Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
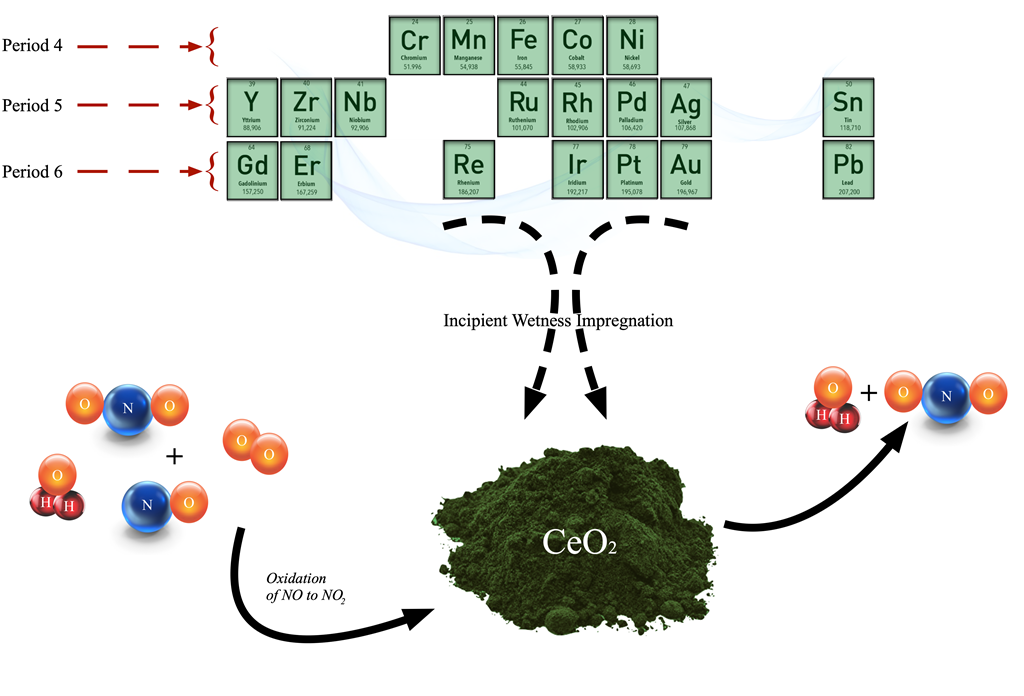
2.1. Catalyst Preparation
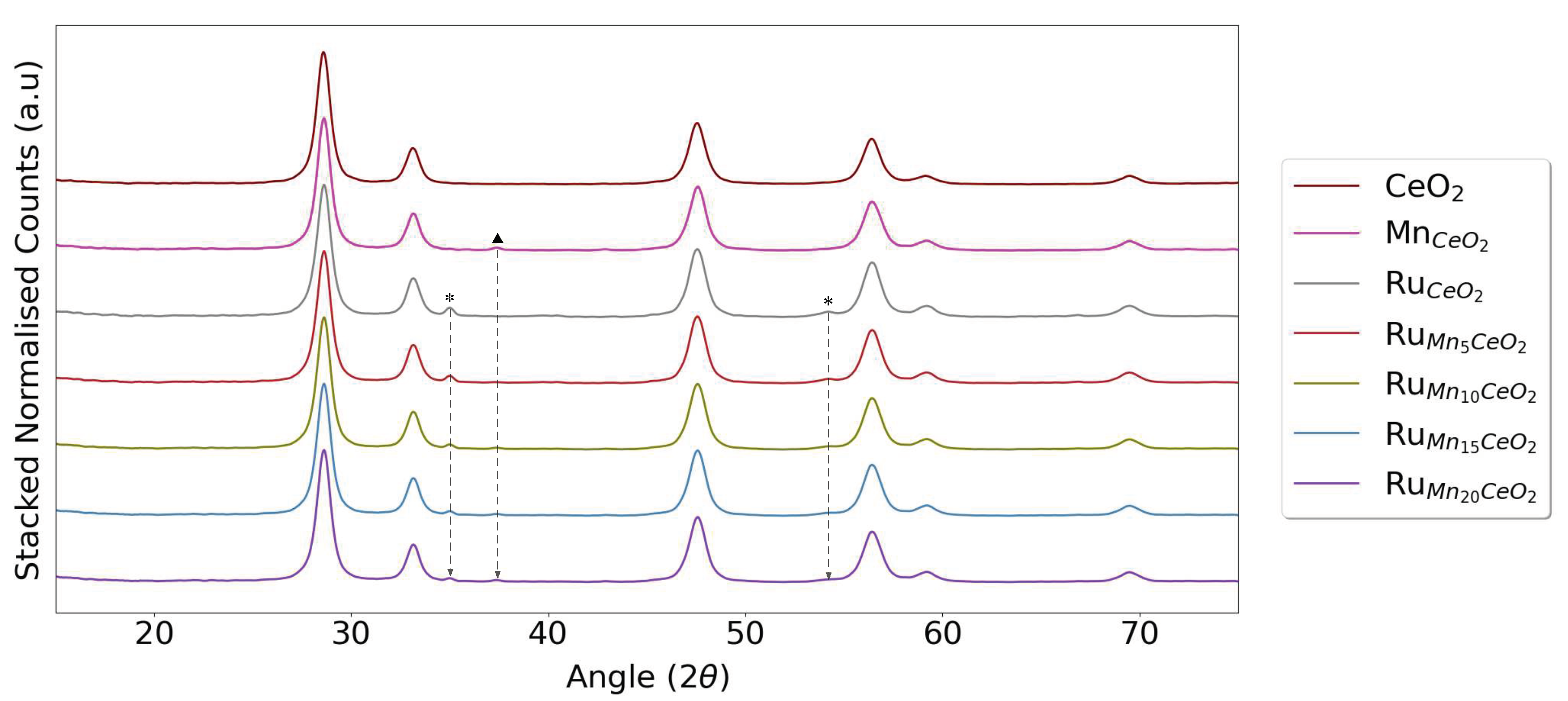
2.2. Characterization
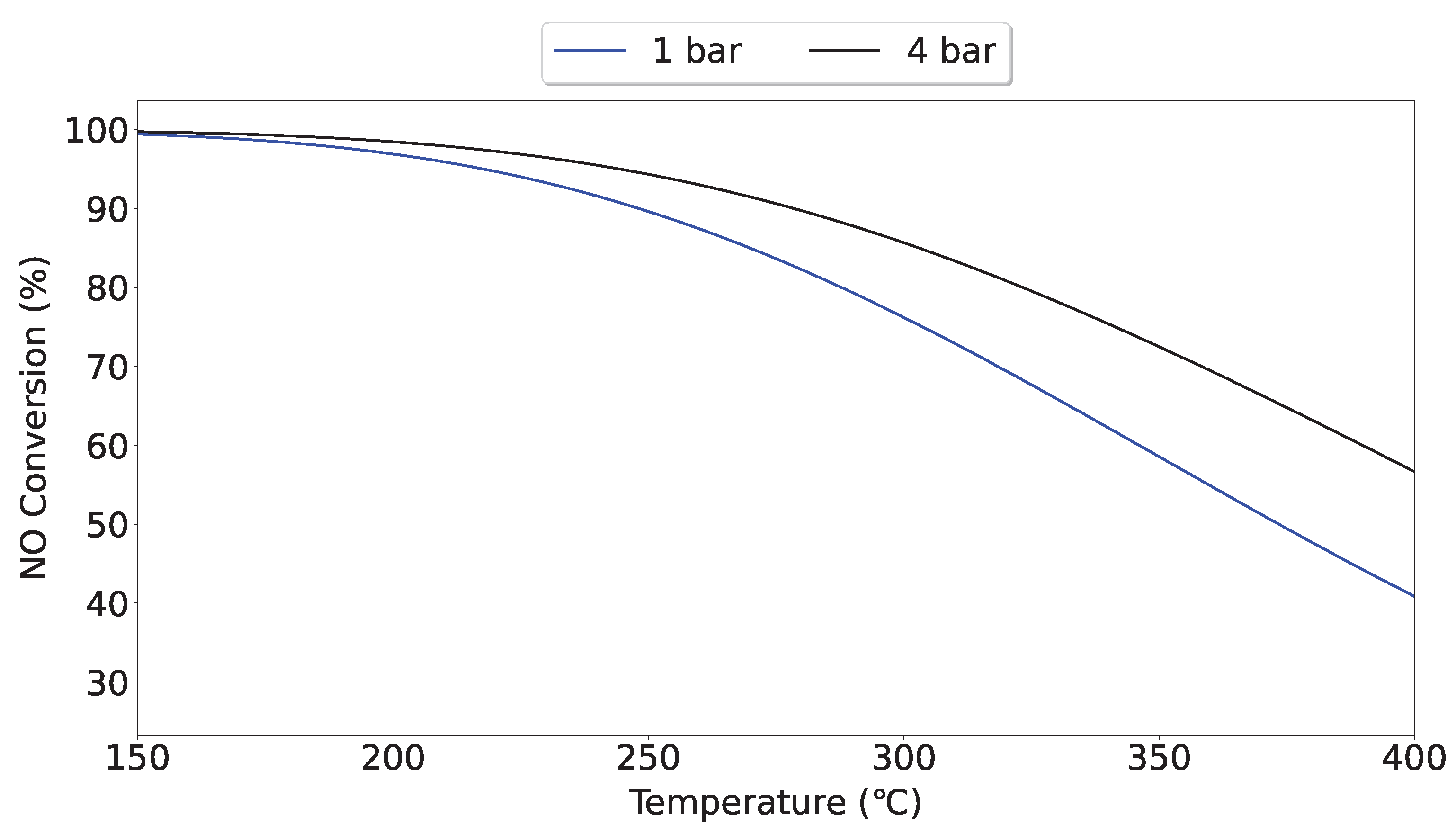
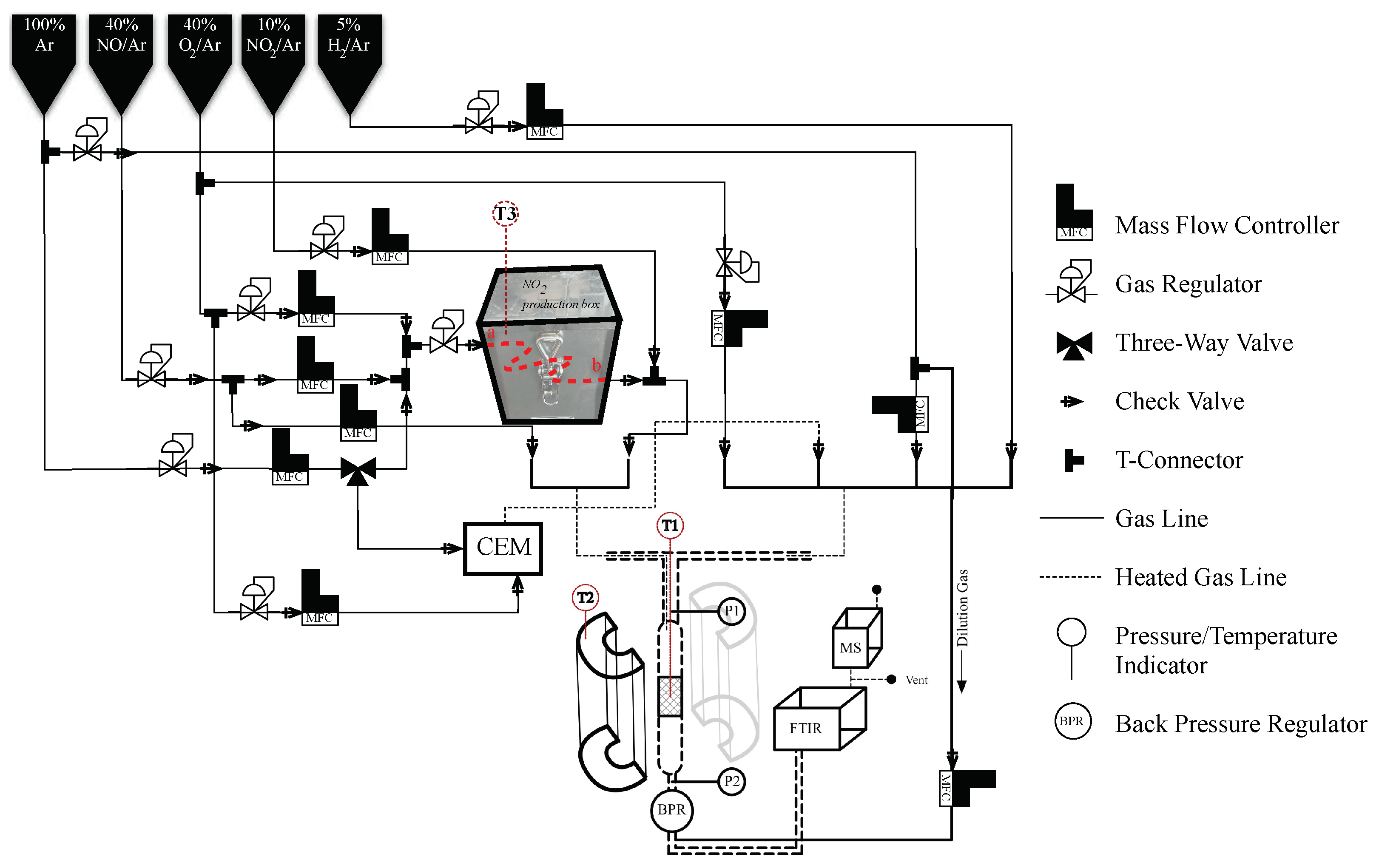
2.3. Activity Testing
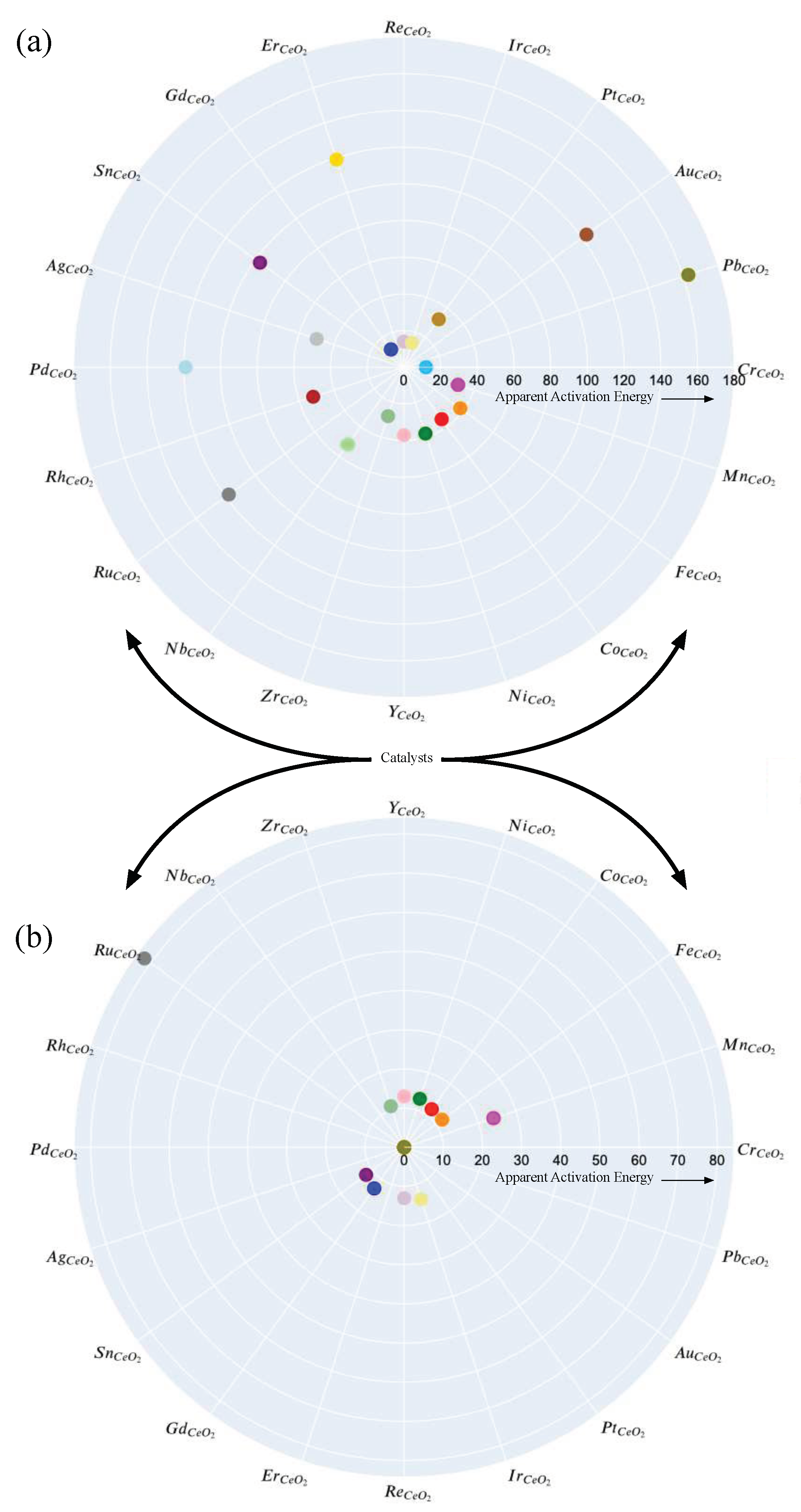
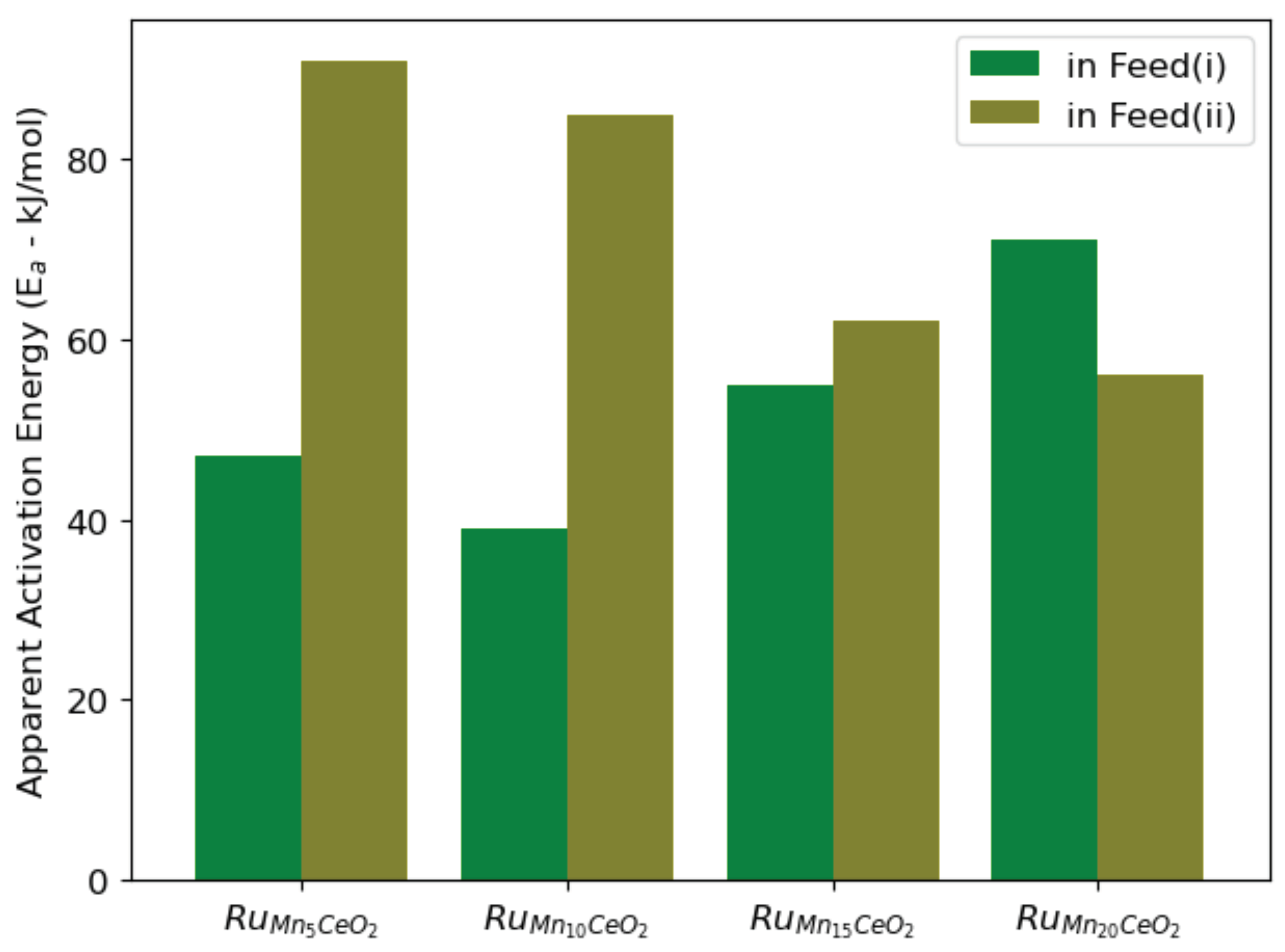
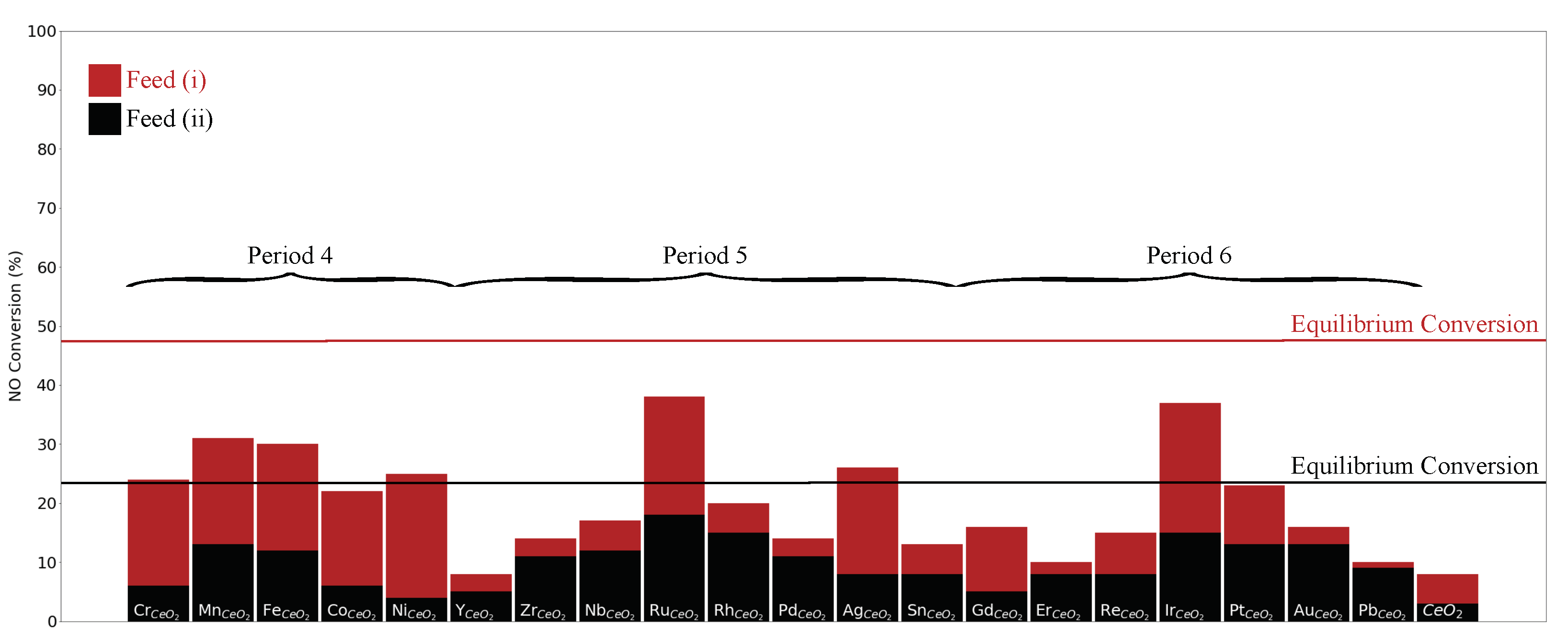
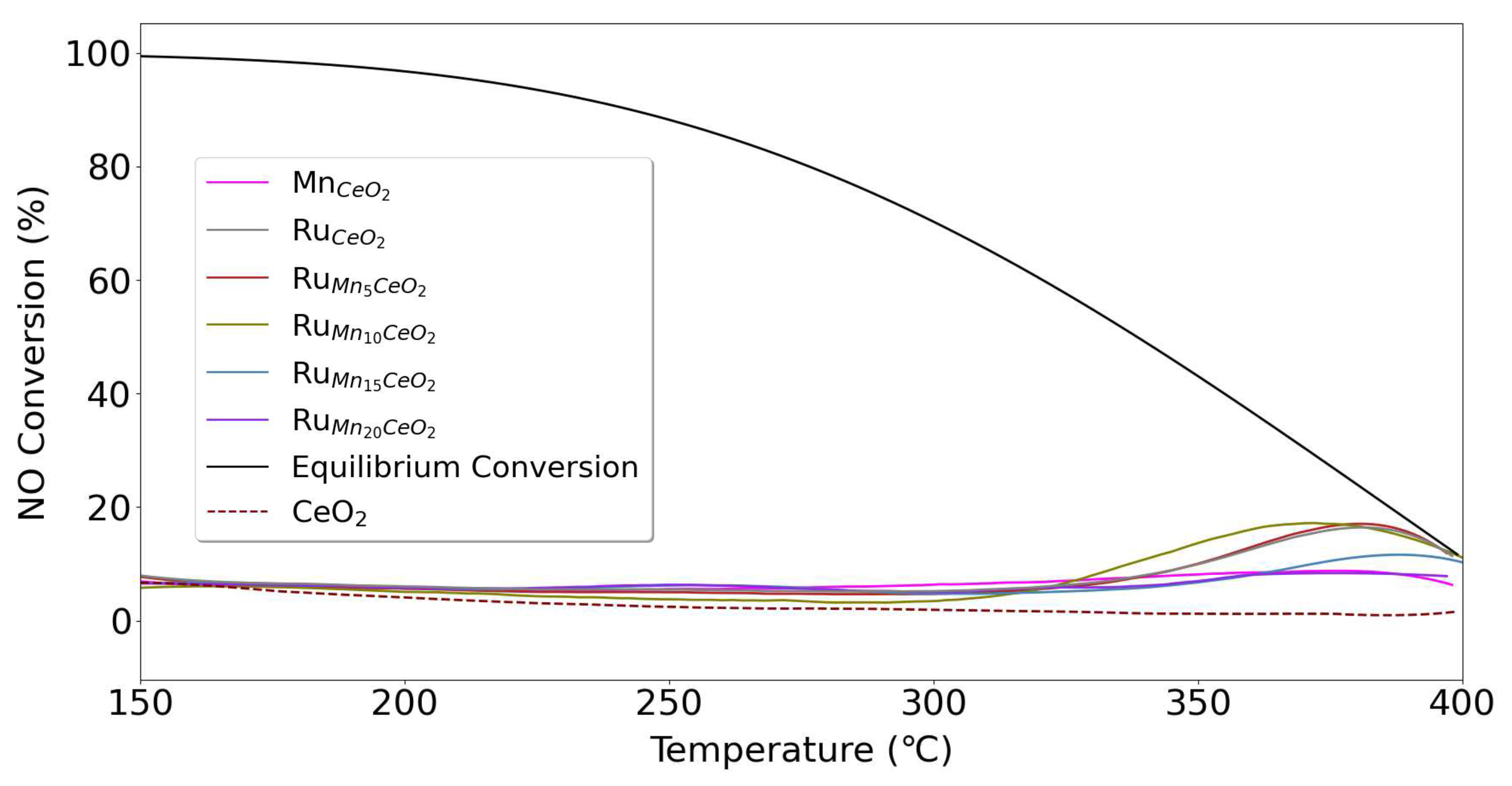
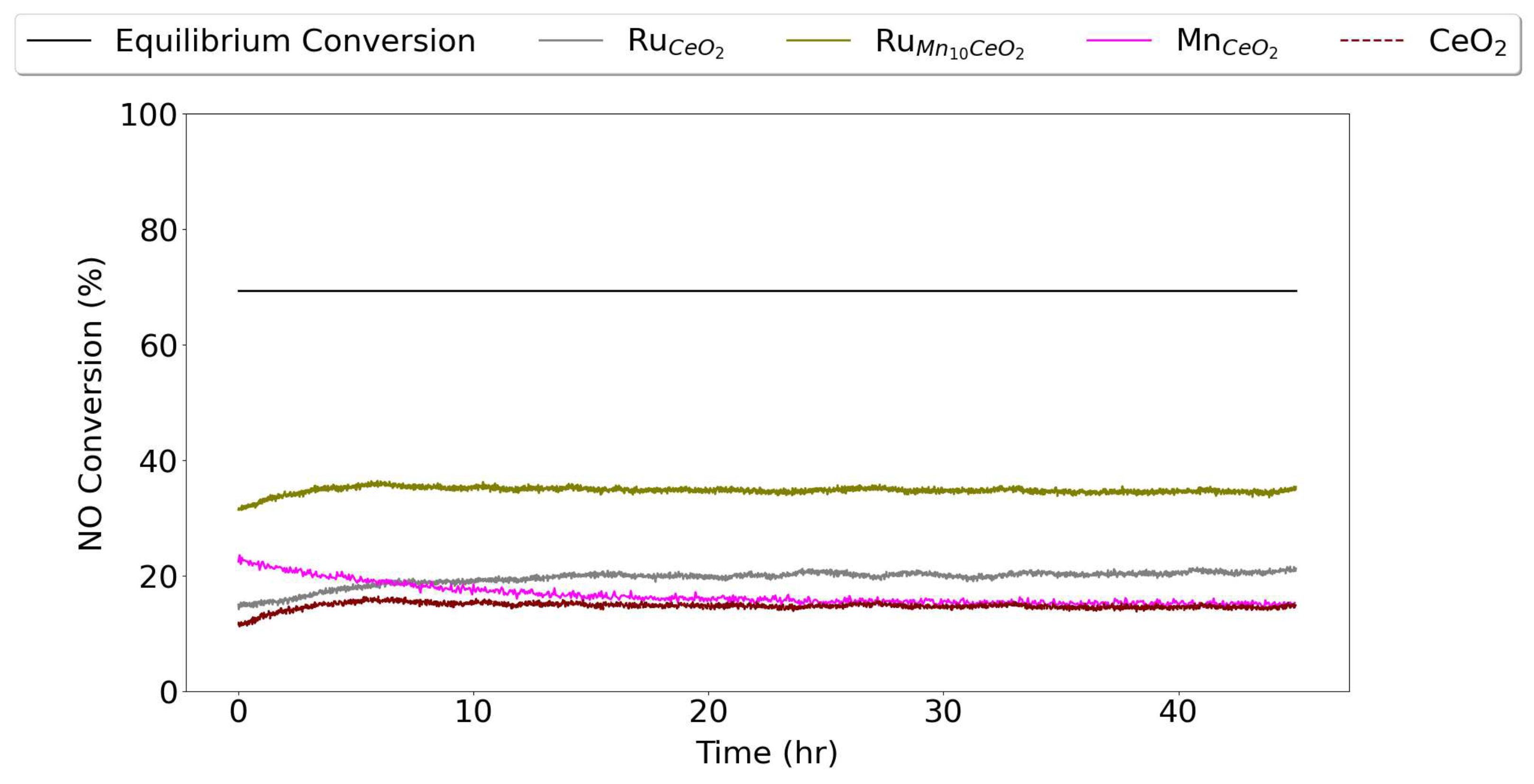
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAPEX | Capital Expenditure |
| PGM | Platinum Group Metals |
| WHSV | Weight Hourly Space Velocity |
References
- Honti, G.D. The Nitrogen Industry, 1976 ed.; Vol. 48, Akadémiai Kiadó, 1976. [CrossRef]
- Travis, A.S. Nitrogen Capture; Springer International Publishing: Cham, 2018. [Google Scholar] [CrossRef]
- Moulijn, J.A. Chemical process technology. Choice Reviews Online 2013, 51, 51–2107. [Google Scholar] [CrossRef]
- Grande, C.A.; Andreassen, K.A.; Cavka, J.H.; Waller, D.; Lorentsen, O.A.; Øien, H.; Zander, H.J.; Poulston, S.; García, S.; Modeshia, D. Process Intensification in Nitric Acid Plants by Catalytic Oxidation of Nitric Oxide. Industrial and Engineering Chemistry Research 2018, 57, 10180–10186. [Google Scholar] [CrossRef]
- YAO, H. Ceria in automotive exhaust catalysts I. Oxygen storage. Journal of Catalysis 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Su, E.; Montreuil, C.; Rothschild, W. Oxygen storage capacity of monolith three-way catalysts. Applied Catalysis 1985, 17, 75–86. [Google Scholar] [CrossRef]
- Courtois, X.; Bion, N.; Marécot, P.; Duprez, D. Chapter 8 The role of cerium-based oxides used as oxygen storage materials in DeNOx catalysis; 2007; pp. 235–259. [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO 2 -Based Materials. Chemical Reviews 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Inger, M.; Rajewski, J.; Ruszak, M.; Wilk, M. The influence of NOx presence on the catalytic N2O decomposition over the supported double-promoted cobalt spinel catalyst. Chemical Papers 2019, 73, 1979–1986. [Google Scholar] [CrossRef]
- Yung, M.M.; Holmgreen, E.M.; Ozkan, U.S. Cobalt-based catalysts supported on titania and zirconia for the oxidation of nitric oxide to nitrogen dioxide. Journal of Catalysis 2007, 247, 356–367. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Lv, Y.K. Catalytic oxidation of NO over MnO2 with different crystal structures. RSC Advances 2016, 6, 54032–54040. [Google Scholar] [CrossRef]
- Salman, A.U.R.; Hyrve, S.M.; Regli, S.K.; Zubair, M.; Enger, B.C.; Lødeng, R.; Waller, D.; Rønning, M. Catalytic Oxidation of NO over LaCo1-xBxO3 (B = Mn, Ni) Perovskites for Nitric Acid Production. Catalysts 2019, 9, 429. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Applied Catalysis B: Environmental 2013, 134-135, 251–257. [Google Scholar] [CrossRef]
- Olsson, L.; Persson, H.; Fridell, E.; Skoglundh, M.; Andersson, B. A Kinetic Study of NO Oxidation and NO x Storage on Pt/Al2O3 and Pt/BaO/Al2O3. The Journal of Physical Chemistry B 2001, 105, 6895–6906. [Google Scholar] [CrossRef]
- Mulla, S.S.; Chen, N.; Delgass, W.N.; Epling, W.S.; Ribeiro, F.H. NO 2 inhibits the catalytic reaction of NO and O 2 over Pt. Catalysis Letters 2005, 100, 267–270. [Google Scholar] [CrossRef]
- Smeltz, A.; Getman, R.; Schneider, W.; Ribeiro, F. Coupled theoretical and experimental analysis of surface coverage effects in Pt-catalyzed NO and O2 reaction to NO2 on Pt(111). Catalysis Today 2008, 136, 84–92. [Google Scholar] [CrossRef]
- Xue, E.; Seshan, K.; Ross, J.R.H. Roles of supports , Pt loading and Pt dispersion in the oxidation of NO to NO , and of SO , to SO , Applied Catalysis B: Environmental 1996, 11, 65–79. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Li, X. Catalytic oxidation of nitric oxide (NO) over different catalysts: an overview. Catalysis Science & Technology 2017, 7, 3440–3452. [Google Scholar] [CrossRef]
- Gopakumar, J.; Benum, P.M.; Svenum, I.H.; Enger, B.C.; Waller, D.; Rønning, M. Redox transformations of Ru catalyst during NO oxidation at industrial nitric acid production conditions. Chemical Engineering Journal 2023, 146406. [Google Scholar] [CrossRef]
- Gopakumar, J.; Vold, S.; Enger, B.C.; Waller, D.; Vullum, P.E.; Rønning, M. Catalytic oxidation of NO to NO 2 for industrial nitric acid production using Ag-promoted MnO2/ZrO2 catalysts. Catalysis Science & Technology 2023, 13, 2783–2793. [Google Scholar] [CrossRef]
- Salman, A.u.R.; Enger, B.C.; Auvray, X.; Lødeng, R.; Menon, M.; Waller, D.; Rønning, M. Catalytic oxidation of NO to NO2 for nitric acid production over a Pt/Al2O3 catalyst. Applied Catalysis A: General 2018, 564, 142–146. [Google Scholar] [CrossRef]
- William, C. Klingelhoefer, S. Nitric Oxide Oxidation, 1938.
- Heilig, M.L. Process of Oxidizing Gases, 1994. [CrossRef]
- Andersen, H.C.; Haley, A.J. Process for the oxidation of nitric oxide, 1963.
- Robertus J. M. Klein Gebbink.; Marc-Etienne Moret. Non-Noble Metal Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Critical Raw Materials Factsheets ( 2020). Technical report, 2020. [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering; 2006.
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.M.; Stockenhuber, M. On the Chemistry of Iron Oxide Supported on γ-Alumina and Silica Catalysts. ACS Omega 2018, 3, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Taher, N.M.; Mahmoudi, M.; Sajjadivand, S.S. Cobalt Catalysts Preparation and Characterization over Alumina Support for Fischer Tropsch Synthesis. Biofuels Engineering 2017, 2, 51–61. [Google Scholar] [CrossRef]
- Robert, C. Reuel.; Calvin H. Bartholomew. The stoichiometries of H2 and CO adsorptions on cobalt: Effects of support and preparation. Journal of Catalysis 1984, 85, 63–77. [Google Scholar] [CrossRef]
- Niu, J.; Liland, S.E.; Yang, J.; Rout, K.R.; Ran, J.; Chen, D. Effect of oxide additives on the hydrotalcite derived Ni catalysts for CO2 reforming of methane. Chemical Engineering Journal 2019, 377, 119763. [Google Scholar] [CrossRef]
- Kim, H.B.; Park, E.D. Ammonia decomposition over Ru catalysts supported on alumina with different crystalline phases. Catalysis Today 2023, 411-412, 113817. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Yang, X.; Duan, H.; Qi, H.; Su, Y.; Liang, B.; Tao, H.; Liu, B.; Chen, D.; Su, X.; Huang, Y.; Zhang, T. Tuning reactivity of Fischer–Tropsch synthesis by regulating TiOx overlayer over Ru/TiO2 nanocatalysts. Nature Communications 2020, 11, 3185. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.K.S.; Chen, L.; Du, Y.; Schreyer, M.; Daniel Ong, S.W.; Poh, C.K.; Hong, L.; Borgna, A. The role of metal–support interaction for CO-free hydrogen from low temperature ethanol steam reforming on Rh–Fe catalysts. Physical Chemistry Chemical Physics 2017, 19, 4199–4207. [Google Scholar] [CrossRef]
- Guerra-Que, Z.; Torres-Torres, G.; Pérez-Vidal, H.; Cuauhtémoc-López, I.; Espinosa de los Monteros, A.; Beltramini, J.N.; Frías-Márquez, D.M. Silver nanoparticles supported on zirconia–ceria for the catalytic wet air oxidation of methyl tert-butyl ether. RSC Advances 2017, 7, 3599–3610. [Google Scholar] [CrossRef]
- MCVICKER, G. Chemisorption properties of iridium on alumina catalysts. Journal of Catalysis 1980, 65, 207–220. [Google Scholar] [CrossRef]
- Bus, E.; Miller, J.T.; van Bokhoven, J.A. Hydrogen Chemisorption on Al2O3-Supported Gold Catalysts. The Journal of Physical Chemistry B 2005, 109, 14581–14587. [Google Scholar] [CrossRef]
- Kühn, F.E. Catalysis. From Principles to Applications. By Matthias Beller, Albert Renken and Rutger A. van Santen. Angewandte Chemie International Edition 2013, 52, 2650–2650. [Google Scholar] [CrossRef]
- Larsen, G. Principles and Practice of Heterogeneous Catalysis By J. M. Thomas (University of Cambridge) and W. J. Thomas (University of Bath). VCH: Weinheim, 1997. xxiii + 669 pp. DM88.00. ISBN 3-527-29239-X. Journal of the American Chemical Society 1997, 119, 11560–11560. [Google Scholar] [CrossRef]
- Bruneau, C.; Dixneuf, P.H. Ruthenium in catalysis; Vol. 48, 2014. [CrossRef]
- LI, H.; TANG, X.; YI, H.; YU, L. Low-temperature catalytic oxidation of NO over Mn-Ce-Ox catalyst. Journal of Rare Earths 2010, 28, 64–68. [Google Scholar] [CrossRef]
- Li, K.; Tang, X.; Yi, H.; Ning, P.; Kang, D.; Wang, C. Low-temperature catalytic oxidation of NO over Mn-Co-Ce-Ox catalyst. Chemical Engineering Journal 2012, 192, 99–104. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Lyu, Y.K. High catalytic activity of Mn-based catalyst in NO oxidation at low temperature and over a wide temperature span. Molecular Catalysis 2018, 454, 21–29. [Google Scholar] [CrossRef]
- Johnstone, A.H. CRC Handbook of Chemistry and Physics-69th Edition Editor in Chief R. C. Weast, CRC Press Inc., Boca Raton, Florida, 1988, pp. 2400, price £57.50. ISBN 0-8493-0369-5. Journal of Chemical Technology & Biotechnology 2007, 50, 294–295. [Google Scholar] [CrossRef]
- Takashima, T.; Hashimoto, K.; Nakamura, R. Mechanisms of pH-dependent activity for water oxidation to molecular oxygen by MnO2 electrocatalysts. Journal of the American Chemical Society 2012, 134, 1519–1527. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Q.; Chen, J.; Wang, L.; Lin, Y.; Wang, H.; Liu, X.; Shen, X.; Zhang, W.; Liu, W.; Qi, Z.; Jiang, Z.; Yang, J.; Yao, T. Dynamic oxygen adsorption on single-atomic Ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nature Communications 2019, 10. [Google Scholar] [CrossRef]

| Catalyst Name | Metal Precursor | Commercial Supplier |
|---|---|---|
| Cr | Cr(NO).9HO | Sigma Aldrich |
| Mn | Mn(NO).4HO | Sigma Aldrich |
| Fe | FeCl | Sigma Aldrich |
| Co | Co(NO).6HO | Sigma Aldrich |
| Ni | Ni(NO).6HO | Sigma Aldrich |
| Y | Y(NO).6HO | Sigma Aldrich |
| Zr | ZrO(NO).xHO | Sigma Aldrich |
| Nb | NbCl | Sigma Aldrich |
| Ru | RuCl.xHO | Sigma Aldrich |
| Ru | RuCl.xHO,Mn(NO).4HO | Sigma Aldrich |
| Ru | RuCl.xHO,Mn(NO).4HO | Sigma Aldrich |
| Ru | RuCl.xHO,Mn(NO).4HO | Sigma Aldrich |
| Ru | RuCl.xHO,Mn(NO).4HO | Sigma Aldrich |
| Rh | RhCl | Sigma Aldrich |
| Pd | PdCl | Sigma Aldrich |
| Ag | AgNO | Alfa Aesar |
| Sn | SnCl | Sigma Aldrich |
| Re | ReCl | Sigma Aldrich |
| Ir | IrCl | Merck |
| Pt | (Pt(NO)) | Alfa Aesar |
| Au | (HAuCl) | Sigma Aldrich |
| Pb | PbCl | Sigma Aldrich |
| Gd | Gd(NO).6HO | Alfa Aesar |
| Er | ClEr.6HO | Sigma Aldrich |
| Catalyst | Surface | Dispersion | Metal:Probe | Probe Gas |
|---|---|---|---|---|
| Area [m/g] | [%] | Specie | Uptake [mol g] | |
| CeO | 92 | − | − | − |
| Cr | 78 | − | − | − |
| Mn | 82 | − | − | − |
| Fe | 82 | 3% | 1:1 - Fe:H [29] | 2 |
| Co | 80 | 10% | 1:1 - Co:H [30,31] | 27 |
| Ni | 79 | 11% | 1:1 - Ni:H [32] | 33 |
| Y | 85 | − | − | − |
| Zr | 86 | − | − | − |
| Nb | 79 | − | − | − |
| Ru | 72 | 41% | 1:1 - Ru:CO [33,34] | 39 |
| Ru | 71 | 39% | 1:1 - Ru:CO [33,34] | 35 |
| Ru | 65 | 32% | 1:1 - Ru:CO [33,34] | 31 |
| Ru | 55 | 19% | 1:1 - Ru:CO [33,34] | 18 |
| Ru | 48 | 13% | 1:1 - Ru:CO [33,34] | 12 |
| Rh | 74 | 38% | 1:1 - Rh:H [35] | 39 |
| Pd | 72 | − | − | − |
| Ag | 70 | 29% | 1:1 - Ag:H [36] | 24 |
| Sn | 80 | − | − | − |
| Re | 75 | − | − | − |
| Ir | 76 | 37% | 1:1 - Ir:CO [37] | 35 |
| Pt | 75 | 43% | 1:1 - Pt:CO [21] | 42 |
| Au | 72 | 2% | 1:1 - Au:H [38] | 2 |
| Pb | 70 | − | − | − |
| Gd | 81 | − | − | − |
| Er | 77 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





