Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
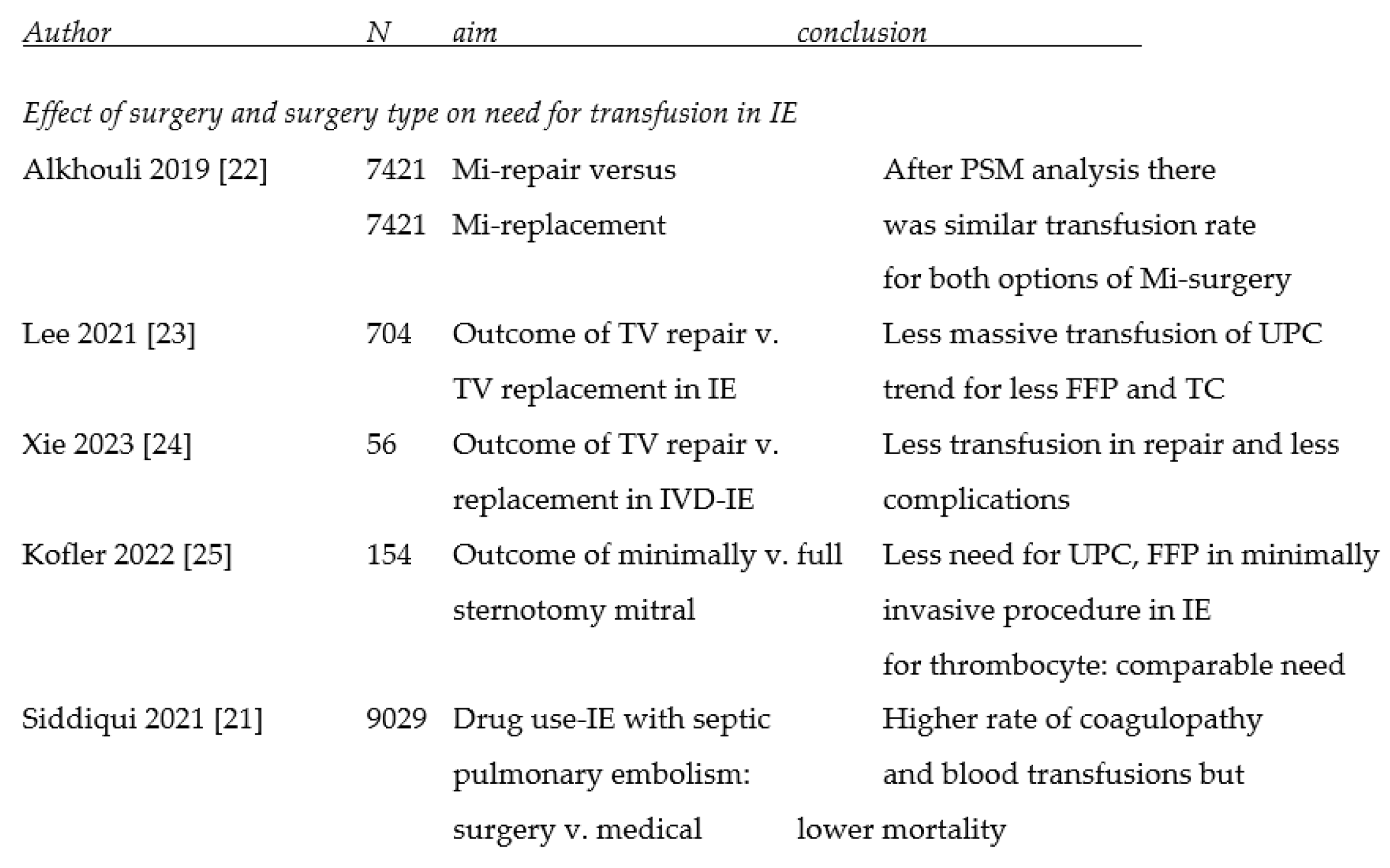
3.1. Effect of surgery, surgical technique and mode of access on the need for transfusion
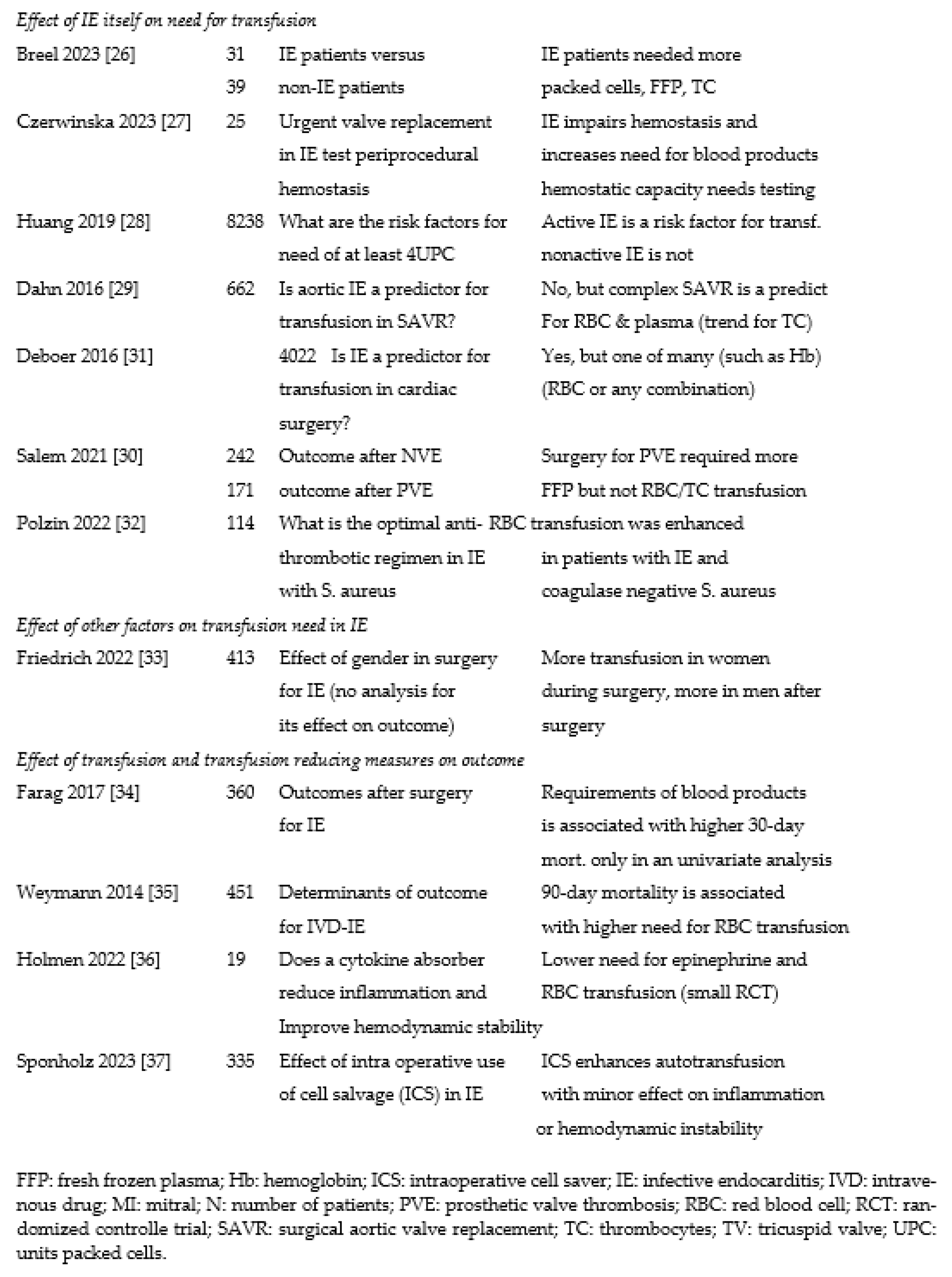
3.2. Effect of infective endocarditis on transfusion need
3.3. Effect of transfusion (and measures to reduce this) on outcome
 |
 |
4. Discussion
4.1. Endocarditis, complexity of surgery and coagulation
4.2. Effect of mode of access and surgical techniques on transfusion need
4.3. Effect of IE on transfusion needs In six series of varying size,
4.4. Effect of transfusion on outcome of IE and techniques to improve outcome
4.5. Alternative approaches to deal with the need for transfusion and transfusion reaction
5. Conclusions
Funding
Conflicts of Interest
References
- Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: A review. JAMA 2018;320:72-83. [CrossRef]
- Mistiaen WP, Gebruers N. How to manage patients in whom malignancy and infective endocarditis are associated: a review. Scand Cardiovasc J. 2020;54:70-76. [CrossRef]
- Mostaghim AS, Lo HYA, Khardori N. A retrospective epidemiologic study to define risk factors, microbiology, and clinical outcomes of infective endocarditis in a large tertiary-care teaching hospital. SAGE Open Med 2017;5:2050312117741772. [CrossRef]
- Mistiaen WP. What are the main predictors of in-hospital mortality in patients with infective endocarditis: a review. Scand Cardiovasc J. 2018;52:58-68. [CrossRef]
- Bennett-Guerrero E, Zhao Y, O’Brien SM, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568-1575. [CrossRef]
- Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44:1453-1462. [CrossRef]
- Durante-Mangoni ER, Molaro R, Iossa D. The role of hemostasis in infective endocarditis. Curr Infect Dis Rep 2014;16:435. [CrossRef]
- Grottke O, Mallaiah S, Karkouti K, et al. Fibrinogen supplementation and its indications. Semin Thromb Hemost 2020;46:38-49. [CrossRef]
- Koltsova EM, Sorokina MA, Pisaryuk AS, et al. Hypercoagulation detected by routine and global laboratory hemostasis assays in patients with infective endocarditis. PLoS One 2021;16:e0261429. [CrossRef]
- Bartoszko J, Karkouti K. Managing the coagulopathy associated with cardiopulmonary bypass. J Thromb Haemost. 2021;19:617-632. [CrossRef]
- Hendrickson JE, Roubinian NH, Chowdhury D, et al. Incidence of transfusion reactions: A multicenter study utilizing systematic active surveillance and expert adjudication. Transfusion 2016;56:2587-2596. [CrossRef]
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA 2010;304:1559-1567. [CrossRef]
- Vlaar AP, Hofstra JJ, Determann RM, et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: A prospective nested case-control study. Blood 2011;117:4218-4225. [CrossRef]
- Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317-26. doi: 10.1001/jama.2014.2726. Erratum in: JAMA. 2014;312:2045. [CrossRef]
- Simancas-Racines D, Osorio D, Martí-Carvajal AJ, Arevalo-Rodriguez I. Leukoreduction for the prevention of adverse reactions from allogeneic blood transfusion. Cochrane Database Syst Rev. 2015(12):CD009745. [CrossRef]
- Remy KE, Hall MW, Cholette J, et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion 2018;58:804-815. [CrossRef]
- Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or liberal red cell transfusion for cardiac surgery. N Engl J Med 2017;377:2133-2144. [CrossRef]
- Youssef LA, Spitalnik SL. Transfusion-related immunomodulation: A reappraisal. Curr Opin Hematol 2017;24:551-557. [CrossRef]
- Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 2008;48:1053-1060. [CrossRef]
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229-1239. [CrossRef]
- Siddiqui E, Alviar CL, Ramachandran A, Flattery E, Bernard S, Xia Y, Nayar A, Keller N, Bangalore S. Outcomes After Tricuspid Valve Operations in Patients With Drug-Use Infective Endocarditis. Am J Cardiol. 2022;185:80-86. [CrossRef]
- Alkhouli M, Alqahtani F, Berzingi C, Cook CC. Contemporary trends and outcomes of mitral valve surgery for infective endocarditis. J Card Surg. 2019;34:583-590. Epub 2019 Jun 18. PMID: 31212382. [CrossRef]
- Lee HA, Chou AH, Wu VC, Chan YS, Cheng YT, Chang CH, Chang SH, Hung KC, Chu PH, Chen SW. Nationwide cohort study of tricuspid valve repair versus replacement for infective endocarditis. Eur J Cardiothorac Surg. 2021;59:878-886. [CrossRef] [PubMed]
- Xie L, Chen X, He J, Lin S, Chen X, Wu Q, Chen L, Zhuang J, Qiu Z, Chen L. Comparison of valvuloplasty and replacement for surgical treatment of tricuspid infective endocarditis. BMC Cardiovasc Disord. 2023;23:213. [CrossRef] [PubMed]
- Kofler M, Van Praet KM, Schambach J, Akansel S, Sündermann S, Schönrath F, Jacobs S, Falk V, Kempfert J. Minimally invasive surgery versus sternotomy in native mitral valve endocarditis: a matched comparison. Eur J Cardiothorac Surg. 2021;61:189-194. [CrossRef] [PubMed]
- Breel JS, Wensing AGCL, Eberl S, Preckel B, Schober P, Müller MCA, Klautz RJM, Hollmann MW, Hermanns H. Patients with infective endocarditis undergoing cardiac surgery have distinct ROTEM profiles and more bleeding complications compared to patients without infective endocarditis. PLoS One. 2023;18:e0284329. [CrossRef]
- Czerwińska-Jelonkiewicz K, Sanetra K, Buszman PP, Gryszko L, Wood A, Crescenzi O, Milewski K, Buszman PE. Hemostatic disorders in patients with infective endocarditis undergoing urgent surgical valve replacement - Rethinking current beliefs. Int J Cardiol. 2023;388:131112. [CrossRef]
- Huang D, Chen C, Ming Y, Liu J, Zhou L, Zhang F, Yan M, Du L. Risk of massive blood product requirement in cardiac surgery: A large retrospective study from 2 heart centers. Medicine. 2019;98:e14219. [CrossRef]
- Dahn H, Buth K, Legare JF, Mingo H, Kent B, Whynot S, Scheffler M. Endocarditis is not an Independent Predictor of Blood Transfusion in Aortic Valve Replacement Patients With Severe Aortic Regurgitation. J Cardiothorac Vasc Anesth. 2016;30:687-691. [CrossRef]
- Salem M, Friedrich C, Saad M, Frank D, Salem M, Puehler T, Schoettler J, Schoeneich F, Cremer J, Haneya A. Active Infective Native and Prosthetic Valve Endocarditis: Short- and Long-Term Outcomes of Patients after Surgical Treatment. J Clin Med. 2021;10:1868. [CrossRef]
- de Boer WJ, Visser C, Ganushchak YM. Preoperative hemoglobin level: the best predictor of transfusion of packed red cells. Perfusion. 2016;31:691-698. [CrossRef]
- Polzin A, Dannenberg L, M’Pembele R, Mourikis P, Naguib D, Zako S, et al. Staphylococcus aureus increases platelet reactivity in patients with infective endocarditis. Sci Rep. 2022;12:12933. [CrossRef]
- Friedrich C, Salem M, Puehler T, Panholzer B, Herbers L, Reimers J, Hummitzsch L, Cremer J, Haneya A. Sex-Specific Risk Factors for Short- and Long-Term Outcomes after Surgery in Patients with Infective Endocarditis. J Clin Med. 2022;11:1875. [CrossRef]
- Farag M, Borst T, Sabashnikov A, Zeriouh M, Schmack B, Arif R, Beller CJ, Popov AF, Kallenbach K, Ruhparwar A, Dohmen PM, Szabó G, Karck M, Weymann A. Surgery for Infective Endocarditis: Outcomes and Predictors of Mortality in 360 Consecutive Patients. Med Sci Monit. 2017;23:3617-3626. [CrossRef]
- Weymann A, Borst T, Popov AF, Sabashnikov A, Bowles C, Schmack B, Veres G, Chaimow N, Simon AR, Karck M, Szabo G. Surgical treatment of infective endocarditis in active intravenous drug users: a justified procedure? J Cardiothorac Surg. 2014;9:58. [CrossRef]
- Holmén A, Corderfeldt A, Lannemyr L, Dellgren G, Hansson EC. Whole Blood Adsorber During CPB and Need for Vasoactive Treatment After Valve Surgery in Acute Endocarditis: A Randomized Controlled Study. J Cardiothorac Vasc Anesth. 2022;36[8 Pt B]:3015-3020. [CrossRef]
- Sponholz C, Sommerfeld O, Moehl C, Lehmann T, Franz M, Bauer M, Doenst T, Faerber G, Diab M. Intraoperative Cell Savage, Infection and Organ Failure in Infective Endocarditis Patients-A Retrospective Single Center Evaluation. J Clin Med. 2023;12:382. [CrossRef]
- Henderson RA, Judd M, Strauss ER, et al. Hematologic evaluation of intraoperative autologous blood collection and allogeneic transfusion in cardiac surgery. Transfusion 2021;61:788-798. [CrossRef]
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force, 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ferraris VA, Brown JR, Despotis GJ, et al. Ann Thorac Surg 2011;91:944-982. [CrossRef]
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Ferraris SP, Saha SP, et al.; Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion; Spiess BD, Shore-Lesserson L, Stafford-Smith M, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007 May;83(5 Suppl):S27-86. [CrossRef]
- Zhou Z-F, Jia X-P, Sun K, et al. Mild volume acute normovolemic hemodilution is associated with lower intraoperative transfusion and postoperative pulmonary infection in patients undergoing cardiac surgery - a retrospective, propensity matching study. BMC Anesthesiol 2017;17:13. [CrossRef]
- Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:79-111. [CrossRef]
- Benjamin RJ. Transfusion-related sepsis: A silent epidemic. Blood 2016;127:380-381. [CrossRef]
- Spaulding AR, Salgado-Pabon W, Kohler PL, et al. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 2013;26:422-447. [CrossRef]
- Konrad FM, Mik EG, Bodmer SI, et al. Acute normovolemic hemodilution in the pig is associated with renal tissue edema, impaired renal microvascular oxygenation, and functional loss. Anesthesiology 2013;119:256-269. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





