1. Introduction
Pyrethroids are highly efficient and broad-spectrum insecticides that have gained widespread use due to their effectiveness and low toxicity. Bifenthrin (BF) is a common pyrethroid insecticide mainly used for the prevention and control of plant diseases and insect pests in arbor forests, tea trees, and cotton (Yang et al., 2018). Despite the relatively rapid degradation of pyrethroids compared to other pesticides, their extensive use has cause the high accumulation in water sources through surface runoff, spraying, and rainwater scouring (Akan et al., 2014; Fernández-Ramos et al., 2014; Ge et al., 2010), even widely detected in kinds of organisms, such as fish, amphibian and humans (Garí et al., 2018; Rawn et al., 2010). Chronic exposure may cause adverse reactions, including tissue structure lesions, neurotoxicity, behavioral changes, endocrine disorders, decreased immune ability, and decreased growth and development (Awoyemi et al., 2019; Baruah and Chaurasia, 2020; Jin et al., 2013; Syed et al., 2018). This is also one of the important reasons for the decline of amphibian populations (Hayes et al., 2010; Hof et al., 2011).
The metabolism of pyrethroids, such as BF, may lead to intracellular reactive oxygen species (ROS) levels rise (R. Reed et al., 2011). Excessive ROS can trigger oxidative stress reactions (Jiang et al., 2022), leading to oxidative DNA and tissue damage, causing tissue structural abnormalities and behavioral damage, and triggering inflammation and apoptosis (Choi et al., 2020; Park et al., 2020; Yu et al., 2018). Therefore, identify the effects of BF on oxidative stress in aquatic organisms is important to clarify the non-targeted effects of pyrethroids and other pesticides. Catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) are important indicators of biological peroxidation injury and 8-hydroxydeoxyguanosine (8-OHdG) has been widely involved in the detection of DNA damage]and oxidative stress (Farag et al., 2021; Liu et al., 2020; Papadimitriou and Loumbourdis, 2002; Prokić et al., 2019; Wang et al., 2021). Furthermore, RNA-seq technology is a useful tool for in-depth developing of how contaminants cause biological toxicity. Rapid, comprehensive, and detailed analysis of non-model biological genomes and transcripts is facilitated by the development and application of RNA-seq, which is widely used to assess transcriptome changes in target tissues or cells, including splicing, novel transcripts, and gene annotation (Collins et al., 2008; Liu et al., 2018; Zhang et al., 2019).
The Chinese giant salamander (CGS), Andrias davidianus, are amphibians whose larvae have a similar lifestyle to fish. The CGS have high permeability bare skin and highly sensitive to environmental changes, can be used as water quality bio-indicator (Langlois, 2021; Porte Visa et al., 2005). However, limited studies on the toxic effects of pyrethroids and other pesticides on amphibians. Especially the molecular toxicological effects of external compounds on the CGS, a non-model experimental organism lacking complete genomic information, has not been fully elucidated. In present study, we utilized the CGS as target organism to investigate the toxic effect of bifenthrin on CGS, comparing behavioral changes, liver histopathology, oxidative stress factors, DNA damage effects, and transcriptome differences between the control and bifenthrin treatments. The results would provide theoretical basis for the rational use of pyrethrins and environmental risks, and scientific guidance for the conservation of amphibian populations.
2. Materials and Methods
2.1. Animals, chemicals, and experimental design
BF and dimethyl sulfoxide (DMSO) used in this study was purchased from Aladdin Bio-Chem Technology (Shanghai, China) and TCI chemicals (Shanghai, China), respectively. The study was conducted in the College of Life Science, Anqing Normal University (Anqing, China). 100 CGSs larvae at 8-month-old were purchased from a CGS farm (Anqing, China) and acclimated in a 100 L aquarium for 1 week, using dechlorinated tap water at 18 ± 2℃. CGSs had initial average body weight at 5.7 ± 0.3 g and a total length of 85 ± 10 mm. All CGSs were not fed during the entire experiment, including domestication period and exposure period.
An acute-exposure experiment was conducted to determine the semi-lethal dose of BF. CGS larvae were randomly grouped and placed in 1 L aquarium and exposed to either control or BF for 1 week. Each group consisted of 4 animals, and there were 3 groups per treatment. Nominal exposure concentrations of BF dissolved in DMSO were: 6.25, 12.5, 25, and 50 μg/L, with a maximum solvent concentration of 0.001% (v/v). The experiment was conducted at 16.5 ± 0.5℃ under a 10 h light: 14 h dark photoperiod; 50% of the water was changed in each treatment every 12 h. CGS were observed for poisoning symptoms after 24, 48, 72 and 96 hours of treatment and any dead CGS were taken out of experimental tank at those times. Concerning the Acute Toxicity Classification Standard (GB/T 31270.12,2014) and classification test method for acute toxicity of dangerous chemicals to fish (GB/T 21281–2007), the following acute toxicity grades were established: acute toxicity grade I was obtained when 96 h LC50 ≤ 1 mg /L, acute toxicity grade II was obtained when 1 mg/L < 96 h LC50 ≤ 10 mg/L, and acute toxicity grade III was obtained when 10 mg/L < 96 h LC50 ≤ 100 mg/L.
Based on the determined semi-death dose, another 1-week experiment was conducted to observe the acute toxic effects of BF on CGS larvae. The experimental design was the same as that described for the acute exposure experiment except that CGSs were exposed to either the control or BF for 1 week, with nominal exposure concentrations of 0.04 and 4 μg/L of BF in DMSO. At the end of the experiment, all CGS larvae were euthanized using an overdose of 2-phenoxyethanol, followed by cervical transection. The gonads were removed, weighed, and fixed in Bouin’s fixative solution (Phygene Biotechnology Co., Ltd, Fuzhou, CN) for 24 h. These samples will be stored in 70 % ethanol until histological observation. The brain, liver, and kidneys were stored at -80℃ for subsequent study.
All experimental procedures involving animals were conducted in compliance with the Animal Care and Use Committee of the College of Life Science,Anqing Normal University,China (Permit Number #2-01).
2.2. Physiological and biochemical indicator detection
Liver, brain, and kidney tissues were homogenized and centrifuged (3500 rpm, 15 min, 4℃). The supernatant was retained for analysis. Total protein, ROS, GSH, MDA, SOD, and ATPase activity were measured using commercial colorimetric kits. 8-OHdG content was measured using a corresponding fish-specific ELISA kit. The above kits were obtained from the Nanjing Jiancheng Institute of Bioengineering (Nanjing, China). All indicators were measured with a Spark 10M microplate reader (Tecan Trading AG, Mannedorf, Switzerland) in accordance with the manufacturer’s instructions.
2.3. Histological Evaluation
Paraffin-embedded fixed liver tiusse were sectioned into 5 μm slices and stained with Mayer’s Hematoxylin and Eosin Y (H-E staining). Gonads histological observation were conducted by a light microscope (40X) and classified (Chen et al., 2019).
2.4. RNA isolation and sequencing
In present study, liver tissue was selected for RNA isolation and sequencing. TRIzolTM Reagent kit (Invitrogen, Carlsbad, California, USA) was used to extract total RNA. The quality of RNA was evaluated on an Agilent 2100 Bioanalyzer. (Agilent Technologies, lnc., Palo Alto, California, USA) and verified by agarose gel electrophoresis without RNase. After total RNA extraction, mRNA was isolated and purified using oligomeric (dT) magnetic beads, buffered to short fragments, and reverse transcribed into cDNA using a NEBNext Ultra RNA library Prep Kit for Illumina (NEB #7530, New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instructions. Purified double-stranded cDNA fragments were subjected to end-repaired, a base was added, and then ligated to Illumina sequencing linker. The ligation reaction was purified using AMPure XP Beads (1.0X) and a polymerase chain reaction (PCR) was performed. The cDNA library was sequenced on an Illumina Novaseq6000 (Gene Denovo Biotechnology Co., Guangzhou, China).
2.5. Transcriptome assembly, annotation and analysis
Transcriptome assembly was performed using Trinity software (Broad Institute, 2.6). Evaluation of the quality of assembly results on the basis of N50 values, serial length and benchmark universal single-copy orthologs (
http://busco.ezlab.org/). Single gene serialization using NR, SwissProt, KEGG, and COG/KOG databases (E-value <:0.00001) to select the protein with the highest serial similarity to a given single gene to obtain protein function annotation information for a single gene. Gene-level stratified clustering and log2(FPKM 1) were calculated for all sampled genes. Differentially expressed genes (DEGs) were selected using edgeR’s general filtering criteria (log2|fold change|>1, false discovery rate [FDR] <0.05) filtered by FPKM data. Analyse DEG temporal expression profiles and perform KEGG enrichment for each spectrum. Select and compare the desired genes to determine how they change when expressed.
2.6. Statistical analysis
Means ± standard error (SE) are reported for physiological and biochemical indicators. Statistical analyses were performed using SPSS (IBM, 23.0, Armonk, NY, USA). The significance level was set at
p <0.05. The normality (Shapiro-Wilk test) and the homogeneity of variance (Levene’s test) of the data were checked before the statistical analysis. When normality and homogeneity of variance were achieved, a one-way analysis of variance (
p < 0.05) followed by Duncan’s multiple range test was used to analyze the effect of BF on CGS larvae. If the normality and homogeneity of variance could not be obtained, nonparametric methods were used. Statistical analyses on DEGs were performed using OmicShare tools (
https://www.omicsmart.com).
3. Results
3.1. Acute toxicity effects of BF on CGS larvae
Table 1 presents the results of the acute toxicity of BF on CGS larvae. The lethal concentrations of BF killed half of CGS larvae (LC50) at 24, 48, 72, and 96 h were 50, 27.4, 12.5, and 9.9 µg/L, respectively, and the safe concentration (SC) was identified at 2.47 µg/L. According to the classifications outlined in section 2.1, BF was classified as acute grade I (highly toxic) to CGS larvae.
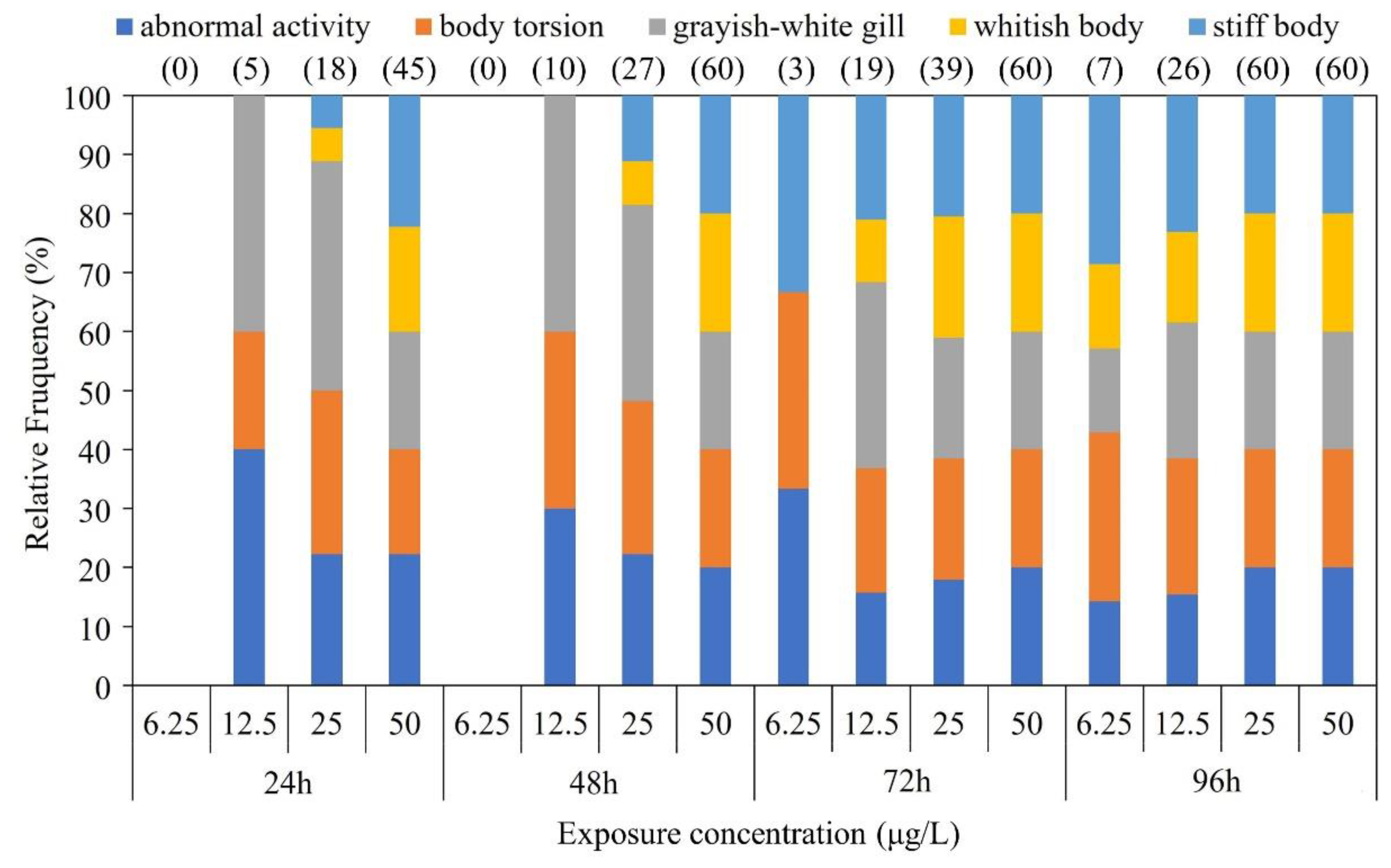
The CGS exhibited a stress response to BF exposure on an individual behavioral level (
Figure 1). CGS larvae exhibited grayish-white gill, abnormal activity, body torsion, and imbalance. Particularly in high BF treatments, most CGS larvae also exhibited whitish bodies, slow movement and stiff bodies.
3.2. Oxidative stress injury of BF exposure on CGS larvae
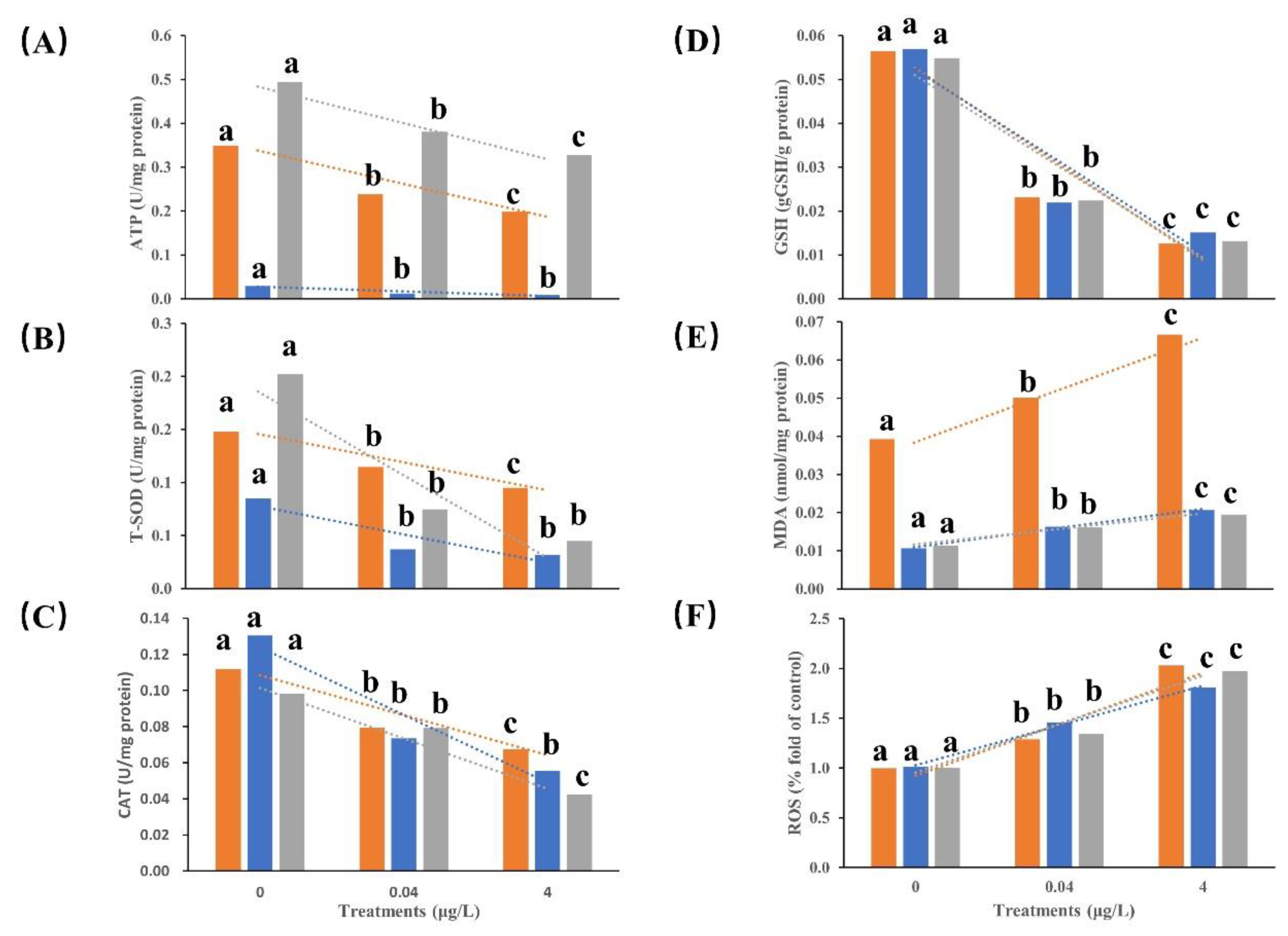
Based on the results of acute BF toxicity tests,
Figure 2 and
Table 2 exhibited the 1-week BF exposure (0, 0.04 and 4 μg/L) effects on oxidative stress in various tissues of the CGS larvae. Significant changes in the oxidative stress factors were detected in various tissues of the CGS larvae (
p <0.05). Compared with the control group, the concentrations of ATP, GSH, SOD, and CAT in the brain, liver, and kidney in all BF exposure groups all decreased significantly with BF increasing. The concentrations of ATP and SOD in the liver were significantly lower than those in the brain and kidney in all BF treatments (
p < 0.05), however, no significant difference (
p > 0.05) of GSH and CAT were detected among the three tissues. Conversely, MDA and ROS concentrations significantly increased with BF concentration increasing and substantial difference were shown between the BF treatments (
p < 0.05). The kidney exhibited a more pronounced downward trend in SOD concentration compared to the liver and brain with BF concentration increasing.
3.3. DNA damage of BF exposure on CGS larvae
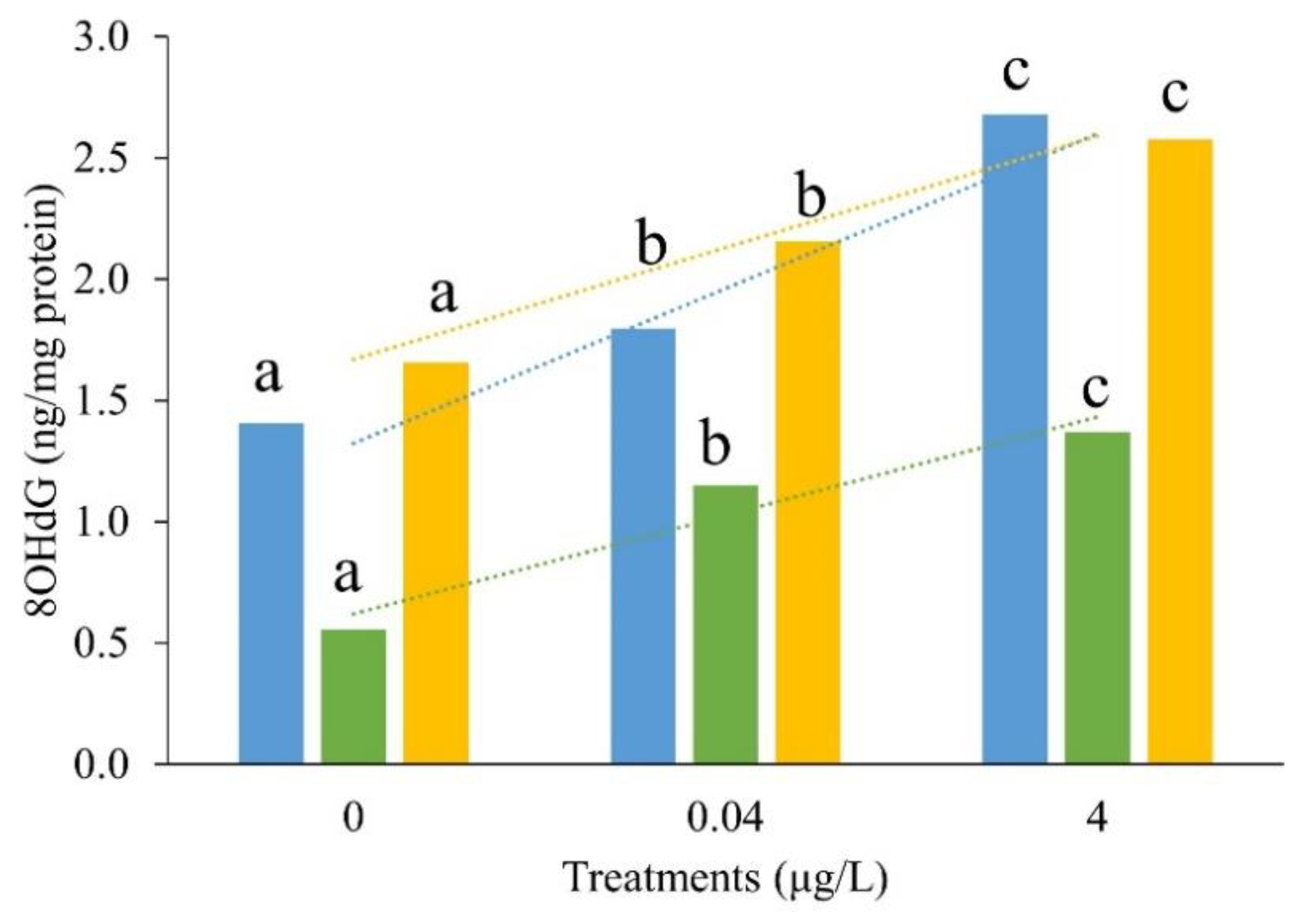
Based on the results of acute BF toxicity tests,
Figure 3 and
Table 2 exhibited the DNA damage effects of 1-week BF exposure on CGS larvae. Even the concentration of 8-OHdG in the liver was lower than that in the brain and kidneys in all BF treatments, the 8-OHdG concentrations were significantly increased in the brain, liver, and kidney with increasing BF concentration (
p < 0.05).
3.4. Effects of BF on liver tissue of CGS
Treated by BF, the liver tissue, which showed the lowest toxicity effects based on observed antioxidant parameters, was selected as a sensitive sample for further study on BF toxicity effects. The pathological analysis of the CGS liver tissue exposed to BF is shown in
Figure 4. In the control group, the liver structure was normal and red blood cells, hepatocytes and hepatic sinusoids were clearly visible. A few cells in the livers of the 0.04 µg/L BF exposed CGS were enlarged, accompanied by vacuolation and some dilated and congested hepatic sinusoids. In the CGS larvae exposed to 4 µg/L BF, the liver exhibited an apparent nuclear shift and atrophy; hepatomegaly increased, there were larger vacuoles, and the boundary between hepatocytes was blurred.
3.5. Transcriptome sequencing and sequence assembly
Compared with brain and kidney, the liver tissue was selected as a sensitive sample in present study for further study on BF toxicity effects.
Table 3 presents the cDNA libraries constructed from liver samples of the control and BF treatments. After filtering the low mass readings, 593.22–865.21 million clean reads were obtained with Q20 and Q30 values of 97.28% and 92.63%, respectively. The GC content ranged from 47.44–48.96%, indicating a good sequencing quality.
3.6. Annotation and classification of functional genes
To clarify gene function in liver of CGS larvae, genes were annotated in four databases (NR, SwissProt, KEGG, and KOG) (
Table 4). A total of 87,958 unigenes were annotated in these four major databases, of which 56,230 genes (63.93%) were annotated to at least one gene in all databases. A total of 30,931, 29,913, 15,458, and 19,999 genes were respectively annotated in the NR, KEGG, KOG, and SwissProt databases, accounting for 35.17, 34.01, 17.57, and 22.74% of the genes. A total of 31,728 genes were annotated in all databases, accounting for 36.07% of the total genes.
3.7. Effects of BF on gene expression and functional enrichment of CGS larvae
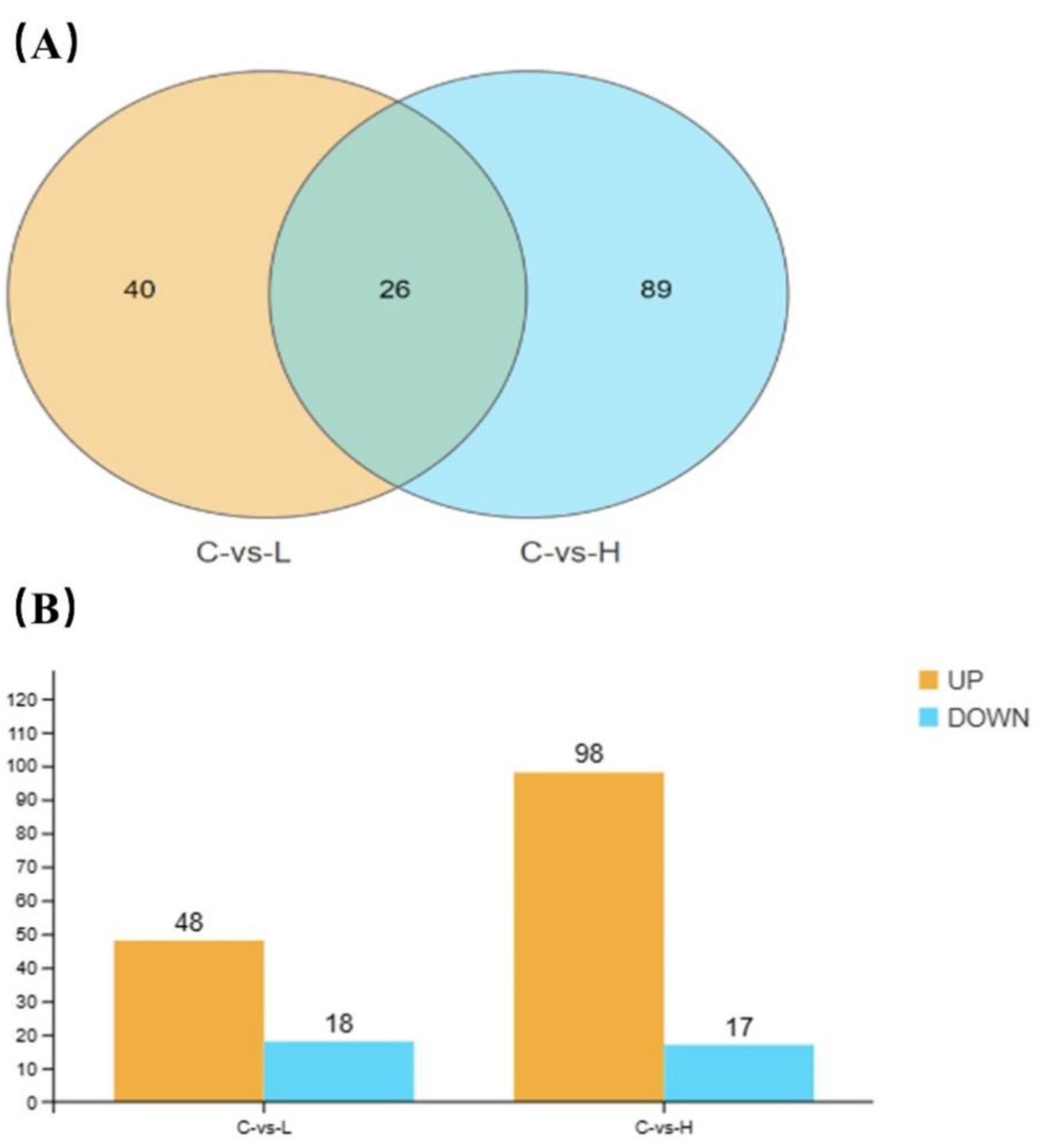
Poisson distribution analysis was used to identify DEGs with FDR ≤0.001 and absolute log2 ratio >1. A total of 66 and 115 DEGs were respectively identified in the 0.04 µg/L and 4 µg/L BF groups, of which 26 genes were co-differentially expressed (
Figure 5A). Especially, 48 and 98 DEGs were up-regulated and 18 and 17 DEG were down-regulated in the 0.04 µg/L and 4 µg/L BF groups, respectively (
Figure 5B).
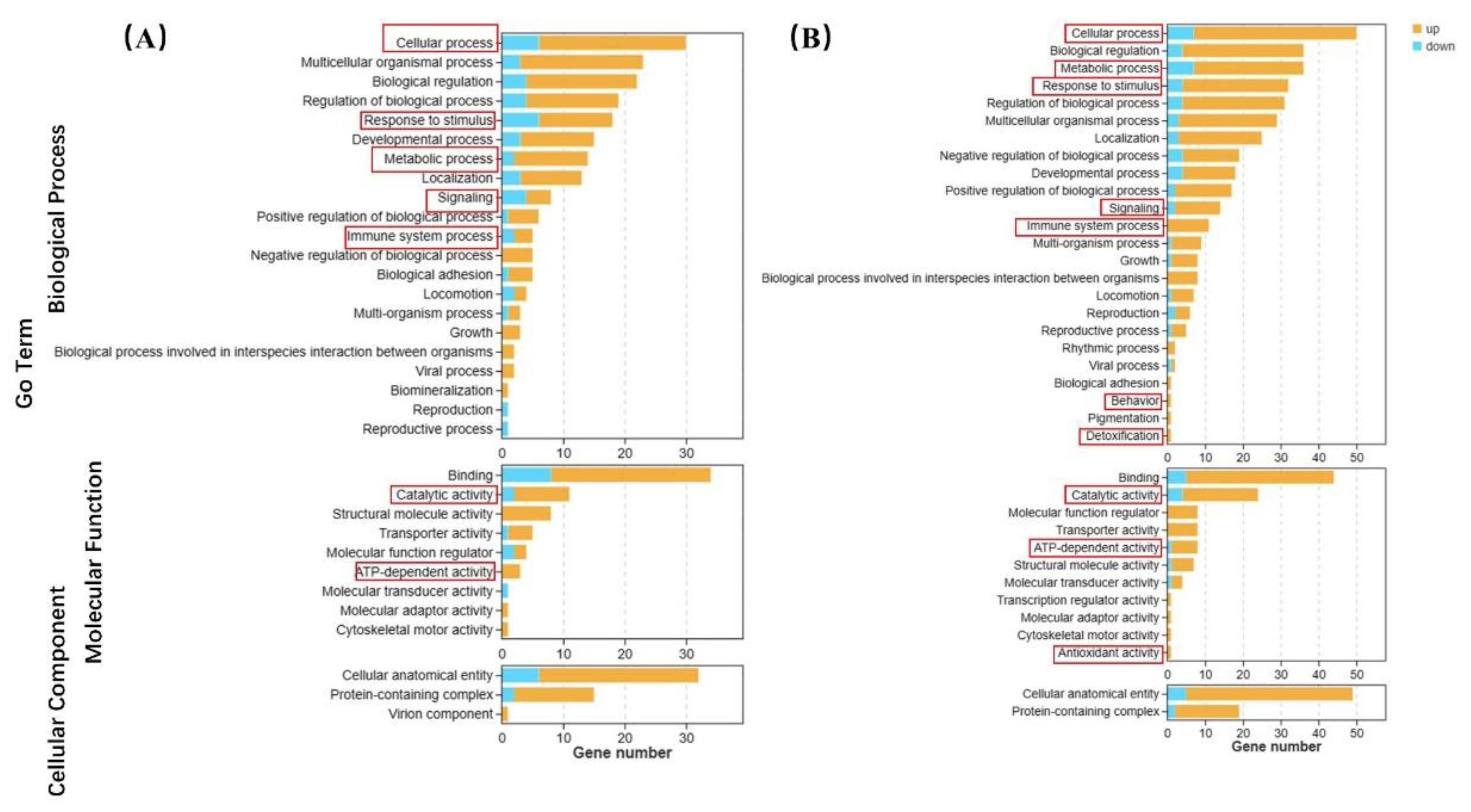
The GO terms are shown in
Figure 6. In the 0.04 μg/L group, there were 24, 12, 12, 4, and 3 up-regulated biological process genes related to cellular process, response to stimulus, metabolic process, signal transduction, and the immune system compared to the control group, respectively. Molecular function genes related to catalytic activity and ATP-dependent activity were up-regulated in 9 and 3, respectively (
Figure 6A). In the 4 μg/L exposure group, there were 43, 29, 28, 12, and 11 up-regulated genes related to cellular processes, metabolic processes, responses to stimuli, signal transduction, and the immune system compared to the control group, respectively. In particular, one of antioxidant activity, behavior related and detoxification genes up-regulated in high BF treatment. Genes with molecular functions related to catalytic activity and ATP-dependent activity were up-regulated in 20 and 7, respectively (
Figure 6B).
KEGG enrichment analysis showed that the top five enriched pathways were calcium signaling, dilated cardiomyopathy, rheumatoid arthritis, cardiac muscle contraction, and NF-Kappa B signaling in 0.04 μg/L BF treated CGS (
Figure 7A). In contrast, combining the top 20 GO and KEGG enriched pathways, the most enriched pathways with 4 g/L BF treatment were in the following five categories: calcium signaling, fat digestion and absorption, PPAR signaling, Staphylococcus aureus infection, and NF-kappa B signaling (
Figure 7B).
4. Discussion
BF is widely used in agriculture and accumulated in aquatic environment, sediments, and organism(Liu et al., 2020; Yang et al., 2018). It can disrupt the levels of antioxidant enzymes in organisms, causing tissue lesion and behavioral abnormalities (Awoyemi et al., 2019; Baruah and Chaurasia, 2020; Jin et al., 2013; Syed et al., 2018). In this study, we observed abnormal expression of oxidative stress factors, liver histopathology, behavioral changes, gene expression, and pathway regulation in CGS larvae after 1 week BF exposure, indicating multiple toxicity.
4.1. Effects of BF on the acute toxicity of CGS larvae
Pyrethroid insecticide exposure is highly toxic to fish, partly due to the high absorption and slow hydrolysis rates in fish gills, but mainly due to the hypersensitivity of the nervous systems of fish to those pesticides (Aydın et al., 2005). In present study, CGS larvae exposed to BF exhibited grayish-white gill, extremely active behavior at the beginning of exposure, body convulsions and imbalances. Moreover, exposed CGS larvae moved slowly, bodies twisted and stiffened in the later stages of the experiment. The zebrafish exposed to decabromodiphenyl ethane (DBDPE) showed similar phenomenon of significant decreases in moving distance and moving speed, suggesting that the calcium homeostasis in vivo was damaged, which affected the calcium signaling pathway and the changes in muscle related proteins, interfered with the muscle contraction function, and then changed the locomotion behavior of zebrafish (Sun et al., 2022). In the present study, BF induced the gene enrichment in CGS larvae liver related to metabolic, signaling, and immune system processes, especially high BF accelerate the abnormal upregulation of behavioral and detoxification genes, resulting in the abnormal gene enrichment related to calcium signaling pathways. Although only liver samples were used for transcriptome analysis, unable directly react the causes of body convulsions and torsion stiffness in CGS larvae affected by BF. However, a large number of enriched genes related to the regulation of muscle contractions were detected in liver tissue, suggesting that BF might be one of the reasons for the abnormal behavior of CGS larvae. Overall, this study highlights the toxic effects of BF exposure on CGS larvae and suggests potential mechanisms underlying the observed behavioral abnormalities. Further research is needed to elucidate the direct causes of body convulsions and torsion stiffness and to explore the effects of BF exposure on CGS larvae more comprehensively.
4.2. Effects of BF on oxidative stress in CGS larvae
In general, the generation and elimination of ROS in organisms maintain a dynamic balance, abnormal ROS causing oxidative stress (Chauhan et al., 1997; Lushchak, 2011). The ability of SOD and CAT to remove ROS is limited and excess ROS inhibits the activity of these enzymes. The enzyme levels in vivo, such as SOD and CAT, are typically used to evaluate oxidative stress (Fatima et al., 2007; Wang et al., 2021). In present study, ROS levels in the brain, liver, and kidney of larvae CGS significantly increased with BF increasing, while the SOD and CAT levels significantly decreased. This may be due to the disorder dynamic balance between the production and elimination of free radicals induced by BF exposure. Similar phenomena were observed in fish against to pesticides induced oxidative stress, speculating that pesticides accumulation promote ROS production and impair the catalytic effect of antioxidant enzymes (Bhatia et al., 2014; Jin et al., 2013; Yan et al., 2015). Those results inferred that BF could stimulate oxidative stress in CGS larvae, especially high BF exposure directly inhibit the catalytic activities of SOD and CAT, leading to enormous injure to the antioxidant system with increasing BF exposure.
To gain deeper insight in the BF antioxidant toxic effect on CGS larvae at molecular levels, the transcriptome analysis was conducted in present study. The results revealed upregulated expression of genes related to catabolic and ATP-dependent activities in vivo, particularly the genes related to antioxidant activity were downregulated at high BF exposure levels. The differentially expressed genes were enriched in calcium signaling pathway, PPAR signaling pathway and other pathways, which ultimately interfered with the response to reactive oxygen. Some aquatic organisms, such as Corbicula fluminea and zebrafish, exposed to pyrethroids showed a similar phenomenon, the genes which correlate to ROS production and ATP synthesis were significantly downregulated in vivo, affecting the balance between oxidants and antioxidants (Awoyemi et al., 2019; H. Zhang et al., 2020). In summary, the abnormal antioxidant indexes of CGS larvae caused by BF may be due to abnormal production and accumulation of ROS, causing abundant upregulation of molecular functional genes such as catalytic activity and ATP-dependent activity, and abnormal regulation of the calcium signaling pathway, PPAR signaling pathway, ect. Furthermore, the upregulation of the antioxidant activity gene in the high BF exposure group reflects the emergency response of CGS larvae to toxicity inhibited catalytic activity.
4.3. Effects of BF on DNA damage in CGS larvae
Novel pesticides not only induce the increasing of ROS and MDA concentration in vivo, which are also two main causes of DNA damage, suggesting that oxidative stress is closely related to DNA damage (Cao et al., 2016; Mai et al., 2010; Selvaraj et al., 2013; Song et al., 2019; C. Zhang et al., 2020). The 8-OHdG, as a biomarker of DNA oxidative damage, showing a significant increase similarly as ROS that observed in a variety of fish cells exposed to pollutants, which is also consistent with our results. Those results speculated that pollutants may induce endogenous or exogenous ROS to attack proteins and nucleic acids in living cells, leading to oxidative DNA damage. In addition, PPARα is a sensor of lipid synthesis and is considered to be a key factor in liver and lipid metabolism (Franklin et al., 2017; Tanaka et al., 2017). The excessive accumulation of ROS can destroy the structural stability of cell membranes and form the lipid peroxide MDA (Du et al., 2015). Similar results were observed in the present study, ROS levels of the CGS larvae increased with BF increasing, and the MDA level increasing accordingly. Transcriptome analysis also showed that increasing BF concentrations interfered with the lipid metabolism of CGS larvae. Many DEGs were significantly enriched in the PPAR signaling pathway and the fat digestion and absorption pathways (Gao et al., 2021), the alteration of which can cause diseases related to oxidative DNA damage, including neurodegenerative (such as Alzheimer’s disease and Parkinson’s disease) and autoimmune diseases (such as rheumatoid arthritis and systemic lupus erythematosus) (Dizdaroglu, 2012; Valavanidis et al., 2009). Our study also found that not only ROS, MDA and 8-OHdG contents in CGS larvae significantly increased with BF increasing, but also exhibited symptoms of rheumatoid arthritis and the viral myocarditis and systemic lupus erythematosus pathways were significantly enriched in the liver by BF exposure. It is possible that BF caused oxidative stress and DNA damage in CGS larvae that was the result of the combined action of abnormally high ROS and MDA. In summary, when the production of free radicals exceeds the potential of the self-defense system, it can cause different degrees of oxidative damage and lipid peroxidation to occur, leading to oxidative stress, abnormal lipid metabolism, and DNA damage (Ullah et al., 2017).
4.4. Effects of BF on the live tissue of CGS larvae
The liver is the main organ that for metabolism, detoxification, and excretion of harmful substances (El-Sayed and Saad, 2007; Ullah et al., 2018; H. Zhang et al., 2020). Liver histopathological changes are key biomarkers to judge the toxic effects caused by exogenous poisons, environmental stressors, and sudden harmful environmental changes (Ullah et al., 2018; Velisek et al., 2006). Various studies have shown that pyrethroid pesticides can cause liver cell degeneration and necrosis, hepatocyte walls disappearance, fat vacuole formation increase, and focal coagulative necrosis in fish, such as Labeo Roxita, Oncorhynchus Mykis, and Ctenopharyngodon Idella (El-Sayed and Saad, 2007; Tilak et al., 2003; Velisek et al., 2006). In this study, we found that the liver tissues of CGS larvae exposed to BF exhibited pathological damage, low BF leaded to cell vacuolation, nuclear atrophy and a shift in liver tissues, and high BF can cause severe liver inflammation. Transcriptome analysis showed that the B-cell receptor signaling pathway was significantly enriched induce by low BF exposure, which affected cell metabolism, gene expression, and cytoskeletal structure (Harwood and Batista, 2008; Kurosaki et al., 2010). This may be the influencing factor of liver tissue appearing vacuolation, nuclear shift, and even atrophy. BF exposure resulted in the significant upregulation of genes involved in immune system processes and signaling, with the significant enrichment appeared in the PPAR and NF-kB signaling pathways. The PARP-1/PPARα/SIRT1 pathway can induce liver inflammation by activating NF-kB signaling pathway or cooperating with NF-kB signaling pathway (Du et al., 2015). It is speculated that the highly inflammatory response exhibited by CGS larvae may be due to the combined action of the PPAR and NF-kB signaling pathways. The severe histological lesions observed in the liver of CGS larvae may related to the high production of ROS against to BF exposure, which are known to cause oxidative damage to organism and chronic toxicity.
5. Conclusions
In conclusion, this present study demonstrates that BF induces free radical production in CGS surpassing the capacity of the self-defense system. This induces to the significant upregulation of molecular function genes associated with catalytic activity, ATP-dependent activity, metabolic processes, signaling and immune system processes, behavior and detoxification. Consequently, disrupted the regulation of the calcium signaling, PPAR, and NF-kB signaling pathways, which were significantly enriched. The imbalance between free radicals and antioxidant defenses results in varying degrees of oxidative damage and lipid peroxidation in CGS larvae, causing oxidative stress, abnormal lipid metabolism, DNA damage, tissue inflammation, and abnormal behavior. The results in present study provide a foundation for further study on the molecular mechanisms of pyrethrins to amphibian, a theoretical basis for the rational use of pyrethrins and environmental risks, and scientific guidance for the conservation of amphibian populations.
Abbreviations
BF, bifenthrin; ROS, reactive oxygen species; CGS, Chinese giant salamanders; ATP, adenosine-triphosphate; SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; MDA, malondialdehyde; 8-OHdG, 8-hydroxydeoxyguanosine; DMSO, dimethyl sulfoxide; DEG, differentially expressed gene; LC50, 50% lethal concentration; SC, safe concentration; PPAR, peroxisome proliferator-activated receptor
References
- Akan, J.; Sodipo, O.; Mohammed, Z.; Abdulrahman, F. Determination of Organochlorine, Organophosphorus and Pyrethroid Pesticide Residues in Water and Sediment Samples by High Performance Liquid Chromatography (HPLC) with UV/visible Detector. J. Anal. Bioanal. Tech. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Awoyemi, O.M.; Kumar, N.; Schmitt, C.; Subbiah, S.; Crago, J. Behavioral, molecular and physiological responses of embryo-larval zebrafish exposed to types I and II pyrethroids. Chemosphere 2019, 219, 526–537. [Google Scholar] [CrossRef]
- Aydın, R.; Köprücü, K.; Dörücü, M.; Köprücü, S.; Pala, M. Acute Toxicity of Synthetic Pyrethroid Cypermethrin on the Common Carp (Cyprinus carpio L.) Embryos and Larvae. Aquac. Int. 2005, 13, 451–458. [Google Scholar] [CrossRef]
- Baruah, P.; Chaurasia, N. Ecotoxicological effects of alpha-cypermethrin on freshwater alga Chlorella sp.: Growth inhibition and oxidative stress studies. Environ. Toxicol. Pharmacol. 2020, 76, 103347. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.; Kumar, A.; Ogino, Y.; Gregg, A.; Chapman, J.; McLaughlin, M.J.; Iguchi, T. Di-n-butyl phthalate causes estrogenic effects in adult male Murray rainbowfish (Melanotaenia fluviatilis). Aquat. Toxicol. 2014, 149, 103–115. [Google Scholar] [CrossRef]
- Cao, F.; Liu, X.; Wang, C.; Zheng, M.; Li, X.; Qiu, L. Acute and short-term developmental toxicity of cyhalofop-butyl to zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2016, 23, 10080–10089. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Pandey, P.; Ogata, A.; Teoh, G.; Krett, N.; Halgren, R.; Rosen, S.; Kufe, D.; Kharbanda, S.; Anderson, K. Cytochrome c-dependent and -independent Induction of Apoptosis in Multiple Myeloma Cells. J. Biol. Chem. 1997, 272, 29995–29997. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.C.-W.; Hu, C.; Tsui, M.M.P.; Lam, P.K.S.; Zhou, B. Perfluorobutanesulfonate Exposure Skews Sex Ratio in Fish and Transgenerationally Impairs Reproduction. Environ. Sci. Technol. 2019, 53, 8389–8397. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Hong, S.H.; Park, J.-W. Evaluation of microplastic toxicity in accordance with different sizes and exposure times in the marine copepod Tigriopus japonicus. Mar. Environ. Res. 2020, 153, 104838. [Google Scholar] [CrossRef]
- Collins, L.J.; Biggs, P.J.; Voelckel, C.; Joly, S. An approach to transcriptome analysis of non-model organisms using short-read sequences. Genome Inform. 2008, 21, 3–14. [Google Scholar]
- Dizdaroglu, M. Oxidatively induced DNA damage: Mechanisms, repair and disease. Cancer Lett. 2012, 327, 26–47. [Google Scholar] [CrossRef]
- Du, J.; Zhao, M.; Li, J. Determination of water quality criteria for three synthetic pyrethroids for the protection of aquatic life. Int. J. Geol. Agric. Environ. Sci. 2015, 3, 21–28. [Google Scholar]
- El-Sayed, Y.S.; Saad, T.T. Subacute Intoxication of a Deltamethrin-Based Preparation (Butox®5% EC) in Monosex Nile Tilapia, Oreochromis niloticus L. Basic Clin. Pharmacol. Toxicol. 2007, 102, 293–299. [Google Scholar] [CrossRef]
- Farag, M.R.; Mahmoud, H.K.; El-Sayed, S.A.; Ahmed, S.Y.; Alagawany, M.; Abou-Zeid, S.M. Neurobehavioral, physiological and inflammatory impairments in response to bifenthrin intoxication in Oreochromis niloticus fish: Role of dietary supplementation with Petroselinum crispum essential oil. Aquat. Toxicol. 2021, 231, 105715. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Mandiki, S.; Douxfils, J.; Silvestre, F.; Coppe, P.; Kestemont, P. Combined effects of herbicides on biomarkers reflecting immune–endocrine interactions in goldfish: Immune and antioxidant effects. Aquat. Toxicol. 2007, 81, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, C.; Šatínský, D.; Solich, P. New method for the determination of carbamate and pyrethroid insecticides in water samples using on-line SPE fused core column chromatography. Talanta 2014, 129, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.P.; Sathyanarayan, A.G.M. Acyl-CoA Thioesterase 1 (ACOT1) Regulates PPARa to couple fatty acid flux with oxidative capacity during fasting. Diabetes 2017, 66, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hua, R.; Hu, K.; Wang, Z. Carbohydrates deteriorate fatty liver by activating the inflammatory response Yuqi. Nutr. Res. Rev. 2021, 35, 41–48. [Google Scholar]
- Garí, M.; González-Quinteiro, Y.; Bravo, N.; Grimalt, J.O. Analysis of metabolites of organophosphate and pyrethroid pesticides in human urine from urban and agricultural populations (Catalonia and Galicia). Sci. Total. Environ. 2018, 622-623, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cong, J.; Sun, Y.; Li, G.; Zhou, Z.; Qian, C.; Liu, F. Determination of Endocrine Disrupting Chemicals in Surface Water and Industrial Wastewater from Beijing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 401–405. [Google Scholar] [CrossRef]
- Harwood, N.E.; Batista, F.D. New Insights into the Early Molecular Events Underlying B Cell Activation. Immunity 2008, 28, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.B.; Falso, P.; Gallipeau, S.; Stice, M. The cause of global amphibian declines: a developmental endocrinologist's perspective. J. Exp. Biol. 2010, 213, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 1998, 120, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Jiang, N.; Song, P.; Li, X.; Zhu, L.; Wang, J.; Yin, X.; Wang, J. Dibutyl phthalate induced oxidative stress and genotoxicity on adult zebrafish (Danio rerio) brain. J. Hazard. Mater. 2022, 424, 127749. [Google Scholar] [CrossRef]
- Jin, Y.; Pan, X.; Cao, L.; Ma, B.; Fu, Z. Embryonic exposure to cis-bifenthrin enantioselectively induces the transcription of genes related to oxidative stress, apoptosis and immunotoxicity in zebrafish (Danio rerio). Fish Shellfish. Immunol. 2013, 34, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Shinohara, H.; Baba, Y. B Cell Signaling and Fate Decision. Annu. Rev. Immunol. 2010, 28, 21–55. [Google Scholar] [CrossRef]
- Langlois, V.S. Amphibian Toxicology: A Rich But Underappreciated Model for Ecotoxicology Research. Arch. Environ. Contam. Toxicol. 2021, 80, 661–662. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Z.-Z.; Song, J.; Zhu, X.-Y.; Liu, Q.-N.; Zhang, D.-Z.; Tang, B.-P.; Zhou, C.-L.; Dai, L.-S. Transcriptome Analysis Reveals Potential Antioxidant Defense Mechanisms in Antheraea pernyi in Response to Zinc Stress. J. Agric. Food Chem. 2018, 66, 8132–8141. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Mai, W.-J.; Yan, J.-L.; Wang, L.; Zheng, Y.; Xin, Y.; Wang, W.-N. Acute acidic exposure induces p53-mediated oxidative stress and DNA damage in tilapia (Oreochromis niloticus) blood cells. Aquat. Toxicol. 2010, 100, 271–281. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Loumbourdis, N.S. Exposure of the Frog Rana ridibunda to Copper: Impact on Two Biomarkers, Lipid Peroxidation, and Glutathione. Bull. Environ. Contam. Toxicol. 2002, 69, 885–891. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-Y.; Park, H.; Song, G.; Lim, W. Bifenthrin induces developmental immunotoxicity and vascular malformation during zebrafish embryogenesis. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2020, 228, 108671. [Google Scholar] [CrossRef]
- Porte Visa, C.; van den Brink, N.; van der Oost, R. Biomarkers in environmental assessment. In Biological Testing in Marine & Freshwaters: Trends, Relevance and Linkages to Ecosystem Health; 2005; pp. 87–152. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Reed, J.R.; Cawley, G.F.; Backes, W.L. Inhibition of cytochrome P450 1A2-mediated metabolism and production of reactive oxygen species by heme oxygenase-1 in rat liver microsomes. Drug Metab. Lett. 2011, 5, 6–16. [Google Scholar] [CrossRef]
- Rawn, D.F.K.; Judge, J.; Roscoe, V. Application of the QuEChERS method for the analysis of pyrethrins and pyrethroids in fish tissues. Anal. Bioanal. Chem. 2010, 397, 2525–2531. [Google Scholar] [CrossRef]
- Selvaraj, V.; Armistead, M.Y.; Cohenford, M.; Murray, E. Arsenic trioxide (As2O3) induces apoptosis and necrosis mediated cell death through mitochondrial membrane potential damage and elevated production of reactive oxygen species in PLHC-1 fish cell line. Chemosphere 2013, 90, 1201–1209. [Google Scholar] [CrossRef]
- Song, P.; Gao, J.; Li, X.; Zhang, C.; Zhu, L.; Wang, J.; Wang, J. Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Environ. Int. 2019, 129, 10–17. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, B.; Ling, S.; Yan, B.; Wang, X.; Jia, S.; Martyniuk, C.J.; Zhang, W.; Yang, L.; Zhou, B. Decabromodiphenyl Ethane Mainly Affected the Muscle Contraction and Reproductive Endocrine System in Female Adult Zebrafish. Environ. Sci. Technol. 2022, 56, 470–479. [Google Scholar] [CrossRef]
- Syed, F.; Awasthi, K.K.; Chandravanshi, L.P.; Verma, R.; Rajawat, N.K.; Khanna, V.K.; John, P.J.; Soni, I. Bifenthrin-induced neurotoxicity in rats: involvement of oxidative stress. Toxicol. Res. 2018, 7, 48–58. [Google Scholar] [CrossRef]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Tilak, K.S.; Veeraiah, K.; Vardhan, K.S. Toxicity and residue studies of fenvalerate to the freshwater fish Channa punctatus (Bloch). Bull. Environ. Contam. Toxicol. 2003, 71, 1207–1212. [Google Scholar] [CrossRef]
- Ullah, S.; Hasan, Z.; Zorriehzahra, M.J.; Ahmad, S. Diagnosis of endosulfan induced DNA damage in rohu (Labeo rohita, Hamilton) using comet assay. Iran. J. Fish. Sci. 2017, 16, 138–149. [Google Scholar] [CrossRef]
- Ullah, S.; Li, Z.; Hasan, Z.; Khan, S.U.; Fahad, S. Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicol. Environ. Saf. 2018, 161, 270–280. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Heal. Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Velisek, J.; Wlasow, T.; Gomulka, P.; Svobodova, Z.; Dobsikova, R.; Novotny, L.; Dudzik, M. Effects of cypermethrin on rainbow trout (Oncorhynchus mykiss). Vet. Med. (Praha). 2006, 51, 469–476. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Wu, L.; Ma, T. Effects of 4-epianhydrotetracycline on oxidative stress in zebrafish (Danio rerio) embryos. Sci. Total. Environ. 2021, 796, 149047. [Google Scholar] [CrossRef]
- Yan, S.H.; Wang, J.H.; Zhu, L.S.; Chen, A.M.; Wang, J. Thiamethoxam induces oxidative stress and antioxidant response in zebrafish (DanioRerio) livers. Environ. Toxicol. 2016, 31, 2006–2015. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Wang, C. Toxicity of the pyrethroid bifenthrin insecticide. Environ. Chem. Lett. 2018, 16, 1377–1391. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Zhu, L.; Du, Z.; Wang, J.; Wang, J.; Li, B.; Yang, Y. Fluoxastrobin-induced effects on acute toxicity, development toxicity, oxidative stress, and DNA damage in Danio rerio embryos. Sci. Total. Environ. 2020, 715, 137069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, X.; Yan, S.; Zha, J.; Qin, J. Environmentally relevant concentrations of bifenthrin induce changes in behaviour, biomarkers, histological characteristics, and the transcriptome in Corbicula fluminea. Sci. Total. Environ. 2020, 728, 138821. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Z.; Zheng, X.; Fan, J.; Wang, S.; Wei, Y.; Yang, L.; Wang, P.; Guo, S. Transcriptome analysis of response mechanism to ammonia stress in Asian clam (Corbicula fluminea). Aquat. Toxicol. 2019, 214, 105235. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).