Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Recruitment
2.2. Study Protocol
2.2.1. Patient Disease Assessment
2.2.2. FDG-PET-CT Equipment, Protocol, and Interpretation
2.3. Statistical Analysis
3. Results
3.1. Differences in Significant Extravascular Sites of FDG Uptake between Patients with LVV-GCA Clinically Diagnosed with PMR or Not
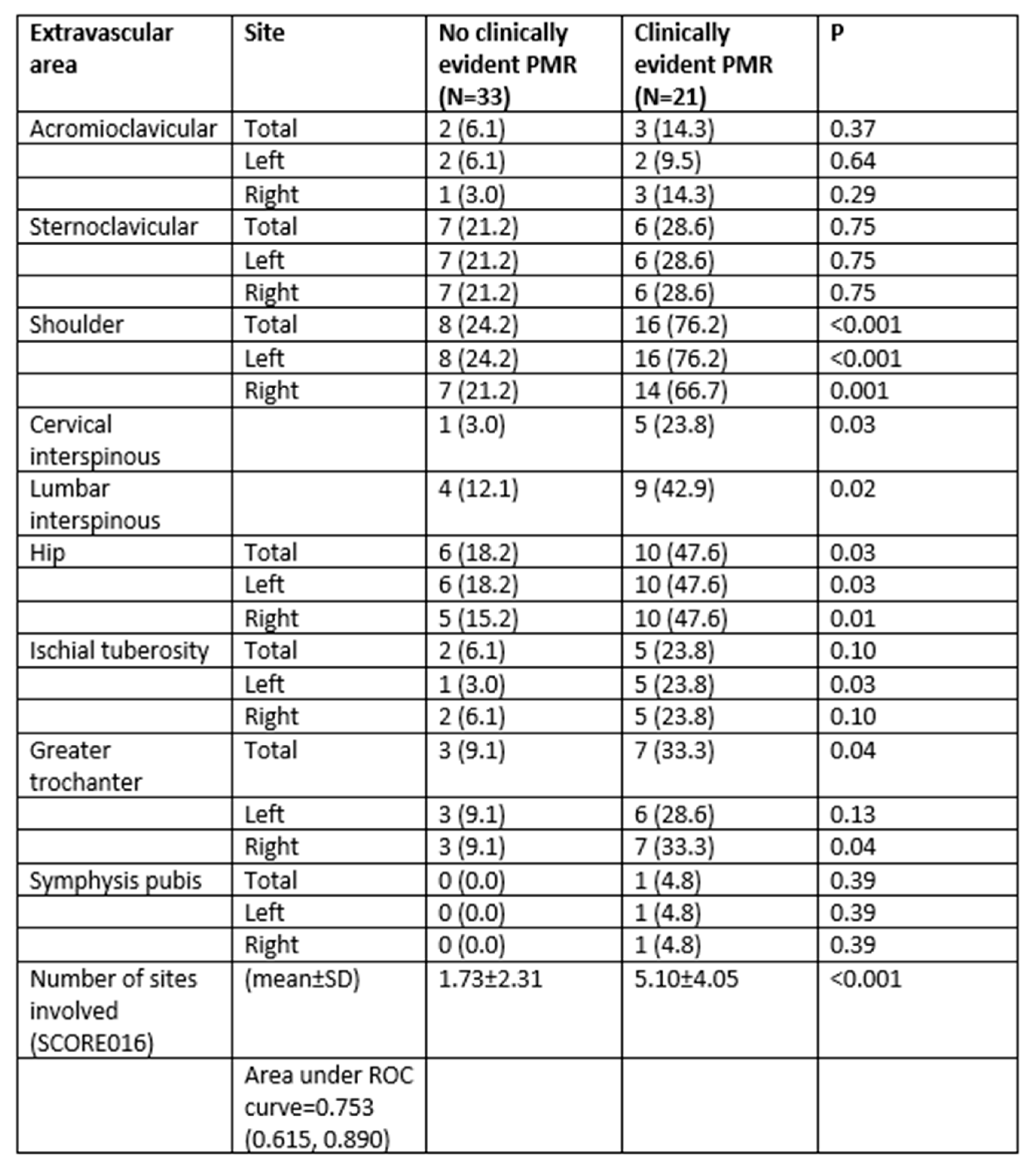
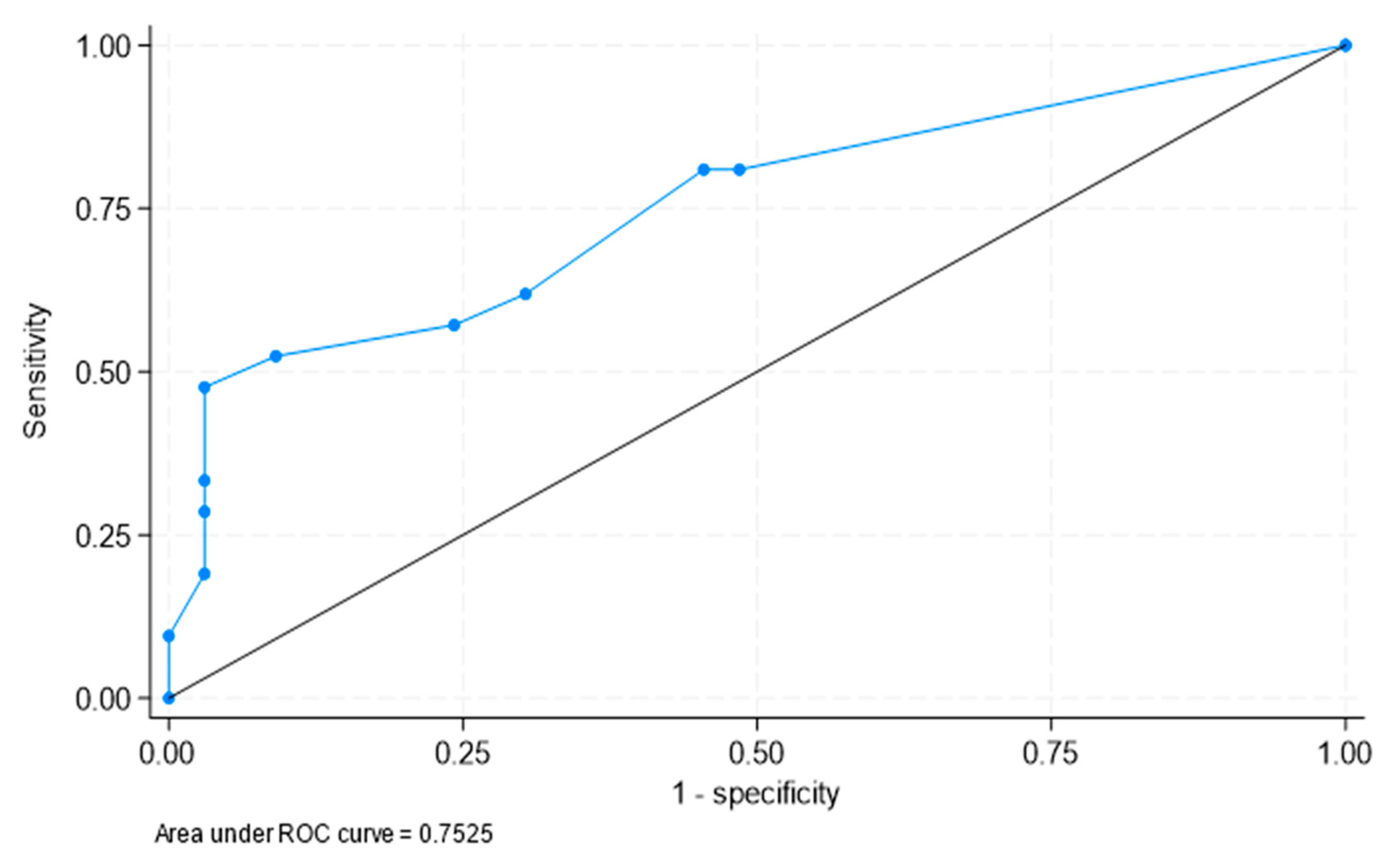
3.2. Determination of the Best Cut-Offs for the Diagnosis of PMR in Patients with LVV-GCA
3.3. Best Areas of Significant FDG Uptake to Clinically Identify PMR in Patients with LVV-GCA
4. Discussion
5. Significance and Innovation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Lopez-Diaz, M.J.; Miranda-Filloy, J.A.; Gonzalez-Juanatey, C.; Martin, J.; Llorca, J. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Care Res. 2009, 61, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- A González-Gay, M.; Matteson, E.L.; Castañeda, S. Polymyalgia rheumatica. Lancet 2017, 390, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Pipitone, N.; Versari, A.; Hunder, G.G. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat. Rev. Rheumatol. 2012, 8, 509–521. [Google Scholar] [CrossRef]
- Serling-Boyd, N.; Stone, J.H. Recent advances in the diagnosis and management of giant cell arteritis. Curr. Opin. Rheumatol. 2020, 32, 201–207. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Castañeda, S.; Martínez-Rodríguez, I.; Atienza-Mateo, B.; Blanco, R.; González-Gay, M.A. Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. J. Clin. Med. 2021, 10, 3704. [Google Scholar] [CrossRef]
- van der Geest, K.S.; Slijkhuis, B.G.; Tomelleri, A.; Gheysens, O.; Jiemy, W.F.; Piccolo, C.; Nienhuis, P.; Sandovici, M.; Brouwer, E.; Glaudemans, A.W.; et al. Positron Emission Tomography Imaging in Vasculitis. Cardiol. Clin. 2023, 41, 251–265. [Google Scholar] [CrossRef]
- Germanò G, Versari A, Muratore F, Pipitone N, Bajocchi GL, Catanoso MG, Salvarani C. Isolated vasculitis of the lower extremities in a patient with polymyalgia rheumatica and giant cell arteritis. Clin Exp Rheumatol. 2011, 29, S138–S139. [PubMed]
- Duftner, C.; Dejaco, C.; Sepriano, A.; Falzon, L.; Schmidt, W.A.; Ramiro, S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018, 4, e000612. [Google Scholar] [CrossRef]
- Schäfer, V.S.; Jin, L.; Schmidt, W.A. Imaging for Diagnosis, Monitoring, and Outcome Prediction of Large Vessel Vasculitides. Curr. Rheumatol. Rep. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Cantini, F.; Salvarani, C.; Niccoli, L.; Nannini, C.; Boiardi, L.; Padula, A.; Olivieri, I.; Valentino, M.; Barozzi, L. Fat suppression magnetic resonance imaging in shoulders of patients with polymyalgia rheumatica. J Rheumatol. 2004, 31. [Google Scholar]
- Cantini, F.; Salvarani, C.; Olivieri, I.; Niccoli, L.; Padula, A.; Macchioni, L.; Boiardi, L.; Ciancio, G.; Mastrorosato, M.; Rubini, F.; et al. Shoulder ultrasonography in the diagnosis of polymyalgia rheumatica: a case-control study. J Rheumatol. 2001, 28, 1049–55. [Google Scholar]
- Cantini F, Salvarani C, Olivieri I, Niccoli L, Padula A, Bozza A. Hip bursitis in active polymyalgia rheumatica: report of a case. Clin Exp Rheumatol. 1999, 17, 512–513. [PubMed]
- Salvarani, C.; Barozzi, L.; Cantini, F.; Niccoli, L.; Boiardi, L.; Valentino, M.; Pipitone, N.; Bajocchi, G.; Macchioni, P.; Catanoso, M.G.; et al. Cervical interspinous bursitis in active polymyalgia rheumatica. Rheumatol. 2008, 67, 758–761. [Google Scholar] [CrossRef]
- Dasgupta, B.; A Cimmino, M.; Maradit-Kremers, H.; A Schmidt, W.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S.; et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Rheumatol. 2012, 71, 484–492. [Google Scholar] [CrossRef]
- Sondag, M.; Guillot, X.; Verhoeven, F.; Blagosklonov, O.; Prati, C.; Boulahdour, H.; Wendling, D. Utility of 18F-fluoro-dexoxyglucose positron emission tomography for the diagnosis of polymyalgia rheumatica: a controlled study. Rheumatol. 2016, 55, 1452–1457. [Google Scholar] [CrossRef]
- Henckaerts, L.; Gheysens, O.; Vanderschueren, S.; Goffin, K.; Blockmans, D. Use of 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of polymyalgia rheumatica—A prospective study of 99 patients. Rheumatol. 2017, 57, 1908–1916. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Treglia, G.; Glaudemans, A.W.J.M.; Brouwer, E.; Jamar, F.; Slart, R.H.J.A.; Gheysens, O. Diagnostic value of [18F]FDG-PET/CT in polymyalgia rheumatica: a systematic review and meta-analysis. Eur. J. Nucl. Med. 2020, 48, 1876–1889. [Google Scholar] [CrossRef]
- Moya-Alvarado, P.; Leon, A.F.; Corica, M.E.; Marti, V.C.; López-Mora, D.A.; Castellví, I.; Corominas, H. "The added value of 18f-FDG PET/CT in the assessment of onset and steroid resistant polimyalgia rheumatica". PLOS ONE 2021, 16, e0255131. [Google Scholar] [CrossRef]
- Rehak, Z.; Vasina, J.; Nemec, P.; Fojtik, Z.; Koukalova, R.; Bortlicek, Z.; Rehakova, D.; Adam, J.; Vavrusova, A.; Adam, Z. Various forms of 18F-FDG PET and PET/CT findings in patients with polymyalgia rheumatica. Biomed. Pap. 2015, 159, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Peña, D.; Martínez-Rodríguez, I.; Loricera, J.; Banzo, I.; Calderón-Goercke, M.; Calvo-Río, V.; González-Vela, C.; Corrales, A.; Castañeda, S.; Blanco, R.; et al. Predictors of positive 18F-FDG PET/CT-scan for large vessel vasculitis in patients with persistent polymyalgia rheumatica. Semin. Arthritis Rheum. 2019, 48, 720–727. [Google Scholar] [CrossRef]
- Heras-Recuero, E.; Landaeta-Kancev, L.C.; de Bourio-Allona, M.M.; Torres-Rosello, A.; Blázquez-Sánchez, T.; Ferraz-Amaro, I.; Castañeda, S.; Martínez-López, J.A.; Martínez-Dhier, L.; Largo, R.; et al. Positron Emission Computed Tomography Spectrum of Large Vessel Vasculitis in a Tertiary Center: Differences in 18F-fluorodeoxyglucose Uptake between Large Vessel Vasculitis with Predominant Cranial and Extracranial Giant Cell Arteritis Phenotypes. J. Clin. Med. 2023, 12, 6164. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Rodríiguez-Valverde, V.; Blanco, R.; Fernández-Sueiro, J.L.; Armona, J.; Figueroa, M.; Martínez-Taboada, V.M. Polymyalgia Rheumatica Without Significantly Increased Erythrocyte Sedimentation Rate. Arch. Intern. Med. 1997, 157, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Cantini F, Salvarani C, Olivieri I, Niccoli L, Macchioni P, Boiardi L, et al. Inflamed shoulder structures in polymyalgia rheumatica with normal erythrocyte sedimentation rate. Arthritis Rheum. 2001, 44, 1155–1159. [CrossRef] [PubMed]
- A Gonzalez-Gay, M.; Garcia-Porrua, C.; Salvarani, C.; Olivieri, I.; Hunder, G.G. The spectrum of conditions mimicking polymyalgia rheumatica in Northwestern Spain. J Rheumatol. 2000, 27, 2179–2184. [Google Scholar]
- Paltta, J.; Suuronen, S.; Pirilä, L.; Palomäki, A. Differential diagnostics of polymyalgia rheumatica in a university hospital in Finland. Scand. J. Rheumatol. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Writing Group. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Casadepax-Soulet, C.; Benali, K.; Crestani, B.; Piekarski, E.; Mahida, B.; Ebstein, E.; Juge, P.-A.; Forien, M.; Dieudé, P.; Ottaviani, S. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in polymyalgia rheumatica: an observational study. Rheumatol. 2022, 41, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kubota, K.; Takahashi, Y.; Minaminoto, R.; Morooka, M.; Ito, K.; Kano, T.; Kaneko, H.; Takashima, H.; Mimoiri, A. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod. Rheumatol. 2011, 22, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Salvarani C, Barozzi L, Boiardi L, Pipitone N, Bajocchi GL, Macchioni PL, et al. Lumbar interspinous bursitis inactive polymyalgia rheumatica. Clin Exp Rheumatol. 2013, 31, 526–531. [PubMed]
- Owen, C.E.; Poon, A.M.T.; Yang, V.; McMaster, C.; Lee, S.T.; Liew, D.F.L.; Leung, J.L.; Scott, A.M.; Buchanan, R.R.C. Abnormalities at three musculoskeletal sites on whole-body positron emission tomography/computed tomography can diagnose polymyalgia rheumatica with high sensitivity and specificity. Eur. J. Nucl. Med. 2020, 47, 2461–2468. [Google Scholar] [CrossRef]
- Rehak, Z.; Vasina, J.; Nemec, P.; Fojtik, Z.; Koukalova, R.; Bortlicek, Z.; Rehakova, D.; Adam, J.; Vavrusova, A.; Adam, Z. Various forms of 18F-FDG PET and PET/CT findings in patients with polymyalgia rheumatica. Biomed. Pap. 2015, 159, 629–636. [Google Scholar] [CrossRef]
- Flaus, A.; Amat, J.; Prevot, N.; Olagne, L.; Descamps, L.; Bouvet, C.; Barres, B.; Valla, C.; Mathieu, S.; Andre, M.; et al. Decision Tree With Only Two Musculoskeletal Sites to Diagnose Polymyalgia Rheumatica Using [18F]FDG PET-CT. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A. Giant cell arteritis and polymyalgia rheumatica: two different but often overlapping conditions. Semin. Arthritis Rheum. 2004, 33, 289–293. [Google Scholar] [CrossRef]
- Salvarani, C.; Padoan, R.; Iorio, L.; Tomelleri, A.; Terrier, B.; Muratore, F.; Dasgupta, B. Subclinical giant cell arteritis in polymyalgia rheumatica: Concurrent conditions or a common spectrum of inflammatory diseases? Autoimmun. Rev. 2023, 103415. [Google Scholar] [CrossRef] [PubMed]
- A González-Gay, M.; García-Porrúa, C.; Vázquez-Caruncho, M. Polymyalgia rheumatica in biopsy proven giant cell arteritis does not constitute a different subset but differs from isolated polymyalgia rheumatica. J Rheumatol. 1998, 25, 1750–1755. [Google Scholar]
- Muratore, F.; Kermani, T.A.; Crowson, C.S.; Green, A.B.; Salvarani, C.; Matteson, E.L.; Warrington, K.J. Large-vessel giant cell arteritis: a cohort study. Rheumatology 2014, 54, 463–470. [Google Scholar] [CrossRef]
- Farina, N.; Tomelleri, A.; Campochiaro, C.; Dagna, L. Giant cell arteritis: Update on clinical manifestations, diagnosis, and management. Eur. J. Intern. Med. 2022, 107, 17–26. [Google Scholar] [CrossRef]
- Therkildsen, P.; de Thurah, A.; Nielsen, B.D.; Hansen, I.T.; Eldrup, N.; Nørgaard, M.; Hauge, E.-M. Increased risk of thoracic aortic complications among patients with giant cell arteritis: a nationwide, population-based cohort study. Rheumatol. 2021, 61, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Moreel, L.; Coudyzer, W.; Boeckxstaens, L.; Betrains, A.; Molenberghs, G.; Vanderschueren, S.; Claus, E.; Van Laere, K.; Blockmans, D. Association Between Vascular 18F-Fluorodeoxyglucose Uptake at Diagnosis and Change in Aortic Dimensions in Giant Cell Arteritis. Ann. Intern. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; Vicente-Rabaneda, E.F.; Heras-Recuero, E.; Castañeda, S. Polymyalgia rheumatica: when should we suspect an underlying large vessel vasculitis? Rheumatology 2023, 41, 774–776. [Google Scholar] [CrossRef]
- Desvages, A.; Hives, F.; Deprez, X.; Pierache, A.; Béhal, H.; Flipo, R.-M.; Paccou, J. Usefulness of 18F-Fluorodeoxyglucose Positron Emission Tomography in Diagnosing Polymyalgia Rheumatica and Large-Vessel Vasculitis: A Case-Control Study. J. Clin. Med. 2023, 12, 2844. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




