Submitted:
08 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
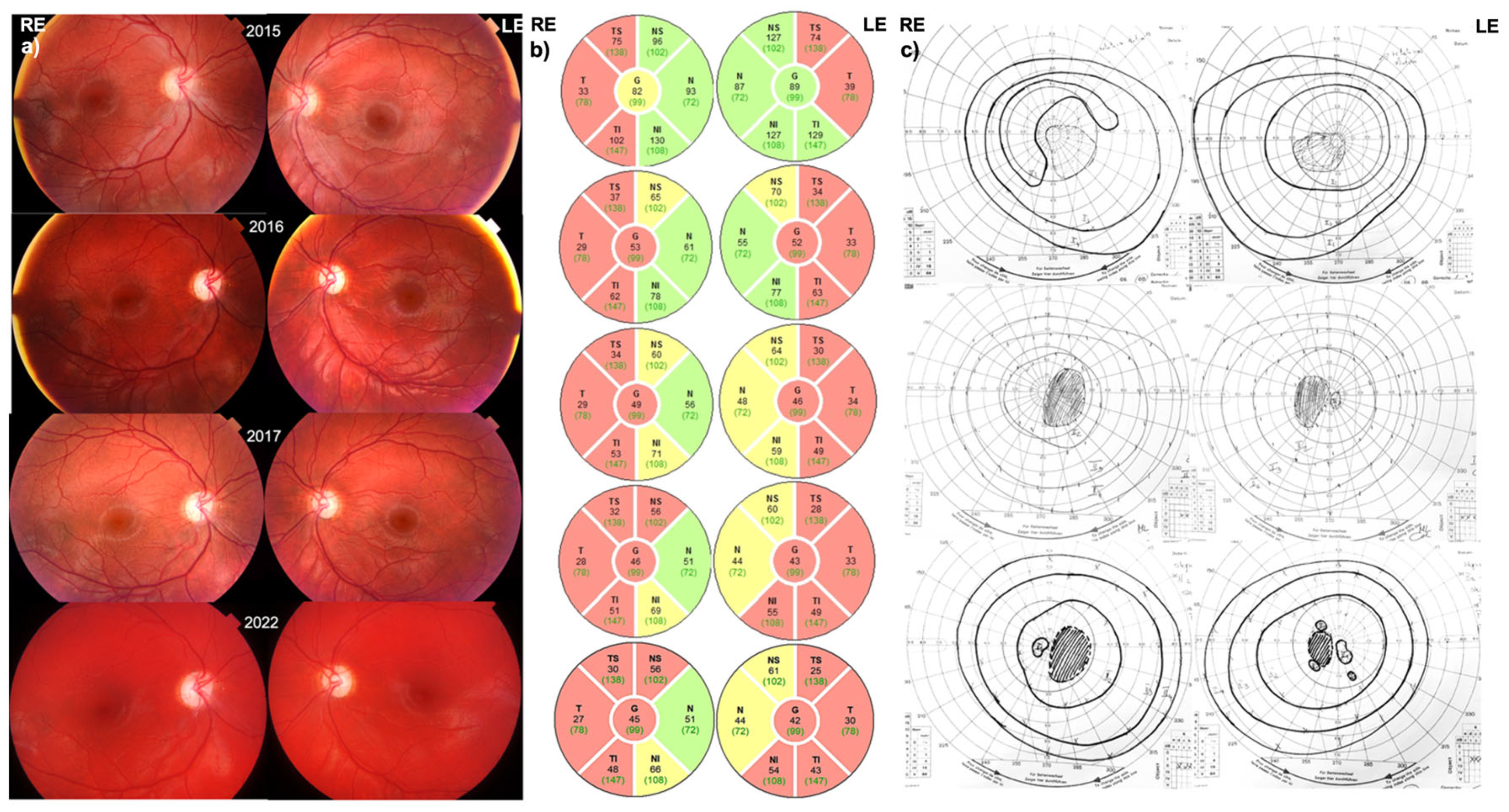
3.1. Patient 1 (MT-ND1:m.3700G>A)
3.1.1. Disease Onset
3.1.2. Follow-Up
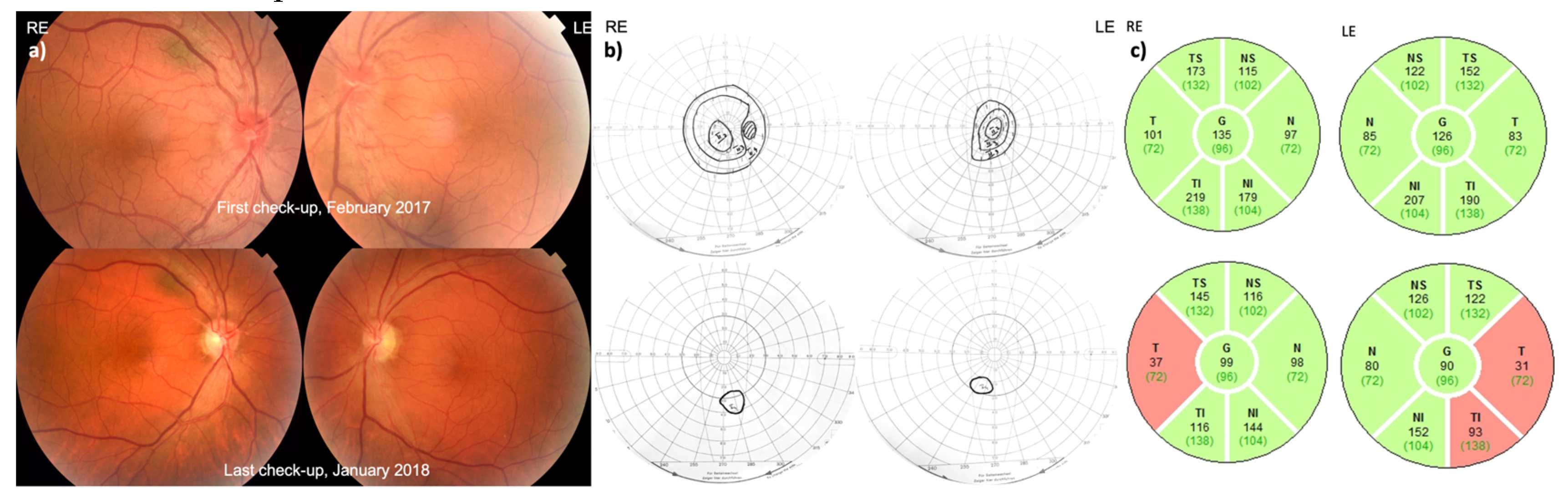
3.2. Patient 2 (MT-ND4:m.11778G>A)
3.2.1. Disease Onset
3.2.2. Follow-Up
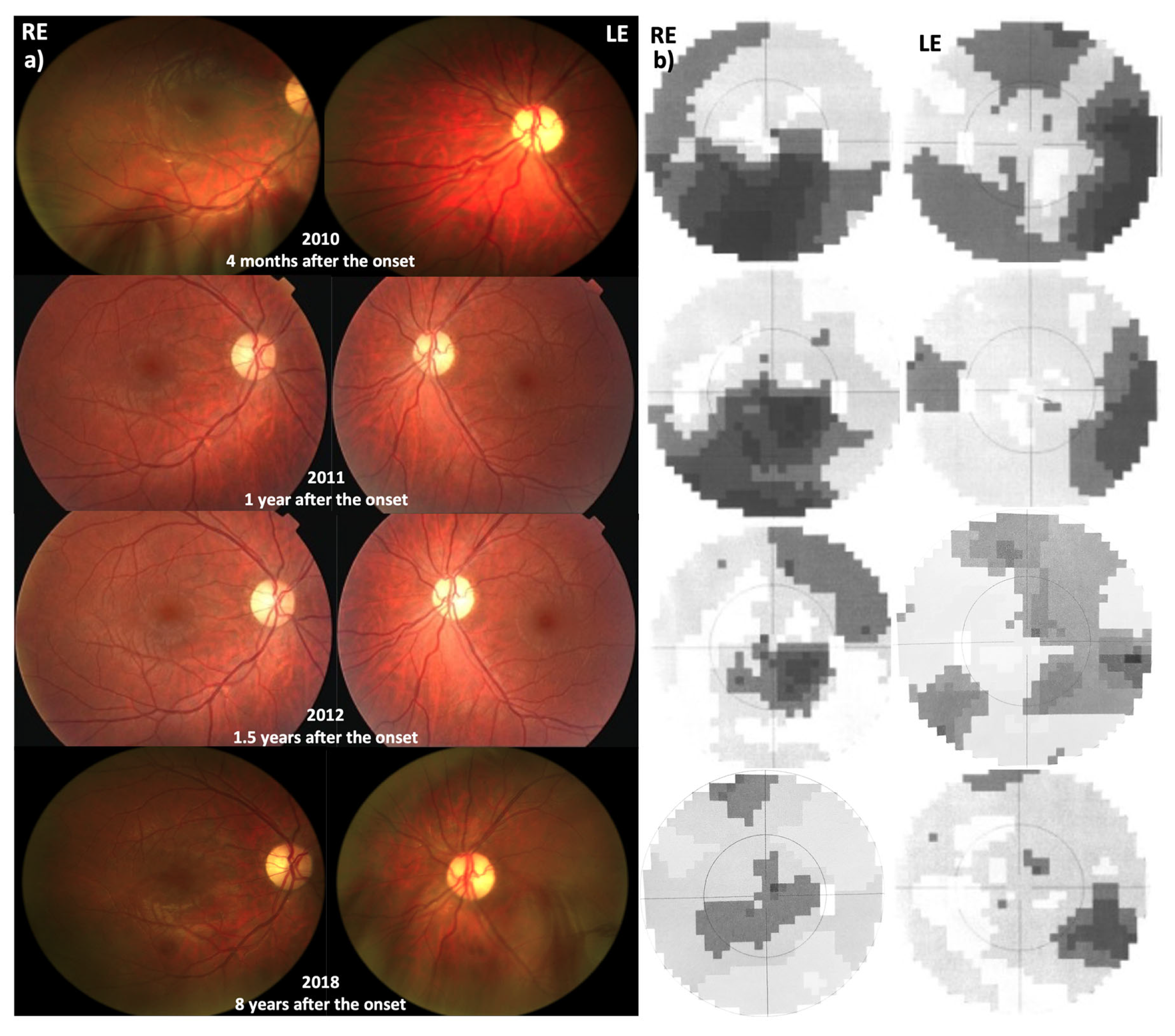
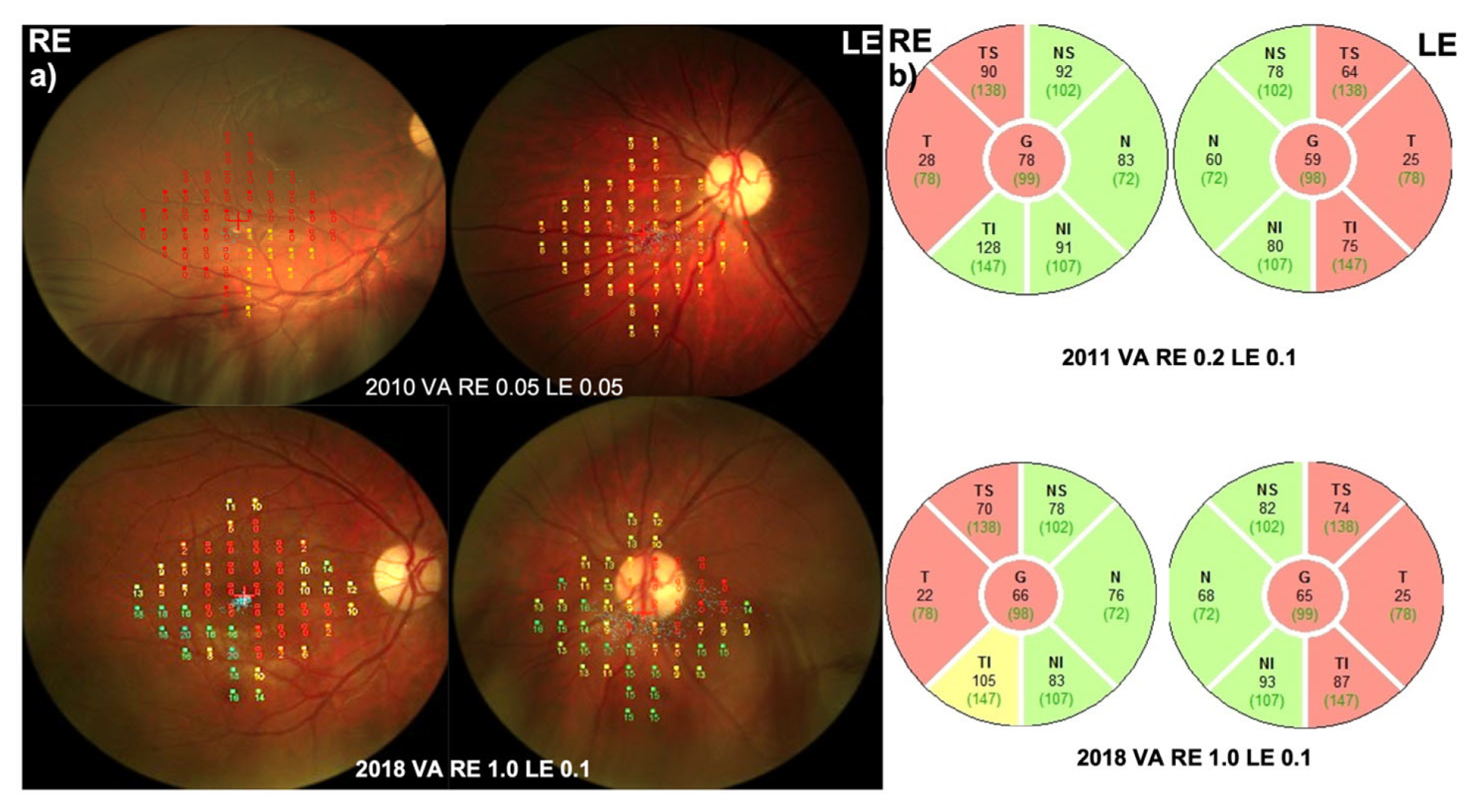
3.3. Patient 3 (m14484 T>C, p.Met64Val)
3.3.1. Disease Onset
3.3.2. Follow-Up
3.4. Segmentation Analysis and pRNFL
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu-Wai-Man, P.; Griffiths, P.G.; Hudson, G.; Chinnery, P.F. Inherited Mitochondrial Optic Neuropathies. J. Med. Genet. 2008, 46, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Newman, N.J. Hereditary Optic Neuropathies: From the Mitochondria to the Optic Nerve. Am. J. Ophthalmol. 2005, 140, 517–e1. [Google Scholar] [CrossRef] [PubMed]
- Sugisaka, E.; Ohde, H.; Shinoda, K.; Mashima, Y. Clinical Case Notes: Woman with Atypical Unilateral Leber’s Hereditary Optic Neuropathy with Visual Improvement. Clin. Experiment. Ophthalmol. 2007, 35, 868–870. [Google Scholar] [CrossRef]
- Nagai, A.; Nakamura, M.; Kusuhara, S.; Kanamori, A.; Negi, A. Unilateral Manifestation of Leber?S Hereditary Optic Neuropathy After Blunt Ocular Trauma. Jpn. J. Ophthalmol. 2005, 49, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, F.K.; Leo-Kottler, B.; Mittelviefhaus, K.; Zrenner, E.; Meyer, J.; Pusch, C.M.; Wissinger, B. Segregation Patterns and Heteroplasmy Prevalence in Leber’s Hereditary Optic Neuropathy. 2001, 42, 7.
- Dandekar, S.S.; Graham, E.M.; Plant, G.T. Ladies with Leber’s Hereditary Optic Neuropathy: An Atypical Disease. Eur. J. Ophthalmol. 2002, 12, 537–541. [Google Scholar] [CrossRef]
- Giordano, C.; Iommarini, L.; Giordano, L.; Maresca, A.; Pisano, A.; Valentino, M.L.; Caporali, L.; Liguori, R.; Deceglie, S.; Roberti, M.; et al. Efficient Mitochondrial Biogenesis Drives Incomplete Penetrance in Leber’s Hereditary Optic Neuropathy. Brain 2014, 137, 335–353. [Google Scholar] [CrossRef]
- Guy, J.; Feuer, W.J.; Porciatti, V.; Schiffman, J.; Abukhalil, F.; Vandenbroucke, R.; Rosa, P.R.; Lam, B.L. Retinal Ganglion Cell Dysfunction in Asymptomatic G11778A: Leber Hereditary Optic Neuropathy. Investig. Opthalmology Vis. Sci. 2014, 55, 841. [Google Scholar] [CrossRef]
- Goyal, S.; Riordan-Eva, P.; Coakes, R.L. Late Onset of Leber’s Hereditary Optic Neuropathy Precipitated by Anaemia. Eye 2004, 18, 1017–1018. [Google Scholar] [CrossRef]
- Majander, A.; Bowman, R.; Poulton, J.; Antcliff, R.J.; Reddy, M.A.; Michaelides, M.; Webster, A.R.; Chinnery, P.F.; Votruba, M.; Moore, A.T.; et al. Childhood-Onset Leber Hereditary Optic Neuropathy. Br. J. Ophthalmol. 2017, 101, 1505–1509. [Google Scholar] [CrossRef]
- Barboni, P.; La Morgia, C.; Cascavilla, M.L.; Hong, E.H.; Battista, M.; Majander, A.; Caporali, L.; Starace, V.; Amore, G.; Renzo, A.D.; et al. Childhood-Onset Leber Hereditary Optic Neuropathy—Clinical and Prognostic Insights. Am. J. Ophthalmol. 2023, 249, 99–107. [Google Scholar] [CrossRef]
- Ohden, K.L.; Tang, P.H.; Lilley, C.C.; Lee, M.S. Atypical Leber Hereditary Optic Neuropathy: 18 Year Interval Between Eyes. J. Neuroophthalmol. 2016, 36, 304–304. [Google Scholar] [CrossRef] [PubMed]
- Nikoskelainen, E.K.; Huoponen, K.; Juvonen, V.; Lamminen, T.; Nummelin, K.; Savontaus, M.-L. Ophthalmologic Findings in Leber Hereditary Optic Neuropathy, with Special Reference to mtDNA Mutations. Ophthalmology 1996, 103, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Barboni, P.; Savini, G.; Valentino, M.L.; La Morgia, C.; Bellusci, C.; De Negri, A.M.; Sadun, F.; Carta, A.; Carbonelli, M.; Sadun, A.A.; et al. Leber’s Hereditary Optic Neuropathy with Childhood Onset. Investig. Opthalmology Vis. Sci. 2006, 47, 5303. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Park, Y.; Shin, S.Y.; Chae, H.; Kim, M.; Park, S.H. Genotypic and Phenotypic Characteristics of Korean Children with Childhood-Onset Leber’s Hereditary Optic Neuropathy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- DuBois, L.G.; Feldon, S.E. Evidence for a Metabolic Trigger for Leber’s Hereditary Optic Neuropathy. A Case Report. J. Clin. Neuroophthalmol. 1992, 12, 15–16. [Google Scholar]
- Weiner, N.C.; Newman, N.J.; Lessell, S.; Johns, D.R.; Lott, M.T.; Wallace, D.C. Atypical Leber’s Hereditary Optic Neuropathy With Molecular Confirmation. Arch. Neurol. 1993, 50, 470–473. [Google Scholar] [CrossRef]
- Moorman, C.M.; Elston, J.S.; Matthews, P. Leber’s Hereditary Optic Neuropathy as a Cause of Severe Visual Loss in Childhood. Pediatrics 1993, 91, 988–989. [Google Scholar] [CrossRef]
- Thieme, H.; Wissinger, B.; Jandeck, C.; Christ-Adler, M.; Kraus, H.; Kellner, U.; Foerster, M.H. A Pedigree of Leber’s Hereditary Optic Neuropathy with Visual Loss in Childhood, Primarily in Girls. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 714–719. [Google Scholar] [CrossRef]
- Balayre, S.; Gicquel, J.-J.; Mercie, M.; Dighiero, P. [Childhood Leber hereditary optic neuropathy. A case of a 6-year-old girl with loss of vision]. J. Fr. Ophtalmol. 2003, 26, 1063–1066. [Google Scholar]
- von Noorden, G.K. Amblyopia: A Multidisciplinary Approach. Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 1985, 26, 1704–1716. [Google Scholar]
- Karanjia, R.; Berezovsky, A.; Sacai, P.Y.; Cavascan, N.N.; Liu, H.Y.; Nazarali, S.; Moraes-Filho, M.N.; Anderson, K.; Tran, J.S.; Watanabe, S.E.; et al. The Photopic Negative Response: An Objective Measure of Retinal Ganglion Cell Function in Patients With Leber’s Hereditary Optic Neuropathy. Investig. Opthalmology Vis. Sci. 2017, 58, BIO300. [Google Scholar] [CrossRef] [PubMed]
- Moussa, G.; Bassilious, K.; Mathews, N. A Novel Excel Sheet Conversion Tool from Snellen Fraction to LogMAR Including ‘Counting Fingers’, ‘Hand Movement’, ‘Light Perception’ and ‘No Light Perception’ and Focused Review of Literature of Low Visual Acuity Reference Values. Acta Ophthalmol. (Copenh.) 2021, 99. [Google Scholar] [CrossRef]
- Kinyoun, J.; Barton, F.; Fisher, M.; Hubbard, L.; Aiello, L.; Ferris, F. Detection of Diabetic Macular Edema. Ophthalmology 1989, 96, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Hee, M.R.; Puliafito, C.A.; Duker, J.S.; Reichel, E.; Coker, J.G.; Wilkins, J.R.; Schuman, J.S.; Swanson, E.A.; Fujimoto, J.G. Topography of Diabetic Macular Edema with Optical Coherence Tomography. Ophthalmology 1998, 105, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Fauser, S.; Luberichs, J.; Besch, D.; Leo-Kottler, B. Sequence Analysis of the Complete Mitochondrial Genome in Patients with Leber’s Hereditary Optic Neuropathy Lacking the Three Most Common Pathogenic DNA Mutations. Biochem. Biophys. Res. Commun. 2002, 295, 342–347. [Google Scholar] [CrossRef]
- Tinuper, P.; Plazzi, G.; Provini, F.; Cerullo, A.; Leonardi, M.; Agati, R.; Righini, A.; Montagna, P. Facial Asymmetry in Partial Epilepsies. Epilepsia 1992, 33, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.I.; Sadun, A.A.; Lee, H. Vasculature and Morphometry of the Optic Canal and Intracanalicular Optic Nerve. J. Neuro-Ophthalmol. Off. J. North Am. Neuro-Ophthalmol. Soc. 1995, 15, 186–190. [Google Scholar] [CrossRef]
- Sidikaro, Y.; von Noorden, G.K. Observations in Sensory Heterotropia. J. Pediatr. Ophthalmol. Strabismus 1982, 19, 12–19. [Google Scholar] [CrossRef]
- Hamm, L.; Chen, Z.; Li, J.; Black, J.; Dai, S.; Yuan, J.; Yu, M.; Thompson, B. Interocular Suppression in Children with Deprivation Amblyopia. Vision Res. 2017, 133, 112–120. [Google Scholar] [CrossRef]
- Meier, K.; Giaschi, D. Unilateral Amblyopia Affects Two Eyes: Fellow Eye Deficits in Amblyopia. Investig. Opthalmology Vis. Sci. 2017, 58, 1779. [Google Scholar] [CrossRef]
- Lawwill, T. Electrophysiologic Aspects of Amblyopia. Ophthalmology 1978, 85, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Black, J.; Lun, V.; Phillips, G.; Thompson, B. Colour Vision in Adult Amblyopia. Acta Ophthalmol. (Copenh.) 2011, 89, 0–0. [Google Scholar] [CrossRef]
- Espinosa, N.; Pinto, I.; Arias, A.; Toro, L.; DiazGranados, J.; Solano, A. Evaluation of Visual Function in Patients with Cataract, Amblyopia, Optic Neuritis, and Retinopathies. Pan-Am. J. Ophthalmol. 2020, 2, 21. [Google Scholar] [CrossRef]
- Taşkıran Çömez, A.; Şanal Ulu, E.; Ekim, Y. Retina and Optic Disc Characteristics in Amblyopic and Non-Amblyopic Eyes of Patients with Myopic or Hyperopic Anisometropia. Türk Oftalmol. Derg. 2017, 47, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-Y.; Cheng, C.-Y.; Wang, A.-G. Retinal Nerve Fiber Layer Thickness in Unilateral Amblyopia. Investig. Opthalmology Vis. Sci. 2004, 45, 2224. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Goodfellow, G.W.; Allison, C.; Block, S.; Frantz, K.A. A Prospective Study of Macular Thickness in Amblyopic Children with Unilateral High Myopia. Investig. Opthalmology Vis. Sci. 2011, 52, 2444. [Google Scholar] [CrossRef]
- Petrovic Pajic, S.; Lapajne, L.; Vratanar, B.; Fakin, A.; Jarc-Vidmar, M.; Sustar Habjan, M.; Volk, M.; Maver, A.; Peterlin, B.; Hawlina, M. The Relative Preservation of the Central Retinal Layers in Leber Hereditary Optic Neuropathy. J. Clin. Med. 2022, 11, 6045. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




