Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
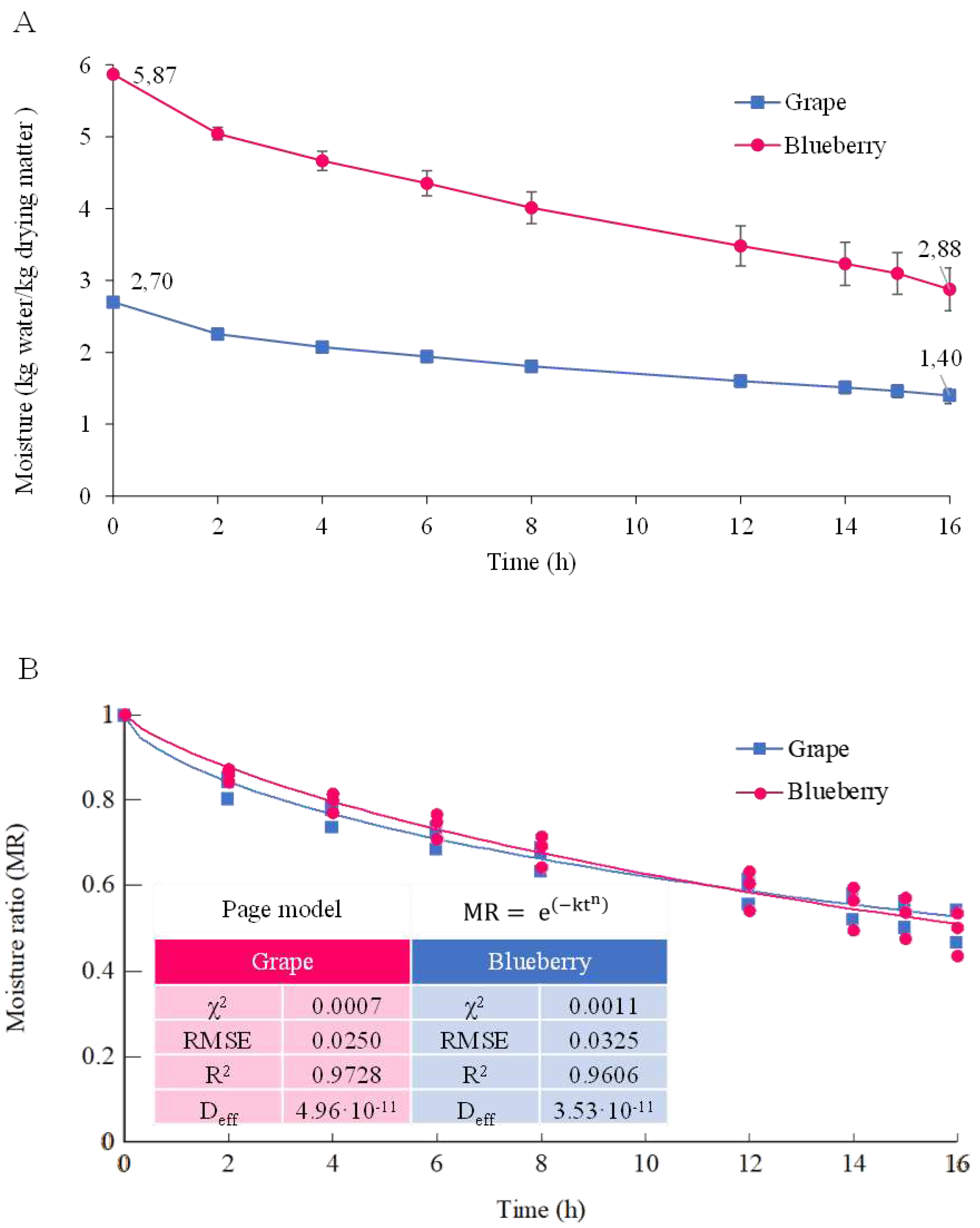
2.2. Dehydration process
2.3. Selection and preparation of yeast inocula
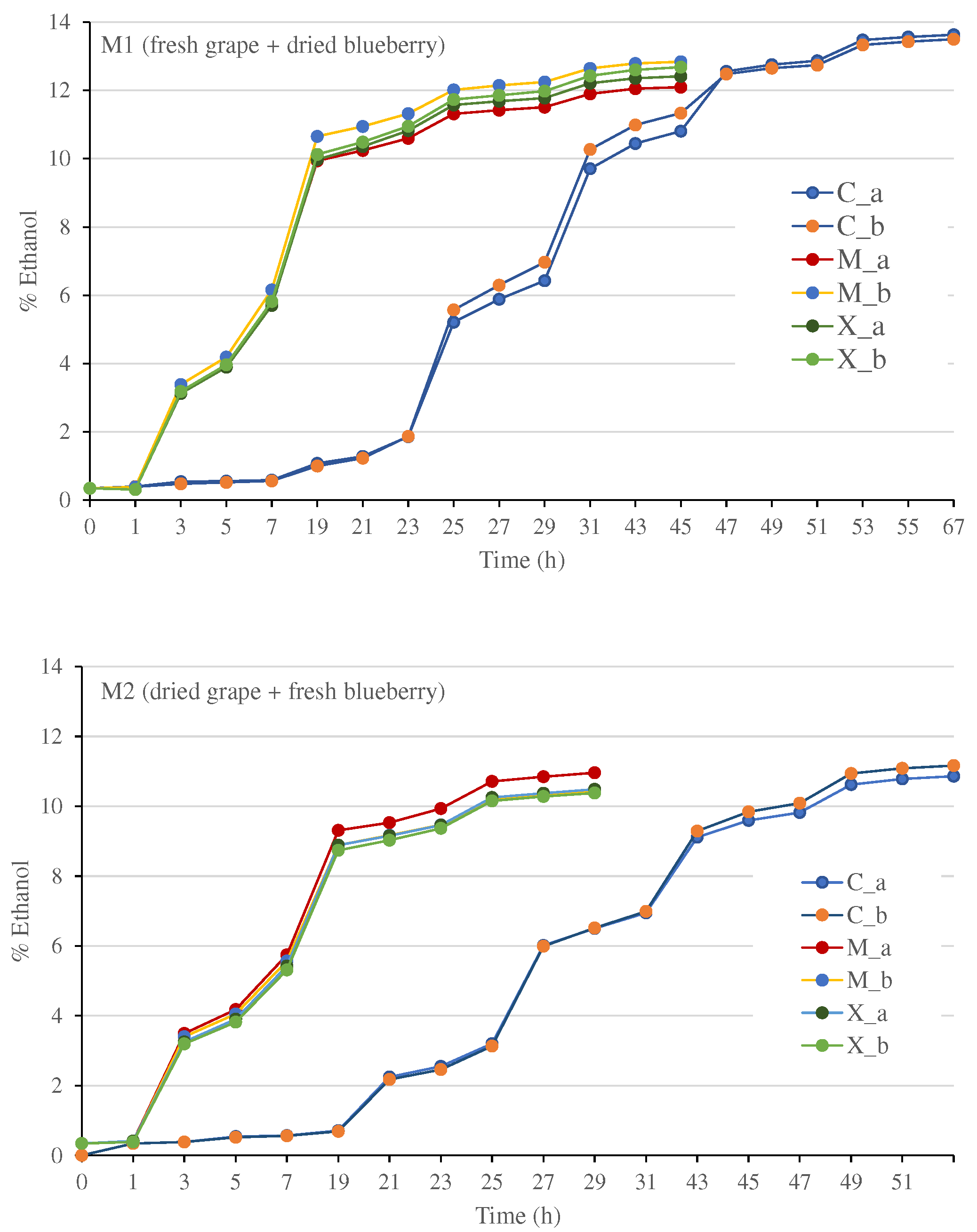
2.4. Fermentation process
| W1 | W2 | |||
| a | b | a | b | |
| Control | W1C_a | W1C_b | W2C_a | W2C_b |
| M05 Mead yeast | W1M_a | W1M_b | W2M_a | W2M_b |
| X5 yeast | W1X_a | W1X_b | W2X_a | W2X_b |
2.5. Alcohol content
2.6. Volatile acidity
2.7. Spectrophotometric determinations
2.8. Total phenolic compounds
2.9. Total flavonoids
2.10. Total anthocyanins
2.11. Antioxidant activity
2.11.1. DPPH assay
2.11.2. ABTS assay
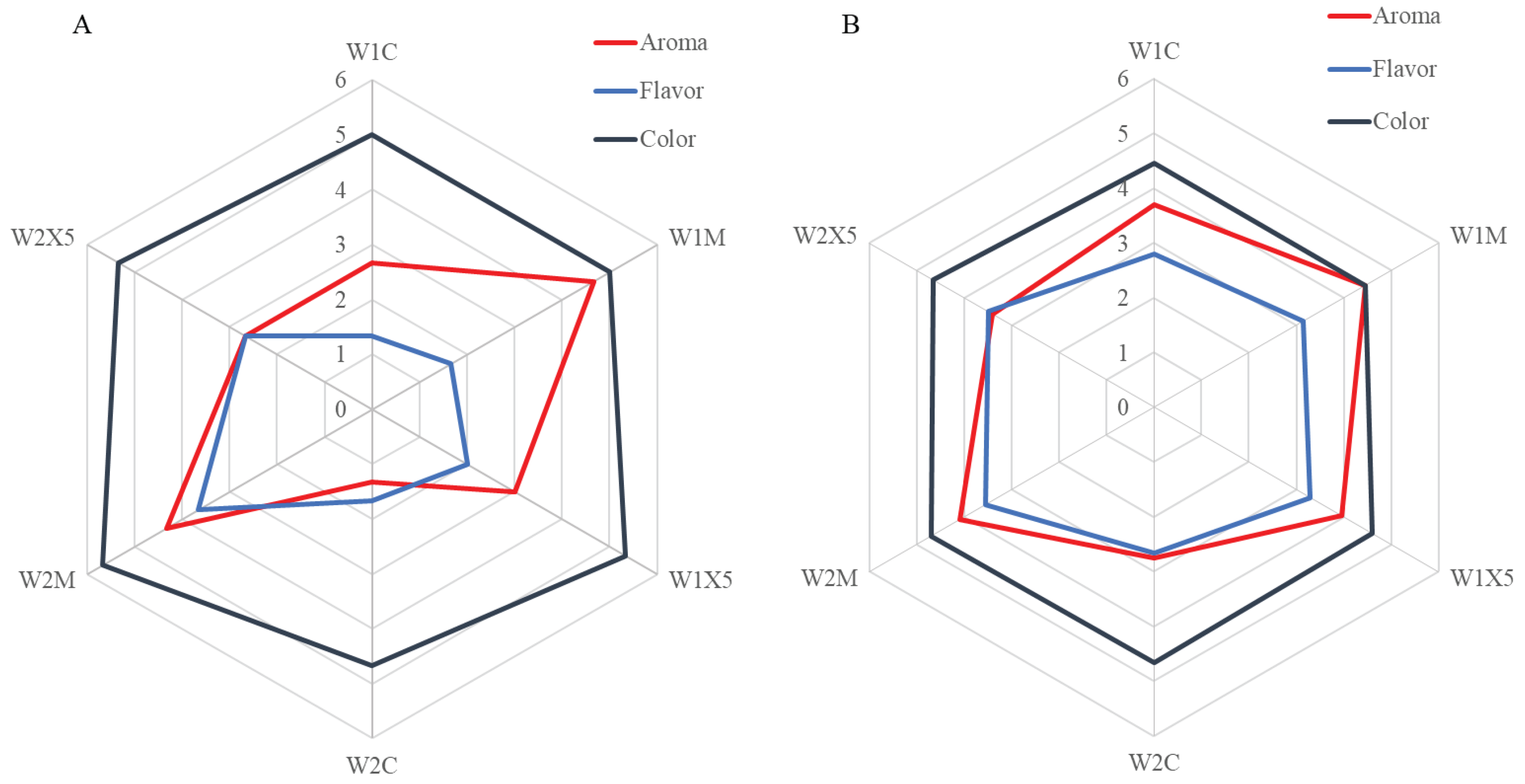
2.12. Sensory analysis
2.13. Statistical analysis
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Casagrande, M.; Zanela, J.; Pereira, D.; de Lima, V.A.; Oldoni, T.L.C.; Carpes, S.T. Optimization of the Extraction of Antioxidant Phenolic Compounds from Grape Pomace Using Response Surface Methodology. Journal of Food Measurement and Characterization 2019, 13, 1120–1129. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Matos, S.; Gonçalves, F.J.; Costa, D.; Mendes, M. Evaluation of Phenolic Compounds and Antioxidant Activity of Blueberries and Modelization by Artificial Neural Networks. International Journal of Fruit Science 2018, 18, 199–214. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a Polyphenol-Rich Grape and Blueberry Extract (MemophenolTM) on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Study. Front Psychol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit Rev Food Sci Nutr 2018, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int J Mol Sci 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry Fruit Valorization and Valuable Constituents: A Review. Int J Food Microbiol 2022, 381. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, Q.; Jiao, J.; Kelanne, N.; Kortesniemi, M.; Xu, X.; Zhu, B.; Laaksonen, O. Exploring the Sensory Properties and Preferences of Fruit Wines Based on an Online Survey and Partial Projective Mapping. Foods 2023, 12. [Google Scholar] [CrossRef]
- Johnson, M.H.; de Mejia, E.G.; Fan, J.; Lila, M.A.; Yousef, G.G. Anthocyanins and Proanthocyanidins from Blueberry–Blackberry Fermented Beverages Inhibit Markers of Inflammation in Macrophages and Carbohydrate-utilizing Enzymes in Vitro. Mol Nutr Food Res 2013, 57, 1182–1197. [Google Scholar] [CrossRef]
- Su, M.-S.; Chien, P.-J. Antioxidant Activity, Anthocyanins, and Phenolics of Rabbiteye Blueberry (Vaccinium Ashei) Fluid Products as Affected by Fermentation. Food Chem 2007, 104, 182–187. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, L.; He, B.; Yue, P.; Gao, X. Analysis of Organic Acids in Blueberry Juice and Its Fermented Wine by High Performance Liquid Chromatography. Advance Journal of Food Science and Technology 2015, 9, 127–134. [Google Scholar] [CrossRef]
- Santos, R.O.; Trindade, S.C.; Maurer, L.H.; Bersch, A.M.; Sautter, C.K.; Penna, N.G. Physicochemical, Antioxidant and Sensory Quality of Brazilian Blueberry Wine. An Acad Bras Cienc 2016, 88, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Gao, X. Phenolic Compounds and Antioxidant Activity of Wines Fermented Using Ten Blueberry Varieties. Am J Food Technol 2016, 11, 291–297. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Weng, L.; Zou, L.; Jiang, H.; Qiu, J.; Fu, J. Analysis of Sucrose Addition on the Physicochemical Properties of Blueberry Wine in the Main Fermentation. Front Nutr 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Cui, M.-Y.; Fu, Y.; Wang, X.-H.; Yang, Q.; Zhu, Y.; Yang, X.-H.; Bi, H.-J.; Gao, X.-L. Aroma Enhancement of Blueberry Wine by Postharvest Partial Dehydration of Blueberries. Food Chem 2023, 426, 136593. [Google Scholar] [CrossRef]
- Marquez, A.; Perez-Serratosa, M.; Varo, M.A.; Merida, J. Effect of Temperature on the Anthocyanin Extraction and Color Evolution during Controlled Dehydration of Tempranillo Grapes. J Agric Food Chem 2014, 62, 7897–7902. [Google Scholar] [CrossRef] [PubMed]
- Serratosa, M.P.; Lopez-Toledano, A.; Medina, M.; Merida, J. Drying of Pedro Ximenez Grapes in Chamber at Controlled Temperature and with Dipping Pretreatments. Changes in the Color Fraction. J Agric Food Chem 2008, 56, 10739–10746. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in Color and Phenolic Compounds during the Raisining of Grape Cv. Pedro Ximenez. J Agric Food Chem 2008, 56, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Serratosa, M.P.; Lopez-Toledano, A.; Millan, C.; Medina, M.; Merida, J. Changes of Ochratoxin A in Grapes Inoculated with Aspergillus Carbonarius and Subjected to Chamber-Drying under Controlled Conditions. J Agric Food Chem 2010, 58, 11907–11912. [Google Scholar] [CrossRef]
- Marquez, A.; Serratosa, M.P.; Lopez-Toledano, A.; Merida, J. Colour and Phenolic Compounds in Sweet Red Wines from Merlot and Tempranillo Grapes Chamber-Dried under Controlled Conditions. Food Chem 2012, 130, 111–120. [Google Scholar] [CrossRef]
- Martin-Gomez, J.; Varo, M.; Merida, J.; Serratosa, M.P. Bioactive Compounds of Chamber-Dried Blueberries at Controlled Temperature and Wines Obtained from Them. J Chem 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Ángeles Varo, M.; Mérida, J.; Serratosa, M.P. The Influence of Berry Perforation on Grape Drying Kinetics and Total Phenolic Compounds. J Sci Food Agric 2019. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, T.; Peinado, R.A.; Maestre, O.; Moreno, J.; Mauricio, J.C. Must Fermentation Containing High Sugar Concentration by Yeast Bioimmobilization. bulletin de l’OIV 2008, 81, 559–568. [Google Scholar]
- Crowell, E.A.; Ough, C.S. Research Note a Modified Procedure for Alcohol Determination by Dichromate Oxidation. Am J Enol Vitic 1979, 30, 61–63. [Google Scholar] [CrossRef]
- 25. OIV, Collection of International Methods of Analysis of Wines and Musts.
- Varo, M.A.; Jacotet-Navarro, M.; Serratosa, M.P.; Mérida, J.; Fabiano-Tixier, A.-S.; Bily, A.; Chemat, F. Green Ultrasound-Assisted Extraction of Antioxidant Phenolic Compounds Determined by High Performance Liquid Chromatography from Bilberry (Vaccinium Myrtillus L.) Juice By-Products. Waste Biomass Valorization 2018, 10, 1945–1955. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J AOAC Int 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 Medicinal Plant Extracts for Antioxidant Capacity and Total Phenols. Food Chem 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gómez, J.; Varo, M.Á.; Mérida, J.; Serratosa, M.P. Influence of Drying Processes on Anthocyanin Profiles, Total Phenolic Compounds and Antioxidant Activities of Blueberry ( Vaccinium Corymbosum ). LWT - Food Science and Technology 2020, 120. [Google Scholar] [CrossRef]
- BOE Boletín Oficial Del Estado 08/09/2012. 2012, 242, 71773–71777, doi:BOE-A-2012-5403.
- Soares-da-Silva, F.A.G.; Campos, F.M.; Ferreira, M.L.; Ribeiro, N.; Amaral, B.; Simões, T.; Silva, C.L.M. Colour Profile Analysis of Port Wines by Various Instrumental and Visual Methods. J Sci Food Agric 2019, 99, 3563–3571. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skračić, Ž.; Kokeza, A.; Soldo, B.; Ljubenkov, I.; Banović, M.; Skroza, D. Effect of Winemaking on Phenolic Profile, Colour Components and Antioxidants in Crljenak Kaštelanski (Sin. Zinfandel, Primitivo, Tribidrag) Wine. J Food Sci Technol 2019, 56, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Heras, J.M.; Callejo, M.J.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Yeast Influence on the Formation of Stable Pigments in Red Winemaking. Food Chem 2016, 197, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Su, M.S.; Chien, P.J. Antioxidant Activity, Anthocyanins, and Phenolics of Rabbiteye Blueberry (Vaccinium Ashei) Fluid Products as Affected by Fermentation. Food Chem 2007, 104, 182–187. [Google Scholar] [CrossRef]
- Johnson, M.H.; Gonzalez de Mejia, E. Comparison of Chemical Composition and Antioxidant Capacity of Commercially Available Blueberry and Blackberry Wines in Illinois. J Food Sci 2012, 77, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Lucius, A.; Meyer, T.; Gonzalez De Mejia, E. Cultivar Evaluation and Effect of Fermentation on Antioxidant Capacity and in Vitro Inhibition of α-Amylase and α-Glucosidase by Highbush Blueberry (Vaccinium Corombosum). J Agric Food Chem 2011, 59, 8923–8930. [Google Scholar] [CrossRef]
- Bakuradze, T.; Tausend, A.; Galan, J.; Maria Groh, I.A.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative Activity and Health Benefits of Anthocyanin-Rich Fruit Juice in Healthy Volunteers. Free Radic Res 2019, 0, 1–11. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Elez Garofulić, I.; Descours, E.; Ščetar, M.; Galić, K. Comparison of Protective Supports and Antioxidative Capacity of Two Bio-Based Films with Revalorised Fruit Pomaces Extracted from Blueberry and Red Grape Skin. Food Packag Shelf Life 2019, 20, 1–21. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J Agric Food Chem 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Chaves, V.C.; Boff, L.; Vizzotto, M.; Calvete, E.; Reginatto, F.H.; Simões, C.M.O. Berries Grown in Brazil: Anthocyanin Profiles and Biological Properties. J Sci Food Agric 2018, 98, 4331–4338. [Google Scholar] [CrossRef]
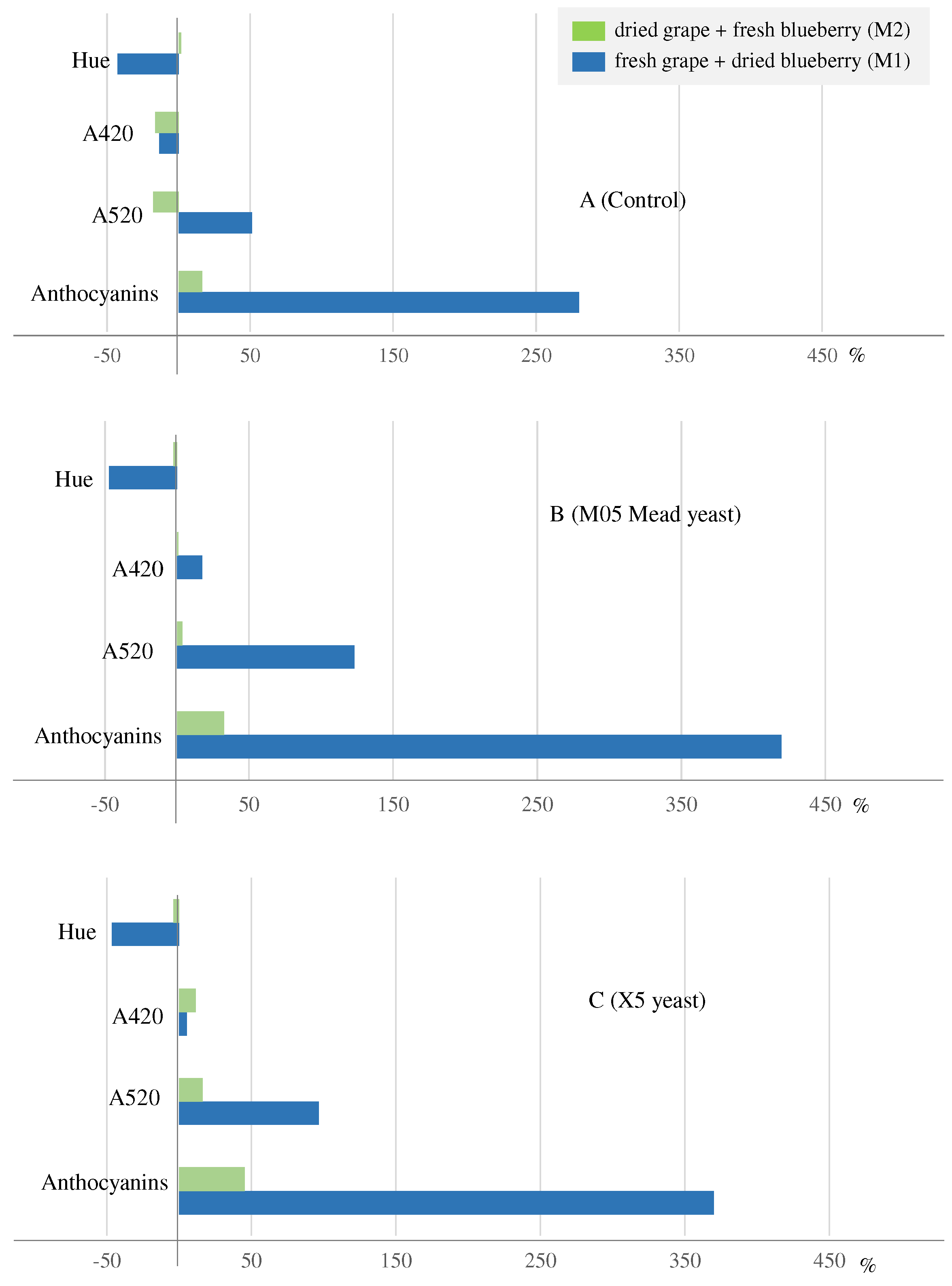
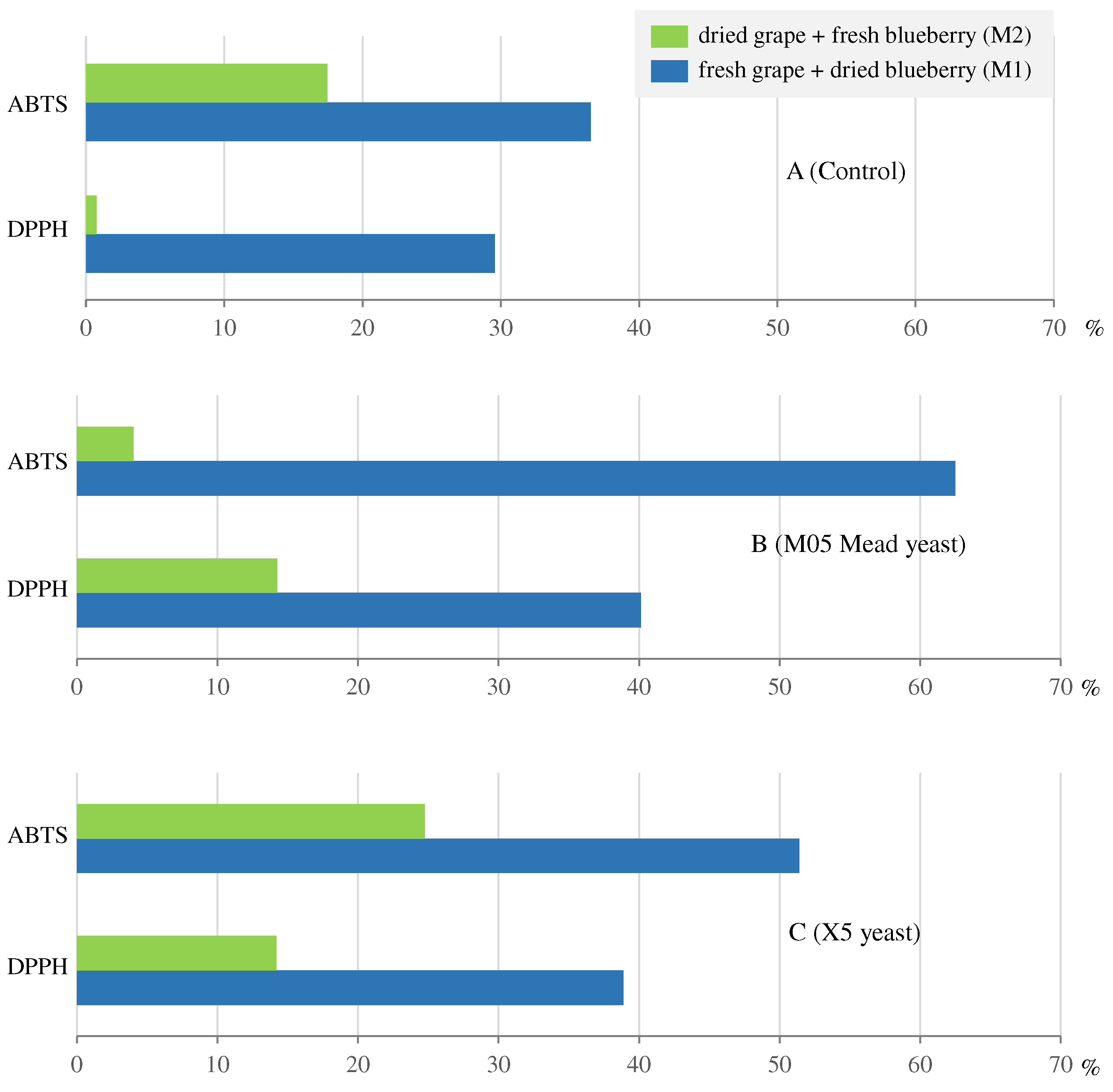
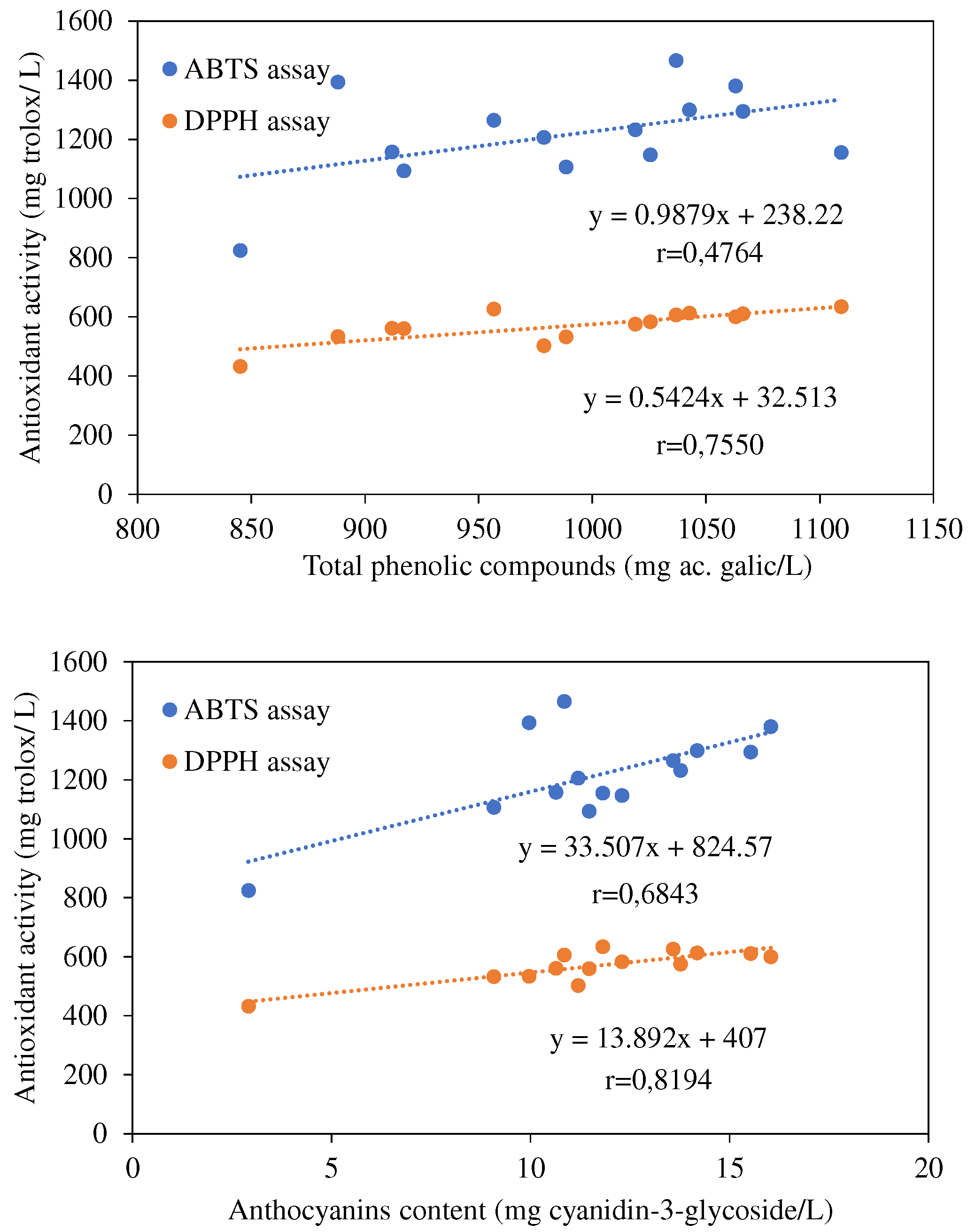
| Ethanol | Volatile acidity | Total phenolic compounds | Total flavonoids | Total Anthocyanins |
Antioxidant activity | ||
|---|---|---|---|---|---|---|---|
| DPPH assay | ABTS assay | ||||||
| M1 | 0 | 0 | 845±2.28 | 27.9±2.62 | 2.91±0.094 | 432±2.72 | 824±43.1 |
| W1C_a | 11.4±0.012 | 7.64±0.246 | 917±0.228 | 37.5±0.131 | 11.47±0.155 | 560±13.3 | 1093±3.29 |
| W1C_b | 11.3±0.067 | 6.17±0.246 | 912±4.53 | 39.4±1.19 | 10.64±0.106 | 561±28.0 | 1157±15.2 |
| W1M_a | 10.5±0.056 | 3.73±0.248 | 1043±7.09 | 41.1±0.758 | 14.18±0.149 | 612±8.52 | 1300±15.9 |
| W1M_b | 11.6±0.281 | 3.95±0.000 | 1063±4.48 | 47.9±0.845 | 16.04±0.291 | 600±3.84 | 1380±2.86 |
| W1X_a | 10.8±0.080 | 1.74±0.248 | 957±2.00 | 40.3±1.22 | 13.58±0.235 | 626±2.84 | 1264±25.0 |
| W1X_b | 10.8±0.080 | 1.74±0.248 | 1019±4.33 | 40.4±0.675 | 13.77±0.072 | 575±15.5 | 1231±23.7 |
| M2 | 0 | 0 | 988±3.22 | 24.1±0.611 | 9.08±0.384 | 532±0.620 | 1106±9.61 |
| W2C_a | 9.6±0.059 | 7.15±0.246 | 988±1.70 | 30.6±0.007 | 9.96±0.181 | 533±1.43 | 1393±13.4 |
| W2C_b | 9.4±0.065 | 7.40±0.000 | 979±4.74 | 33.1±0.801 | 11.19±0.073 | 540±0.562 | 1205±10.9 |
| W2M_a | 10.5±0.106 | 2.73±0.248 | 1109±6.48 | 36.4±0.420 | 11.81±0.127 | 634±4.20 | 1155±23.2 |
| W2M_b | 10.3±0.049 | 2.96±0.000 | 1025±51.8 | 33.1±0.210 | 12.29±0.068 | 583±0.580 | 1146±11.2 |
| W2X_a | 10.4±0.039 | 1.74±0.248 | 1037±5.96 | 34.9±0.250 | 10.84±0.162 | 606±0.926 | 1466±40.5 |
| W2X_b | 10.5±0.112 | 1.99±0.000 | 1066±9.15 | 36.5±1.230 | 15.53±1.399 | 610±9.38 | 1294±9.3 |
| A420 | A520 | A620 | Color intensity | Hue | |
|---|---|---|---|---|---|
| M1 | 1.85±0.010 | 1.68±0.003 | 0.410±0.003 | 3.94±0.009 | 1.10±0.004 |
| W1C_a | 1.59±0.005 | 2.58±0.000 | 0.358±0.003 | 4.52±0.002 | 0.615±0.002 |
| W1C_b | 1.61±0.001 | 2.50±0.005 | 0.345±0.005 | 4.45±0.001 | 0.646±0.001 |
| W1M_a | 2.11±0.008 | 3.66±0.023 | 0.497±0.004 | 6.26±0.034 | 0.578±0.001 |
| W1M_b | 2.23±0.029 | 3.83±0.043 | 0.526±0.020 | 6.59±0.092 | 0.583±0.001 |
| W1X_a | 2.05±0.004 | 3.47±0.011 | 0.511±0.001 | 6.03±0.015 | 0.592±0.001 |
| W1X_b | 1.84±0.004 | 3.14±0.006 | 0.436±0.002 | 5.41±0.008 | 0.586±0.000 |
| M2 | 1.47±0.007 | 2.30±0.034 | 0.400±0.003 | 4.17±0.044 | 0.642±0.006 |
| W2C_a | 1.23±0.004 | 1.84±0.019 | 0.272±0.001 | 3.34±0.024 | 0.667±0.005 |
| W2C_b | 1.24±0.006 | 1.94±0.021 | 0.253±0.002 | 3.43±0.029 | 0.641±0.004 |
| W2M_a | 1.50±0.007 | 2.32±0.015 | 0.316±0.004 | 4.13±0.025 | 0.643±0.001 |
| W2M_b | 1.49±0.002 | 2.44±0.011 | 0.343±0.002 | 4.27±0.015 | 0.607±0.002 |
| W2X_a | 1.65±0.005 | 2.64±0.017 | 0.385±0.001 | 4.67±0.023 | 0.622±0.002 |
| W2X_b | 1.65±0.001 | 2.70±0.007 | 0.419±0.001 | 4.76±0.005 | 0.611±0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





