Submitted:
04 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cell surface receptors (c-fms and RANK) and osteoclastogenesis
2.1. Colony-stimulating factor 1 receptor (c-fms)
2.2. RANK receptors
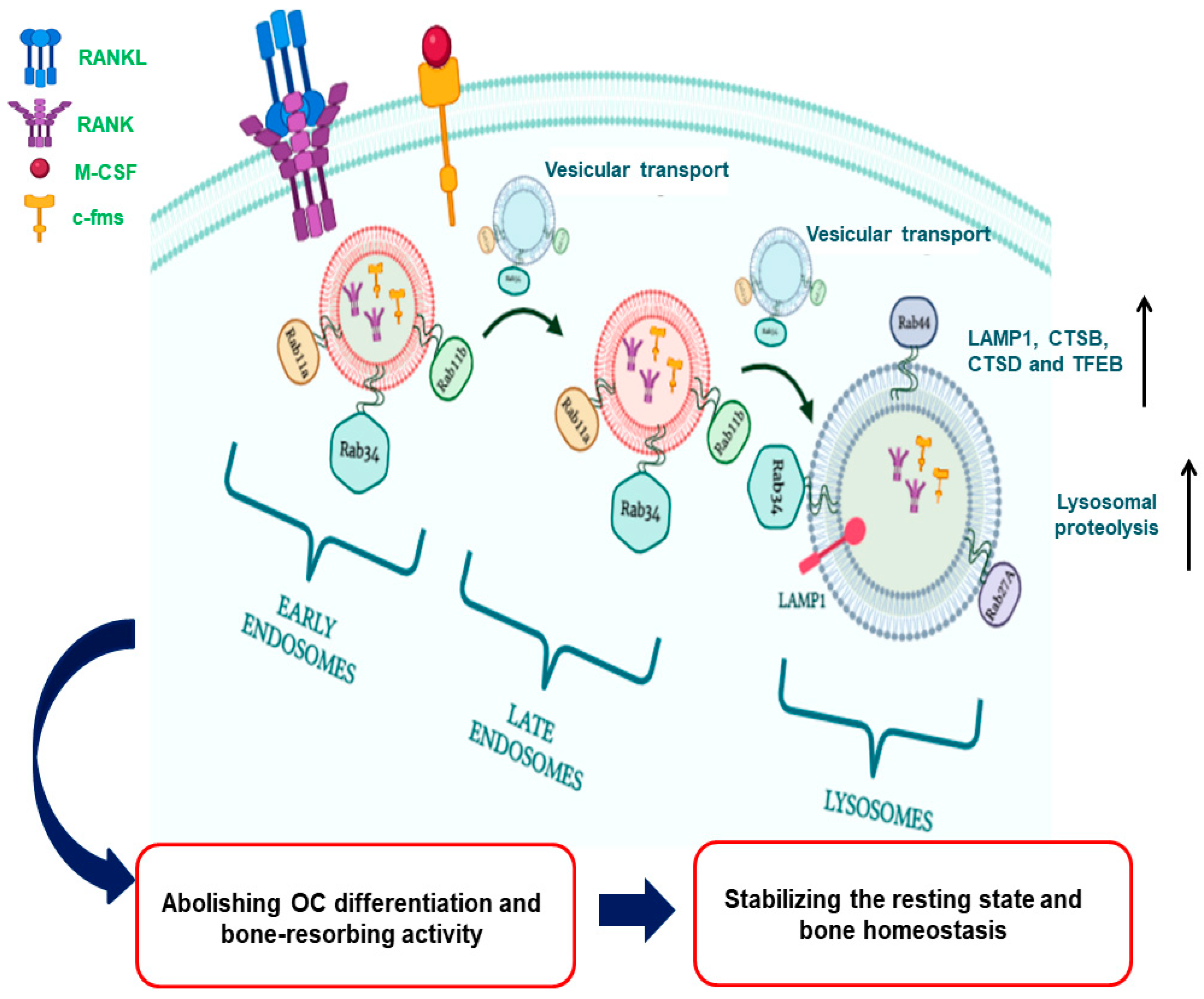
3. Rab11, lysosomes and osteoclastogenesis
3.1. Lysosomes
3.2. The axis of c-fms and RANK receptors-Rab GTPases-Lysosomes
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. Journal of Cell Science 2000, 113, 377-381. [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337-342. [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed Res Int 2020, 2020, 6910312. [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008, 473, 139-146. [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 2006, 12, 17-25. [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 2021, 39, 19-26. [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol Cells 2017, 40, 706-713. [CrossRef]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Suzuki, H. Regulatory mechanisms of RANKL presentation to osteoclast precursors. Curr Osteoporos Rep 2014, 12, 115-120. [CrossRef]
- Vääräniemi, J.; Halleen, J.M.; Kaarlonen, K.; Ylipahkala, H.; Alatalo, S.L.; Andersson, G.; Kaija, H.; Vihko, P.; Väänänen, H.K. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J Bone Miner Res 2004, 19, 1432-1440. [CrossRef]
- Lacombe, J.; Karsenty, G.; Ferron, M. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle 2013, 12, 2744-2752. [CrossRef]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.-H. The M-CSF receptor in osteoclasts and beyond. Experimental & Molecular Medicine 2020, 52, 1239-1254. [CrossRef]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. cmj 2016, 52, 12-17. [CrossRef]
- Takito, J.; Inoue, S.; Nakamura, M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. International Journal of Molecular Sciences 2018, 19, 984.
- Bhuin, T.; Roy, J.K. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res 2014, 328, 1-19. [CrossRef]
- Zhen, Y.; Stenmark, H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015, 128, 3171-3176. [CrossRef]
- Pfeffer, S.R. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 2013, 25, 414-419. [CrossRef]
- Lürick, A.; Gao, J.; Kuhlee, A.; Yavavli, E.; Langemeyer, L.; Perz, A.; Raunser, S.; Ungermann, C. Multivalent Rab interactions determine tether-mediated membrane fusion. Mol Biol Cell 2017, 28, 322-332. [CrossRef]
- Takahashi, S.; Kubo, K.; Waguri, S.; Yabashi, A.; Shin, H.W.; Katoh, Y.; Nakayama, K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci 2012, 125, 4049-4057. [CrossRef]
- Takahashi, S.; Kubo, K.; Waguri, S.; Yabashi, A.; Shin, H.-W.; Katoh, Y.; Nakayama, K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. Journal of Cell Science 2012, 125, 4049-4057. [CrossRef]
- Scapin, S.M.N.; Carneiro, F.R.G.; Alves, A.C.; Medrano, F.J.; Guimarães, B.G.; Zanchin, N.I.T. The crystal structure of the small GTPase Rab11b reveals critical differences relative to the Rab11a isoform. Journal of Structural Biology 2006, 154, 260-268. https://doi.org/.
- Baetz, N.W.; Goldenring, J.R. Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system. Molecular Biology of the Cell 2013, 24, 643-658. [CrossRef]
- Lapierre, L.A.; Kumar, R.; Hales, C.M.; Navarre, J.; Bhartur, S.G.; Burnette, J.O.; Provance, D.W., Jr.; Mercer, J.A.; Bähler, M.; Goldenring, J.R. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell 2001, 12, 1843-1857. [CrossRef]
- Zhang, X.M.; Ellis, S.; Sriratana, A.; Mitchell, C.A.; Rowe, T. Sec15 is an effector for the Rab11 in mammalian cells. J Biol Chem 2004, 279, 43027-43034. [CrossRef]
- Tran, M.T.; Okusha, Y.; Htike, K.; Sogawa, C.; Eguchi, T.; Kadowaki, T.; Sakai, E.; Tsukuba, T.; Okamoto, K. HSP90 drives the Rab11a-mediated vesicular transport of the cell surface receptors in osteoclasts. Cell Biochem Funct 2022, 40, 838-855. [CrossRef]
- Tran, M.T.; Okusha, Y.; Feng, Y.; Sogawa, C.; Eguchi, T.; Kadowaki, T.; Sakai, E.; Tsukuba, T.; Okamoto, K. A novel role of HSP90 in regulating osteoclastogenesis by abrogating Rab11b-driven transport. Biochim Biophys Acta Mol Cell Res 2021, 1868, 119096. [CrossRef]
- Roy, M.; Roux, S. Rab GTPases in Osteoclastic Bone Resorption and Autophagy. International Journal of Molecular Sciences 2020, 21, 7655.
- Roy, M.; Roux, S. Rab GTPases in Osteoclastic Endomembrane Systems. Biomed Res Int 2018, 2018, 4541538. [CrossRef]
- Ushach, I.; Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. Journal of Leukocyte Biology 2016, 100, 481-489. https://doi.org/.
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med 1999, 190, 1741-1754. [CrossRef]
- Le Beau, M.M.; Pettenati, M.J.; Lemons, R.S.; Diaz, M.O.; Westbrook, C.A.; Larson, R.A.; Sherr, C.J.; Rowley, J.D. Assignment of the GM-CSF, CSF-1, and FMS genes to human chromosome 5 provides evidence for linkage of a family of genes regulating hematopoiesis and for their involvement in the deletion (5q) in myeloid disorders. Cold Spring Harb Symp Quant Biol 1986, 51 Pt 2, 899-909. [CrossRef]
- Hoggan, M.D.; Halden, N.F.; Buckler, C.E.; Kozak, C.A. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J Virol 1988, 62, 1055-1056. [CrossRef]
- Rojo, R.; Pridans, C.; Langlais, D.; Hume, D.A. Transcriptional mechanisms that control expression of the macrophage colony-stimulating factor receptor locus. Clin Sci (Lond) 2017, 131, 2161-2182. [CrossRef]
- Stanley, E.R.; Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 2014, 6. [CrossRef]
- Liu, H.; Leo, C.; Chen, X.; Wong, B.R.; Williams, L.T.; Lin, H.; He, X. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim Biophys Acta 2012, 1824, 938-945. [CrossRef]
- Yu, W.; Chen, J.; Xiong, Y.; Pixley, F.J.; Yeung, Y.G.; Stanley, E.R. Macrophage proliferation is regulated through CSF-1 receptor tyrosines 544, 559, and 807. J Biol Chem 2012, 287, 13694-13704. [CrossRef]
- Mancini, A.; Niedenthal, R.; Joos, H.; Koch, A.; Trouliaris, S.; Niemann, H.; Tamura, T. Identification of a second Grb2 binding site in the v-Fms tyrosine kinase. Oncogene 1997, 15, 1565-1572. [CrossRef]
- Dai, X.M.; Zong, X.H.; Akhter, M.P.; Stanley, E.R. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2004, 19, 1441-1451. [CrossRef]
- Yoshida, H.; Hayashi, S.; Kunisada, T.; Ogawa, M.; Nishikawa, S.; Okamura, H.; Sudo, T.; Shultz, L.D.; Nishikawa, S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990, 345, 442-444. [CrossRef]
- Insogna, K.L.; Sahni, M.; Grey, A.B.; Tanaka, S.; Horne, W.C.; Neff, L.; Mitnick, M.; Levy, J.B.; Baron, R. Colony-stimulating factor-1 induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J Clin Invest 1997, 100, 2476-2485. [CrossRef]
- Nakamura, I.; Duong, L.T.; Rodan, S.B.; Rodan, G.A. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab 2007, 25, 337-344. [CrossRef]
- McHugh, K.P.; Hodivala-Dilke, K.; Zheng, M.H.; Namba, N.; Lam, J.; Novack, D.; Feng, X.; Ross, F.P.; Hynes, R.O.; Teitelbaum, S.L. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest 2000, 105, 433-440. [CrossRef]
- Faccio, R.; Zou, W.; Colaianni, G.; Teitelbaum, S.L.; Ross, F.P. High dose M-CSF partially rescues the Dap12-/- osteoclast phenotype. J Cell Biochem 2003, 90, 871-883. [CrossRef]
- Tsuda, E.; Goto, M.; Mochizuki, S.; Yano, K.; Kobayashi, F.; Morinaga, T.; Higashio, K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 1997, 234, 137-142. [CrossRef]
- Nakashima, T.; Kobayashi, Y.; Yamasaki, S.; Kawakami, A.; Eguchi, K.; Sasaki, H.; Sakai, H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun 2000, 275, 768-775. [CrossRef]
- Anderson, D.M.; Maraskovsky, E.; Billingsley, W.L.; Dougall, W.C.; Tometsko, M.E.; Roux, E.R.; Teepe, M.C.; DuBose, R.F.; Cosman, D.; Galibert, L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390, 175-179. [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep 2014, 15, 46-61. [CrossRef]
- McManus, S.; Roux, S. The adaptor protein p62/SQSTM1 in osteoclast signaling pathways. J Mol Signal 2012, 7, 1. [CrossRef]
- Kishida, S.; Sanjo, H.; Akira, S.; Matsumoto, K.; Ninomiya-Tsuji, J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells 2005, 10, 447-454. [CrossRef]
- Jimi, E.; Katagiri, T. Critical Roles of NF-κB Signaling Molecules in Bone Metabolism Revealed by Genetic Mutations in Osteopetrosis. Int J Mol Sci 2022, 23. [CrossRef]
- Garces de Los Fayos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers (Basel) 2018, 10. [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 2011, 75, 50-83. [CrossRef]
- Tsukuba, T.; Sakai, E.; Nishishita, K.; Kadowaki, T.; Okamoto, K. New functions of lysosomes in bone cells. Journal of Oral Biosciences 2017, 59, 92-95. https://doi.org/.
- Toyomura, T.; Murata, Y.; Yamamoto, A.; Oka, T.; Sun-Wada, G.-H.; Wada, Y.; Futai, M. From Lysosomes to the Plasma Membrane: LOCALIZATION OF VACUOLAR TYPE H+-ATPase WITH THE a3 ISOFORM DURING OSTEOCLAST DIFFERENTIATION*. Journal of Biological Chemistry 2003, 278, 22023-22030. https://doi.org/.
- Tran, M.T.; Okusha, Y.; Feng, Y.; Morimatsu, M.; Wei, P.; Sogawa, C.; Eguchi, T.; Kadowaki, T.; Sakai, E.; Okamura, H.; et al. The Inhibitory Role of Rab11b in Osteoclastogenesis through Triggering Lysosome-Induced Degradation of c-Fms and RANK Surface Receptors. International Journal of Molecular Sciences 2020, 21, 9352.
- Okusha, Y.; Tran, M.T.; Itagaki, M.; Sogawa, C.; Eguchi, T.; Okui, T.; Kadowaki, T.; Sakai, E.; Tsukuba, T.; Okamoto, K. Rab11A Functions as a Negative Regulator of Osteoclastogenesis through Dictating Lysosome-Induced Proteolysis of c-fms and RANK Surface Receptors. Cells 2020, 9, 2384.
- Feng, Y.; Tran, M.T.; Lu, Y.; Htike, K.; Okusha, Y.; Sogawa, C.; Eguchi, T.; Kadowaki, T.; Sakai, E.; Tsukuba, T.; et al. Rab34 plays a critical role as a bidirectional regulator of osteoclastogenesis. Cell Biochem Funct 2022, 40, 263-277. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




