Submitted:
06 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling Site Selection
2.2. Sampling Methods
2.3. Chemical Analysis
3. Results and Discussion
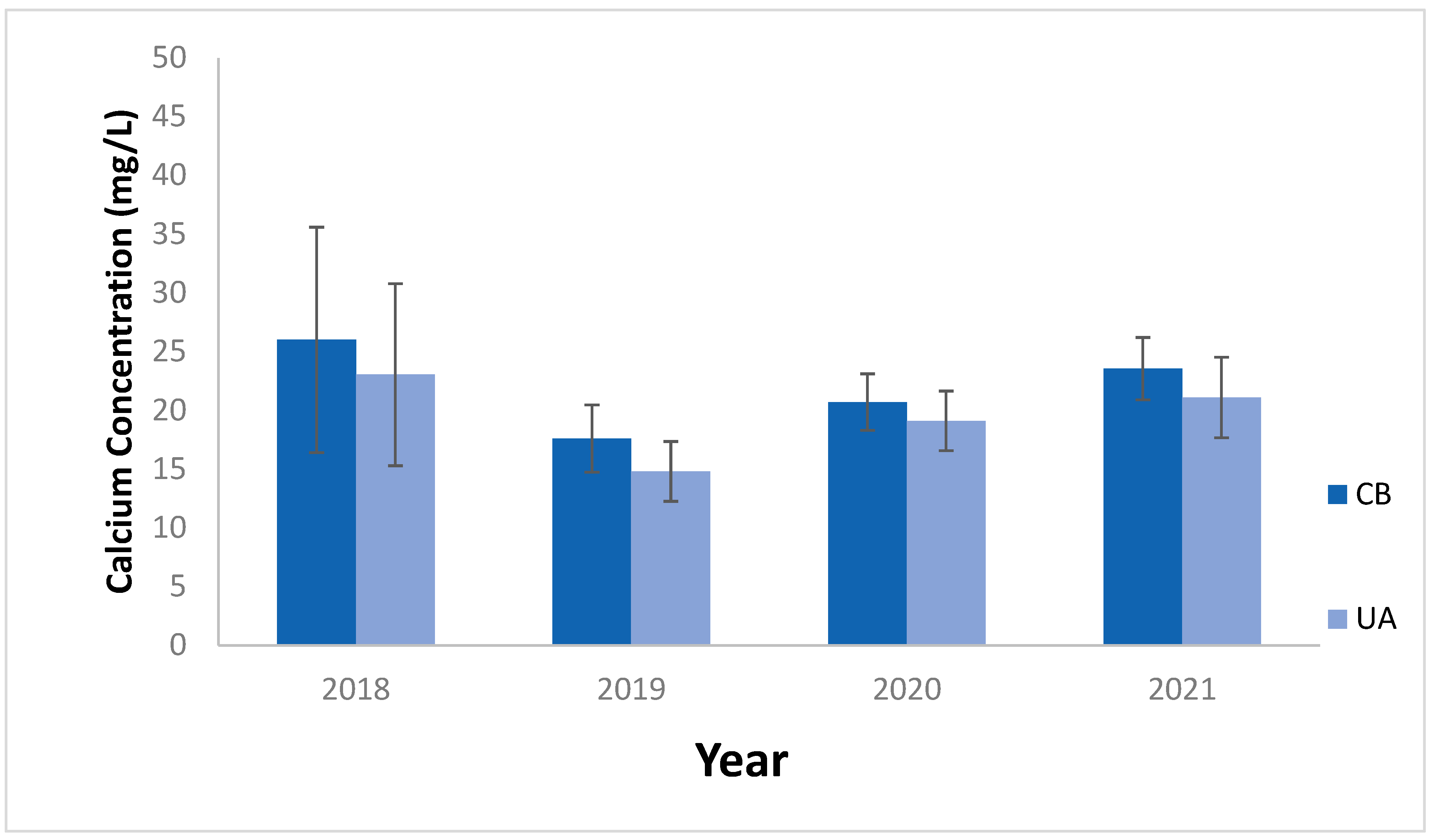
3.1. Dissolved Calcium Concentrations in Tributary Streams and Embayments
3.2. Dissolved Calcium Concentrations in Kentucky Lake
3.2.1. Spatial and Temporal Variation in Dissolved Calcium Concentrations in Kentucky Lake
3.3. Dissolved Calcium Concentrations in Ohio River
3.4. Sources of Calcium in Kentucky Lake Waters
3.5. Zebra Mussel Occurrence and Calcium Threshold Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Hagihara, T.; Mano, H.; Miura, T.; Hasebe, M.; Toytota, M. Calcium-mediated rapid movements defend against herbivores insects in Mimosa pudica. Nature Commun. 2022, 13, 6412. [Google Scholar] [CrossRef]
- Jaiswal, J.K. Calcium- how and why? J. Bio. Sci. 2001, 26, 37–363. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why calcium? How calcium became the best communicator? J. Biol. Sci. 2016, 291, 20849–20857. [Google Scholar] [CrossRef]
- Berridge, M.; Lipp, P.; Bootman, M. Calcium signaling. Curr. Bio. 1999, 9, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Dolmetsch, R.E.; Lewis, R.S.; Goodnow, C.C.; Healy, J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997, 386, 855–858. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Xu, K.; Lewis, R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998, 392, 933–936. [Google Scholar] [CrossRef]
- Mooren, F.C.; Kinne, R.K.H. Cellular calcium in health and disease. Bichim. Biophys. Acta. 1998, 1406, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Bevelander, G. Calcification in molluscs. III. Intake and deposition of Ca and P in relation of shell formation. Biol. Bull. 1952, 102, 9–15. [Google Scholar] [CrossRef]
- Greenaway, P. Calcium regulation in the freshwater mollusk, Limnaea stagnalis (L) (Gastropoda: Pulmonata). J. Exp. Biol. 1971, 54, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C. The dangers of ocean acidification. Sci. Amer.. 2006, 294, 59–65. [Google Scholar] [CrossRef]
- Marshall, D.J.; Santos, J.H.; Leung, K.M.Y.; Chak, W.H. Correlation between gastropod shell dissolution and water chemical properties in a tropical estuary. Mar. Environ. Res. 2008, 66, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Meybeck, M. Global occurrence of major elements in rivers. In. Surface and ground water, weathering, and soils, Ed. J.I. Drever, Elsevier, 2003, Vol. 5, pp. 207–223.
- Verpoorter, C.; Kutser, T.; Seckel, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Letters 2014, 6396–6402. [Google Scholar] [CrossRef]
- Meybeck. M. Global chemical-weathering of surficial rock estimated from dissolved loads. Amer. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- McMahon, R.F. The physiological ecology of the zebra mussel (Dreissena polymorpha), in North America and Europe. Amer, Zool. 1996, 36, 339–363. [Google Scholar] [CrossRef]
- Jones, S. Invasive Work Group Report on Zebra Mussels. Dane County Lakes and Watershed Commission. Madison, WI. 2002, pp. 12.
- Whittier, T.R.; Ringold, P.L.; Herlihy, A.T.; Pierson, S.M. A calcium-based invasion risk assessment for zebra and quagga mussels (Dreissena Spp). Front. Ecol. Environ. 2008, 6, 180–184. [Google Scholar] [CrossRef]
- Baker, S.M.; Hornbach, D.J. Acute physiological effects of zebra mussel (Dreissena polymorpha) infestation on two unionid mussels, Actinonaias ligmentina, and Amblema plicata. Can. J. Fish Aquatic. Sci. 1997, 54, 512–519. [Google Scholar] [CrossRef]
- Benson, A. 2022. NAS – Nonindigenous Aquatic Species, Dreissena polymorpha (zebra mussel) – Species Profile. U.S. Geological Survey. https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=5 (Accessed 4/18/2022).
- Boelman, S.F.; Neilson, F.M.; Dardeans, E.A.; Cross, T. Zebra Mussel (Dreissena polymorpha) Control Handbook for Facility Operators, First Edition. Zebra Mussel Research Program. The US. Army Corps of Engineers. Misc. Paper El-97-1. 1997, pp. 78.
- Fanlow, D.L. , Nalepa, T.F.; Lang, G.A. Filtration rates of zebra mussels (Dreissena polymorpha) on natural seston from Saginaw Bay, Lake Huron. J. Great Lakes Res, 1995; 21, 489–500. [Google Scholar]
- Simberloff, D.; Rejmanek, M. 100 of the world’s worst invasive alien species: A selection from the Global Invasive Species Database. In. Encyclopedia of biological invasions (Eds. D. Simberloff, M. Rejmanek). 2011, pp. 715–716. University of California Press.
- IUCN Invasive Species Specialist Group (2022). Global invasive species database. International Union for Conservation of Nature. http://www.iucngisd.org/gisd/ (accessed on 8-8-2023).
- Read, D.P. Spawning and larval development in Zebra mussel (Dreissena polymorpha) in Tennessee and Ohio River. M.S. Thesis. Murray State University. Murray, KY. 83pp. 2002.
- Hagg, W.R. North American Freshwater Mussels: Natural History, Ecology & Conservation. Cambridge University Press, New York., 2012. pp. 493.
- Ricciardi, A., R. J. Neves, and J.B. Rasmussen. 1998. Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. Journal of Animal Ecology.
- Schloesser, D.W., J. L. Metacalfe-Smith, W.P. Kovalak, G.D. Longton, and R.D. Smithee. Extirpation of freshwater mussels (Bivalvia: Unionidae) following the invasion of dreissenid mussels in an interconnecting river of the Laurentian Great Lakes. American Midland Naturalist. 2006, 155, 307–320. 155.
- Carriker, N. E.; Cox, J. P. 1984. Technical Report Series: Kentucky Lake Reservoir Water Quality-1982.
- Lapviboonsuk, J.; Loganathan, B.G. Polynuclear aromatic hydrocarbons in sediments and mussel tissue from the lowermost Tennessee River and Kentucky Lake. J. Ky. Acad. Sci. 2007, 68, 186–197. [Google Scholar]
- Tennessee Valley Authority. Quality of water in Kentucky Reservoir. Water Quality Branch. Chattanooga, TN. Tennessee Valley Authority. Quality of water in Kentucky Reservoir. Water Quality Branch. Chattanooga, TN. 1974, pp. 86.
- TVA. Zebra mussel found in Tennessee River. News Release from Tennessee Valley Authority. Knoxville, TN. 18 September 1991.
- Cohen, A.N.; Weinstein, A. Zebra mussel’s threshold and implications for its potential distribution in North America. National Fish and Wildlife Foundation- Project #: R/C-31PD-The California Sea Grant College Program- San Francisco Estuary Institute, Richmond, CA. 2001. pp. 44.
- White, D.S.; Johnston, K.L.; Rice, G.T. 2007. The Center for Reservoir Research over its first twenty years with special reference to the long-term monitoring program. J. Ky.Acad. Sci. 2007, 68, 2–10. [Google Scholar]
- US EPA. Recommended guidelines for measuring metals in Puget Sound marine water, sediment and tissue samples. The United States Environmental Protection Agency (US EPA) Puget Sound Water Quality Action Team, Olympia, Washington, USA. 1997, pp 59.
- APHA, Standard Methods for the Examination of Water and Wastewater. Greenberg, A.E., Clesceri, L.S., and Eaton A.D., Eds. 18th ed., Part 3000, Method 3030A. American Public Health Association, Washington, DC. 1992.
- Bentley, E.M.; Lee, G.F. Determination of calcium in natural water by atomic absorption spectrophotometry. Env. Sci. Technol. 1967, 1, 721–724. [Google Scholar] [CrossRef]
- Federal Register, Appendix B to part 139. Definition and procedure for the determination of detection limit, 1984, Vol. 49, p 209.
- Fryar, A.E., Thompson, K.E., Hendricks, S.P., and White, D.S. Groundwater flow and reservoir management in a tributary watershed along Kentucky Lake. Journal of the Kentucky Academy of Science 2007, 68, 11–23. [CrossRef]
- Trifonov, D.N.; Trifonov, V.D. Chemical elements: How they were discovered? MIR Publishers. Moscow. 1982, pp. 116–117.
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry- An Analysis of Global Change. Academic Press, Elsevier. 2013. pp.672.
- Cañedo-Argϋelles, M.; Hawkins, C.P.; Kefford, B.J.; et al. Saving freshwater from salts. Science. 2016, 351, 914–916. [Google Scholar] [CrossRef]
- Dougan, H.A. Salting our freshwater lakes. Proc. Natl. Acad,. Sci. USA 2017, 114, 4453–4458. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pce, M.L.; et al. 2018. Freshwater salinization syndrome on a continental scale. PNAS. [CrossRef]
- Kaushal, S.S.; Goffman, P.M.; Likens, G.E.; Fisher, G.T. Increased salinization of freshwater in the northeastern United States. Proc. Natl. Acad. Sci. USA 2005, 102, 13517–13520. [Google Scholar] [CrossRef]
- Coldsnow, K.D.; Relyea, R.A. Toxicity of various road-deicing salts to Asian clams (Corbicula fluminea). Environ. Toxicol. Chem. 2018, 37, 1839–1845. [Google Scholar] [CrossRef]
- Lambert, M.R.; Stoler, A.B.; Smylie, M.S.; Relyea, R.A.; Skelly, D.K. Interactive effect of road salt and leaf litter on wood frog sex ratios and sexual size dimorphism. Can. J. Fish. Aquat. Sci. 2017, 74, 141–146. [Google Scholar] [CrossRef]
- Harris, F.E.; Tucker, E.M. Minerals Yearbook 1945: Salt. US Government Printing Office, 1947, Washington, DC.
- Bolen, W.P. 2014 Minerals Yearbook: Salt. US Geological Survey, Reston, VA, USA, 2016, 63, 1–22.
- Weyhenmeyer, G.A.; Hartmann, J.; Hessen, D.O.; Kopacek, J.; Hejzlar, J.; Jacquet, S.; Hamilton, S.K.; Verburg, P.; Leach, T.H.; Schmid, M.; Flaim, G.; Noges, T.; Noges, P.; Wentzky, V.C.; et al. Widespread diminishing anthropogenic effects on calcium in freshwaters. Nature 2019, 9, 10450. [Google Scholar] [CrossRef]
- Mellina, E.; Rasmussen, J.B. Patterns in the distribution and abundance of zebra mussel (Dreissena polymorpha) in rivers and lakes in relation to substrate and other physicochemical factors. Can. J. Fish. Aquat. Sci. 1994, 51, 1024–1036. [Google Scholar] [CrossRef]
- O’Neill, C.R. Jr. The Zebra Mussel: Impacts and Control. New York Sea Grant, Cornell University, Ithaca, NY. Cornell Cooperative Extension Information Bulletin. 1996, No. 238.
- Ramachandran, C.W.; Padilla, D.K.; Dodson, S.I. Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Can. J. Fish. Aquat. Sci. 1992, 49, 2611–2620. [Google Scholar] [CrossRef]
- Padilla, D.K. Presentation at the Seventh International Zebra Mussel Conference. April 1997, New Orleans, LA. [Google Scholar]
- Karatayev, A. Factors determining the distribution and abundance of Dreissena polymorpha in lakes, dam reservoirs, and channels. In. Proceedings of the Fifth International Zebra Mussel and Other Aquatic Nuisance Organisms Conference, February 1995, Toronto, ON. pp 227-243.
- Strayer, D.L. Projected distribution of the zebra mussel, Dreissena polymorpha, in North America. Can. J. Fish. Aquat. Sci. 1991, 48, 1389–1395. [Google Scholar] [CrossRef]
- Hincks, S.S.; Mackie, G.L. Effects of pH, calcium, alkalinity, hardness, and chlorophyll on the survival, growth, and reproductive success of zebra mussel (Dreissena polymorpha) in Ontario lakes. Can. J. Fish. Aquat. Sci. 1997, 54, 2049–2057. [Google Scholar] [CrossRef]
- Roy. H.E.; Pauchard, A.; Stoett, P.; Truong, T.R.; Bacher, S.; Galil, B.S.; Hulme, P.E.; Ikeda, T.; Kavileveetil, S.; McGeoch, M.A.; Meyerson, L.A.; Nunez, M.A.; Ordonez, A.; Rahlao, S.J.; Schwindt, E.; Seebens, H.; Sheppard, A.W.; Vandvik, V. The Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES) Assessment Report on Invasive Alien Species and their Control. Advanced United Version. September 4 2023. Pp. 41. IPBES Secretariat, Bonn, Germany.
- Cuthbert, R.N.; Pattison, G.; Taylor, N.G.; Verbrugge, L.; Diagne, C.; Ahmed, D.A.; Leroy, B.; Angulo, E.; Briski, E.; Capinha, C.; Catford, J.A.; Dalu, T.; Essl, F.; Gozlan, R.E.; Haubrock, P.J.; Kourantidou, M.; Kramer, A.M.; Renault, D.; Wasserman, R.J.; Courchamp, F. Global economic costs of aquatic invasive alien species. Sci. Total Environ. 2021, 775, 145238. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H. ; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Kleunen, M.v.; Kuhn, I.; Jeschke, J.M.; Lenzner, B.; Liebhold, A.M.; Pattison, Z.; Pergl, J.; Pysek, P.; Winter, M.; Essl, F. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




