1. Introduction
Demethyleneberberine is one of the main metabolites of berberine in animals and humans [
1]. Berberine and demethyleneberberine are the main active components of Cortex Phellodendri, which is a traditional Chinese medicinal material derived from the bark of the Rutaceae plant, Winged Bark. This plant is commonly medicine and food homology. It can effectively promote the secretion of bile and pancreatic juice, facilitating the rapid excretion of bilirubin from the body. Thus, it can be used to treat and protect against liver diseases [
2]. Moreover, Phellodendron extract also exhibits antibacterial [
3,
4], anti-inflammatory activities [
5,
6,
7] and demonstrates various pharmacological effects, such as reducing the risk of diabetes [
8] and protecting gastric ulcers [
9].
In recent years, numerous studies have shown that berberine has a variety of pharmacological activities, including antibacterial [
10] and anti-inflammatory [
11,
12]. Additionally, research has increasingly indicated that berberine can effectively lower blood lipids to reduce the incidence of cardiovascular diseases [
13,
14] and prevent the development of atherosclerosis [
15,
16]. It provides cardiovascular protection and prevents the development of atherosclerosis. Interestingly, the gut microbiota plays a crucial role in mediating the cardiovascular protection provided by berberine [
17,
18,
19]. Importantly, berberine has demonstrated promising potential effects in various areas, such as anti-cancer [
20,
21,
22], anti-diabetic [
23,
24,
25], anti-rheumatoid [
26], anti-obesity [
27,
28] and pro-bone regeneration [
29,
30]. It also exhibits certain protective effects on the liver [
31,
32], lung function [
33,
34] and nerves [
35,
36]. Studies have found that despite berberine’s significant therapeutic potential, its plasma bioavailability is extremely low, ranging from 0.37%~0.68% [
37,
38]. First-pass elimination and malabsorption may be some of the factors that contribute to the low bioavailability of ingested berberine [
39]. However, demethyleneberberine, the primary metabolite of berberine in vivo, has frequently been found at high plasma concentrations in studies, which has led to increased attention for berberine metabolites. Therefore, further research is underway to explore new ways to overcome the challenge of low bioavailability of berberine.
Demethyleneberberine, a metabolite of berberine, also exhibits similar anti-inflammatory activity [
40,
41,
42]. According to research reports, in vivo and in vitro, demethyleneberberine exhibits significant antioxidant effects and exerts hepatoprotective and anti-fibrosis effects through various pathways [
43,
44,
45]. Additionally, demethyleneberberine has significant therapeutic effects on pulmonary fibrosis in mice [
46]. Moreover, demethyleneberberine can also promote cancer cell apoptosis, inhibit cell proliferation [
47,
48], and performs better than berberine in interacting with LOX-5/COX-2 to improve benign prostatic hyperplasia [
49]. Furthermore, demethyleneberberine can activate AMP-activated protein kinase (AMPK) and up-regulate the expression of hepatic low-density lipoprotein receptor, thereby achieving lipid-lowering effects [
50,
51,
52]. Notably, studies at the cellular level have demonstrated that demethyleneberberine has pharmacological activity on the central nervous system through several signaling pathways (such as NF-κB, MAPK and AMPK) [
53,
54]. Therefore, demethyleneberberine has significant research value. However, there is still a lack of comprehensive pharmacokinetic data on demethyleneberberine absorption, distribution and excretion in animals. To fully comprehend the biological role of demethyleneberberine in vivo, our study aimed to evaluate the absorption of demethyleneberberine in rats and mice via single oral administration and tail vein injection. Meanwhile, the tissue distribution and excretion of demethyleneberberine in rats and mice were also investigated after oral administration.
2. Results and Discussion
2.1. Method validation
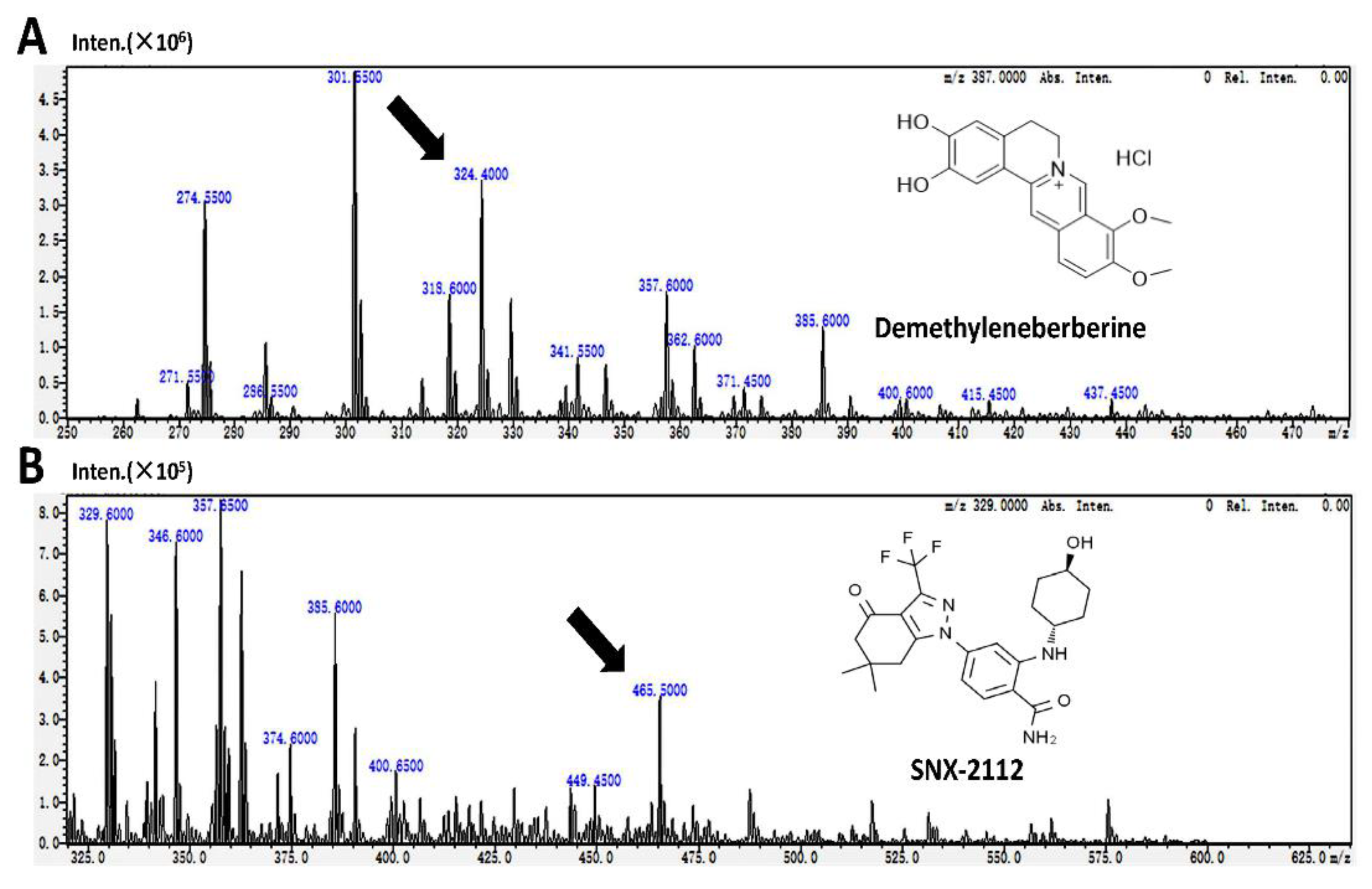
The detection of demethyleneberberine and SNX-2112 (internal standard, IS) was accomplished at ion pairs m/z 324.4 → 308.35 and m/z 465.5 → 350.35 respectively (
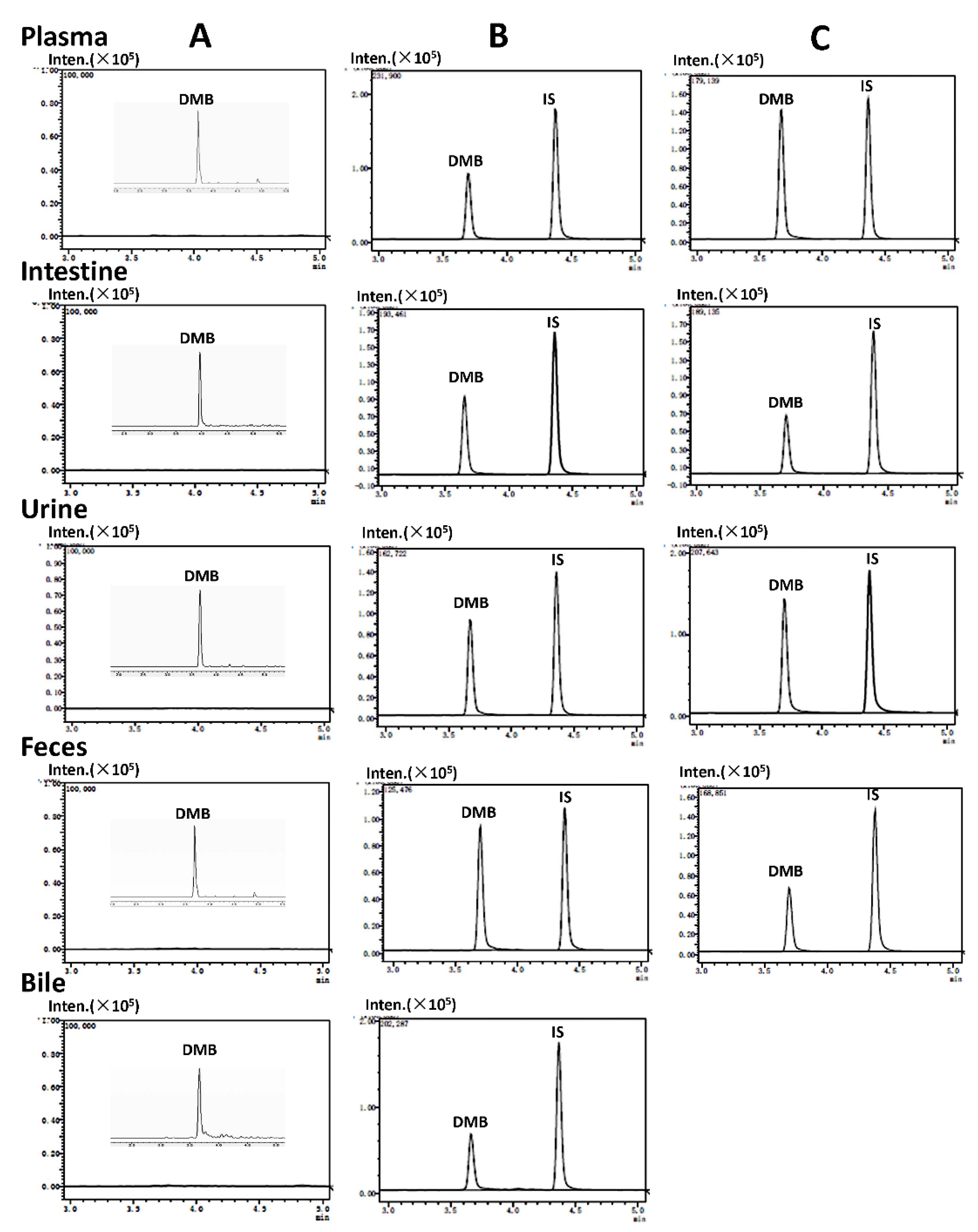
Figure 1), with subsequent quantitative analysis. The research results showed that demethyleneberberine and SNX-2112 could be effectively separated, and no endogenous interfering substances were found at the retention time of demethyleneberberine or SNX-2112. The multiple reaction monitoring (MRM) mass spectrum of the blank biological sample spiked with demethyleneberberine and SNX-2112 is shown in
Figure 2. The calibration curves, correlation coefficients and linear ranges of demethyleneberberine in plasma, various tissues and excreta are listed in
Table 1. The standard curve formed between the peak area ratio (Y) of demethyleneberberine to SNX-2112 and the concentration (X) of demethyleneberberine was well linear in all biological samples. The regression coefficients (r
2) were all higher than 0.9911. The limit of detection (LOD) and limit of quantitation (LOQ) of demethyleneberberine were 0.1 and 0.5 ng/mL in plasma and 0.1 and 3 ng/mL in other biological samples.
The corresponding quality control (QC) samples at three concentration levels (10 ng/ml, 200 ng/ml, 800 ng/ml) were utilized to determine the precision of intraday and interday measurements (RSD), extraction recovery, as well as matrix effect. The method presented in this study was evaluated in plasma, tissue, bile, urine and feces. The measurement results of demethyleneberberine are shown in
Table 2. The intra-day RSD ranged from 0.49% to 10.39%, while the inter-day RSD ranged from 0.50% to 14.19%. The intra-day and inter-day accuracies of all samples were within ± 15%. The extraction recoveries of all analytes in all samples were between 75.52% and 98.97%, with the RSD within ± 7.60%. Except for the bile, the matrix recovery of all analytes in all samples ranged from 82.95% to 113.51%, with the RSD within ± 9.68% (
Table 3). Potential inhibition of demethyleneberberine ionization by bile components or other factors should be considered. However, the matrix effect of each concentration of bile is similar, with the RSD within ± 1.28%, indicating that the determination of demethyleneberberine concentration in bile is not impacted and can be accurately measured. These results indicated that the method is suitable for the detection of demethyleneberberine in plasma, tissue, urine, feces and bile.
The stability study was conducted for the analysis of the extracted samples under different conditions, including room temperature, three freeze-thaw cycles, long-term (30 days) storage at −80 °C, in the auto-sampler (30℃) for 12 h, and on ice for 2 h. All sample concentrations, except for the extracted samples of intestine and feces at room temperature, were less than ± 15% of their nominal values. This may be due to the presence of some metabolic enzymes or intestinal microorganisms in the intestine, urine and feces, which can easily degrade demethyleneberberine at room temperature. Therefore, the determination of demethyleneberberine in biological samples requires sample pretreatment on ice and rapid processing (2 h deviation less than ± 15%), indicating that the samples were stable and could meet the requirements of the analysis.
2.2. Pharmacokinetic Parameters and Bioavailability
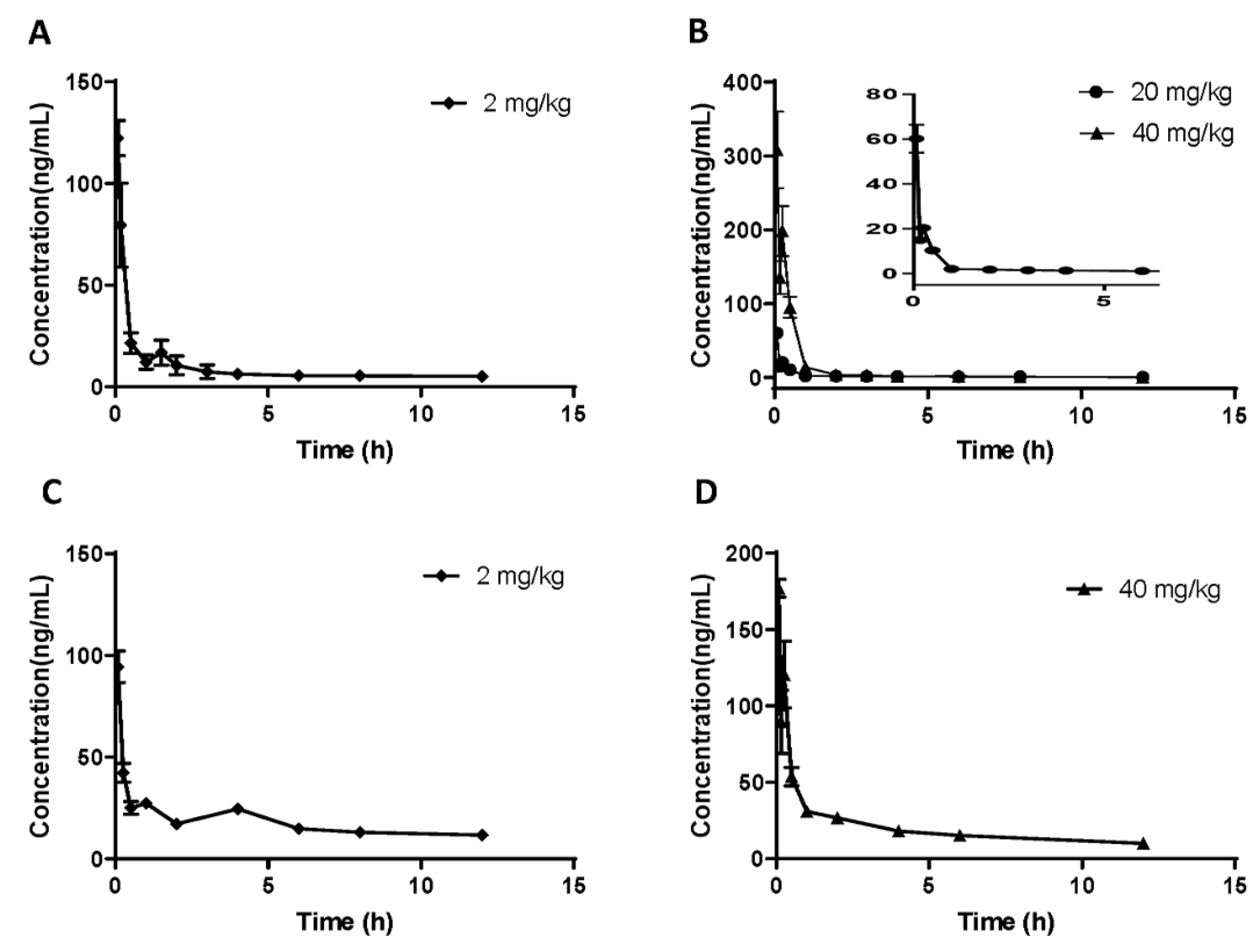
In order to determine the oral bioavailability of demethyleneberberine, three different doses (i. v. 2 mg/kg and i.g. 20 and 40 mg/kg) were administered to rats; while C57BL/6J mice were given two different doses (i. v. 2 mg/kg and i.g. 40 mg/kg). The plasma concentration-time curves of demethyleneberberine administration were recorded and analyzed (
Figure 3).
The corresponding pharmacokinetic parameters for rats and mice, fitted using non-compartmental analysis, were listed in
Table 4 and
Table 5, respectively. After administering 20 and 40 mg/kg of demethyleneberberine to rats intragastrically, its Peak Concentration (C
max) and Area Under Curve (AUC
0-∞) were 60.22 ± 12.53 ng/mL and 29.83 ± 2.14 ng•h/mL for the 20 mg/kg group and 308.25 ± 103.86 ng/mL and 133.36 ± 33.20 ng•h/mL for the 40 mg/kg group, respectively. Similarly, after administering 40 mg/kg to mice, its C
max and AUC
0-∞ were respectively 177.15 ± 11.73 ng/mL and 264.61 ± 25.01 ng•h/mL, overall, which showed nonlinear pharmacokinetic characteristics. The intragastric (i.g.) administration of 20 mg/kg of the drug was found to have a half-life (t
1/2) of 4.26 ± 1.48 h in rats; whereas the t
1/2 of the 40 mg/kg group was 3.14 ± 0.42 h in rats and 4.10 ± 0.81 h in mice. Additionally, the t
1/2 of the 2 mg/kg dose group was 5.57 ± 0.83 h in rats and 6.80 ± 1.31 h in mice.
These results suggest that demethyleneberberine is rapidly absorbed from the gastrointestinal tract and rapidly cleared from plasma. It has been reported that after oral administration of berberine (at a dosage of 48.2 mg/kg) [
37], relatively rapid berberine absorption (T
max, 2.75 ± 2.95 h) was observed. Demethyleneberberine is absorbed significantly faster from the gastrointestinal tract (T
max, 0.08 ± 0.00 h) than berberine. In addition, the oral bioavailability of demethyleneberberine is relatively low, with a value of 2.44% at 20 mg/kg in rats, 5.92% at 40 mg/kg, and 4.47% at 40 mg/kg in mice. The results showed that the bioavailability of rats and mice was comparable. Compared with berberine (0.37%~0.68%) [
37,
38], the bioavailability of demethyleneberberine was relatively higher. If necessary, additional chemical structural modifications and pharmacological studies may be performed to increase intestinal absorption and improve demethyleneberberine bioavailability. The plasma concentration-time curves of demethyleneberberine in both rats and mice have double peaks, indicating that there may be a phenomenon of enterohepatic circulation. Related studies have found that berberine metabolites can be reabsorbed through hepatic circulation, which once again confirms the phenomenon of enterohepatic circulation [
1].
2.3. Tissue distribution study
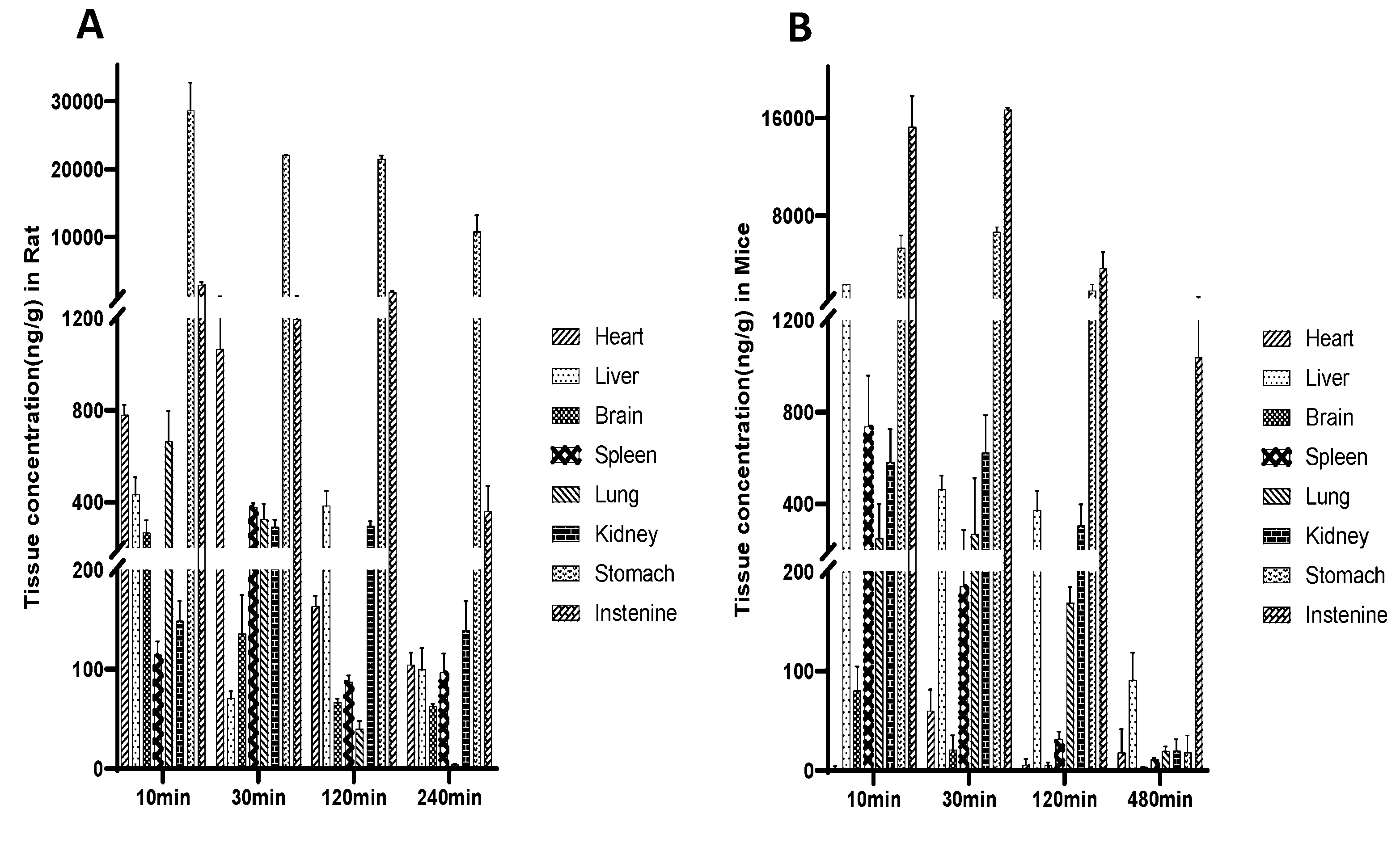
To investigate the distribution of demethyleneberberine in different tissues, we conducted a tissue distribution study in rats and mice following a single oral dose of 40 mg/kg. The concentration of demethyleneberberine in various tissues of rats was measured at 10 min, 30 min, 120 min and 240 min after administration. The concentration of demethyleneberberine in different tissues was measured at 10 min, 30 min, 120 min and 480 min after administration in mice. The results are presented in
Figure 4.
Demethyleneberberine was rapidly distributed to all examined tissues in rats within 10 min of administration, and its highest concentrations were detected in the stomach (28585.86 ng/g) and intestine (2934.97 ng/g). In addition to the gastrointestinal tract, the peak concentration of demethyleneberberine in other tissues is the heart (1066.17 ng/g), lung (664.85 ng/g), liver (432.50 ng/g), spleen (378.39 ng/g), kidney (294.99 ng/g), and brain (267.56 ng/g). After administration in mice, the order of demethyleneberberine concentrations was as follows: intestine (15254.14 ng/g), stomach (5347.30 ng/g), liver (2371.47 ng/g), spleen (737.43 ng/g), kidney (623.73 ng/g), lung (268.53 ng/g), brain (80.30 ng/g),and heart (60.02 ng/g).
We found that, regardless of rats or mice, the Cmax of demethyleneberberine in the digestive tract was much higher than that in other organs, indicating that the gastrointestinal tract may be the main organ of absorption. However, we cannot rule out the possibility that the higher Cmax in the digestive tract was partly due to the presence of undigested demethyleneberberine in the lumen. The results of our experiment showed that in both rats and mice, demethyleneberberine could pass through the blood-brain barrier (BBB) and enter the brain, further confirming the pharmacological activity of demethyleneberberine in the central nervous system [
54]. In rats, the concentration of demethyleneberberine in the heart peaked at 30 min and reached a lower level after 240 min, while other tissues also reached a lower level after 240 min. In mice, the liver reached the peak at 10 min, and reached a lower level after 480 min, while other tissues reached very low levels after 480 min. Whether it was a rat or a mouse, as the administration time was prolonged, the concentration in each tissue has begun to decrease at 30 min after administration and has significantly decreased at 120 min. Thus, the results indicate that there is no long-term accumulation of demethyleneberberine in various tissues.
In rat tissue distributions, demethyleneberberine is concentrated in the heart, lung and liver. The results showed that demethyleneberberine concentration in the heart was high within half an hour, Previous studies have shown a cardiovascular protective effect of berberine and it is possible that demethyleneberberine also has a similar effect, but no such report has emerged [
14]. Moreover, the high concentration state of lung tissue may serve as a crucial factor in determining the pharmacological effects related to the lungs [
46]. The changes in the concentration of demethyleneberberine in the liver over time showed a bimodal trend, supporting the bimodal phenomenon of demethyleneberberine in plasma and confirming the possible existence of enterohepatic circulation. In mice, slightly different from rats, in addition to the gastrointestinal tract, demethyleneberberine is concentrated in the liver, spleen and kidneys, and the highest distribution in the liver (2371.47 ng/g) was more than three times that of other tissues, which may be associated with the hepatoprotective effects of demethyleneberberine on treating hepatitis and preventing liver fibrosis in mice [
43,
44,
45]. Admittedly, it is not excluded that the strong metabolism of demethyleneberberine in the liver, which causes the first-pass effect to reduce the absorption of drugs in plasma. The difference in tissue distribution between rats and mice may be caused by species differences or by inconsistent distribution of enzymes and transporters.
2.4. Excretion study
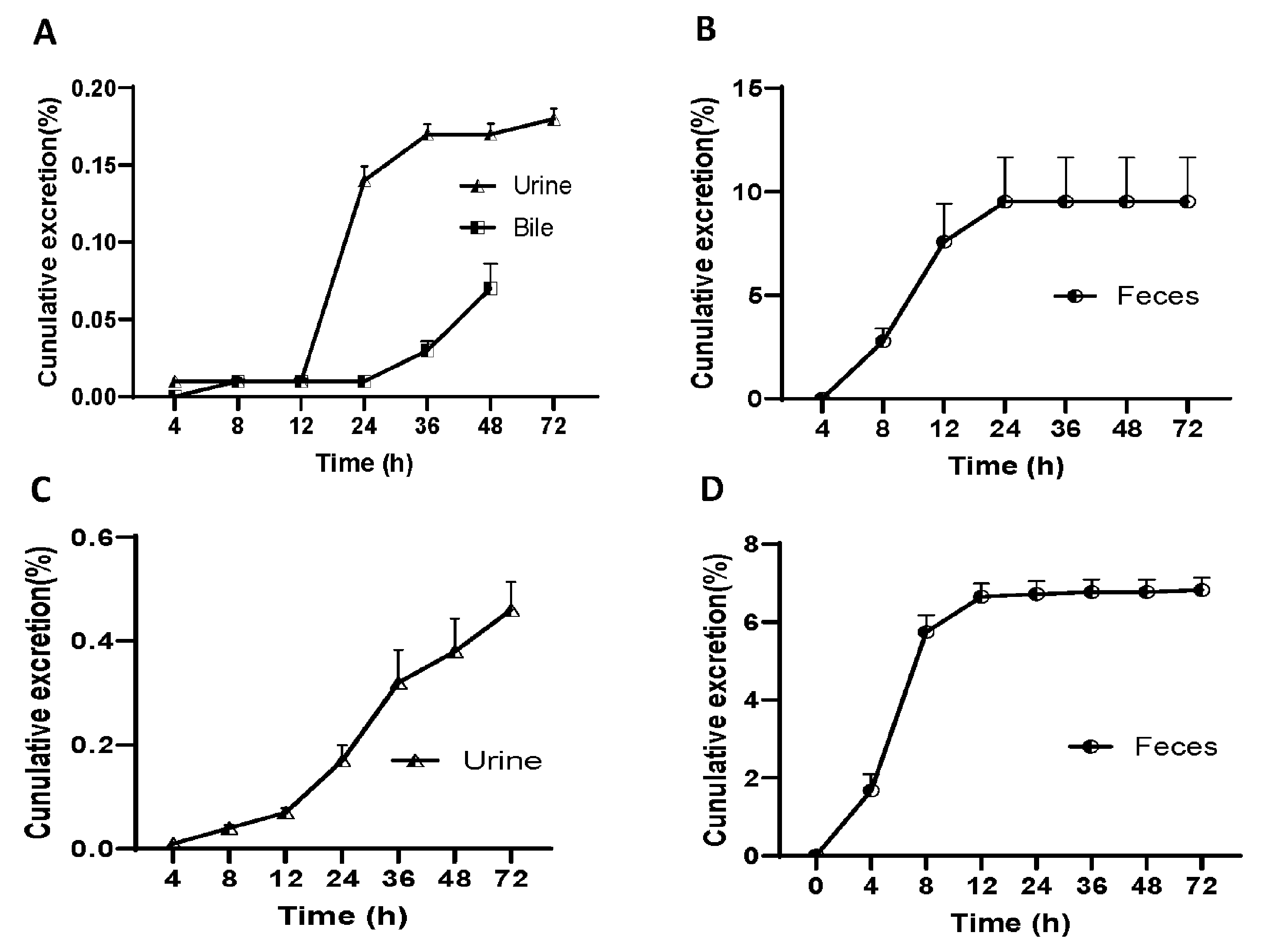
In order to study the elimination of demethyleneberberine, we conducted an experiment on rats by administering a single oral dose of demethyleneberberine (40 mg/kg) to measure its excretion in bile, urine, and feces, and the results are shown in
Figure 5.
At different time intervals, the results of the experiment demonstrated that the concentration of demethyleneberberine in urine samples peaked at 12-24 h and gradually decreased to 42μg/mL at 36-72 h. The highest concentration of demethyleneberberine in stool samples was found around 8-12 h. The total excretion of demethyleneberberine in urine, feces and bile was 9.77% (0.18%, 9.52% and 0.07% for urine, feces and bile, respectively). We also conducted a similar experiment on mice and measured their urine and feces. Like the results from rats, the concentration of demethyleneberberine in urine samples reached the peak at 12-24 h and gradually decreased to 163μg/mL at 36-72 h. However, the highest concentration of demethyleneberberine in stool samples was found around 4-8 hours, which was faster than observed in rats. The total excretion of demethyleneberberine in urine and feces was 7.28% (0.46% and 6.82% for urine and feces, respectively).
The results showed that the recovery rate of demethyleneberberine from rat feces was <10%, and the recovery rate from mouse feces was <8%, which was similar in both rats and mice. This indicates a lower absorption and permeability of demethyleneberberine, with the possibility that it may not be fully absorbed. Furthermore, the low blood concentration of demethyleneberberine and the low urine drug recovery rate further confirm the likelihood of malabsorption. The lower recovery rate in bile suggests that demethyleneberberine is likely to be affected by the first-pass effect, with some of the absorbed demethyleneberberine metabolized to other forms in the liver. This finding is consistent with a higher distribution in the stomach, intestine and liver. Therefore, these above results suggest that the liver may play an important role in the elimination of demethyleneberberine, but further studies are needed to determine whether drug metabolism is the main reason for the low recovery of demethyleneberberine.
3. Materials and Methods
3.1. Chemicals and reagents
Demethyleneberberine was obtained from Dr. Tao's laboratory [
54] and SNX-2112 was purchased from Aladdin (China). Acetonitrile (Thermo Fisher Scientific, US), methanol (Thermo Fisher Scientific, US), formic acid (Macklin, China), and dimethyl sulfoxide (DMSO, Aladdin, China) were of chromatographically pure grade, while the rest were of analytical grade.
3.2. Animals
Healthy male SD rats (220-250 g) and C57BL/6 mice (18-25 g), 6-week-old, were purchased from Guangdong Weitong Lihua Experimental Animal Technology Co., Ltd. (Foshan, China). The objective of this study was to investigate the pharmacokinetics, distribution and excretion of demethyleneberberine. All animals were maintained in a controlled environment (temperature 22 ± 3°C, 40-60% relative humidity, 12 h light-dark cycle, 06:00-18:00). All experimental animals were housed under the above conditions for a week to adapt to the environment. Furthermore, the animals were subjected to an overnight fasting period before the experiment, while water intake remained unrestricted. The experimental protocol was approved by the National Laboratory Animal Center (Guangdong Medical University, Dongguan, Guangdong), and the care of the animals complied with the Guangdong government's Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by Guangdong Medical University.
3.3. Instruments and analytical conditions
Samples were analyzed by LC-MS/MS-8045 (Shimadzu, Japan) using Labsolutions LC-MS/MS Ver.5.1 collection workstation software. The separation was performed on a Shim-pack GIST C-18 chromatographic column (2.1 × 100 mm, 3 μm). The sample was eluted with a mixture of 0.1% formic acid (A) and acetonitrile (B). The gradient was maintained at 20% B for 1 min, then increased linearly from 20 to 90% B over 1.5 min and maintained at 90% B for 2.5 min. The temperature of the column oven was 30°C. The flow rate was 0.3 mL/min, and the injection volume was 1 μL. The autosampler performed a needle wash for 5 s after each injection. MS detection was performed in positive ionization mode with an electrospray ionization (ESI) source. High-purity nitrogen was used as the nebulizing and drying gas. The positive ion electrospray ionization mode was employed in the detection process using multiple reaction monitoring (MRM) technology. Other conditions were defined as follows: desolvation gas flow rate 10 L/min; ion spraying voltage 4000 V; interface temperature 300 °C.
3.4. Standard and sample preparation
3.4.1. Preparation of stock and working solutions
Stock solutions of demethyleneberberine (1 mg/mL) and SNX-2112 (1 mg/mL) were prepared in acetonitrile-water (50/50, v/v), respectively. To prepare the working standard solution, a series of standard solutions at 0.003, 0.01, 0.03, 0.10, 0.3, 1.00, 3.00, 10.00, 30.00, and 50.00 μg/mL were prepared by diluting the stock solution of demethyleneberberine with acetonitrile-water (50/50, v/v). The stock solution of SNX-2112 was diluted with acetonitrile-water (50/50, v/v) to prepare a 0.04 μg/mL solution for protein precipitation. All solution of demethyleneberberine was stored in the dark at -20°C, and the solution of SNX-2112 was sealed and stored at 4°C.
3.4.2. Preparation of standard and quality control (QC) samples
Appropriate amounts of working solutions were added to blank biological matrices. Three QC samples (high: 800 ng/mL; middle: 200 ng/mL; low: 10 ng/mL) were independently prepared for each sample. Standards and QC samples should be freshly prepared before use to ensure accuracy.
3.5. Methodology Validation
Method validation tests were performed in accordance with the currently recognized Chinese State Food and Drug Administration (SFDA) bioanalytical method validation guidelines [
55]. The biological matrix used a mixed matrix of both rats and mice. The tests included investigation of selectivity, linearity, precision, accuracy, extraction recovery, matrix effect and stability. Each was evaluated individually to confirm the validity of the method.
The selectivity of the method was assessed by analyzing five different sources of the blank biological matrix, including plasma, intestine, urine, feces and bile. The calibration curves, described as Y = aX + b, were assessed by plotting the peak-area ratios of demethyleneberberine. The limits of detection (LOD, S/N ≥3) and quantification (LOQ, S/N ≥10) were determined at the lowest detectable and quantifiable concentrations of the calibration curve. Precision (the relative standard deviation, RSD) and accuracy (the relative error, RE) were evaluated by analyzing QC samples at three concentration levels (low, medium and high concentrations) by the use of five replicates within three consecutive days. The extraction recovery (more than 75%) and the matrix effects (deviation between 80% and 120%) of demethyleneberberine were evaluated by comparing the peak areas of QC samples and the blank bio-samples which were firstly extracted with methanol and then spiked with standards, as well as comparing the peak areas of QC samples and dissolved in water at the three same concentrations. The stability study assessed for the dilution effect of the extracted samples under different conditions (deviation between 85% and 115%), including room temperature, three freeze-thaw cycles, long-term (30 days) storage at −80 °C, in the auto-sampler (30℃) for 12 h and on ice for 2 h.
3.6. Experimental
3.6.1. Pharmacokinetics and Bioavailability
For the pharmacokinetic study, SD rats (five per group) were divided into three groups. The first and second groups were given 40 mg/kg and 20 mg/kg of demethyleneberberine by intragastric administration respectively, and the third group was given 2 mg/kg of demethyleneberberine by tail vein injection. Demethyleneberberine was suspended in 5% carboxymethylcellulose sodium (CMC-Na) for oral administration, and was dissolved in saline containing 10% Tween-80 and 5% DMSO for intravenous injection. After oral administration of demethyleneberberine, an aliquot of blood was collected from blood from each animal at the scheduled time points: 0.083, 0.167, 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h. For the intravenous injection group, the scheduled time points were 0.083, 0.167, 0.25, 0.5, 1, 2, 3, 4, 6, 8 and 12 h. Blood samples were collected through the orbital venous plexus of the same animal and transferred to heparinized tubes. After centrifugation at 8,000 g for 10 min at 4 °C, the supernatant (i.e., plasma) was collected and stored in -80 °C refrigerator.
The mouse pharmacokinetic experiment was carried out in the same way. C57BL/6 mice (fifteen per group) were divided into two groups. The mice were administered demethyleneberberine by intragastric 40 mg/kg or intravenous 2 mg/kg. After oral administration at the scheduled time points: 0.083, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h. For the intravenous injection group, the scheduled time points were 0.083, 0.167, 0.25, 0.5, 1, 2, 4, 6, 8 and 12 h. Blood was collected through the orbital venous plexus of mice, and then operated in the same way as rats.
50 μL plasma sample was mixed with 250 μL acetonitrile containing 0.04 μg/mL SNX-2112 (internal standard, IS) for protein precipitation, vortex for 2 min. After centrifugation at 12,000 g for 15 min at 4 °C, the collected supernatant was transferred to a new EP tube, and the solvent was evaporated using a Concentrator plus (Eppendorf, Deutschland). The dried supernatant was redissolved in 200 μL methanol/water (50/50, v/v) mixture, vortexed for 2 min, and then centrifuged at 12,000 g for 10 min at 4°C. The supernatant was transferred to a liquid phase vial prior to high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Pharmacokinetic parameters were calculated using DAS 2.0 software (Mathematical Pharmacology Professional Committee of China). The absolute oral bioavailability was calculated by comparing the AUC0-t of demethyleneberberine after oral administration and intravenous injection.
3.6.2. Distribution experiment
In the distribution experiment, SD rats (five per group) were divided into four groups. Rats were orally given demethyleneberberine 40 mg/kg (suspended in 0.5% CMC-Na). After the blood was cleared via the aorta, the rats were sacrificed within 10 min, 30 min, 120 min and 240 min. The tissue samples, including the heart, spleen, lung, liver, kidney, brain, stomach and small intestine, were collected, rinsed with normal saline, and blotted dry. Tissue samples were stored at -80°C until analysis. For extraction, the organ was cut into small pieces, weighed before being homogenized in 2 volumes (v/w) of normal saline. The homogenized rat tissue was ground using a JXFSTPRP-24L (Shanghai Jingxin, China) at 60 HZ for 120 s.
The mouse distribution experiment was carried out in the same way, mice (five per group) were divided into four groups. And mice were orally given demethyleneberberine 40 mg/kg. The mice were sacrificed within 10 min, 30 min, 120 min and 480 min. The same method was performed as the rats.
50 μL tissue homogenate was mixed with 250 μL acetonitrile containing 0.04 μg/mL SNX-2112 for protein precipitation. Individual tissue samples were processed using similar procedures as those used for plasma samples, and then subjected to LC-MS/MS analysis.
3.6.3. Excretion experiment
For the excretion experiments, rats (n=5) were orally given demethyleneberberine 40 mg/kg (suspended in 0.5% CMC-Na) and housed individually in metabolic cages. Urine and fecal samples were collected at the following time intervals: 0-4, 4-8, 8-12, 12-24, 24-36, 36-48 and 48-72 h. For the bile collection experiment, 5 rats were given demethyleneberberine 40 mg/kg by intragastric administration, and the rats underwent bile duct cannulation to collect bile. Bile samples were collected at the following intervals: 0-4, 4-8, 8-12, 12-24, 24-36 and 36-48 h. Measurements were taken of the volume of urine and bile, as well as the weight of feces samples at each time point prior to extraction. Each sample was stored at -80°C until reprocessing.
The mouse excretion experiment was conducted in the same manner as before. Nine mice were administered orally with demethyleneberberine at a dosage of 40 mg/kg. They were randomly divided into three groups (three per group) and housed in metabolic cages. Urine and stool samples were collected at the following intervals: 0-4, 4-8, 8-12, 12-24, 24-36, 36-48 and 48-72 h. Each sample was stored at -80°C until reprocessing.
Fecal samples were allowed to dry before pulverization, weighed before being homogenized in 5 volumes (v/w) of 50% methanol aqueous solution. The fecal samples were ground at 60 HZ for 120 s. Then, they underwent ultrasonic extraction for 10 minutes and centrifugation at 12,000 g for 15 min at 4°C, and the supernatant was collected and transferred to a new tube. 50 μL sample solution was mixed with 250 μL acetonitrile containing 0.04 μg/mL SNX-2112 for protein precipitation. Individual tissue samples were processed using similar procedures as those used for plasma samples, and then subjected to LC-MS/MS analysis.
4. Conclusions
In conclusion, our study established and verified the LC-MS/MS method for the determination of demethyleneberberine, and investigated the pharmacokinetics, tissue distribution and excretion of demethyleneberberine in rats and mice. This study provides a reliable and cost-effective method for the determination of demethyleneberberine in a variety of biological matrices. The pharmacokinetic study investigated the concentration of demethyleneberberine at different time intervals after intragastric administration or intravenous injection. The absolute oral bioavailability of demethyleneberberine in rats ranged from 2.44% to 5.92%, and that in mice was 4.47%. Tissue distribution analysis revealed rapid and widespread distribution of demethyleneberberine in the tissues of both rats and mice. In rats and mice, a significant amount of demethyleneberberine was distributed in the gastrointestinal tissue, which showed that demethyleneberberine may be absorbed through the gastrointestinal tissue. Moreover, in addition to the gastrointestinal tract, demethyleneberberine was found to be primarily concentrated in the heart, kidney and liver of rats, and in the liver of mice. In addition, less than 10% of demethyleneberberine was excreted in bile, urine or feces. Collectively, the present findings enhance understanding of the biological functions of demethyleneberberine in vivo. These insights can be helpful not only in further deepening our understanding of the potential applications of desmethylberberine in treating certain diseases, but the research also plays a critical role in evaluating if the effects in vitro of desmethylberberine can be successfully translated in vivo.
5. Abbreviations
LC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; LC, high- performance liquid chromatography; MRM, multiple reaction monitoring; IS, internal standard; AUC, Area Under Curve; RSD, Relative standard deviation; LOD, limit of detection; LOQ, limit of quantitation; t1/2, Half-life; Tmax, peaktime; Cmax, Peak Concentration.
Author Contributions
Participated in research design: J.L., C.L. and Y.T.; Conducted experiments: J.L., Q.Z. and Y.C.; Performed data analysis: J.L., Q.Z. and Y.T.; Wrote or contributed to the writing of the manuscript: J.L. and Y.T.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81903905), and Doctoral Start-up Foundation of Guangdong Medical University (No. 4SG22211G) and Province Universities and Colleges Pearl River Scholar Fund (No. 4SG22006G).
Informed Consent Statement
The animal study protocol was approved by Guangdong Medical University (Dongguan, Guangdong) Laboratory Animal Welfare Ethical Review Committee.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of demethyleneberberine were available from Dr. Tao Cheng, College of Pharmacy, Guangdong Medical University.
References
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug metabolism and disposition: the biological fate of chemicals 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhu, J. X.; Luo, G. M.; Li, L.; Zhu, Y. Y.; Zeng, J. X.; Wang, X. Y.; Wu, B. [Study on the liver-protective and choleretic effect of zhizi baipi soup and its disassembled prescription]. Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials 2013, 36, 1132–1135. [Google Scholar]
- Xu, B.; Yan, Y.; Huang, J.; Yin, B.; Pan, Y.; Ma, L. Cortex Phellodendri extract's anti-diarrhea effect in mice related to its modification of gut microbiota. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2020, 123, 109720. [Google Scholar]
- Yin, M. C.; Chang, C. H.; Su, C. H.; Yu, B.; Hsu, Y. M. Pteris multifida, Cortex phellodendri, and probiotics attenuated inflammatory status and immunity in mice with a Salmonella enterica serovar Typhimurium infection. Bioscience, biotechnology, and biochemistry 2018, 82, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y. Y.; Kim, M. H.; Han, J. M.; Hong, J.; Lee, T. H.; Kim, S. H.; Yang, W. M. The anti-inflammatory potential of Cortex Phellodendron in vivo and in vitro: down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. International immunopharmacology 2014, 19, 214–220. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Wang, W.; Wang, Q.; Kuang, H.; Wang, Q. Characterizing metabolites and potential metabolic pathways changes to understanding the mechanism of medicinal plant Phellodendri Amurensis cortex against doxorubicin-induced nephritis rats using UPLC-Q/TOF-MS metabolomics. Journal of pharmaceutical and biomedical analysis 2020, 188, 113336. [Google Scholar] [CrossRef]
- Jung, H. W.; Kim, K. H.; Park, Y. K. Inhibitory effect of the extract of Phellodendron amurense ruprecht root on collagen-induced arthritis in mice. Chinese journal of integrative medicine 2017, 23, 755–762. [Google Scholar] [CrossRef]
- Kim, H. J.; Kong, M. K.; Kim, Y. C. Beneficial effects of Phellodendri Cortex extract on hyperglycemia and diabetic nephropathy in streptozotocin-induced diabetic rats. BMB reports 2008, 41, 710–715. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhu, X. M.; Liu, Y. Q.; Du, W. J.; Ji, J.; He, X.; Zhang, C. F.; Li, F.; Guo, C. R.; Wang, C. Z.; Yuan, C. S. Gastroprotective Effect of Alkaloids from Cortex Phellodendri on Gastric Ulcers in Rats through Neurohumoral Regulation. Planta medica 2017, 83, 277–284. [Google Scholar] [CrossRef]
- Petronio Petronio, G.; Cutuli, M. A.; Magnifico, I.; Venditti, N.; Pietrangelo, L.; Vergalito, F.; Pane, A.; Scapagnini, G.; Di Marco, R. In Vitro and In Vivo Biological Activity of Berberine Chloride against Uropathogenic E. coli Strains Using Galleria mellonella as a Host Model. Molecules (Basel, Switzerland) 2020, 25, 5010. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Zhang, Y.; Huang, Z.; Yan, W.; Zhou, T.; Wang, Z.; Liao, L.; Cao, H.; Tan, B. Therapeutic Effects of Berberine Hydrochloride on Stress-Induced Diarrhea-Predominant Irritable Bowel Syndrome Rats by Inhibiting Neurotransmission in Colonic Smooth Muscle. Frontiers in pharmacology 2021, 12, 596686. [Google Scholar] [CrossRef]
- Wei, W.; Zeng, Q.; Wang, Y.; Guo, X.; Fan, T.; Li, Y.; Deng, H.; Zhao, L.; Zhang, X.; Liu, Y.; Shi, Y.; Zhu, J.; Ma, X.; Wang, Y.; Jiang, J.; Song, D. Discovery and identification of EIF2AK2 as a direct key target of berberine for anti-inflammatory effects. Acta pharmaceutica Sinica. B 2023, 13, 2138–2151. [Google Scholar] [CrossRef]
- Gu, S.; Cao, B.; Sun, R.; Tang, Y.; Paletta, J. L.; Wu, X.; Liu, L.; Zha, W.; Zhao, C.; Li, Y.; Ridlon, J. M.; Hylemon, P. B.; Zhou, H.; Aa, J.; Wang, G. A metabolomic and pharmacokinetic study on the mechanism underlying the lipid-lowering effect of orally administered berberine. Molecular bioSystems 2015, 11, 463–474. [Google Scholar] [CrossRef]
- Qing, Y.; Dong, X.; Hongli, L.; Yanhui, L. Berberine promoted myocardial protection of postoperative patients through regulating myocardial autophagy. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018, 105, 1050–1053. [Google Scholar]
- Song, T.; Chen, W. D. Berberine inhibited carotid atherosclerosis through PI3K/AKTmTOR signaling pathway. Bioengineered 2021, 12, 8135–8146. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(-/-) mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef]
- Huang, Z.; Li, M.; Qin, Z.; Ma, X.; Huang, R.; Liu, Y.; Xie, J.; Zeng, H.; Zhan, R.; Su, Z. Intestines-erythrocytes-mediated bio-disposition deciphers the hypolipidemic effect of berberine from Rhizoma Coptidis: A neglected insight. Journal of ethnopharmacology 2023, 314, 116600. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, Y.; Zhang, Y.; Yang, Y.; Su, W.; Yang, Y.; Sun, L.; Zhang, F.; Yu, J.; Wang, Y.; Guo, P.; Zhu, B.; Wu, S. Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR's cholesterol-decreasing efficacy in patients. Journal of advanced research 2022, 37, 197–208. [Google Scholar] [CrossRef]
- Ma, S. R.; Tong, Q.; Lin, Y.; Pan, L. B.; Fu, J.; Peng, R.; Zhang, X. F.; Zhao, Z. X.; Li, Y.; Yu, J. B.; Cong, L.; Han, P.; Zhang, Z. W.; Yu, H.; Wang, Y.; Jiang, J. D. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal transduction and targeted therapy 2022, 7, 207. [Google Scholar] [CrossRef]
- Zhao, Z.; Meng, M.; Yao, J.; Zhou, H.; Chen, Y.; Liu, J.; Wang, J.; Liu, Y.; Qiao, Y.; Zhang, M.; Qi, J.; Zhang, T.; Zhou, Z.; Jiang, T.; Shang, B.; Zhou, Q. The long non-coding RNA keratin-7 antisense acts as a new tumor suppressor to inhibit tumorigenesis and enhance apoptosis in lung and breast cancers. Cell death & disease 2023, 14, 293. [Google Scholar]
- Chen, H.; Ye, C.; Wu, C.; Zhang, J.; Xu, L.; Wang, X.; Xu, C.; Zhang, J.; Guo, Y.; Yao, Q. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. International journal of biological sciences 2023, 19, 2097–2113. [Google Scholar] [CrossRef]
- Xiong, R. G.; Huang, S. Y.; Wu, S. X.; Zhou, D. D.; Yang, Z. J.; Saimaiti, A.; Zhao, C. N.; Shang, A.; Zhang, Y. J.; Gan, R. Y.; Li, H. B. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules (Basel, Switzerland) 2022, 27, 4523. [Google Scholar] [CrossRef]
- He, Q.; Dong, H.; Guo, Y.; Gong, M.; Xia, Q.; Lu, F.; Wang, D. Multi-target regulation of intestinal microbiota by berberine to improve type 2 diabetes mellitus. Frontiers in endocrinology 2022, 13, 1074348. [Google Scholar] [CrossRef]
- Guo, H. H.; Shen, H. R.; Wang, L. L.; Luo, Z. G.; Zhang, J. L.; Zhang, H. J.; Gao, T. L.; Han, Y. X.; Jiang, J. D. Berberine is a potential alternative for metformin with good regulatory effect on lipids in treating metabolic diseases. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2023, 163, 114754. [Google Scholar]
- Ren, G.; Ding, Y. W.; Wang, L. L.; Jiang, J. D. Berberine stimulates lysosomal AMPK independent of PEN2 and maintains cellular AMPK activity through inhibiting the dephosphorylation regulator UHRF1. Frontiers in pharmacology 2023, 14, 1148611. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Wang, Z.; Fan, Q.; Lin, Z.; Tao, X.; Wu, J.; Liu, Z.; Lin, R.; Zhao, C. Berberine inhibits RA-FLS cell proliferation and adhesion by regulating RAS/MAPK/FOXO/HIF-1 signal pathway in the treatment of rheumatoid arthritis. Bone & joint research 2023, 12, 91–102. [Google Scholar]
- Li, C.; Leng, Q.; Li, L.; Hu, F.; Xu, Y.; Gong, S.; Yang, Y.; Zhang, H.; Li, X. Berberine Ameliorates Obesity by Inducing GDF15 Secretion by Brown Adipocytes. Endocrinology 2023, 164, bqad035. [Google Scholar] [CrossRef]
- Rong, Q.; Han, B.; Li, Y.; Yin, H.; Li, J.; Hou, Y. Berberine Reduces Lipid Accumulation by Promoting Fatty Acid Oxidation in Renal Tubular Epithelial Cells of the Diabetic Kidney. Frontiers in pharmacology 2021, 12, 729384. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Z. Berberine Promotes the Proliferation and Osteogenic Differentiation of Alveolar Osteoblasts through Regulating the Expression of miR-214. Pharmacology 2021, 106, 70–78. [Google Scholar] [CrossRef]
- Chen, Q. C.; Pu, Y. L.; Bi, J.; Zhang, Y. Protective effects of berberine on senile osteoporosis in mice. Journal of bone and mineral metabolism 2021, 39, 748–756. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, J. J.; Li, H. D.; Li, J. J.; Cheng, M.; Niu, X. N.; Jia, P. C.; Liu, J. Y.; Huang, C.; Lv, X. W.; Li, J. Berberine Ameliorates Abnormal Lipid Metabolism via the Adenosine Monophosphate-Activated Protein Kinase/Sirtuin 1 Pathway in Alcohol-Related Liver Disease. Laboratory investigation; a journal of technical methods and pathology 2023, 103, 100041. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, Y.; Lin, S.; Chen, Y.; Wang, Z.; Feng, H.; Fang, G.; Quan, S. Berberine Ameliorates Metabolic-Associated Fatty Liver Disease Mediated Metabolism Disorder and Redox Homeostasis by Upregulating Clock Genes: Clock and Bmal1 Expressions. Molecules (Basel, Switzerland) 2023, 28, 1874. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Wang, X.; Lu, Z.; Ye, Y. Berberine Alleviates Acute Lung Injury in Septic Mice by Modulating Treg/Th17 Homeostasis and Downregulating NF-κB Signaling. Drug design, development and therapy 2023, 17, 1139–1151. [Google Scholar] [CrossRef]
- Ahmedy, O. A.; Kamel, M. W.; Abouelfadl, D. M.; Shabana, M. E.; Sayed, R. H. Berberine attenuates epithelial mesenchymal transition in bleomycin-induced pulmonary fibrosis in mice via activating A(2a)R and mitigating the SDF-1/CXCR4 signaling. Life sciences 2023, 322, 121665. [Google Scholar] [CrossRef]
- Ding, W.; Gu, Q.; Liu, M.; Zou, J.; Sun, J.; Zhu, J. Astrocytes-derived exosomes pre-treated by berberine inhibit neuroinflammation after stroke via miR-182-5p/Rac1 pathway. International immunopharmacology 2023, 118, 110047. [Google Scholar] [CrossRef]
- Zhang, R. L.; Lei, B. X.; Wu, G. Y.; Wang, Y. Y.; Huang, Q. H. Protective effects of berberine against β-amyloid-induced neurotoxicity in HT22 cells via the Nrf2/HO-1 pathway. Bioorganic chemistry 2023, 133, 106210. [Google Scholar] [CrossRef]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Frontiers in pharmacology 2020, 11, 594852. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y. Q.; Fan, D. J.; Yang, S. S.; Lin, X.; Meng, L. K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef]
- Liu, Y. T.; Hao, H. P.; Xie, H. G.; Lai, L.; Wang, Q.; Liu, C. X.; Wang, G. J. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug metabolism and disposition: the biological fate of chemicals 2010, 38, 1779–1784. [Google Scholar] [CrossRef]
- Chen, Y. Y.; Li, R. Y.; Shi, M. J.; Zhao, Y. X.; Yan, Y.; Xu, X. X.; Zhang, M.; Zhao, X. T.; Zhang, Y. B. Demethyleneberberine alleviates inflammatory bowel disease in mice through regulating NF-κB signaling and T-helper cell homeostasis. Inflammation research: official journal of the European Histamine Research Society... [et al.] 2017, 66, 187–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, P.; Luan, H.; Jiang, H.; Xu, Y.; Zhang, Y.; Zhang, Y.; Li, R. Demethyleneberberine alleviated the inflammatory response by targeting MD-2 to inhibit the TLR4 signaling. Frontiers in immunology 2023, 14, 1130404. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, P.; Zhang, Y.; Jiang, H.; Luan, H.; Xu, Y.; Zhang, Y.; Li, R. Demethyleneberberine blocked the maturation of IL-1β in inflammation by inhibiting TLR4-mitochondria signaling. International immunopharmacology 2022, (Pt A), 109319. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Yan, Y.; Qiang, X.; Zhou, C.; Li, R.; Chen, H.; Zhang, Y. Demethyleneberberine Protects against Hepatic Fibrosis in Mice by Modulating NF-κB Signaling. International journal of molecular sciences 2016, 17, 1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Q.; Zhou, C.; Zhao, Y.; Li, R.; Zhang, Y. Demethyleneberberine attenuates concanavalin A-induced autoimmune hepatitis in mice through inhibition of NF-κB and MAPK signaling. International immunopharmacology 2020, 80, 106137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qiang, X.; Zhang, M.; Ma, D.; Zhao, Z.; Zhou, C.; Liu, X.; Li, R.; Chen, H.; Zhang, Y. Demethyleneberberine, a natural mitochondria-targeted antioxidant, inhibits mitochondrial dysfunction, oxidative stress, and steatosis in alcoholic liver disease mouse model. The Journal of pharmacology and experimental therapeutics 2015, 352, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, C.; Han, Y. To methylene berberine hydrochloride in the preparation of the application of the treatment of pulmonary fibrosis drug. CN115192573A, 18 October 2022. [Google Scholar]
- Liu, J.; Huang, X.; Liu, D.; Ji, K.; Tao, C.; Zhang, R.; Chen, J. Demethyleneberberine induces cell cycle arrest and cellular senescence of NSCLC cells via c-Myc/HIF-1α pathway. Phytomedicine : international journal of phytotherapy and phytopharmacology 2021, 91, 153678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Shi, B.; Sun, X.; Xie, Y.; Yang, H.; Zi, C.; Wang, X.; Sheng, J. Demethyleneberberine promotes apoptosis and suppresses TGF-β/Smads induced EMT in the colon cancer cells HCT-116. Cell biochemistry and function 2021, 39, 763–770. [Google Scholar] [CrossRef]
- Wang, S.; Lee, D. Y.; Shang, Y.; Liao, J.; Cao, X.; Xie, L.; Zhang, T.; Liu, J.; Dai, R. The bioactive alkaloids identified from Cortex Phellodendri ameliorate benign prostatic hyperplasia via LOX-5/COX-2 pathways. Phytomedicine : international journal of phytotherapy and phytopharmacology 2021, 93, 153813. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y.; Xu, P.; Wang, Y.; Yan, J.; Bin, W.; Qiu, F.; Kang, N. Berberine metabolites exhibit triglyceride-lowering effects via activation of AMP-activated protein kinase in Hep G2 cells. Journal of ethnopharmacology 2013, 149, 576–582. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, S.; Wang, Y.; Xu, P.; Yan, J.; Bin, W.; Qiu, F.; Kang, N. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia 2014, 92, 230–237. [Google Scholar] [CrossRef]
- Qiang, X.; Xu, L.; Zhang, M.; Zhang, P.; Wang, Y.; Wang, Y.; Zhao, Z.; Chen, H.; Liu, X.; Zhang, Y. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochemical and biophysical research communications 2016, 472, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Saklani, P.; Khan, H.; Singh, T. G.; Gupta, S.; Grewal, A. K. Demethyleneberberine, a potential therapeutic agent in neurodegenerative disorders: a proposed mechanistic insight. Molecular biology reports 2022, 49, 10101–10113. [Google Scholar] [CrossRef]
- Tao, C.; Hu, S. Q.; Chen, J.; Chen, Y. J.; Sun, K. H.; Cui, G. Z.; Ma, M.; Wu, Z. Z. Highly efficient synthesis and monoamine oxidase B inhibitory profile of demethyleneberberine, columbamine and palmatine. Neurochemistry international 2020, 139, 104807. [Google Scholar] [CrossRef]
- US Food, Drug Administration, FDA Guidance for Industry: Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, 2001(http://www.accessdata.fda.gov/drugsatfdadocs/appletter/2004/212-77s019,21085s024ltr.pdf).
Figure 1.
Chemical structures of (A) demethyleneberberine and (B) SNX-2112 and their mass spectra of [M + H]+ and product ions.
Figure 1.
Chemical structures of (A) demethyleneberberine and (B) SNX-2112 and their mass spectra of [M + H]+ and product ions.
Figure 2.
Representative extract ion MRM chromatograms of demethyleneberberine (DMB) and IS in plasma, intestine, urine, feces and bile. (A) Blank biological samples and the limit of quantification (LOQ, S/N N>10) of DMB in plasma (0.5 ng/mL), intestine and urine (1 ng/mL) bile, and feces (3 ng/mL); (B) Blank biological samples spiked from rats with DMB (300 ng/mL for plasma, intestine, urine, feces and bile) and IS (40 ng/mL); (C) Blank biological samples spiked from mice with DMB (300 ng/mL for plasma, intestine, urine and feces) and IS (40 ng/mL).
Figure 2.
Representative extract ion MRM chromatograms of demethyleneberberine (DMB) and IS in plasma, intestine, urine, feces and bile. (A) Blank biological samples and the limit of quantification (LOQ, S/N N>10) of DMB in plasma (0.5 ng/mL), intestine and urine (1 ng/mL) bile, and feces (3 ng/mL); (B) Blank biological samples spiked from rats with DMB (300 ng/mL for plasma, intestine, urine, feces and bile) and IS (40 ng/mL); (C) Blank biological samples spiked from mice with DMB (300 ng/mL for plasma, intestine, urine and feces) and IS (40 ng/mL).
Figure 3.
Plasma concentration-time course of demethyleneberberine in rats after a single (A) i.v. dose of 2.0 mg/kg or (B) i.g. dose of 20, 40 mg/kg; in mice after a single (C) i.v. dose of 2.0 mg/kg or (D) i.g. dose of 40 mg/kg. Data are presented as mean ± SD (n =5 or 15).
Figure 3.
Plasma concentration-time course of demethyleneberberine in rats after a single (A) i.v. dose of 2.0 mg/kg or (B) i.g. dose of 20, 40 mg/kg; in mice after a single (C) i.v. dose of 2.0 mg/kg or (D) i.g. dose of 40 mg/kg. Data are presented as mean ± SD (n =5 or 15).
Figure 4.
Tissue distribution of demethyleneberberine, (A) in rats after a single i.g. dose of 40 mg/kg; (B) in mice after a single i.g. dose of 40 mg/kg. Data are presented as mean ± SD (n =5).
Figure 4.
Tissue distribution of demethyleneberberine, (A) in rats after a single i.g. dose of 40 mg/kg; (B) in mice after a single i.g. dose of 40 mg/kg. Data are presented as mean ± SD (n =5).
Figure 5.
(A) Biliary, urinary and (B) fecal cumulative excretion of demethyleneberberine in rats after a single i.g. dose of 40 mg/kg; (C) Urinary and (D) fecal cumulative excretion of demethyleneberberine in mice after a single i.g. dose of 40 mg/kg. Data are presented as mean ± SD. (n =5 or 9).
Figure 5.
(A) Biliary, urinary and (B) fecal cumulative excretion of demethyleneberberine in rats after a single i.g. dose of 40 mg/kg; (C) Urinary and (D) fecal cumulative excretion of demethyleneberberine in mice after a single i.g. dose of 40 mg/kg. Data are presented as mean ± SD. (n =5 or 9).
Table 1.
Regression equations, correlation coefficients and linear ranges of demethyleneberberine in different mixed matrices of rats and mice.
Table 1.
Regression equations, correlation coefficients and linear ranges of demethyleneberberine in different mixed matrices of rats and mice.
| Matrix |
Regression equations |
Correlation coefficient (r2) |
Linear range (ng/mL) |
| Plasma |
Y=227.71 X + 9.788 |
0.9984 |
0.5-1000 |
| Heart |
Y=867.95 X + 15.193 |
0.9994 |
1-1000 |
| Liver |
Y=770.17 X + 6.9004 |
0.9998 |
1-1000 |
| Spleen |
Y=271.01 X + 16.991 |
0.9940 |
1-1000 |
| Lung |
Y=239.7 X - 6.2526 |
0.9983 |
1-1000 |
| Kidney |
Y=560.53 X + 13.146 |
0.9964 |
1-1000 |
| Brain |
Y=198.63 X + 17.806 |
0.9948 |
1-1000 |
| Stomach |
Y=418.44 X - 26.683 |
0.9976 |
3-3000 |
| Intestine |
Y=1379.2 X + 9.7943 |
0.9961 |
1-3000 |
| Urine |
Y=363.85 X + 12.913 |
0.9998 |
1-3000 |
| Feces |
Y = 605.06 X - 61.477 |
0.9930 |
3-5000 |
| Bile |
Y=716.2 X - 18.949 |
0.9911 |
3-5000 |
Table 2.
Intra-and inter-day precision, accuracy, extract recovery and matrix effect of demethyleneberberine in plasma, intestine, bile, urine and feces (n =5).
Table 2.
Intra-and inter-day precision, accuracy, extract recovery and matrix effect of demethyleneberberine in plasma, intestine, bile, urine and feces (n =5).
| Sample |
Analyte concentration
(ng/mL)
|
Precision RSD (%) |
Accuracy (%) |
Extract recovery
(mean ± SD, %)
|
Matrix effect
(mean ± SD, %)
|
| Intra-day |
Inter-day |
Intra-day |
Inter-day |
| Plasma |
10 |
2.57 |
2.58 |
3.11 |
13.43 |
83.17 ± 7.16 |
113.51 ± 0.33 |
| 200 |
1.34 |
0.99 |
0.90 |
8.32 |
84.46 ± 2.06 |
108.25 ± 3.17 |
| 800 |
0.63 |
0.50 |
2.76 |
2.75 |
82.77 ± 2.30 |
89.22 ± 1.02 |
| Intestine |
10 |
3.56 |
2.93 |
11.16 |
2.14 |
80.81 ± 4.82 |
90.91 ± 0.83 |
| 200 |
1.62 |
1.29 |
-0.55 |
-0.55 |
91.90 ± 2.06 |
94.27 ± 4.45 |
| 800 |
0.49 |
0.96 |
13.87 |
9.00 |
82.95 ± 0.61 |
98.07 ± 3.48 |
| Bile |
10 |
8.94 |
4.27 |
1.63 |
-5.02 |
78.92 ± 7.15 |
42.86 ± 2.43 |
| 200 |
2.54 |
6.25 |
-12.58 |
-11.33 |
75.52 ± 5.12 |
41.36 ± 0.87 |
| 800 |
3.10 |
2.85 |
-10.17 |
-5.85 |
76.98 ± 5.21 |
39.73 ± 7.19 |
| Urine |
10 |
4.89 |
7.98 |
14.91 |
10.41 |
90.42 ± 7.60 |
85.86 ± 6.40 |
| 200 |
2.39 |
2.31 |
2.32 |
2.45 |
86.15 ± 1.34 |
83.17 ± 1.76 |
| 800 |
0.76 |
0.79 |
9.88 |
9.23 |
96.66 ± 3.30 |
82.95 ± 1.05 |
| Feces |
10 |
10.39 |
14.19 |
0.57 |
-7.70 |
94.82 ± 2.46 |
104.27 ± 3.82 |
| 200 |
3.91 |
0.77 |
13.28 |
-9.66 |
98.97 ± 3.65 |
98.62 ± 2.83 |
| 800 |
1.29 |
3.81 |
-8.15 |
14.27 |
97.74 ± 5.69 |
100.37 ± 9.68 |
Table 3.
Stability of demethyleneberberine in plasma, intestine, bile, urine and feces under different storage conditions (mean ± SD, %, n =5).
Table 3.
Stability of demethyleneberberine in plasma, intestine, bile, urine and feces under different storage conditions (mean ± SD, %, n =5).
| Sample |
Analyte concentration
(ng/mL) |
Autosampler
(12 h, 20 °C) |
Room temperature (4 h) # |
Three freeze/
thaw cycles# |
Long-term
(30 days, −80 °C) # |
| Plasma |
10 |
90.18 ± 6.86 |
106.98 ± 2.86 |
105.96 ± 3.61 |
101.04 ± 6.64 |
| 200 |
95.39 ± 7.51 |
112.08 ± 0.90 |
109.78 ± 4.25 |
111.33 ± 2.91 |
| 800 |
96.21 ± 6.64 |
90.91 ± 2.29 |
102.05 ± 3.01 |
97.89 ± 1.67 |
| Intestine |
10 |
98.57 ± 2.18 |
101.25 ± 9.02a
|
104.14 ± 3.61 |
94.96 ± 3.47 |
| 200 |
104.19 ± 1.17 |
98.90 ± 1.51a
|
92.55 ± 1.47 |
99.91 ± 1.46 |
| 800 |
101.13 ± 1.06 |
89.16 ± 2.78a
|
95.55 ± 0.51 |
99.35 ± 1.28 |
| Bile |
10 |
88.51 ± 3.93 |
89.00 ± 7.70 |
92.34 ± 12.00 |
85.61 ± 5.23 |
| 200 |
98.53 ± 1.23 |
105.37 ± 4.67 |
88.76 ± 1.43 |
104.95 ± 2.91 |
| 800 |
87.08 ± 1.72 |
87.13 ± 3.38 |
89.22 ± 2.85 |
96.97 ± 2.09 |
| Urine |
10 |
86.36 ± 4.20 |
89.14 ± 11.04a
|
103.46 ± 4.15 |
95.21 ± 3.14 |
| 200 |
85.93 ± 1.33 |
100.78 ± 2.01a
|
95.55 ± 1.38 |
85.15 ± 1.25 |
| 800 |
95.12 ± 0.76 |
87.80 ± 9.98a
|
91.00 ± 0.13 |
107.43 ± 0.64 |
| Feces |
10 |
95.15 ± 6.64 |
95.79 ± 12.71b
|
94.65 ± 6.23 |
113.83 ± 4.19 |
| 200 |
99.11 ± 1.28 |
94.84 ± 4.27b
|
114.04 ± 13.81 |
85.20 ± 4.47 |
| 800 |
93.67 ± 2.55 |
90.58 ± 1.03b
|
97.56 ± 3.69 |
93.90 ± 0.97 |
Table 4.
Non-compartmental pharmacokinetic parameters of demethyleneberberine following a single i.g. (20, 40 mg/kg) and i.v. (2 mg/kg) administration in rats (mean ± SD, n =5).
Table 4.
Non-compartmental pharmacokinetic parameters of demethyleneberberine following a single i.g. (20, 40 mg/kg) and i.v. (2 mg/kg) administration in rats (mean ± SD, n =5).
| PK Parameters |
Unit |
i.v. (mg/kg) |
i.g. (mg/kg) |
| 2 |
20 |
40 |
| AUC0-t
|
h •ng/mL |
111.13 ± 39.71 |
27.14 ± 2.02 |
131.60 ± 33.12 |
| AUC0-∞
|
h •ng/mL |
158.75 ± 95.78 |
29.83 ± 2.14 |
133.36 ± 33.20 |
| Cmax
|
ng/mL |
128.24 ± 15.32 |
60.22 ± 12.53 |
308.25 ± 103.86 |
| Tmax
|
h |
0.08 ± 0.00 |
0.08 ± 0.00 |
0.08 ± 0.00 |
| t1/2
|
h |
5.57 ± 0.83 |
4.26 ± 1.48 |
3.14 ± 0.42 |
| MRT0-∞
|
h |
5.13 ± 2.91 |
4.1 3 ± 1.35 |
1.13 ± 0.40 |
| Bioavailability |
% |
- |
2.44 |
5.92 |
Table 5.
Non-compartmental pharmacokinetic parameters of demethyleneberberine following a single i.g. (40 mg/kg) and i.v. (2 mg/kg) administration in mice (mean ± SD, n =15).
Table 5.
Non-compartmental pharmacokinetic parameters of demethyleneberberine following a single i.g. (40 mg/kg) and i.v. (2 mg/kg) administration in mice (mean ± SD, n =15).
| PK Parameters |
Unit |
i.v. (mg/kg) |
i.g. (mg/kg) |
| 2 |
40 |
| AUC0-t
|
h •ng/mL |
226.16 ± 28.25 |
202.26 ± 14.02 |
| AUC0-∞
|
h •ng/mL |
343.51 ± 34.15 |
264.61 ± 25.01 |
| Cmax
|
ng/mL |
94.50 ± 17.45 |
177.15 ± 11.73 |
| Tmax
|
h |
0.08 ± 0.00 |
0.08 ± 0.00 |
| t1/2
|
h |
6.80 ± 1.31 |
4.10 ± 0.81 |
| MRT0-∞
|
h |
10.54 ± 2.15 |
5.29 ± 1.30 |
| Bioavailability |
% |
- |
4.47 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).