1. Introduction
Metabolic bone diseases cover a broad spectrum of disorders that share alterations of bone metabolism leading to a defective skeleton [
1]. The most prevalent metabolic bone disease worldwide is osteoporosis (OMIM: 166710), which is a chronic, progressive, systemic disease associated with reduced bone mineral density (BMD) and alterations in the microarchitecture of bone tissue [
2,
3]. The underlying mechanism associated with osteoporosis is an imbalance in bone formation and resorption that leads to reduction of BMD and increased bone fragility [
1,
4]. Bone fragility fractures are the most relevant clinical complication of osteoporosis [
5,
6], and have become a public health issue that increases morbidity, disability and mortality [
7].
The most accepted hypothesis of the etiopathogenic of osteoporosis and bone fragility fracture is the combined action of environmental and genetic factors. Many risk factors are involved, including age, physical activity, medication use and coexisting diseases. But one of the most important is a positive family history, as it emphasizes the relevance of genetic predisposition in the pathogenesis of osteoporosis and bone fracture [
8,
9,
10,
11]. Several hundreds of genetic loci have been associated with osteoporosis, low BMD and fragility fractures [
12,
13]. One of the genes so far associated therewith is
TP53 [
14], the Arg72Pro variant of which has been linked to an increased risk of osteoporosis [
15]. TP53 gene encodes protein p53 with 393 amino acids. The principal role of p53 is to promote cell cycle arrest, apoptosis, and cell senescence [
16], but protein p53 also regulates osteoblast differentiation, bone formation, osteoblast-dependent osteoclast differentiation, and bone remodeling [
17,
18]. The Arg72Pro polymorphism is in a proline-rich domain involved in the apoptotic role of p53 [19-21]. It has been reported that the arginine allele of Arg72Pro polymorphism is associated with more apoptosis induction than the proline variant [19-21].
In such a scenario, and a need existing to better understand the genetic factors determining osteoporosis and fragility fractures, this study aimed at determining the influence of the TP53 Arg72Pro genetic variant in bone cells and tissue in an animal model.
2. Results
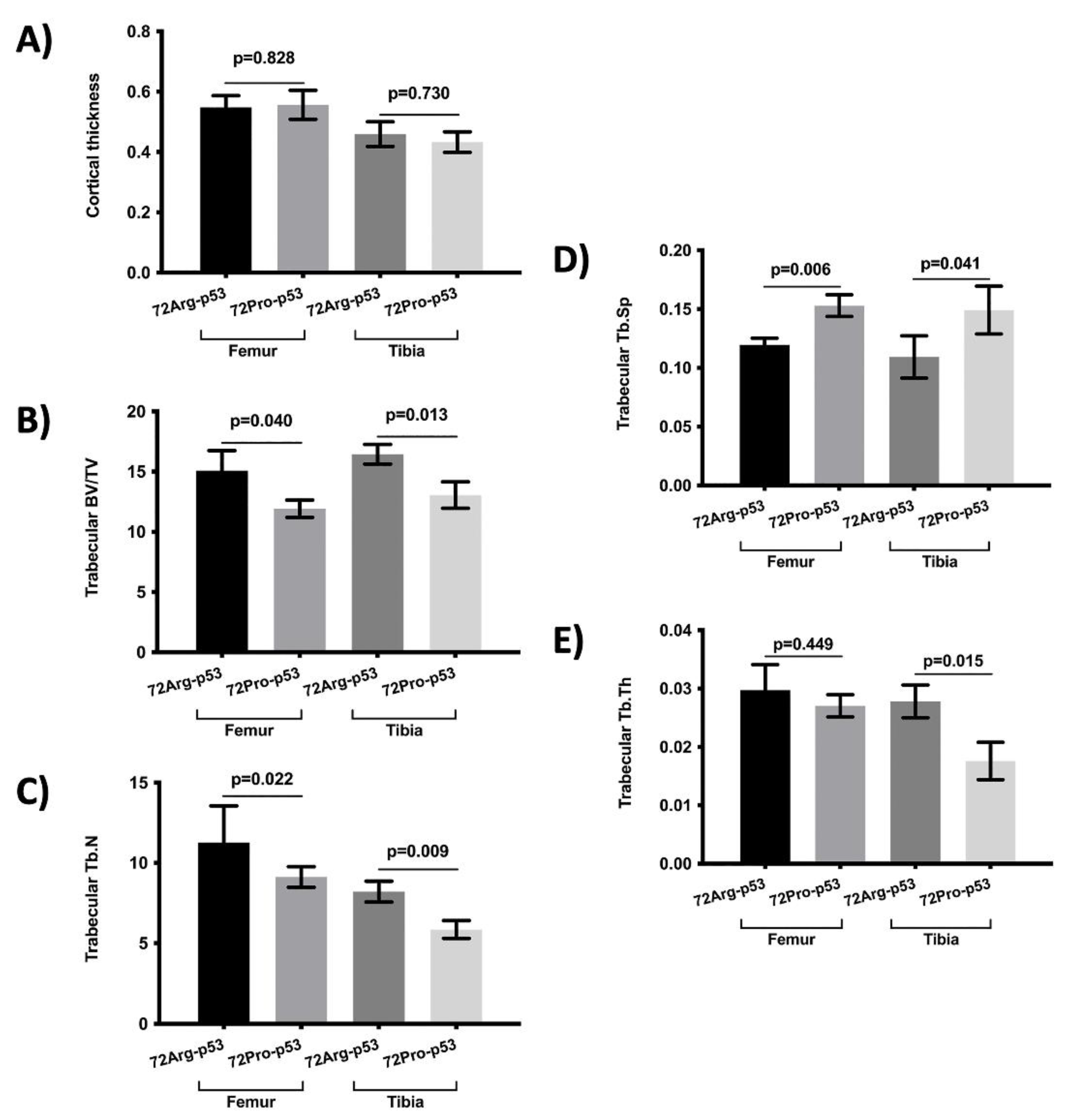
The bone histomorphometry parameters were evaluated in humanized 72Arg-p53 and 72Pro-p53 mice (
Figure 1). The results showed that 72Pro-p53 mice had a lower trabecular bone mass, both at the femur and the tibia, with lower femur and tibia bone volume over total volume (BV/TV), femur and tibia trabecular separation (Tb.Sp) and tibia trabecular thickness (Tb.Th). In addition, 72Pro-p53 mice had lower femur and tibia trabecular number (Tb.N) than 72Arg-p53 mice (
Figure 1). The analysis of the cortical thickness did not show statistical differences between both genotypes (
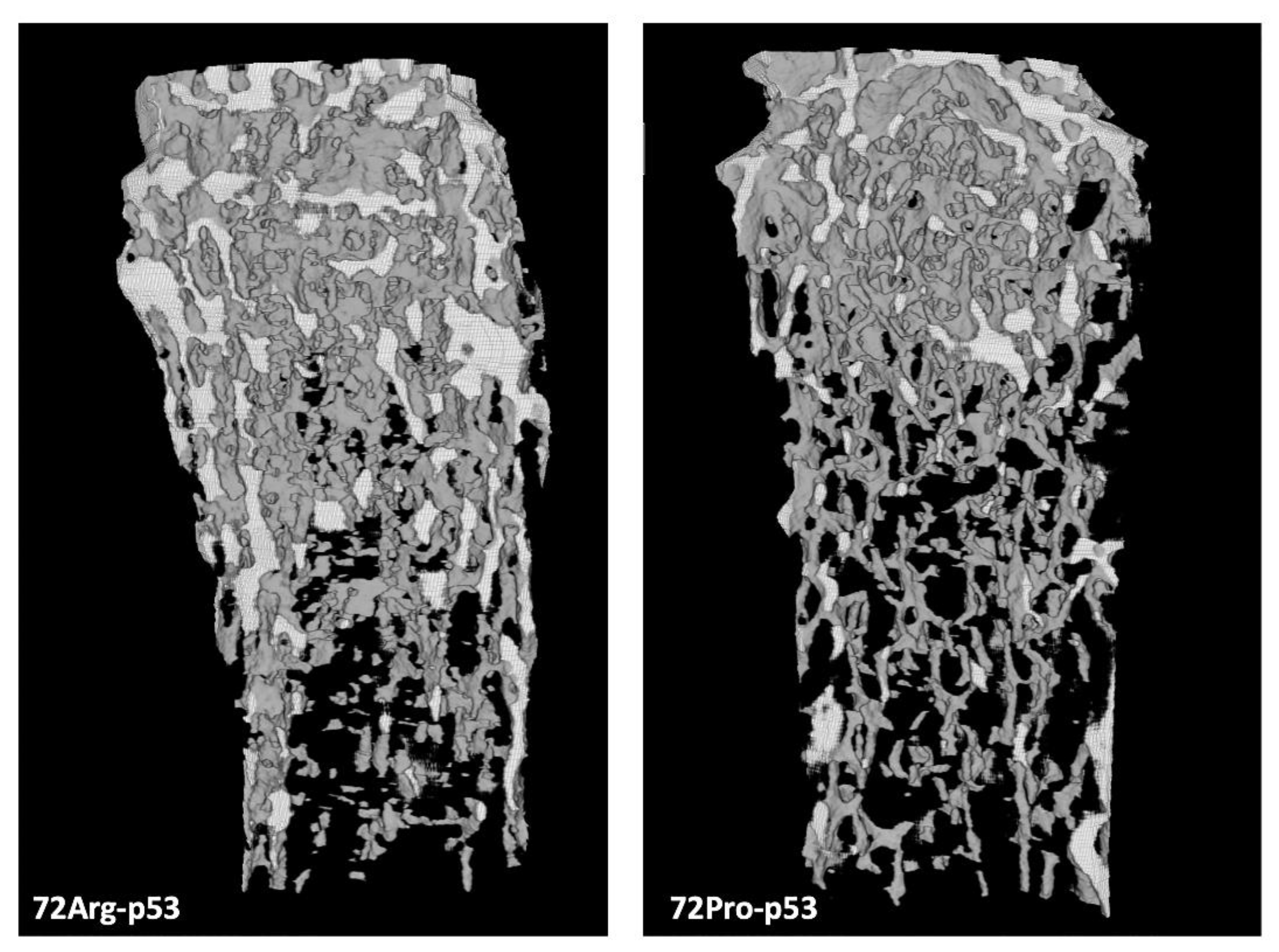
Figure 1). A representative comparison of femur trabecular areas between 72Arg-and 72Pro-p53 mice is shown in
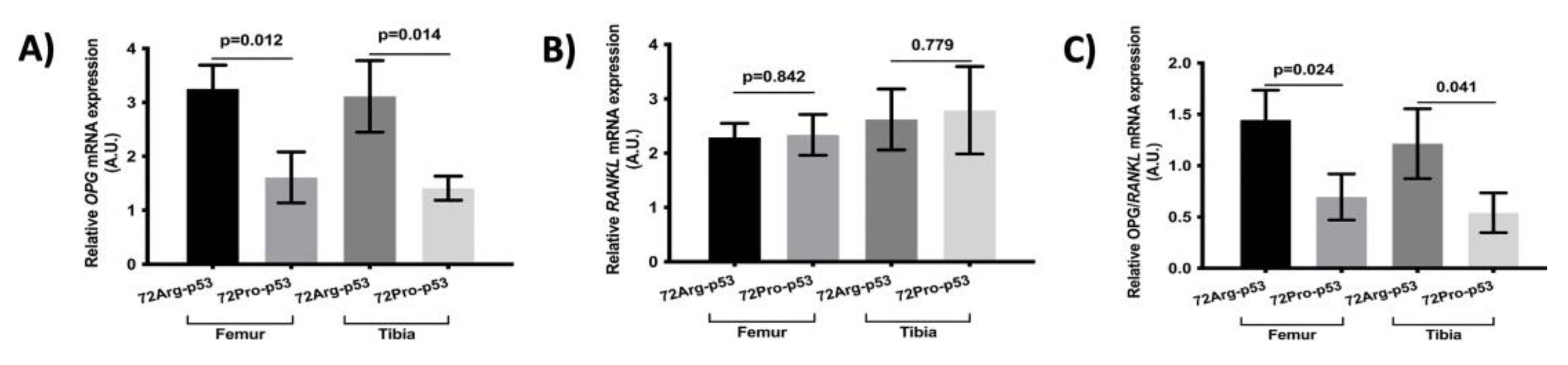
Figure 2, with deterioration in the trabecular area in 72Pro-p53 mice. The results of the analysis of osteoclast maturation and activation related genes showed a lower relative expression of osteoprotegerin (
OPG) in femur and tibia in 72Pro-p53 mice. Also, the
OPG/RANKL ratio was lower in 72Pro-p53 mice (
Figure 3).
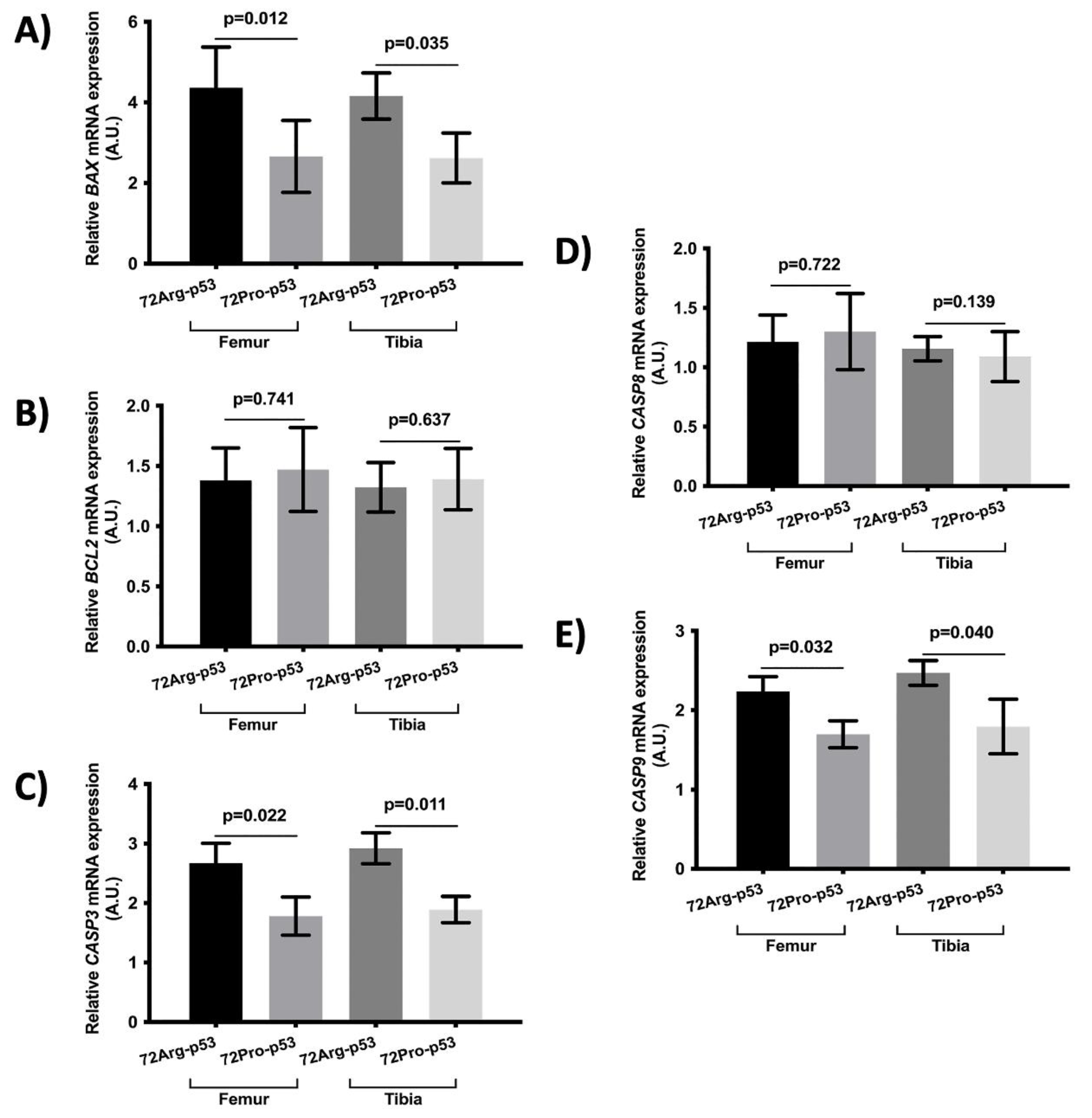
The results also showed statistical differences in the expression of genes involved in apoptosis between 72Arg-p53 and 72Pro-p53 mice. Relative mRNA quantification of gene expression involved in apoptosis showed a lower relative expression of
BAX,
CASP3 and
CASP9 in femur and tibia of 72Pro-p53 mice. The expression of these genes were higher in 72Arg-p53 mice (
Figure 3). The relative expression of
BCL2 and
CASP8 did not show statistical differences between 72Arg-p53 and 72Pro-p53 mice (
Figure 4).
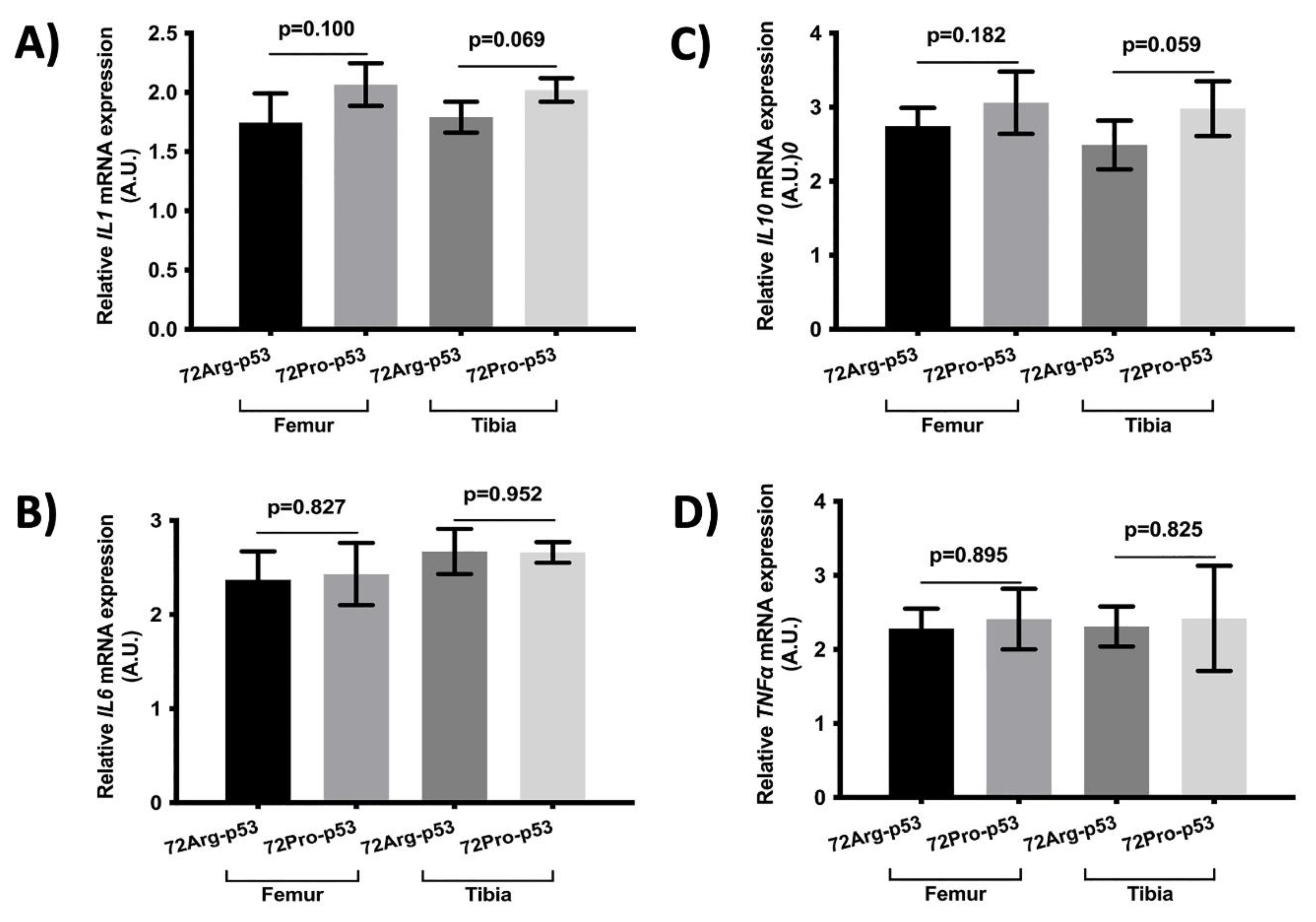
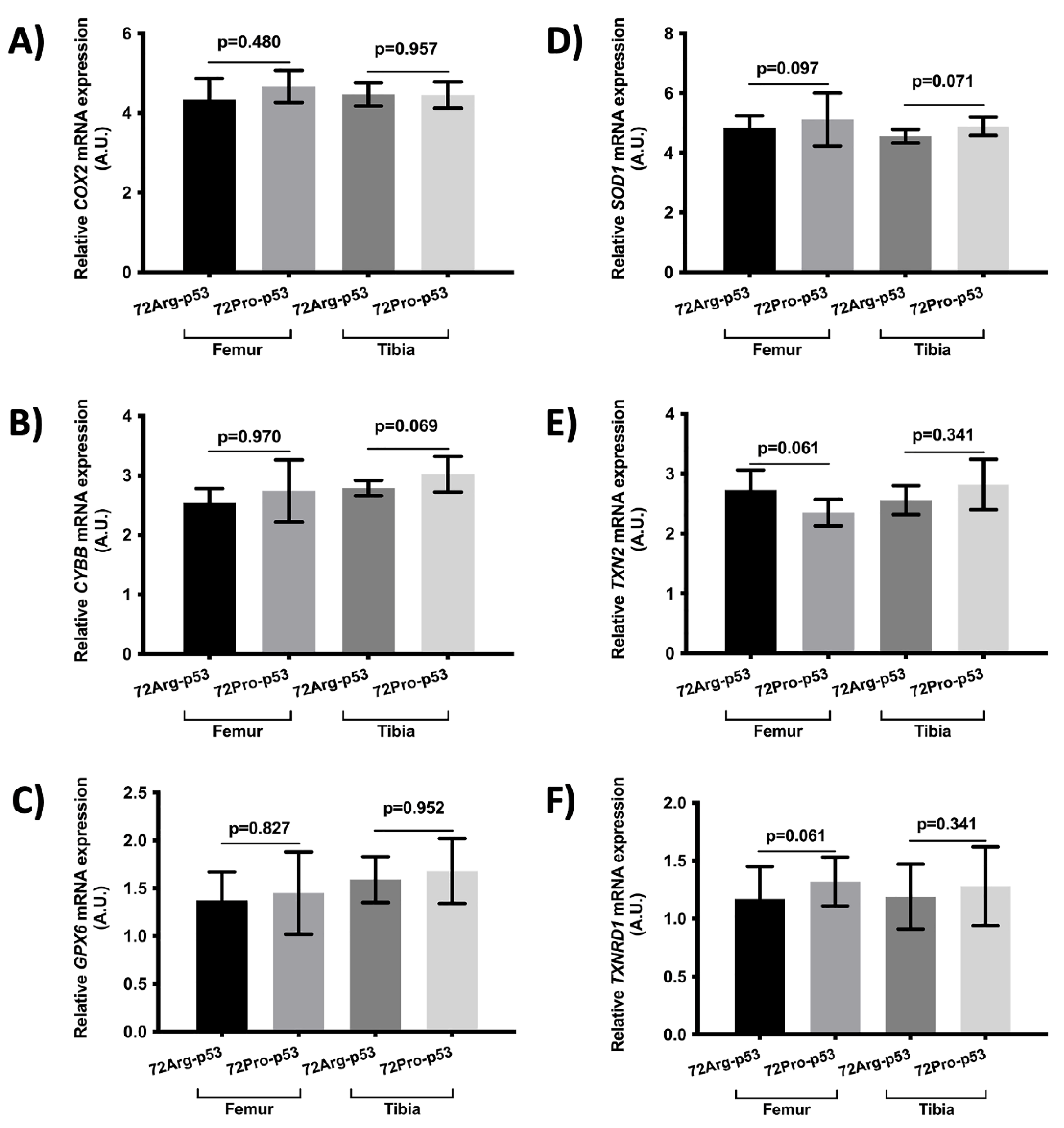
The relative expression of genes involved in inflammation and oxidative stress did not yield statical differences (
Figure 5 and
Figure 6).
3. Discussion
Osteoporosis, characterized by low BMD and alteration of bone microarchitecture, is the most common metabolic bone disease [
2,
3]. Our results are in line with the concept that genetic predisposition could be crucial in its aetiology. The results showed that the proline variant of
TP53 Arg72Pro polymorphism was associated with deteriorated bone tissue, reinforcing the hypothesis that the
TP53 gene could be involved in determining individual osteoporosis susceptibility [
14,
15].
Protein p53 is a tumor suppressor that is a stress sensor inducing apoptosis, cell cycle arrest or senescence [
16]. It has also been published that p53 plays a key role in bone metabolism [
17,
18]. Protein p53 could be involved in osteogenesis [
22], it modulates osteoblastic and osteoclastic differentiation increasing the Notch signaling pathway [
17,
23]. P53 also has been associated with the regulation of the OPG synthesis, it has been reported a negative correlation between
TP53 gene modifications and OPG regulation [
24]. OPG is a soluble member of the tumor necrosis factor receptor super family. It is a decoy receptor for RANKL, due to OPG inhibits osteoclastic bone resorption by interfering with binding of RANKL to RANK [
25,
26]. Multiple single nucleotide polymorphisms (SNPs) have been identified in
TP53 [
27,
28], one of the most studied has been the Arg72Pro variant. This genetic polymorphism has been associated with various cancers, inflammatory diseases, or stroke [
29,
30,
31,
32]. Also, the
TP53 Arg72Pro genetic variant has been involved in osteoporosis; the proline variant has been associated with an increased risk of suffering osteoporosis [
15]. In this sense, our results showed that the proline allele of
TP53 Arg72Pro polymorphism was associated with a higher level of deterioration of bone tissue in trabecular femur and tibia. It addition, we report that proline allele was associated with lower
OPG gene expression and lower
OPG/RANKL ratio in femur and tibia. It has been reported the crucial role of protein p53 in the regulation of OPG status [
24] and therefore in bone metabolism [
18]. Our results support the hypothesis that the
TP53 Arg72Pro variant could be associated with a negative regulation of OPG and, therefore, with increased osteoclastogenesis and, consequently, a more impaired bone microarchitecture.
Also, P53 has a crucial role in apoptosis induction. Arg72Pro SNP is in a proline-rich domain involved in the apoptotic role of p53 [19-21]. In this sense, it has been reported that the arginine allele of Arg72Pro polymorphism is associated with more apoptosis induction than the proline variant [19-21]. Our report showed that the arginine variant could be associated with more apoptosis induction in bone tissue, specifically with the intrinsic apoptotic pathway. Although genetic factors associated to inflammation and oxidative stress have been associated with bone osteoporotic fracture [
33,
34], our results did not show modifications in inflammation and oxidative stress gene expression in bone tissue with regards to
TP53 Arg72Pro polymorphism. Many risk factors have been associated with alterations in bone metabolism and with the risk of suffering bone metabolism diseases [
6]. Our hypothesis is that the metabolic bone response to these risk factors could be different depending on the
TP53 Arg72Pro genetic variant. The results of this work indicate that the proline allele of
TP53 Arg72Pro polymorphism is associated with decreased apoptotic function, lower OPG/RANKL ratio and with worsened bone microarchitecture. It could be speculated that reduced levels of apoptosis may be followed by a more aggressive cellular response and thus more bone alterations. On the other hand and in addition, the proline allele of
TP53 Arg72Pro SNP has been associated with increased activation of the NF-kB pathway [
35], which is crucial in osteoclastogenesis [
36,
37]. Also p53 is involved in the regulation of OPG [
24] which has a crucial role in osteoclastic bone resorption [
25,
26]. In this sense, we report an association between proline allele of
TP53 Arg72Pro variant and
OPG expression, the proline variant was associated with lower gene expression and lower OPG/RANKL ratio. Hence, the proline variant of
TP53 Arg72Pro polymorphism could be associated with more osteoclast maturation and activation and therefore with more bone resorption. This mutation may have special significance in elderly population. The decrease in apoptosis increases the percentage of senescent cells in bone tissue, which are viable cells but with an irreversible arrest of the cell cycle. The p53/21 metabolic pathway plays a role in this arrest. Clinical-functional repercussions increase morbidity and mortality among this population [
38].
The main limitation of the study is that we have not evaluated the influence of the TP53 Arg72Pro genetic variant in bone metabolism under conditions of bone injury. For the first time, this work summarizes the influence of TP53 Arg72Pro polymorphism in OPG gene expression, OPG/RANKL ratio, bone microarchitecture and apoptosis bone status.
In conclusion, we described the influence of TP53 Arg72Pro polymorphism in bone microarchitecture reinforcing the hypothesis that the TP53 Arg72Pro genetic variant could be crucial in osteoporosis risk. The TP53 Arg72Pro variant could be a genetic biomarker to identify individuals with an increased risk of suffering osteoporosis.
4. Materials and Methods
4.1. Animals
Humanized TP53 Arg72Pro knock-in (KI) mice were used. No differences in whole skeleton, body weight and survival between the two genotypes were described. Animals were bred at the Animal Welfare and Research Service of the University of Valladolid in accordance with the Spanish law (RD 53/2013). All experiments were carried out in compliance with the applicable international rules and policies —European Union Directive for Protection of Vertebrates Used for Experimental and Other Scientific Ends (2010/63/EU)—and were reviewed and approved by the University of Valladolid Institutional Committee for Animal Care and Use.
4.2. Micro-Computed Tomography (μCT)
The femur and tibia specimens were scanned using a high-energy micro-computed tomography system 527 (SkyScan 11732, Bruker Micro-CT, Kontich, Belgium) and the Sky-scan1172 µCT data acquisition software. Since the aim was to maximize the resolution of the samples, the pixel size was reduced to the minimum, reaching a pixel size of 6.7 µm and voxel size of 300.76 µm3. Scanning was made at 50 kV and an Al 0.5 mm filter was used to reduce the noise during scanning. During the reconstruction, parameters were used to correct possible beam hardening, ring artifact, and misalignment problems. Maximum and minimum values for the attenuation coefficient were established. The minimum value was set at 0. For the maximum value, the critical section of all scans, the one with the maximum attenuation coefficient value was selected at the operator’s discretion. Once this section was defined, the maximum value within the histogram was determined and a margin of error of 10% was applied. Finally, the cortical and trabecular areas of the tibia and femur were analyzed. The trabecular bone analysis was made in the distal femur and proximal tibia areas. The regions of interest covered a total of 3 mm, specifically 2 mm below the growth plate. For the analysis of the cortical area, 3 mm of the central regions of the femur and tibia were selected at a distance of 15 mm from the growth plate of the tibia and femur. The regions of interest for delineation in each image were fully automated and assessed, as described by Bruker’s instructions [
39,
40]. The scan parameters have been included in
Supplementary Table S1.
The structural parameter of the cortical bone analyzed was cortical thickness. In the trabecular bone, bone volume over total volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were analyzed. The BV/TV parameter indicates the ratio of bone tissue within the whole sample and Tb.N, Tb.Th, and Tb.Sp determine the quality of the trabecular bone.
4.3. Sample Processing and RNA Extraction
Bone tissue was submerged in RNA stabilizing solution (RNAlater, Invitrogen) and stored at -80ºC until RNA extraction, which was performed using Trizol reagent (Invitrogen) and polytron tissue homogenizer. RNA quantity and purity was determined by absorbance in a spectrophotometer (NanoDrop 2000, Thermo, Waltham, MA, USA).
4.4. Reverse Transcription and Real-Time Quantitative PCR
Complementary DNA (cDNA) was synthesized by reverse transcription using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Relative quantitative real-time polymerase chain reaction (qPCR) was performed using SYBR Green PCR master mix (Applied Biosystems) and mice-specific primer sets (
Supplementary Table S2). Relative mRNA expression was analyzed for apoptosis, inflammation, and oxidative stress related genes. The qPCR experiments were conducted using the Applied Biosystems 7500 Real-Time PCR System under the following conditions: 95ºC, 10 minutes; 40 cycles of 95ºC, 15 seconds; 60ºC, 1 minute, and a final melting curve step.
GAPDH gene was used as a housekeeping for normalization of the expression level of mRNA. The threshold cycle was determined for each reaction, and gene expression was quantified using the 2-ΔΔCt method [
41]. All qPCR reactions were performed in triplicate.
4.5. Statistical Analysis
Continuous variables were expressed as mean (standard deviation). The Kolmogorov–Smirnov test was used to analyze the distribution of continuous variables. As for normally distributed variables, the analysis of variance t-test was applied. In the case of non-normally distributed variables, the groups were compared using the Mann–Whitney U-test (two groups) or the Kruskal–Wallis test (more than two groups). A p value < 0.05 was considered significant. All analyses were performed using the SPSS version 22.0 statistical package (SPSS, Chicago, IL, USA).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Table S1. Scan parameters. Supplementary Table S2. Gene-specific primer sequences for real-time quantitative PCR
Author Contributions
Conceptualization: RUM. and JLPC.; methodology: RUM, NGC, SPI, JMFG, ADR, DF, RL, FMR, JAR, AA and JLPC.; formal analysis: RUM, NGC and JLPC.; investigation: RUM, NGC, SPI, JMFG, ADR, DF, RL, FMR, JAR, AA and JLPC ; resources: RUM; writing—original draft preparation: RUM; writing—review and editing: RUM, NGC, SPI, JMFG, ADR, DF, RL, FMR, JAR, AA and JLPC; supervision: RUM and JLPC; project administration: RUM and JLPC; funding acquisition, RUM, SPI, AA and JLPC. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación (Spain Government; PID2020-114585RA-I00 to RUM and SPI) and Instituto de Salud Carlos III (Spain; PI21/00727 to AA).
Informed Consent Statement
Animals were bred at the Animal Welfare and Research Service of the University of Valladolid in accordance with the Spanish law (RD 53/2013). All experiments were carried out in compliance with the applicable international rules and policies —European Union Directive for Protection of Vertebrates Used for Experimental and Other Scientific Ends (2010/63/EU)—and were reviewed and approved by the University of Valladolid Institutional Committee for Animal Care and Use.
Data Availability Statement
All the results are in the main manuscript
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results
References
- Weitzmann, M.N.; Ofotokun, I. Physiological and pathophysiological bone turnover — role of the immune system. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef]
- Kanis, J.A. Diagnosis of osteoporosis. Osteoporos Int. 1997, 7 (Suppl S3), S108–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-L.; Shen, H.; Liu, A.; Dong, S.-S.; Zhang, L.; Deng, F.-Y.; Zhao, Q.; Deng, H.-W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2019, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Siris, E.S.; Miller, P.D.; Barrett-Connor, E.; Faulkner, K.G.; Wehren, L.E.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Identification and Fracture Outcomes of Undiagnosed Low Bone Mineral Density in Postmenopausal Women: Results From the National Osteoporosis Risk Assessment. Obstet. Gynecol. Surv. 2002, 57, 220–221. [Google Scholar] [CrossRef]
- Ja K. Diagnosis of osteoporosis and assessment of fracture risk. Lancet (London, England) [Internet]. 2002 Jan 6 [cited 2023 May 26];359(9321). Available from: https://pubmed.ncbi.nlm.nih.gov/12057569/.
- Adachi, J.D.; Adami, S.; Gehlbach, S.; Anderson, F.A., Jr.; Boonen, S.; Chapurlat, R.D.; Compston, J.E.; Cooper, C.; Delmas, P.; Diez-Perez, A.; et al. Impact of Prevalent Fractures on Quality of Life: Baseline Results From the Global Longitudinal Study of Osteoporosis in Women. Mayo Clin. Proc. 2010, 85, 806–813. [Google Scholar] [CrossRef]
- Kanis, J.; Johansson, H.; Oden, A.; Johnell, O.; De Laet, C.; Eisman, J.; McCloskey, E.; Mellstrom, D.; Melton, L.; Pols, H.; et al. A family history of fracture and fracture risk: a meta-analysis. Bone 2004, 35, 1029–1037. [Google Scholar] [CrossRef]
- Ralston SH, Uitterlinden AG. Genetics of Osteoporosis. Endocr. Rev. 2010 Oct 1;31(5):629–62. [CrossRef]
- Trajanoska, K.; Rivadeneira, F. The genetic architecture of osteoporosis and fracture risk. Bone 2019, 126, 2–10. [Google Scholar] [CrossRef]
- Mitek T, Nagraba Ł, Deszczyński J, Stolarczyk M, Kuchar E, Stolarczyk A. Genetic Predisposition for Osteoporosis and Fractures in Postmenopausal Women. Adv Exp Med Biol. 2019;1211:17–24. [CrossRef]
- Koromani, F.; Trajanoska, K.; Rivadeneira, F.; Oei, L. Recent Advances in the Genetics of Fractures in Osteoporosis. Front. Endocrinol. 2019, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bai, W.; Zheng, H. Twelve years of GWAS discoveries for osteoporosis and related traits: advances, challenges and applications. Bone Res. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Yu, T.; You, X.; Zhou, H.; Kang, A.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; et al. p53 plays a central role in the development of osteoporosis. Aging 2020, 12, 10473–10487. [Google Scholar] [CrossRef] [PubMed]
- Jia F, Sun R, Li J, Li Q, Chen G, Fu W. Interactions of Pri-miRNA-34b/c and TP53 Polymorphisms on the Risk of Osteoporosis. Genet Test Mol Biomarkers. 2016 Jul;20(7):398–401. [CrossRef]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000 Nov;408(6810):307–10. [CrossRef]
- Wang, X.; Kua, H.-Y.; Hu, Y.; Guo, K.; Zeng, Q.; Wu, Q.; Ng, H.-H.; Karsenty, G.; de Crombrugghe, B.; Yeh, J.; et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2005, 172, 115–125. [Google Scholar] [CrossRef]
- Liu, H.; Li, B. p53 control of bone remodeling. J. Cell. Biochem. 2010, 111, 529–534. [Google Scholar] [CrossRef]
- Sakamuro, D.; Sabbatini, P.; White, E.; Prendergast, G.C. The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene 1997, 15, 887–898. [Google Scholar] [CrossRef]
- Bonafé, M.; Salvioli, S.; Barbi, C.; Trapassi, C.; Tocco, F.; Storci, G.; Invidia, L.; Vannini, I.; Rossi, M.; Marzi, E.; et al. The different apoptotic potential of the p53 codon 72 alleles increases with age and modulates in vivo ischaemia-induced cell death. Cell Death Differ. 2004, 11, 962–973. [Google Scholar] [CrossRef]
- Dumont, P.; Leu, J.I.-J.; Pietra, A.C.D., III; George, D.L.; Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003, 33, 357–365. [Google Scholar] [CrossRef]
- Liu W, Qi M, Konermann A, Zhang L, Jin F, Jin Y. The p53/miR-17/Smurf1 pathway mediates skeletal deformities in an age-related model via inhibiting the function of mesenchymal stem cells. Aging (Albany NY). 2015 Mar;7(3):205–18. [CrossRef]
- Yun, J.; Espinoza, I.; Pannuti, A.; Romero, D.; Martinez, L.; Caskey, M.; Stanculescu, A.; Bocchetta, M.; Rizzo, P.; Band, V.; et al. p53 Modulates Notch Signaling in MCF-7 Breast Cancer Cells by Associating With the Notch Transcriptional Complex Via MAML1. J. Cell. Physiol. 2015, 230, 3115–3127. [Google Scholar] [CrossRef] [PubMed]
- Velleri T, Huang Y, Wang Y, Li Q, Hu M, Xie N et al. Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin. Cell Death and Differentiation. 28, 156–169.
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Eeles, R.; Hollstein, M.; Khan, M.A.; Harris, C.C.; Hainaut, P. The IARC TP53 database: New online mutation analysis and recommendations to users. Hum. Mutat. 2002, 19, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, E.C.; Humbey, O.; E Murphy, M. Polymorphisms in the p53 pathway. Oncogene 2006, 25, 1602–1611. [Google Scholar] [CrossRef]
- Tian, X.; Dai, S.; Sun, J.; Jiang, S.; Jiang, Y. The association between the TP53 Arg72Pro polymorphism and colorectal cancer: An updated meta-analysis based on 32 studies. Oncotarget 2016, 8, 1156–1165. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.C.; Delgado-Esteban, M.; Rodriguez-Hernandez, I.; Sobrino, T.; de la Ossa, N.P.; Reverte, S.; Bolaños, J.P.; Gonzalez-Sarmiento, R.; Castillo, J.; Almeida, A. The human Tp53 Arg72Pro polymorphism explains different functional prognosis in stroke. J. Exp. Med. 2011, 208, 429–437. [Google Scholar] [CrossRef]
- Leu, J.I.-J.; E Murphy, M.; George, D.L. The p53 Codon 72 Polymorphism Modifies the Cellular Response to Inflammatory Challenge in the Liver. J. Liver 2013, 02. [Google Scholar] [CrossRef]
- Diakite, B.; Kassogue, Y.; Dolo, G.; Wang, J.; Neuschler, E.; Kassogue, O.; Keita, M.L.; Traore, C.B.; Kamate, B.; Dembele, E.; et al. Arg72Pro polymorphism of P53 and breast cancer risk: a meta-analysis of case-control studies. BMC Med Genet. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Usategui-Martín R, Lendinez-Tortajada V, Pérez-Castrillón JL, Briongos-Figuero L, Abadía-Otero J, Martín-Vallejo J, et al. Polymorphisms in genes involved in inflammation, the NF-kB pathway and the renin-angiotensin-aldosterone system are associated with the risk of osteoporotic fracture. The Hortega Follow-up Study. Bone. 2020 Sep;138:115477. [CrossRef]
- Usategui-Martín, R.; Pérez-Castrillón, J.L.; Mansego, M.L.; Lara-Hernández, F.; Manzano, I.; Briongos, L.; Abadía-Otero, J.; Martín-Vallejo, J.; García-García, A.B.; Martín-Escudero, J.C.; et al. Association between genetic variants in oxidative stress-related genes and osteoporotic bone fracture. The Hortega follow-up study. Gene 2022, 809, 146036. [Google Scholar] [CrossRef]
- Frank AK, Leu JIJ, Zhou Y, Devarajan K, Nedelko T, Klein-Szanto A, et al. The Codon 72 Polymorphism of p53 Regulates Interaction with NF-κB and Transactivation of Genes Involved in Immunity and Inflammation. Mol Cell Biol. 2011 Mar;31(6):1201–13. [CrossRef]
- Novack, DV. Role of NF-κB in the skeleton. Cell Res. 2011 Jan;21(1):169–82. [CrossRef]
- Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol Metab (Seoul). 2015 Mar;30(1):35–44. [CrossRef]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef]
- Campbell, G.M.; Sophocleous, A. Quantitative analysis of bone and soft tissue by micro-computed tomography: applications to ex vivo and in vivo studies. BoneKEy Rep. 2014, 3, 564. [Google Scholar] [CrossRef]
- van ’t Hof RJ. Analysis of bone architecture in rodents using microcomputed tomography. Methods Mol Biol. 2012;816:461–76. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).