1. Introduction
Hair loss is an emblematic human scalp-related condition caused by chemotherapy, chronic diseases and aging. Androgenetic alopecia (AGA) is a genetic disorder of hair loss that has been observed in both sexes (1). At present, it is estimated that approximately 50% of males and 15−30% of females are facing hair loss problems (2). Minoxidil (MXD) is considered a drug of choice for the topical treatment of hair fall in men and women caused by a disorder known as AGA. MXD is a derivative of pyrimidine that acts as a peripheral vasodilating agent, and initially in 1970, it was approved for reducing blood pressure (3-5). MXD opens the potassium channel, releases nitric oxide (NO) and increases the flow of blood towards the follicles of hair, modifying the pathway of prostaglandins and consequently suppressing the pathway of alopecia (4, 5). Chronic oral intake of MXD could cause adverse skin reactions, which cause the discontinuity of therapy and decrease patient adherence to therapy. Similarly, as the water solubility of MXD is very low, ethanol- or propylene glycol-based formulations lead to crystal formation upon evaporation of the solvents, which may induce several adverse effects, such as pruritus, rash, redness of skin, dandruff, dryness of scalp and allergic contact dermatitis (6). Therefore, distinctive, and suitable new formulations are required for the topical application of MXD (5, 7).
To address the issues argued above, previously developed formulations for topical MXD application include 5% minoxidil solution, nanoparticles with penetration enhancers and mucoadhesive polymer-based formulations (5, 8). Plenty of active medicine is lost in the course of the application of MXD solutions, and poor adherence towards therapy is associated with this, which could further lead to ineffective dose regulation (5, 9). Likewise, a penetration enhancer could lead to the accumulation of toxic levels of MXD in the skin tissue, and mucoadhesive formulations cause stickiness over the scalp and area of application (5).
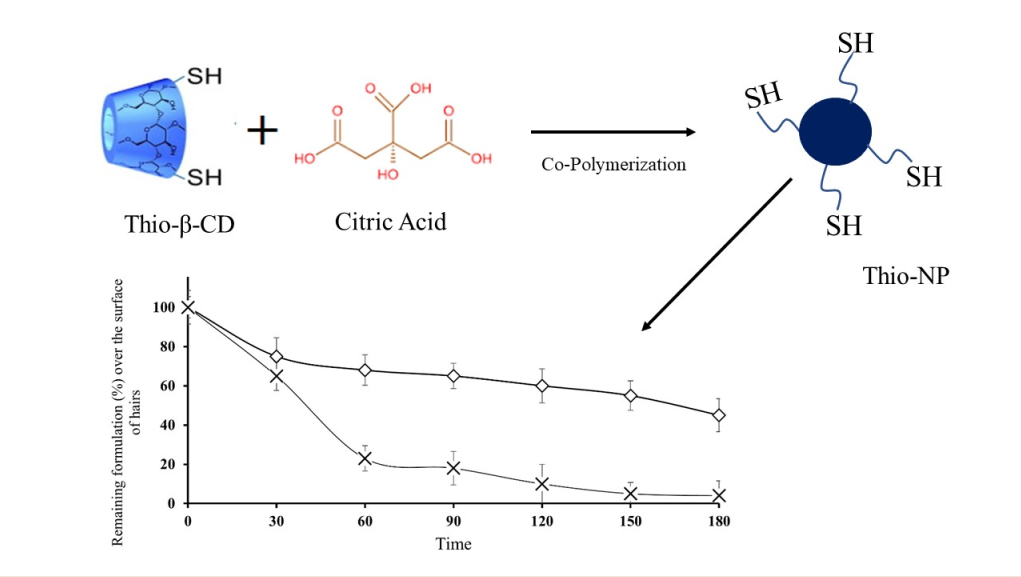
Cyclodextrins (CDs), cyclic polysaccharides with 6-8 units of glucopyranose, are enzymatically produced by the mortification of starch (5, 10). CDs have a lipophilic cavity for the encapsulation of active drug molecules, and CDs encourage the sustained and extended release of encapsulated model drugs. CDs have been recognized as the smallest known drug carriers and are capable of enhancing the dissolvability of numerous drug molecules by the development of comparatively persistent inclusion complexes. CDs have a high affinity for a particular drug molecule because of their carrier system, which equips them with a specific drug release profile (11, 12). Previous studies from our research group have demonstrated that thiolated cyclodextrins provide the advantage of covalent bond formation through thiol/disulfide interchange reactions with cysteine-rich subregions on hair surfaces or other dermal appendages. Therefore, the aim of this research was to develop inclusion complexes of MXD with thiolated β-CD-SH (13). Thiol moieties attached to β-CD-SH develop covalent bonds with the disulfide bonds of alpha keratin. The inclusion complex of MXD with β-cyclodextrin will be formed to enhance its water solubility, which will further improve its activity.
2. Materials and Methods
2.1. Materials
Beta-cyclodextrin, cysteine HCl, Ellman’s reagent (5,5-dithiobis (2-nitro-benzoic acid)), MES hydrate (4-morpholine-ethanesulfonic acid), cysteamine, sodium periodate (NaIO4), sodium cyanoborohydride (NaCNBH3), dimethyl sulfoxide (DMSO), ethylene glycol, minoxidil (MXD), distilled water, chitosan, and sodium phosphate monobasic dihydrate (purity ≥99%) were obtained from Glentham Life Sciences, UK. Analytical grade chemicals and reagents were purchased from International chemical suppliers. Pretreated regenerated cellulose dialysis tubing of 1000-2000 Da was purchased from Spectrum, USA.
2.2. Method
2.2.1. Oxidation
To develop aldehyde functional groups in the structure of β-CD, an oxidation reaction was carried out. Briefly, a 1% solution of β-CD was prepared in 180 mL deionized water in aluminum-wrapped 500 mL Erlenmeyer flasks. Sodium per iodate (NaIO4, 0.8 g) dissolved in 20 mL deionized water was added to the above solution to create di-aldehyde functional groups following the process of oxidation. The solution mixture was vigorously mixed by stirring at 25 °C for almost 2 h, to consume the unreacted NaIO4, 800 µL of ethylene glycol was added. Finally, the reaction mixture was stirred at 25 °C for 1 h, and then the final product (Aldo-β-CD) was isolated via dialysis against distilled water for 72 h. During the dialysis process, the dialyzing water was replaced three times a day. The purified product was freeze-dried to produce the final product (13-15).
2.2.2. Conjugation of Aldo-β-CD with Cysteamine HCl
For the conjugation reaction, 1% Aldo-β-CD was dissolved in 0.1 M buffered solution of 4-morpholine-ethanesulfonic acid (MES) hydrate. After equilibration, 0.5% cysteamine HCl dissolved in deionized water was added. 0.01 M HCl was used to bring the pH of the mixture to 4. The ultimate volume of the mixture was maintained at 100 ml with purified water. The reaction mixtures were stirred in the dark at 25 °C for 3 h. NaCNBH3 (8%) was added, and the reaction was stirred for an additional 72 h beneath a laminar air flow hood. A similar method was used for the conjugation of the fluorescent marker rhodamine-123. Once the reaction was finished, the final solutions were dialyzed almost 6 times at 10 °C by using a dialyzing tube to separate the polymer conjugates and unreacted residues of chemicals. In detail, the mixture was dialyzed 2 times at pH 4.5 against distilled water and then 2 times against distilled water at pH 4.5, but 1% sodium chloride was added to quench the ionic interactions. Finally, samples were dialyzed 2 times against distilled water at pH 4. Finally, the purified solutions obtained after dialysis were frozen at -78 °C and lyophilized under vacuum for 1 day. Dried conjugates were stored at 4 °C in hermetic containers (13).
2.3. Encapsulation of MXD
For the preparation of the inclusion complex of MXD, 25 mg of MXD was dissolved in 5 ml ethanol, and this solution was dispersed in 30 ml of phosphate sodium buffer (0.05 M) containing 75 mg thiolated β-CD (Thio-β-CD) and unmodified β-CD in a molar ratio of 1:1, and the mixture was continuously agitated at 25 °C for 24 h. Finally, the mixture suspensions were strained through a porous cellulose membrane filters with a pore size of 0.45 µm, and the filtrate obtained was freeze and lyophilized (13, 16).
2.4. Preparation of Nanoparticles
The nanoparticles were prepared by using a previously reported method of ionic gelation (17). Briefly, Chitosan (1.5 mg/mL) dissolved in acetic acid (1% v/v) was continuously stirred with 1 mL (30 mg/mL water) solution of inclusion complex of Thio-β-CD. Encapsulated solution was added dropwise into the chitosan solution and continuously stirred. The pH of all mixtures adjusted to 4 and continuously stirred at 37 ◦C for 60 minutes, then sodium tri-polyphosphate TPP (3 mL, 4 mg/mL) was added dropwise. Similarly, blank NP were prepared with unmodified β-CD. The obtained nanomaterials were filtered to separate both insoluble and soluble material. Then, the water phase was neutralized, and dialysis was performed for 24 h. Yellowish colored product was yielded after drying the solution under reduced pressure. Nanoparticles developed by using Thio-β-CD were labeled Thio-NP, and nanoparticles formulated with unmodified CD were labeled blank-NP.
2.5. Determination of the thiol group and disulfide content
For the quantification of thiol groups conjugated on Thio-β-CD, Ellman's reagent was used; likewise, the amount of thiol groups present over the surface of nanoparticles was also determined (18). Similarly, disulfide contents were also determined after reduction with sodium borohydride (NaBH4) and the addition of 5,5′-dithiobis (2-nitrobenzoic acid) (19).
2.6. FTIR Study
FT-IR analysis was used for the confirmation of inclusion complex formation between MXD and CDs. Encapsulation of MXD was confirmed by comparing the variance of peak dimensions, position, and strength. FTIR spectroscopy is also used to confirm the possible physical and chemical interactions among β-CD and MXD in the solid form because complex formation will change the absorption spectrum of MXD (12). FT-IR spectra of powdered Thio-β-CD, MXD encapsulated within Thio-β-CD and free MXD were measured by benchtop FTIR spectroscopy.
2.7. Zeta-potential measurements
The particle size of formulated nanoparticles (Thio-NP, Blank-NP) was measured by using Malvern, Panalytical Zeta Sizer, UK. The size measurement was optimized by software equipped with ZS Xplorer software. For the evaluation of particle size, 50 µg of Thio-NP and Blank-NP were suspended in deionized water. Each sample was run for 30 sec each for ten cycles of measurement. The measurement process for particle size evaluation was repeated three times. All the samples were analyzed at 25 °C, and other parameters, such as the electric field at 13.89 V/cm, refractive index at 1.330, and voltage at 5 V. The mean zeta potential with SD was computed utilizing the ZS Xplorer software. Data were reported from three independent syntheses; each set of measurements had 10 replicates (20).
2.8. Dissolution study
For this purpose, 30 mg of nanoparticles (Thio-NP) containing MXD-loaded thiolated β-CD and blank-NP containing MXD equivalent to 20 mg of pure MXD were weighed and compressed into tablets. Freshly prepared 0.1 M phosphate buffer (pH 6.8) was utilized as a dissolution medium. The samples were transferred to Erlenmeyer flasks containing 10 ml of phosphate buffer. The Erlenmeyer flasks with NP were placed in an incubator on a rocker at 37 °C and at comparative humidity maintained at 100%. The sink condition was retained during the test study. Specimens of 200 µl were withdrawn at predetermined times, and the volume was replaced with an equal volume of fresh phosphate buffer. Centrifugation and filtration of samples was carried out before analysis. All the samples were analyzed using HPLC equipped with a ProntoSIL C-18 column. The quantification of MXD was performed by comprehending peaks, and the concentration of the soluble drug was calculated by approximation from a model curve made of higher strengths of MXD. Collective rectification was made for already drawn samples (13).
2.9. In vitro evaluation of adhesion properties on human hairs
For the adhesive properties of Thio-NP loaded with MXD complexed with thiolated β-CD, human hair cuttings were collected from a barbershop located in Shershah block A. As the hair cuttings at barbershops in Pakistan are generally considered as a waste, therefore ethical committee waived the condition of taking any written or verbal consent. For preparing the hair cutting for adhesive studies, a 100 mM phosphate buffer (pH 6.8) was employed to wash and rinse the hair cuttings continuously. Approximately 30 mg of fluorescently labeled Thio-NP and Blank-NP were equally applied all over the length of hair after a stabilization time of 5 minutes. A peristaltic pump with a persistent surge rate of 1 ml/min was used. After a stabilization time of 5 minutes, sample collection was initiated at fixed intervals of 30, 60, 90, 120, and 180 minutes, and the phosphate buffer that flowed down the hair was gathered. The same procedure was repeated on the hair for 0.2 mg of free rhodamine-123, but without any polymer, and later, to equate with Thio-NP and Blank-NP, 30 mg of thiolated and unmodified β-CD (drug free) was added to the phosphate buffer that had been collected and considered a standard for the measurement of adhesiveness. All the collected specimens were vortexed for 5 minutes, after which 100 µl from every sample was transmitted to a microplate reader, and the absorption was documented at a wavelength of 535 nm and excitation at a wavelength of 485 nm. All experiments will be performed in triplicate (13).
2.10. Skin Tolerance Test
To perform in vivo skin corrosion and irritation tests, healthy rabbits with intact skin were utilized (age = 3.9 months, average weight = 3.0 kg). Ethical approval for conduction of animal study was obtained from COMSATS research ethics and biosafety committee via letter number CUI-REB/23. Twenty-four hours prior to performing the test, an electrical shaver was used to remove hair from 3 areas of an estimated 6 cm2. A pre-weighed quantity of 500 mg nanoparticles (Thio-NP, Blank-NP) was uniformly dispersed on a lint fabric, applied carefully to the shaved area of rabbit, and shielded with a gauze patch, which was kept in position by using a nonirritant flexible patch. If no skin reaction was noticed in the first 180 seconds after applying the NP loaded cloth, the second patch was administered at another shaved area and removed after 60 minutes. If the findings at this point suggest that exposure to nanoparticles for up to 60 minutes was quite normally bearable without any irritation, a third patch was added and removed after 4 hours. The reaction was calculated in accordance with the guidelines (21). At the end of the experiments, nanoparticles were removed from the skin, all the rabbits were active and healthy, moreover, rabbits were kept isolated in the departmental animal house for further 14 days for further observation of any kind of skin allergy.
2.11. Drug uptake through skin
Drug uptake across the bovine ear skin membrane was evaluated by using Franz diffusion cells by following the methods reported in earlier studies, and the thickness of the bovine skin used for permeation ranged from 4 to 6 mm. Bovine ear skin was acquired from Khaliq slaughterhouse, situated at main road, Sher shah Raiwind Road, Lahore. Before the experiment, skin was prepared by removing fats and extra subcutaneous layers by using a scalpel. The final isolated and prepared skin membrane was stored at -20 °C. Briefly, the receptor compartment of the Franz diffusion cell was filled with 8.5 mL of phosphate buffer (pH 6.8) containing 1% Tween 80 to maintain sink conditions. The prepared skin membrane was clamped between the upper and lower compartments of the Franz cell. Fifty milligrams of nanoparticle formulations (Thio-NP and Blank-NP) containing an equivalent quantity of 20 mg MXD and, similarly, ethanolic solution containing 20 mg MXD (Control) were added to the upper donor chamber.
Uptake and drug retention experiments were carried out for 180 minutes. Samples from the receptor compartment were withdrawn at regular intervals of 30 min. Equal volumes of buffer were replaced with each sample withdrawal. At the end of the 3 h experiment, formulations from the donor chamber were removed, and the skin membrane was cleaned with isopropyl alcohol. To evaluate and measure the amount of MXD retained within skin and follicles, differential stripping, and coating of skin with acrylate. During stripping of skin, initial stripping was not considered for analysis. All the tape strips, acrylate coating and skin tissue were dipped in ethanol for extraction of MXD, and all the suspensions and skin tissues were centrifuged for 30 min at 14000 rpm. The concentration of MXD at different intervals and extracted from strips and acrylate coatings was analyzed by HPLC.
2.12. Statistical analysis
Statistical data analysis was executed by utilizing Student's t test with a confidence interval (p < 0.05) for analyzing two test groups.
3. Results and discussion
3.1. Characterization of β-CD-Cys derivatives
To induce the mucoadhesive properties to β-CD oligomers, thiolation was carried out via the oxidation and subsequent reductive deamination of β-CD. Thiolation of β-CD was carried out successfully by the covalent binding of cysteamine to β-CD-CHO. Conjugation of primary amines such as cysteamine depends on the presence of carbonyl groups on β-CD, whereas unmodified β-CD does not contain carbonyl groups necessary for the reaction with primary amines. For the oxidation of β-CD, NaIO4 was used, and the conjugation of cysteamine with β-CD-CHO was achieved by a reducing agent, known as NaCNBH3. Increasing the concentration of NaIO4 could lead to an increase in the oxidation state of β-CD and subsequently increase the number of conjugated cysteamines (13). Thiolated β-CD, obtained after lyophilization, was an amorphous, odorless, and white powder.
3.2. Determination of thiol group and disulfide bond contents
The thiolated conjugates obtained were characterized via Ellman’s reagent, and the results showed 1804.68 ± 25 μmol/g free thiol groups and 902.34 ± 25 μmol/g disulfide bonds, on average, as shown in
Table 1. Likewise, increasing the concentration of cysteamine while keeping the NaIO
4 concentration constant could lead to an increased number of thiol groups attached, as is evident from the research work of previous researchers (13).
3.3. FTIR spectral analysis of thio-β-CD
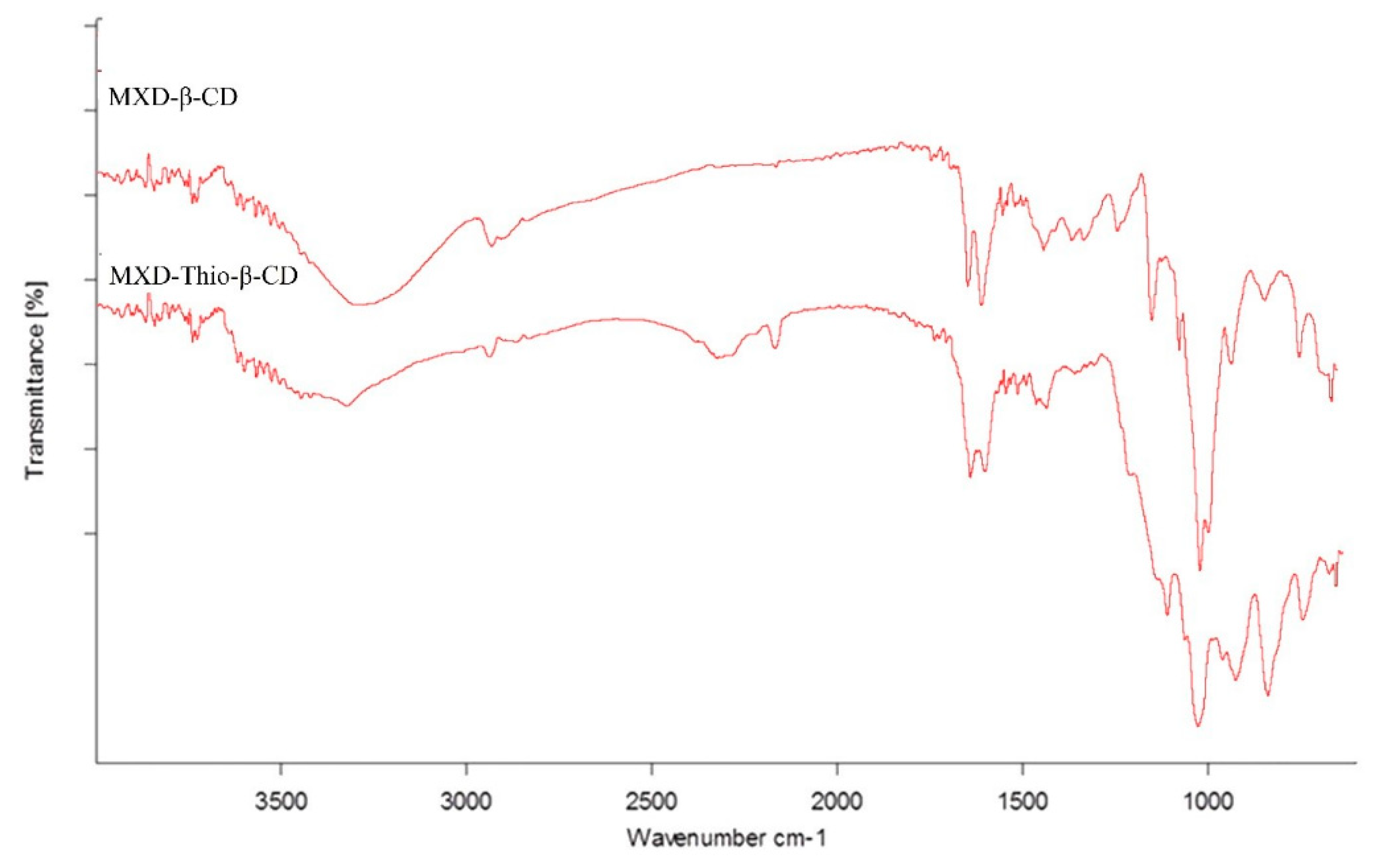
FTIR analysis of the cysteamine conjugation and confirmation of inclusion complexes was carried out. The variation in the shape, shift, and intensity of the IR absorption peaks of the guest or host can provide enough information for the occurrence of the inclusion complex. The presence of thiol groups was established by the results acquired from FTIR spectroscopy. The FTIR spectra of MXD complexed with thio-β-CD and unmodified β-CD are shown in
Figure 1. A small and floppy vibrational stretch witnessed at approximately 2550–2625 cm−1 was assigned to the -SH stretching vibration in thio-β-CD. The broad absorption band at approximately 3100–3550 cm
−1 was attributed to -NH (which also confirms the presence of conjugated cysteamine) and -OH groups co-existing within thio-β-CD (13).
In the case of aldehydic β-CD, the absorption band at 3150–3400 cm
−1 was observed, which characterizes the existence of -OH groups alone. The absorption band at 2900 cm
−1 was assigned to carbon–hydrogen stretching vibrations in both compounds. The absorption band at approximately 1680 cm
−1 was attributed to aldehyde groups of the studied compounds. The absorption band at 1654 cm
−1 vanished or shifted to low wavenumbers in the MXD-β-CD inclusion complex, indicating that the C ̶ O stretching vibration was restricted after the formation of the inclusion complex. 1552 cm
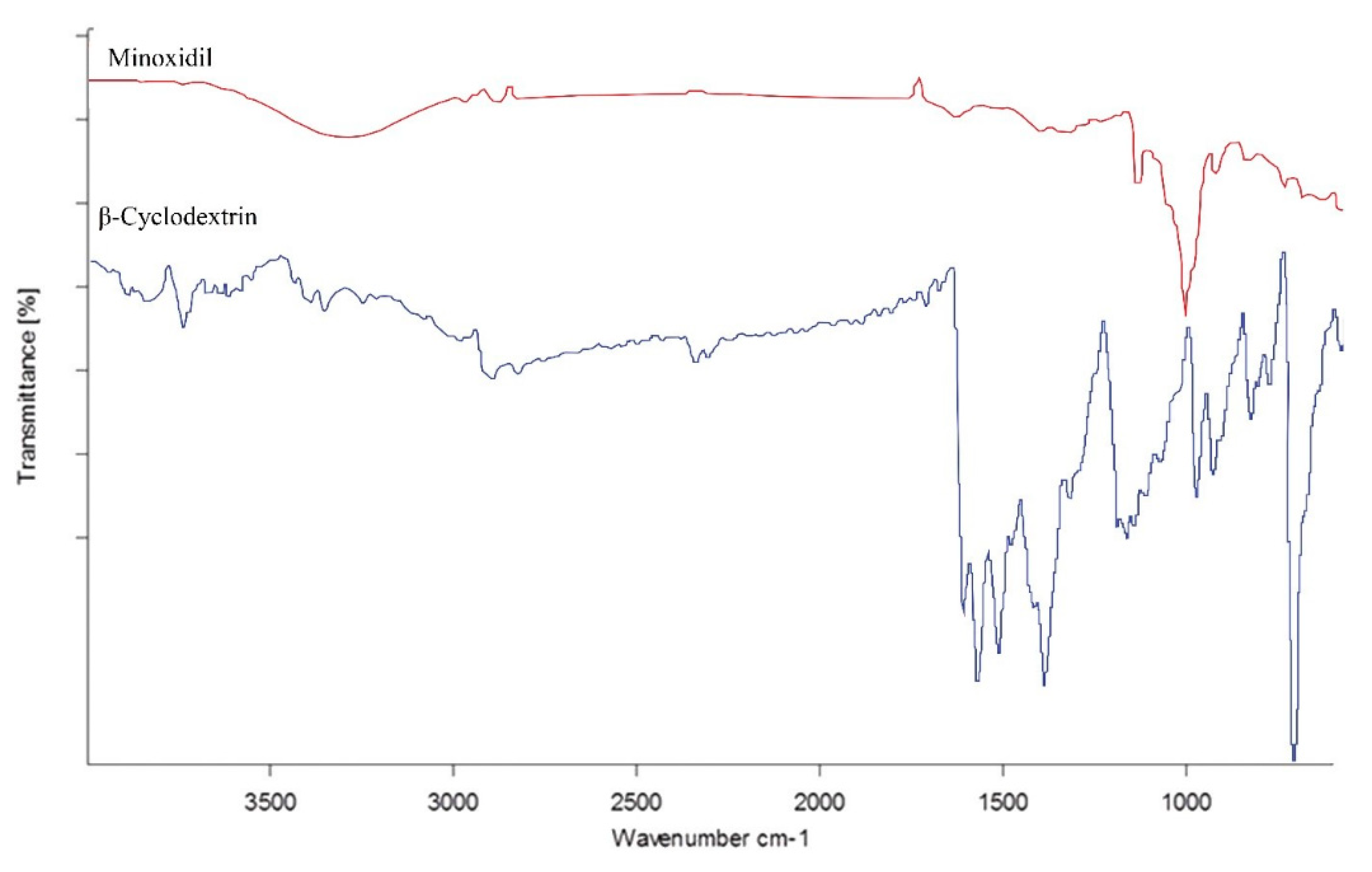
−1 was greatly weakened, indicating that a majority of MXD was included in the complex. The FTIR spectra of free minoxidil and β-CD are presented in
Figure 2 for comparative analysis.
3.4. Nanoparticle size and zeta potential measurements
All the nanoparticles prepared from thiolated (Thio-NP) and unmodified β-CD (Blank-NP) displayed uniform particle sizes within the range of 231 nm to 319 nm. In general, the Z-average diameters for Thio-NP composed of thiolated β-CD were larger than the core size of the nanoparticle (Blank-NP) consisting of unmodified β-CD because of the disulfide bond formation and agglomeration of thiolated β-CD; on the other hand, the nanoparticles composed of unmodified β-CD have a smaller size. Increased size could be the reflection of increased amount of drug loading within the complexes of the thiolated β-CD. Similarly, the zeta potential of all the nanoparticles was in the range of ̶ 8.1 to + 12.0, and the significant shift of negative potential to positive potential seems due to the cat-ion charges of –NH+ present in the amino groups of cysteamine attached to thiolated unmodified β-CD (22).
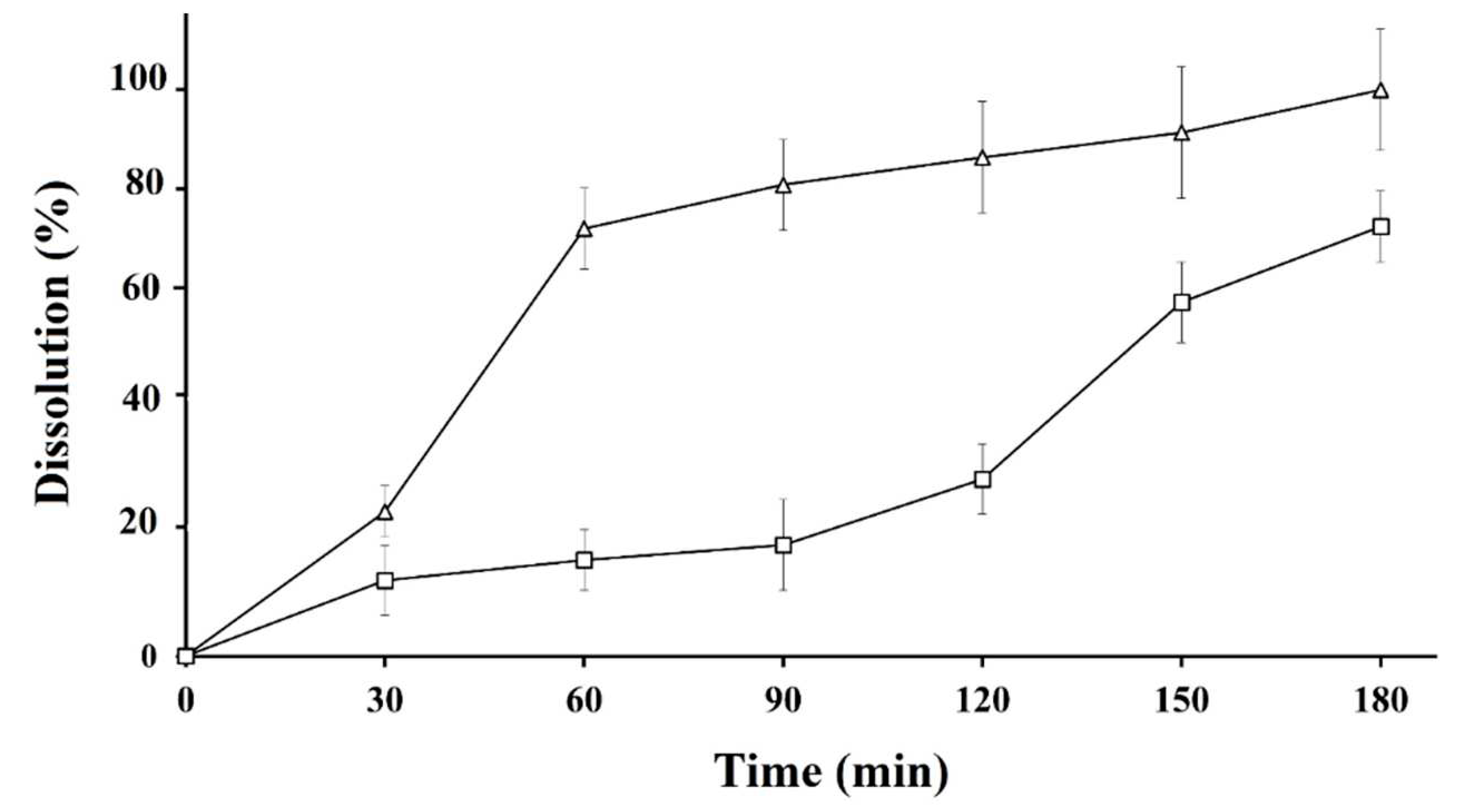
3.5. Drug dissolution studies
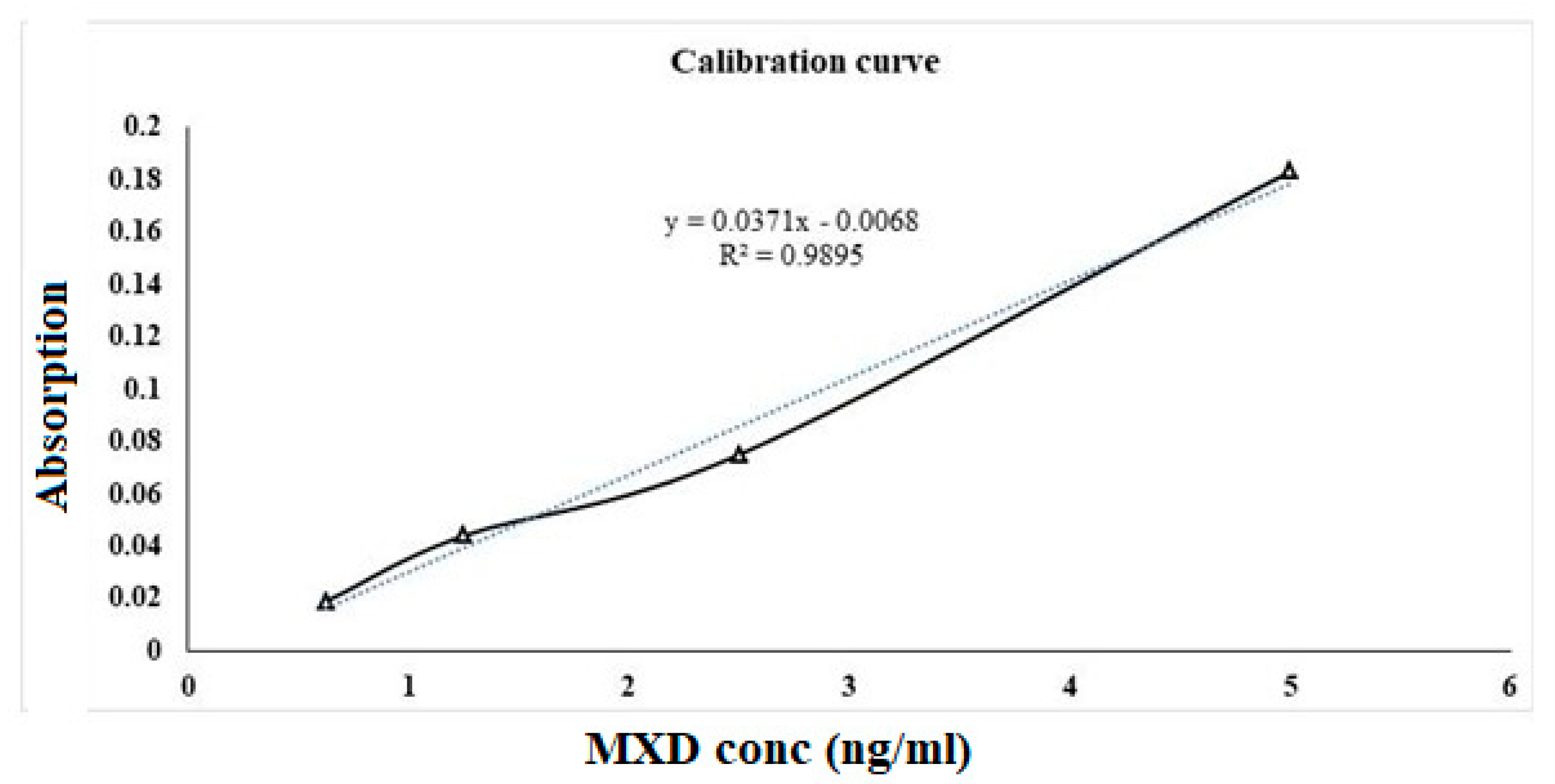
The dissolution profiles of Thio-NP and blank-NP formulations were evaluated and compared with the calibration curve of standard MXD. The drug release profile of Thio-NP tested conceivably validates the impact of the realistic effect of thiolation and the potential for multi purposing of Thio-NP composed of thiolated β-CD and chitosan. The calibration curve shown in
Figure 3 was developed with increasing concentrations of MXD. Notably, this is one of the few studies that has evaluated the topical drug release of nanoparticle formulations. Therefore, the oral formulations and the drug properties control the absorption after oral administration, as evidenced by the incongruity between the plasma concentration of one of the drugs and the in vitro release kinetics (23). In contrast to the available research, it has been revealed here that excipient-free β-CD nanoparticles can be cast off as release-retarding excipients and to prepare nanocarriers with desired release profiles. In the case of Thio-NP, we observed sustained drug release for the first 1–3 h, as shown in
Figure 4, because drug entrapment within the CD adjoining the oligomers prohibited water from penetrating the internal core containing the drug. After the first phase of release due to dissolution of the nanoparticles, the drug-loaded β-CD functioned as a 2-h long stop-gate for the second phase of nanoparticles to dissolve and release the drug (24).
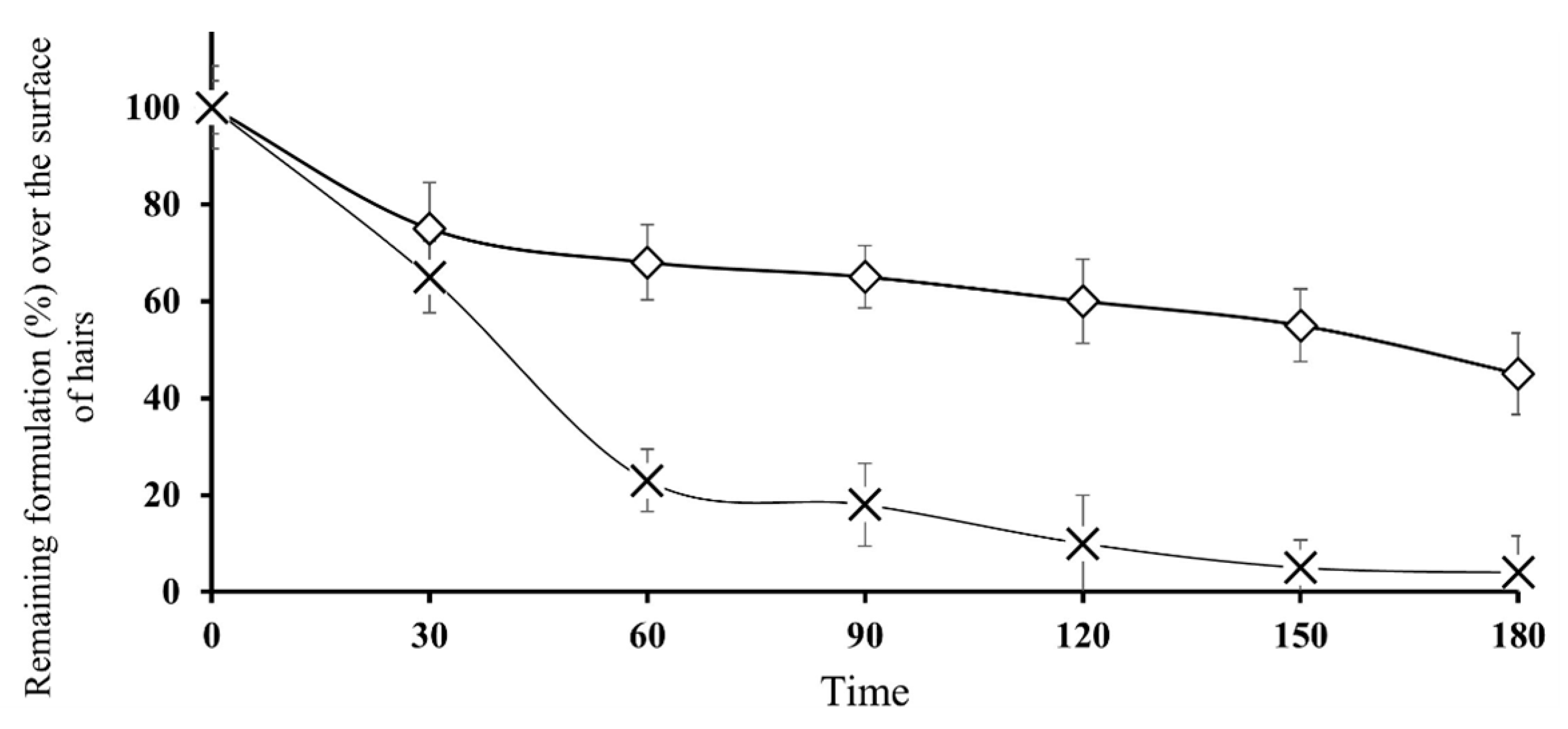
3.6. In vitro evaluation of adhesion properties on human hairs
Topically applied nanoparticles in the form of aqueous suspensions are pharmaceutical persistent release formulations that maneuver in a state firmly attached to human hairs; consequently, adhesion properties are imperative characteristics of this form of application. There are many concepts of adhesion and fundamental forces that appear to act independently (25). In previous adhesion studies over porcine skin/hairs, skin adhesion was measured from the pure unmodified β-CD combinations of straight compressed discs as well as from the final drug composition. The outermost coating of the skin is the stratum corneum, which consists of both lipophilic and hydrophilic domains as well as hair follicles and sweat glands. The hydrophilic parts consist mostly of keratin. The circumstance that high polarity formulations such as hydrogels may also adhere to skin may be in part due to the skin being wet and perhaps due to the presence of hair follicles and sweat glands, which contain aqueous channels. However, in the case of thiolated CDs containing nanoparticles (Thio-NP), disulfide bonds are formed between the hair keratin and free thiol groups attached to the CDs (26). These free thiol groups develop strong covalent bonds with the hair roots, and in this way, nanoparticles reside over hairs for a longer time, as shown in
Figure 5, in which nanoparticles formulated with thiolated CDs were retained for a significantly longer time than nanoparticles consisting of unmodified CDs.
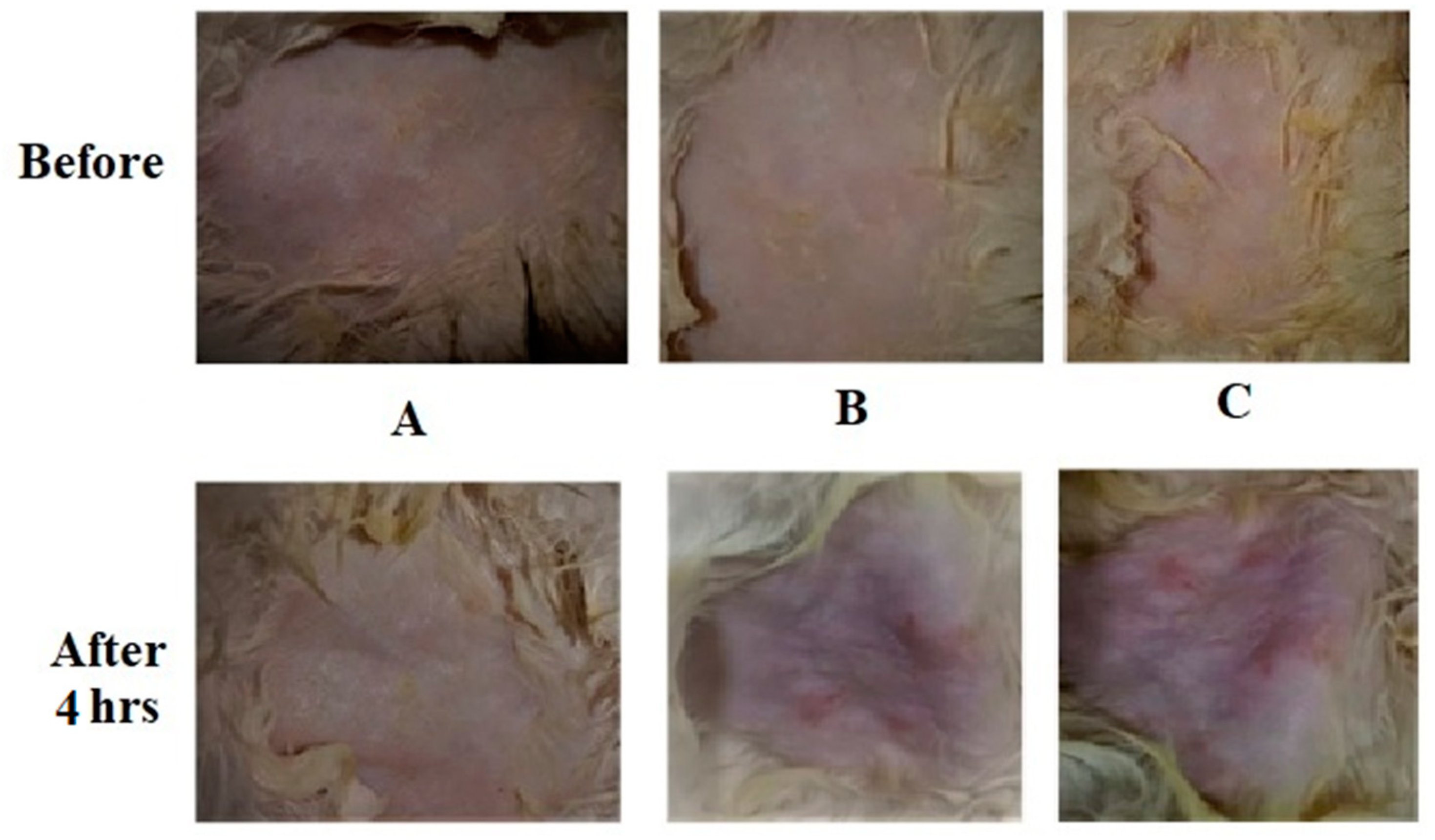
3.7. Skin Tolerance Test
A skin tolerance test was executed on rabbits to appraise whether the application of newly developed adhesive nanoparticle suspensions might cause skin irritation (27). All nanoparticle formulations developed from thiolated (Thio-NP) and unmodified CDs (Blank-NP) showed no significant skin irritations (erythema and edema) on normal rabbit skins.
Figure 6 shows photographs of sections of the hairless skin of rabbits treated with unmodified CDs nanoparticles (blank-NP), skin treated with thiolated CDs nanoparticles (Thio-NP) and skin treated with the strong base NaOH. It was evident that skin samples treated with nanoparticles developed from thiolated CDs did not reveal any marked changes in the normal skin surfaces and no signs of inflammation, guaranteeing good skin tolerability of the developed formulation (28).
3.8. MXD uptake through skin
The amount of drug accumulated in the skin compartments, expressed as a percentage of the dose applied on the skin surface, is shown in
Table 2. Through this experiment, the main objective was to evaluate whether nanoparticles with thiolated CDs (thio-NP) would remain attached for a longer duration at the hair shaft compared with nanoparticles developed with unmodified CDs (Blank-NP). In this context, the Thio-NP demonstrated greater MXD retention in dermal layer compared with the greater penetration of blank NP in the hair follicles, and the least drug penetration was observed for the Thio-NP sample (p < 0.05), which reflects the attachment of thiol groups with keratin (highly rich in disulfide bonds) present in the hair shaft and therefore, increased the retention of MXD at the application side, which led to an increased accumulation of MXD loaded within thiolated nanoparticles (29, 30). The effect of thiol groups present in Thio-NP on hair keratin targeting and the subsequently increased retention effect is already well known (31, 32). Moreover, it is noteworthy that the presence of sebum in the follicle compartment can particularly increase the partition and permeation of (MXD) hydrophobic compounds (33).
Thio-NP and blank-NP provided significantly different transdermal drug permeation rates, with 7.35 ± 1.67 % and 3.56 ± 0.34 % of the total doses of MXD applied on the skin surface, respectively, with significant differences among these results (p > 0.05). The significantly improved MXD dermal layer retention observed for Thio-NP is reflected in MXD dermal accumulation and lesser follicular penetration. Regarding follicular penetration, Thio-NP provided 1.56 ± 0.57 % of the total amount of MXD, whereas Blank-NP provided 8.45 ± 2.69 %. These results show that Thio-NP could retain and maintain higher concentrations of MXD at the hair shaft for at least 3 h, which might provide higher efficacy in the treatment of AGA.
4. Conclusion
This research work has revealed that nanoparticles developed from thiolated CDs can be premeditated to adhere over hair shafts for a prolonged time. CDs anchored with thiol groups when formulated into nanoparticles can bind to sulfur moieties of keratin and are confined on the skin external and near to the skin exterior in the hair follicle. For nanoparticles ornamented with thiolated CDs, roughly half of the thiol groups appeared over the particle surface, so binding to keratin was greatly increased. Nanoparticles developed from thiolated CDs displayed uniform particle size and positive zeta potential, and MXD loaded in the CDs showed sustained release. By controlling the amount of the free thiol groups attached over the CDs, it is reasonable to develop nanoparticle formulations focused over the hair shafts and might be a potential carrier for application for male baldness issues and other skin topical issues.
Funding declaration
There was no funding awarded for this research.
References
- Nagai, N.; Iwai, Y.; Sakamoto, A.; Otake, H.; Oaku, Y.; Abe, A.; Nagahama, T. Drug Delivery System Based On Minoxidil Nanoparticles Promotes Hair Growth In C57BL/6 Mice. Int. J. Nanomed. 2019, ume 14, 7921–7931. [Google Scholar] [CrossRef]
- Yang, G.; Chen, G.; Gu, Z. Transdermal Drug Delivery for Hair Regrowth. Mol. Pharm. 2020, 18, 483–490. [Google Scholar] [CrossRef]
- Tricarico, D.; Maqoud, F.; Curci, A.; Camerino, G.; Zizzo, N.; Denora, N.; Cutrignelli, A.; Laquintana, V.; Lopalco, A.; la Forgia, F.; et al. Characterization of minoxidil/hydroxypropyl-β-cyclodextrin inclusion complex in aqueous alginate gel useful for alopecia management: Efficacy evaluation in male rat. Eur. J. Pharm. Biopharm. 2018, 122, 146–157. [Google Scholar] [CrossRef]
- Chandrashekar, B.; Nandhini, T.; Vasanth, V.; Sriram, R.; Navale, S. Topical minoxidil fortified with finasteride: An account of maintenance of hair density after replacing oral finasteride. Indian Dermatol. Online J. 2015, 6, 17–20. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira-Silva, M.; Guerra, C.; Costa, D.; Peixoto, D.; Pereira, I.; Pita, I.; Ribeiro, A.J.; Veiga, F. Topical Minoxidil-Loaded Nanotechnology Strategies for Alopecia. Cosmetics 2020, 7, 21. [Google Scholar] [CrossRef]
- Mali, N.; Darandale, S.; Vavia, P. Niosomes as a vesicular carrier for topical administration of minoxidil: formulation and in vitro assessment. Drug Deliv. Transl. Res. 2012, 3, 587–592. [Google Scholar] [CrossRef]
- Lopedota, A.; Cutrignelli, A.; Denora, N.; Laquintana, V.; Lopalco, A.; Selva, S.; Ragni, L.; Tongiani, S.; Franco, M. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-β-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev. Ind. Pharm. 2014, 41, 728–736. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: a review. Drug Design, Development and Therapy. 2019, 13, 2777. [Google Scholar] [CrossRef]
- Herrmann, S.; Daniels, R.; Lunter, D. Methods for the determination of the substantivity of topical formulations. Pharm. Dev. Technol. 2016, 22, 487–491. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lochhead, R.; Maibach, H.; Yamashita, Y. Cosmetic science and technology: theoretical principles and applications; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ijaz, M.; Ahmad, M.; Akhtar, N.; Laffleur, F.; Bernkop-Schnürch, A. Thiolated α-Cyclodextrin: The Invisible Choice to Prolong Ocular Drug Residence Time. J. Pharm. Sci. 2016, 105, 2848–2854. [Google Scholar] [CrossRef]
- Ijaz, M.; Matuszczak, B.; Rahmat, D.; Mahmood, A.; Bonengel, S.; Hussain, S.; Huck, C.W.; Bernkop-Schnürch, A. Synthesis and characterization of thiolated β-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery. Carbohydr. Polym. 2015, 132, 187–195. [Google Scholar] [CrossRef]
- Ijaz, M.; Griessinger, J.A.; Mahmood, A.; Laffleur, F.; Bernkop-Schnürch, A. Thiolated Cyclodextrin: Development of a Mucoadhesive Vaginal Delivery System for Acyclovir. J. Pharm. Sci. 2016, 105, 1714–1720. [Google Scholar] [CrossRef]
- Rahmat, D.; Sakloetsakun, D.; Shahnaz, G.; Perera, G.; Kaindl, R.; Bernkop-Schnürch, A. Design and synthesis of a novel cationic thiolated polymer. Int. J. Pharm. 2011, 411, 10–17. [Google Scholar] [CrossRef]
- Binder, C.M.; Dixon, D.D.; Almaraz, E.; Tius, M.A.; Singaram, B. A simple procedure for C–C bond cleavage of aromatic and aliphatic epoxides with aqueous sodium periodate under ambient conditions. Tetrahedron Lett. 2008, 49, 2764–2767. [Google Scholar] [CrossRef]
- Wang, J.-h.; Cai, Z. Investigation of inclusion complex of miconazole nitrate with β-cyclodextrin. Carbohydrate polymers. 2008, 72, 255–60. [Google Scholar] [CrossRef]
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Ma, J.; Zhong, F. pH and temperature stability of (−)-epigallocatechin-3-gallate-β-cyclodextrin inclusion complex-loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 149, 340–347. [Google Scholar] [CrossRef]
- Hombach, J.; Palmberger, T.F.; Bernkop-Schnürch, A. Development and in vitro evaluation of a mucoadhesive vaginal delivery system for nystatin. J Pharm Sci. 2009, 98, 555–64. [Google Scholar] [CrossRef]
- Iqbal, J.; Sarti, F.; Perera, G.; Bernkop-Schnürch, A. Development and in vivo evaluation of an oral drug delivery system for paclitaxel. Biomaterials 2011, 32, 170–175. [Google Scholar] [CrossRef]
- Sun J, Zhou S, Hou P, Yang Y, Weng J, Li X, et al. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. Journal of biomedical materials research Part A. 2007, 80, 333–41.
- Choi, J.; Kim, H.; Choi, J.; Oh, S.M.; Park, J.; Park, K. Skin corrosion and irritation test of sunscreen nanoparticles using reconstructed 3D human skin model. Environ. Heal. Toxicol. 2014, 29, e2014004. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, M.; Knez. ; Novak, Z. Encapsulation and drug release of poorly water soluble nifedipine from bio-carriers. J. Non-Crystalline Solids 2018, 481, 486–493. [Google Scholar] [CrossRef]
- Budai-Szűcs, M.; Kiss, E.L.; Szilágyi, B. .; Szilágyi, A.; Gyarmati, B.; Berkó, S.; Kovács, A.; Horvát, G.; Aigner, Z.; Soós, J.; et al. Mucoadhesive Cyclodextrin-Modified Thiolated Poly(aspartic acid) as a Potential Ophthalmic Drug Delivery System. Polymers 2018, 10, 199. [Google Scholar] [CrossRef]
- Horstmann, M.; Müller, W.; Asmussen, B. Principles of skin adhesion and methods for measuring adhesion of transdermal systems. Drugs and the pharmaceutical sciences. 1999, 98, 175–96. [Google Scholar]
- Grießinger, J.A.; Bonengel, S.; Partenhauser, A.; Ijaz, M.; Bernkop-Schnürch, A. Thiolated polymers: evaluation of their potential as dermoadhesive excipients. Drug Dev. Ind. Pharm. 2016, 43, 204–212. [Google Scholar] [CrossRef]
- Khurana, S.; Bedi, P.; Jain, N. Preparation and evaluation of solid lipid nanoparticles based nanogel for dermal delivery of meloxicam. Chem. Phys. Lipids 2013, 175-176, 65–72. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, W.; Xu, L.; Yu, D. Surface modification of keratin fibers through step-growth dithiol-diacrylate thiol-ene click reactions. Mater. Lett. 2016, 178, 159–162. [Google Scholar] [CrossRef]
- Leichner, C.; Steinbring, C.; Baus, R.A.; Baecker, D.; Gust, R.; Bernkop-Schnürch, A. Reactive keratin derivatives: A promising strategy for covalent binding to hair. J. Colloid Interface Sci. 2018, 534, 533–541. [Google Scholar] [CrossRef]
- Li, B.; Sun, Y.; Yao, J.; Wu, H.; Shen, Y.; Zhi, C.; Li, J. An environment-friendly chemical modification method for thiol groups on polypeptide macromolecules to improve the performance of regenerated keratin materials. Mater. Des. 2022, 217, 110611. [Google Scholar] [CrossRef]
- Al Mahrooqi, J.H.; Khutoryanskiy, V.V.; Williams, A.C. Thiolated and PEGylated silica nanoparticle delivery to hair follicles. International Journal of Pharmaceutics. 2021, 593, 120130. [Google Scholar] [CrossRef]
- Kim, J.-C.; Lee, M.-H.; Rang, M.-J. Minoxidil-containing dosage forms: Skin retention and after-rinsing hair-growth promotion. Drug Deliv. 2003, 10, 119–123. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).