/L, respectively. An increment of the POR for hypertension in those with the top [Cd]b quartile ([Cd]b > 6.92 µg/L) was not statistically significant (p = 0.082).
3.3. Cd-induced eGFR reductions
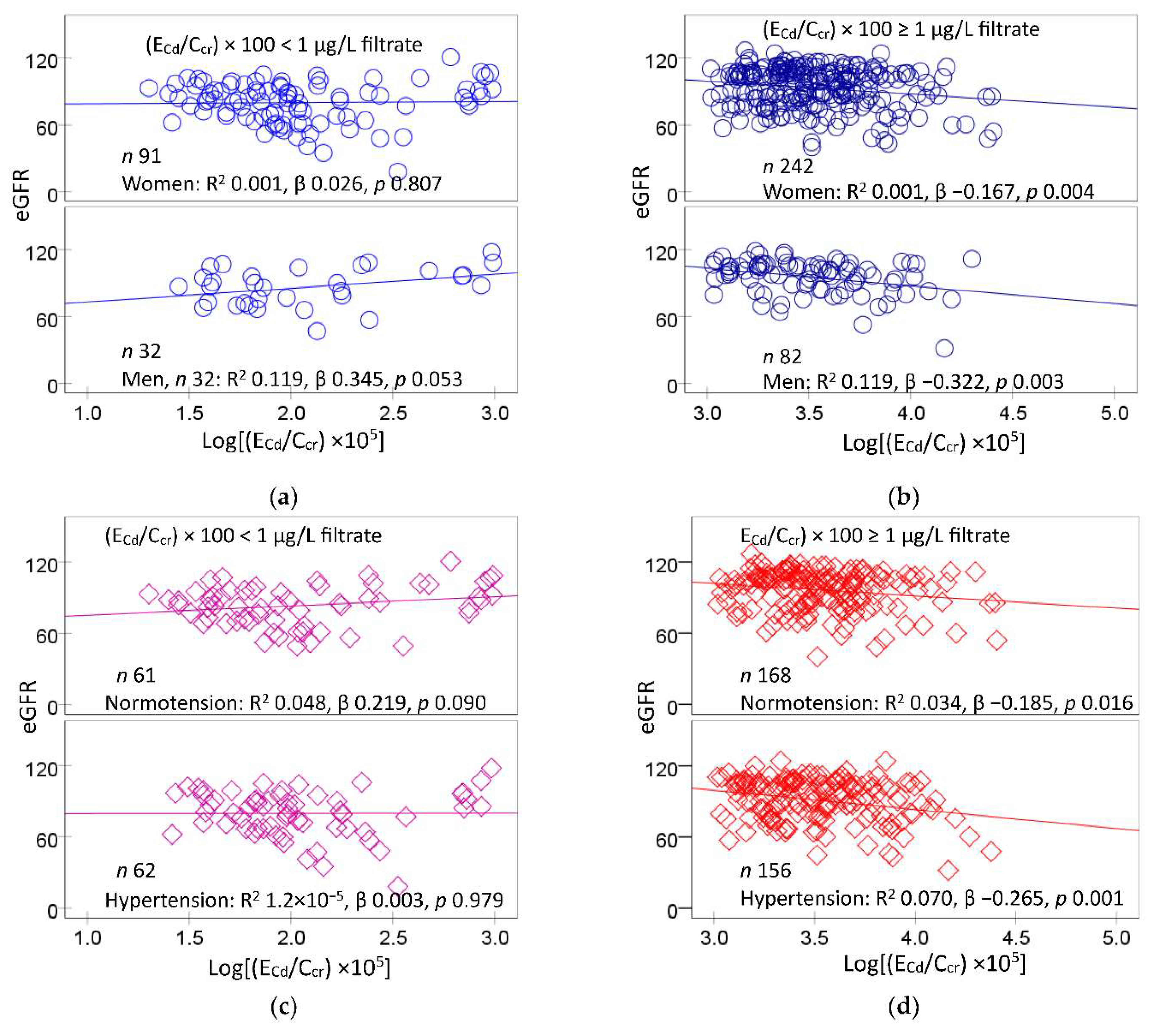
Figure 1 provides scatterplots that related eGFR to the excretion rate of Cd (E
Cd)
A significant inverse dose-response was observed between eGFR and E
Cd/C
cr in women (β = −0.167) and men (β −0.322) with medium plus heavy Cd burdens (
Figure 1b), but not in those a mild Cd burden (
Figure 1a). Similarly, lower eGFR values were associated with higher E
Cd/C
cr values in those with normotension (β = −0.034) and hypertension (β = −0.070) with the medium plus heavy Cd burdens (
Figure 1d). In comparison, eGFR and E
Cd/C
cr were not corelated with each other in the normotensive and the hypertensive groups with a mild Cd burden (
Figure 1c).
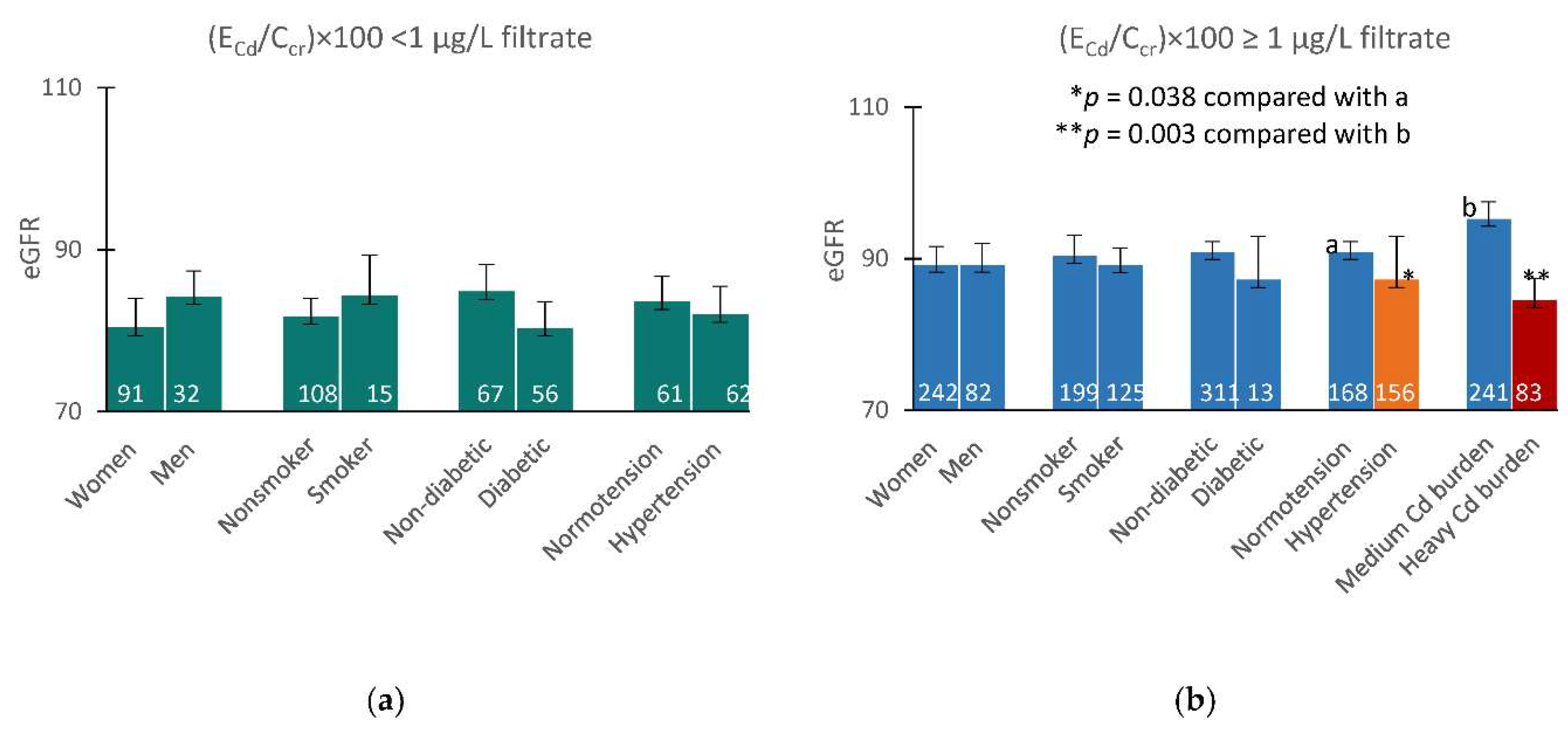
Figure 2 provides results of a quantitative covariance analysis, which indicated that Cd may have decreased eGFR, when (E
Cd/C
cr)×100 rose above 1 µg/L filtrate.
Among those with a mild Cd burden, covariate-adjusted mean eGFR values did not differ in any pairs of subgroups based on gender, smoking status, and diabetes (
Figure 2a). A covariate-adjusted mean eGFR in the hypertensive with medium plus heavy Cd burdens, was 7.5 mL/min/1.73m
2 lower (
p = 0.038), compared with the normotensive who had the same overall Cd burden (
Figure 2b). Furthermore, a covariate-adjusted mean eGFR in those with heavy Cd burden was 10.7 mL/min/1.73m
2 lower, compared with the medium Cd burden group (
p = 0.003) (
Figure 2b).
Table 4 provides results of eGFR regressions that incorporated age, BMI, log
2[(E
Cd/C
cr)×10
5], gender, hypertension, smoking, and diabetes as the independent variables.
Age, BMI, log2[(ECd/Ccr) ×105], gender, hypertension, smoking, and diabetes as contributed, respectively to 27.9%, 24.8%, 31.8% and 24.2% the variation in eGFR in women, men, the normotensive and hypertensive groups.
In women, lower eGFR values were associated with older age (β =−0.528) higher ECd/Ccr (β = −0.121), and diabetes (β = −0.133). In men, lower eGFR values were associated with older age (β =−0.505) and hypertension (β =−0.203). In the normotensive group, eGFR was inversely associated with age (β =−0.559) while showing a positive association with smoking. In the hypertensive group, eGFR was inversely associated with age (β =−0.517), ECd/Ccr (β = −0.177), and diabetes (β = −0.175).
3.4. Inverse relationships between blood pressure and eGFR
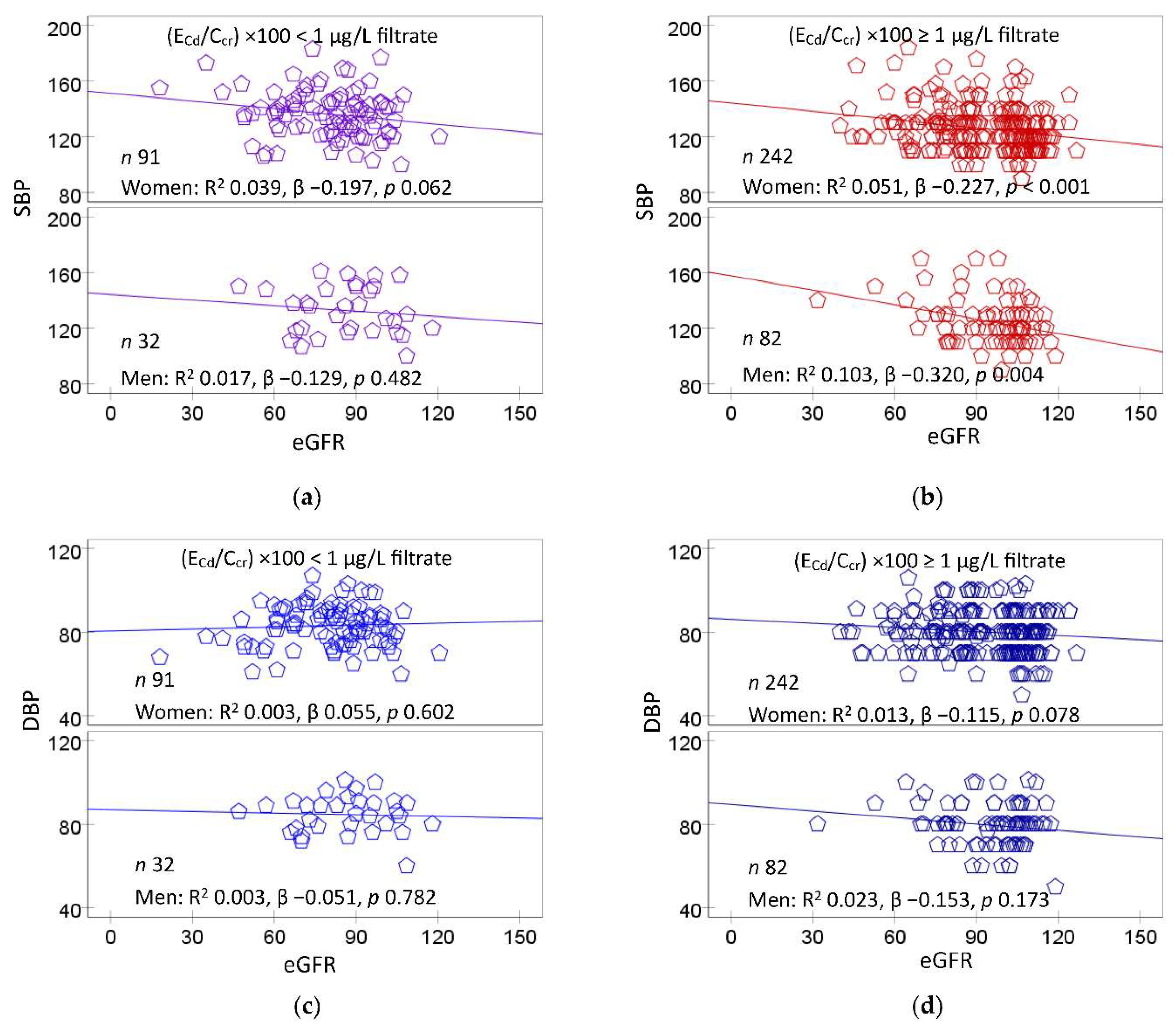
Figure 3 provides scatterplots relating blood pressure measurements to eGFR.
A significant inverse dose-response relationship was observed between SBP and eGFR in women (β = −0.227) and men (β −0.320) of the medium plus heavy Cd burden group (
Figure 3b), but not in those with a mild Cd burden (
Figure 3a). In comparison, DBP did not show a significant correlation with eGFR in women or men in any Cd burden groups (
Figure 3c and
Figure 3d).
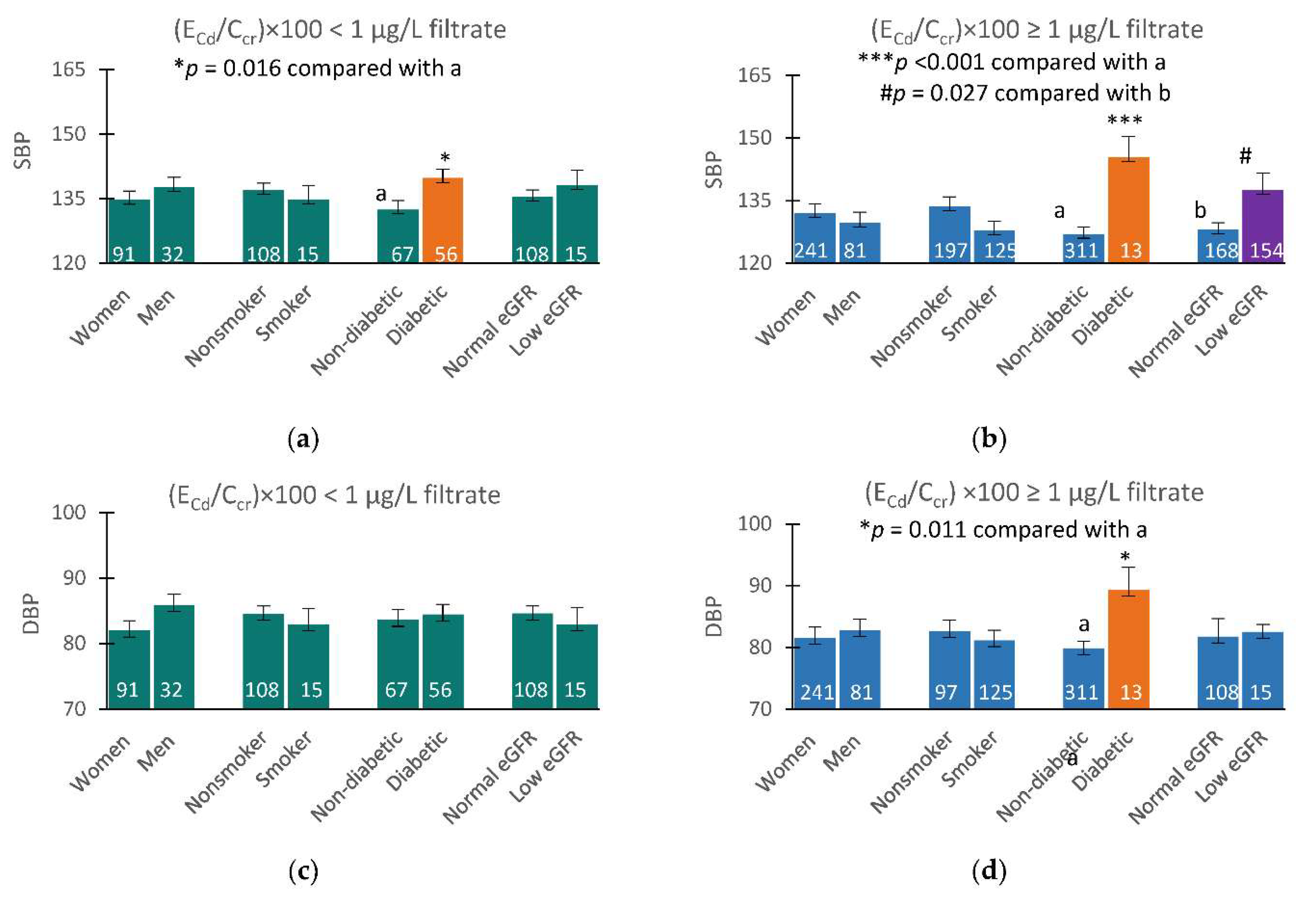
Figure 4 provides results of covariance analysis where an increased mean SBP appeared to be related to Cd burden, eGFR groups, and diabetes.
Mean SBP in those who had low eGFR and medium plus heavy Cd burdens was 9.5 mmHg higher than those with a normal eGFR with medium plus heavy Cd burdens (
Figure 3b). At the mild Cd burden level, mean SBP in those with low and normal eGFR did not differ, but the mean SBP in diabetics was 7.4 mmHg higher than those without diabetes (
Figure 3a). At the medium plus high Cd burden, mean SBP in those with diabetes was increased further to 18.4 mmHg higher than those without diabetes (
Figure 3b).
Mean DBP values among subgroups did not appear to be affected by Cd burden or eGFR groups, but the covariate adjusted mean DBP in diabetics with medium plus high Cd burden was 9.5 mmHg higher, compared with those without diabetes.
3.5. Regression analysis of blood pressure increases
Table 5 provide results of a multiple linear regression analysis to confirm the interrelationships of Cd burden, eGFR, and blood pressure.
In this regression, age, BMI, log2[(ECd/Ccr)×105], eGFR, gender, hypertension, smoking, and diabetes were incorporated as independent variables. These eight variables contributed, respectively to 19.9%, 15.7%, and 15.0% of the variation in SBP in all subjects, the mild Cd burden, and medium plus heavy Cd burden groups. Corresponding fractional DBP variation explained by these independent variables were 4.6%, 0% and 5.8%.
In an inclusive analysis, higher SBP values were associated with older age (β = 0.243), higher BMI (β = 0.113), lower eGFR (β = −0.106) and diabetes (β = 0.216). In the mild Cd burden group, SBP showed positive associations with BMI (β = 0.395) and diabetes (β = 0.202). In the medium plus high Cd burden group, SBP showed a significant association with diabetes (β = 0.265), while showing an inverse association with eGFR (β = −0.176).
In an equivalent inclusive analysis, higher DBP values were associated only with higher BMI values (β = 0.123). In the medium plus high Cd burden group only, higher DBP values were associated with higher BMI (β = 0.123), lower eGFR (β = −0.130), and diabetes (β = 0.193).
4. Discussion
Among 447 study subjects, nearly half (48.8%) had diagnosed hypertension, while 15.4% and 6.9% had diabetes and low eGFR, respectively. The prevalence of low eGFR in our study was in line with a Taiwanese population study (6.3%) [
30]. However, the % of hypertension and diabetes in the present study were higher than those recorded in studies from U.S. where % hypertension and diabetes were 39% and 10.3%-13%, respectively [31, 32]. Mean Cd excretion rate was 3.20 µg/L filtrate, corresponding to 4.03 µg/g creatinine. The mean blood and urinary Cd concentrations were 2.75 and 4.23 µg/L, respectively. High numbers of hypertensive and diabetic cases along with a wide range of Cd exposure levels provided a scenario for a dose-response and mechanistic analyses with a high degree of statistical certainty.
An increased risk of hypertension was associated with both urinary and blood Cd levels (
Table 2 and
Table 3). POR for hypertension rose two-fold in the medium burden group and those with the blood Cd quartiles 2 and 3. In contrast, the increases in POR for hypertension in those with heavy burden of Cd (POR 1.66) and those with the top blood Cd quartile (POR 1.80) were not statistically significant. A stronger effect of a low-dose Cd on blood pressure increases, compared to a high dose, confirm observations made previously, some of which are recapitulated below.
In a Chinese case-control study, a 1.33-fold increase in the risk of hypertension was associated with urinary Cd levels > 1.07 μg/L [
11]. In the present study, a two-fold rise in POR for hypertension was observed in the medium burden group with urinary Cd levels > 0.57 µg/L (
Table 2). A 2.6-fold increase in risk of hypertension was seen in white and Mexican-American women who had blood Cd levels ≥ 0.4 µg/L [
6]. In the present study, an increase in POR for hypertension was found in those who had blood Cd of 0.61−3.38 µg/L (
Table 3). In study from Korea, increases in the prevalence of pre-hypertension and hypertension were associated with doubling blood Cd from 0.62 to 1.33 µg/L in men, and from 0.73 to 1.57 µg/L in women [
13]. In a study of residents in a Cd-polluted area of China, increased risk of hypertension was associated with blood Cd of 1–1.7 µg/L [
10].
In a Canadian study, SBP and DBP were positively associated with blood Cd, but the risk of hypertension fell 52% in women who were current smokers [
9]. A similar observation was made in a U.S. population study, where associations between blood pressure measurements and blood Cd were particularly strong in non-smokers, moderate in former smokers, and weak or negligible in current smokers [
6].
Apparently, urinary and blood Cd levels found to be associated with a significant increase in risk of hypertension varies among populations. These data could be interpreted to suggest differences in susceptibility to hypertension among populations. For instance, white and Mexican-American women were found to be more susceptible to apparent effects of Cd on blood pressure than black women; an increased risk of hypertension was seen in Caucasian (OR 1.54) and Mexican-American women (OR 2.38) who had blood Cd as little as 0.4 µg/L, but not in black women or white, black, or Mexican-American men [
7].
Gender differences in susceptibility to the effect of Cd on blood pressure was also evident in the present study. In a regression analysis (
Table 4), an inverse association of eGFR and Cd burden was found only in women (β = −0.121). This was additional to its inverse associations with age (β =−0.528) and diabetes (β = −0.133). In men, eGFR did not show a significant association with a Cd burden indicator (β = −0.077), but it was inversely associated with age (β =−0.505) and hypertension (β =−0.203). In a Taiwanese study, an association of urinary Cd and a marker of tubular damage, N-acetyl-β-D-glucosaminidase (NAG), was observed in women only [
33]. In another study from Thailand, risk of hypertension rose 11% as urinary Cd increased from 0.39 to 1.12 µg/L, and that risk was further increased from to 20% in those with an elevated NAG excretion level [
34].
The independent effects of Cd exposure, diabetes and hypertension on GFR became apparent in a covariance analysis (
Figure 2). Among those with a mild Cd burden, covariate adjusted mean eGFR did not differ in any pairs of subgroups (
Figure 2a). The effects of hypertension and Cd exposure on eGFR were observed among those with medium and heavy Cd burdens. The hypertensive with medium plus heavy Cd burdens had mean eGFR 7.5 mL/min/1.73 m
2 lower, compared with the normotensive who had the same overall Cd burden (
p = 0.038) (
Figure 2b). Similarly, those with a medium Cd burden had mean eGFR 10.7 mL/min/1.73m
2 lower than those with heavy Cd burden (
p = 0.003).
Of note, blood, urinary Cd levels found to be associated with increased risk of hypertension, detailed above, were in ranges with those showing an association with diabetes. For instance, a dose-response meta-analysis of pooled data from 42 studies indicated that risks of prediabetes and diabetes increased linearly with Cd exposure levels; prediabetes risk reached a plateau at Cd excretion rate of 2 µg/g creatinine, and diabetes risk rose as blood Cd reached 1 µg/L [
35]. A covariance analysis (
Figure 3) has revealed independent effects of Cd and diabetes on blood pressure increases. The mean SBP in diabetics with a Cd burden was 7.4 mmHg higher than those without diabetes all of which had a low Cd burden (
Figure 3a). In comparison, mean SBP in diabetics who had medium plus heavy Cd burdens was 18.4 mmHg higher than those without diabetes (
Figure 3b). Furthermore, while increases in SBP were found in both non-diabetes and diabetes, a significant rise in DBP was seen only in diabetics with medium plus heavy Cd burdens (
Figure 4d). A potential involvement of circulating β
2-microglobulin (β
2M) in the pathogenesis of hypertension in Cd-exposed subjects with diabetes has been identified in a recent case-control study [
36]. In the Framingham Heart Study (
n = 7065), top plasma β
2M quartile was associated with 1.3-fold and 1.6-fold increases in prevalence and incidence of new-onset hypertension, compared with the bottom quartile [
37].
To the best of our knowledge, the present study has provided, for the first time, evidence linking Cd-induced eGFR reduction to a rise in blood pressure, especially SBP (
Figure 3). SBP was inversely associated with eGFR in women (β = −0.227) and men (β = −0.320) who had medium plus heavy Cd burdens. Like SBP, DBP showed an inverse association with eGFR, but such association was weak and statistically insignificant (
Figure 3a,
Figure 3b vs.
Figure 3c,
Figure 3d). A covariance analysis confirmed an inverse relationship between SBP and eGFR (
Figure 4). Subjects with medium plus heavy Cd burdens and low eGFR had mean SBP 9.5 mmHg higher than those with a normal eGFR and the same overall Cd burden (
Figure 3b).
Herein, we have shown that increases in blood pressure may be a consequence of a sustained decrease in GFR due to the cytotoxicity of Cd and nephron destruction. The indispensable role of kidney in blood pressure regulation is well known [
1]; as its function is declining, less water and sodium would be eliminated by the kidney, which may increase blood pressure. Rats with Cd-induced hypertension showed increased sodium retention and reduced sodium excretion [
38,
39,
40]. Thus, increased tubular avidity for filtered sodium appeared to be a possible mechanism by which lifelong, low-dose Cd intake causes hypertension. Previously, we have shown that Cd may increase the synthesis of a second messenger produced in kidneys, 20-hydroxyeicosatetraenoic acid (20-HETE), which regulates salt re-absorption, vascular tone, and volume homeostasis in response to Cd toxic accumulation [41, 42]. However, it remains to be resolved whether 20-HETE is a mediator or a mitigator of Cd-induced hypertension.
Figure 1.
Cadmium as a predictor of eGFR decline. Scatterplots relate eGFR to log[(ECd/Ccr) ×105] in participants grouped by gender (a,b), blood pressure status (c,d), and cadmium burden; (ECd/Ccr)×100 < 1 and ≥ 1 µg/L filtrate. Coefficients of determination (R2) and standardized β-coefficients for all scatterplots, numbers of participants in subgroups, and p-values are provided.
Figure 1.
Cadmium as a predictor of eGFR decline. Scatterplots relate eGFR to log[(ECd/Ccr) ×105] in participants grouped by gender (a,b), blood pressure status (c,d), and cadmium burden; (ECd/Ccr)×100 < 1 and ≥ 1 µg/L filtrate. Coefficients of determination (R2) and standardized β-coefficients for all scatterplots, numbers of participants in subgroups, and p-values are provided.
Figure 2.
Effects of cadmium burden and other variables on eGFR. Bar graphs depict mean eGFR in participants with (ECd/Ccr) × 100 < 1 and ≥ 1 µg/L filtrate (a,b) stratified by gender, smoking status, diabetes, and blood pressure status. Mean eGFR values were obtained via univariate covariance analysis with adjustment for covariates; age, BMI, gender, smoking, hypertension, diabetes and interactions.
Figure 2.
Effects of cadmium burden and other variables on eGFR. Bar graphs depict mean eGFR in participants with (ECd/Ccr) × 100 < 1 and ≥ 1 µg/L filtrate (a,b) stratified by gender, smoking status, diabetes, and blood pressure status. Mean eGFR values were obtained via univariate covariance analysis with adjustment for covariates; age, BMI, gender, smoking, hypertension, diabetes and interactions.
Figure 3.
eGFR as a predictor of blood pressure increases. Scatterplots relate SBP (a,b) and DBP (c,d) to eGFR in women and men with (ECd/Ccr)×100 of < 1 and ≥ 1 µg/L filtrate. Coefficients of determination (R2) and standardized β-coefficients for all scatterplots, numbers of participants in subgroups, and p-values are provided.
Figure 3.
eGFR as a predictor of blood pressure increases. Scatterplots relate SBP (a,b) and DBP (c,d) to eGFR in women and men with (ECd/Ccr)×100 of < 1 and ≥ 1 µg/L filtrate. Coefficients of determination (R2) and standardized β-coefficients for all scatterplots, numbers of participants in subgroups, and p-values are provided.
Figure 4.
Effects of cadmium burden and other variables on blood pressure. Bar graphs represent mean SBP (a,b) and DBP (c,d) in participants with (ECd/Ccr) × 100 < 1 and ≥ 1 µg/L filtrate. Mean SBP and mean DBP values were obtained via univariate covariance analysis with adjustment for covariates; age, BMI, gender, smoking, diabetes, eGFR levels, and interactions.
Figure 4.
Effects of cadmium burden and other variables on blood pressure. Bar graphs represent mean SBP (a,b) and DBP (c,d) in participants with (ECd/Ccr) × 100 < 1 and ≥ 1 µg/L filtrate. Mean SBP and mean DBP values were obtained via univariate covariance analysis with adjustment for covariates; age, BMI, gender, smoking, diabetes, eGFR levels, and interactions.
Table 1.
Descriptive characteristics of study subjects according to cadmium burden tertiles.
Table 1.
Descriptive characteristics of study subjects according to cadmium burden tertiles.
| Parameters |
All, n = 447 |
(ECd/Ccr) ×100 tertiles |
p |
| Low, n =148 |
Middle, n =149 |
High, n = 150 |
| Age, years |
51.1 ± 8.6 |
56.6 ± 9.7 |
48.1 ± 6.9 |
48.7 ± 6.1 |
<0.001 |
| BMI, kg/m2
|
24.8 ± 4.0 |
25.5 ± 4.5 |
24.8 ± 3.8 |
24.0 ± 3.4 |
0.006 |
| eGFR a, mL/min/1.73m2
|
90 ± 18 |
84 ± 18 |
96 ± 17 |
91 ± 18 |
<0.001 |
| % eGFR ≤ 60 mL/min/1.73m2
|
6.9 |
10.3 |
1.3 |
8.7 |
0.005 |
| % Hypertension |
48.8 |
51.4 |
46.3 |
48.7 |
0.685 |
| % Smoking |
31.1 |
16.2 |
34.9 |
42.7 |
<0.001 |
| % Diabetes |
15.4 |
39.2 |
3.4 |
4.0 |
<0.001 |
| Systolic blood pressure, mmHg |
128 ± 17 |
134 ± 17 |
126 ± 16 |
126 ± 16 |
<0.001 |
| Diastolic blood pressure, mmHg |
81 ± 10 |
83 ± 10 |
80 ± 10 |
80 ± 11 |
0.019 |
| [cr]p, mg/dL |
0.82 ± 0.22 |
0.86 ± 0.25 |
0.77 ± 0.17 |
0.83 ± 0.23 |
0.001 |
| [cr]u, mg/dL |
114 ± 74 |
113 ± 72 |
131 ± 72 |
99 ± 75 |
<0.001 |
| [Cd]b, µg/L |
2.75 ± 3.19 |
0.72 ± 0.83 |
2.37 ± 2.06 |
5.14 ± 3.95 |
<0.001 |
| [Cd]u, µg/L |
4.23 ± 5.68 |
0.71 ± 1.20 |
3.91 ± 2.50 |
8.03 ± 7.86 |
<0.001 |
| Normalized to Ecr (ECd/Ecr) b
|
|
|
|
|
|
| ECd/Ecr, µg/g creatinine |
4.03 ± 4.42 |
0.48 ± 0.62 |
3.07 ± 0.93 |
8.48 ± 4.87 |
<0.001 |
| Normalized to Ccr, (ECd/Ccr) c
|
|
|
|
|
|
| (ECd/Ccr) ×100, µg/L filtrate |
3.20 ± 3.73 |
0.38 ± 0.46 |
2.28 ± 0.56 |
6.89 ± 4.31 |
<0.001 |
Table 2.
Prevalence odds ratios for hypertension in relation to relation to cadmium burden and other independent variables.
Table 2.
Prevalence odds ratios for hypertension in relation to relation to cadmium burden and other independent variables.
| Independent Variables/Factors |
Hypertension |
| β coefficients |
POR |
95% CI |
p |
| (SE) |
|
Lower |
Upper |
|
| Age, years |
0.023 (0.014) |
1.024 |
0.997 |
1.051 |
0.085 |
| BMI, kg/m2
|
0.079 (0.027) |
1.082 |
1.027 |
1.140 |
0.003 |
| Gender |
−0.070 (0.260) |
0.932 |
0.560 |
1.551 |
0.788 |
| Smoking |
−0.444 (0.250) |
0.642 |
0.393 |
1.048 |
0.076 |
| Diabetes |
0.575 (0.329) |
1.777 |
0.932 |
3.388 |
0.081 |
| Cd burden a
|
|
|
|
|
|
| Mild |
Referent |
|
|
|
|
| Moderate |
0.748 |
2.114 |
1.049 |
4.260 |
0.036 |
| Heavy |
0.504 |
1.655 |
0.921 |
2.973 |
0.092 |
Table 3.
Prevalence odds ratios for hypertension in relation to blood cadmium quartiles.
Table 3.
Prevalence odds ratios for hypertension in relation to blood cadmium quartiles.
| Independent Variables/Factors |
Hypertension |
| β coefficients |
POR |
95% CI |
p |
| (SE) |
|
Lower |
Upper |
|
| Age, years |
0.018 (0.012) |
1.018 |
0.994 |
1.042 |
0.148 |
| BMI, kg/m2
|
0.080 (0.026) |
1.083 |
1.029 |
1.140 |
0.002 |
| Gender |
−0.050 (0.254) |
0.951 |
0.578 |
1.565 |
0.844 |
| Smoking |
−0.433 (0.255) |
0.649 |
0.394 |
1.069 |
0.089 |
| Diabetes |
0.422 (0.294) |
1.526 |
0.858 |
2.713 |
0.150 |
| Quartile of [Cd]b, µg/L |
|
|
|
|
|
| Q1: < 0.60 |
Referent |
|
|
|
|
| Q2: 0.61−1.69 |
0.748 (0.293) |
2.113 |
1.191 |
3.749 |
0.011 |
| Q3: 1.70−3.38 |
0.606 (0.309) |
1.833 |
1.000 |
3.360 |
0.050 |
| Q4: >3.38 |
0.587 (0.337) |
1.798 |
0.928 |
3.482 |
0.082 |
Table 4.
Comparing inverse associations of eGFR with cadmium excretion rate in subjects grouped by gender and blood pressure status.
Table 4.
Comparing inverse associations of eGFR with cadmium excretion rate in subjects grouped by gender and blood pressure status.
Independent variables/
factors |
eGFR, mL/min/1.73m2
|
Women,
n = 333 |
Men,
n = 114 |
Normotension,
n = 229 |
Hypertension,
n = 218 |
| β |
p |
β |
p |
β |
p |
β |
p |
| Age, years |
−0.528 |
<0.001 |
−0.505 |
<0.001 |
−0.559 |
<0.001 |
−0.517 |
<0.001 |
| BMI, kg/m2
|
−0.050 |
0.308 |
−0.136 |
0.122 |
−0.037 |
0.532 |
−0.077 |
0.216 |
| Log2[(ECd/Ccr)×105], µg/L filtrate |
−0.121 |
0.051 |
−0.077 |
0.463 |
−0.056 |
0.440 |
−0.177 |
0.023 |
| Gender |
− |
− |
− |
− |
−0.017 |
0.787 |
−0.012 |
0.870 |
| Hypertension |
−0.045 |
0.344 |
−0.203 |
0.018 |
− |
− |
− |
− |
| Smoking |
0.031 |
0.533 |
0.043 |
0.624 |
0.152 |
0.020 |
−0.098 |
0.178 |
| Diabetes |
−0.133 |
0.016 |
−0.018 |
0.854 |
−0.049 |
0.445 |
−0.175 |
0.012 |
| Adjusted R2
|
0.279 |
<0.001 |
0.248 |
<0.001 |
0.318 |
<0.001 |
0.242 |
<0.001 |
Table 5.
Multiple linear regression analysis to evaluate association of systolic and diastolic blood pressures with cadmium, eGFR and other variables.
Table 5.
Multiple linear regression analysis to evaluate association of systolic and diastolic blood pressures with cadmium, eGFR and other variables.
Independent
Variables/Factors |
SBP or DBP |
All, n = 447
|
Mild Cd burden a
n = 123 |
Medium + heavy
n = 324 |
| β |
p |
β |
p |
β |
p |
| Model 1: SBP |
|
|
|
|
|
|
| Age, years |
0.243 |
<0.001 |
0.395 |
<0.001 |
0.091 |
0.143 |
| BMI, kg/m2
|
0.113 |
0.013 |
0.081 |
0.361 |
0.097 |
0.084 |
| Log2[(ECd/Ccr)× 105], µg/L filtrate |
0.027 |
0.624 |
0.080 |
0.372 |
−0.051 |
0.352 |
| eGFR, mL/min/1.73m2
|
−0.106 |
0.036 |
0.011 |
0.907 |
−0.176 |
0.004 |
| Gender |
−0.044 |
0.378 |
−0.096 |
0.360 |
−0.024 |
0.688 |
| Smoking |
−0.075 |
0.145 |
−0.176 |
0.093 |
−0.031 |
0.600 |
| Diabetes |
0.216 |
<0.001 |
0.202 |
0.020 |
0.265 |
<0.001 |
| Adjusted R2
|
0.199 |
<0.001 |
0.157 |
<0.001 |
0.150 |
<0.001 |
| Model 2: DBP |
|
|
|
|
|
|
| Age, years |
−0.028 |
0.650 |
0.036 |
0.739 |
−0.081 |
0.213 |
| BMI, kg/m2
|
0.123 |
0.013 |
0.069 |
0.475 |
0.123 |
0.037 |
| Log2[(ECd/Ccr)× 105], µg/L filtrate |
−0.069 |
0.255 |
−0.059 |
0.546 |
−0.025 |
0.660 |
| eGFR, mL/min/1.73m2
|
−0.085 |
0.123 |
0.057 |
0.582 |
−0.130 |
0.041 |
| Gender |
−0.055 |
0.314 |
−0.207 |
0.074 |
−0.003 |
0.968 |
| Smoking |
−0.050 |
0.373 |
−0.209 |
0.068 |
0.008 |
0.897 |
| Diabetes |
0.102 |
0.064 |
0.027 |
0.775 |
0.193 |
0.001 |
| Adjusted R2
|
0.046 |
<0.001 |
−0.005 |
0.498 |
0.058 |
0.001 |