Submitted:
06 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
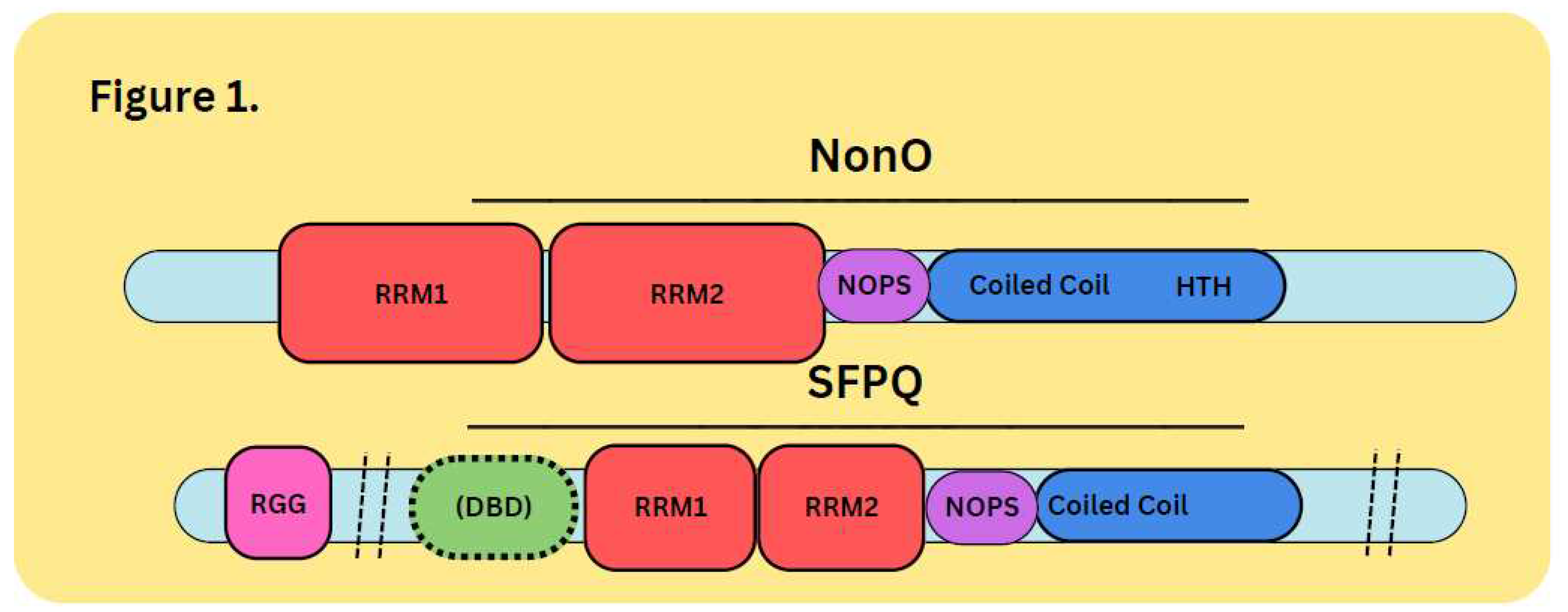
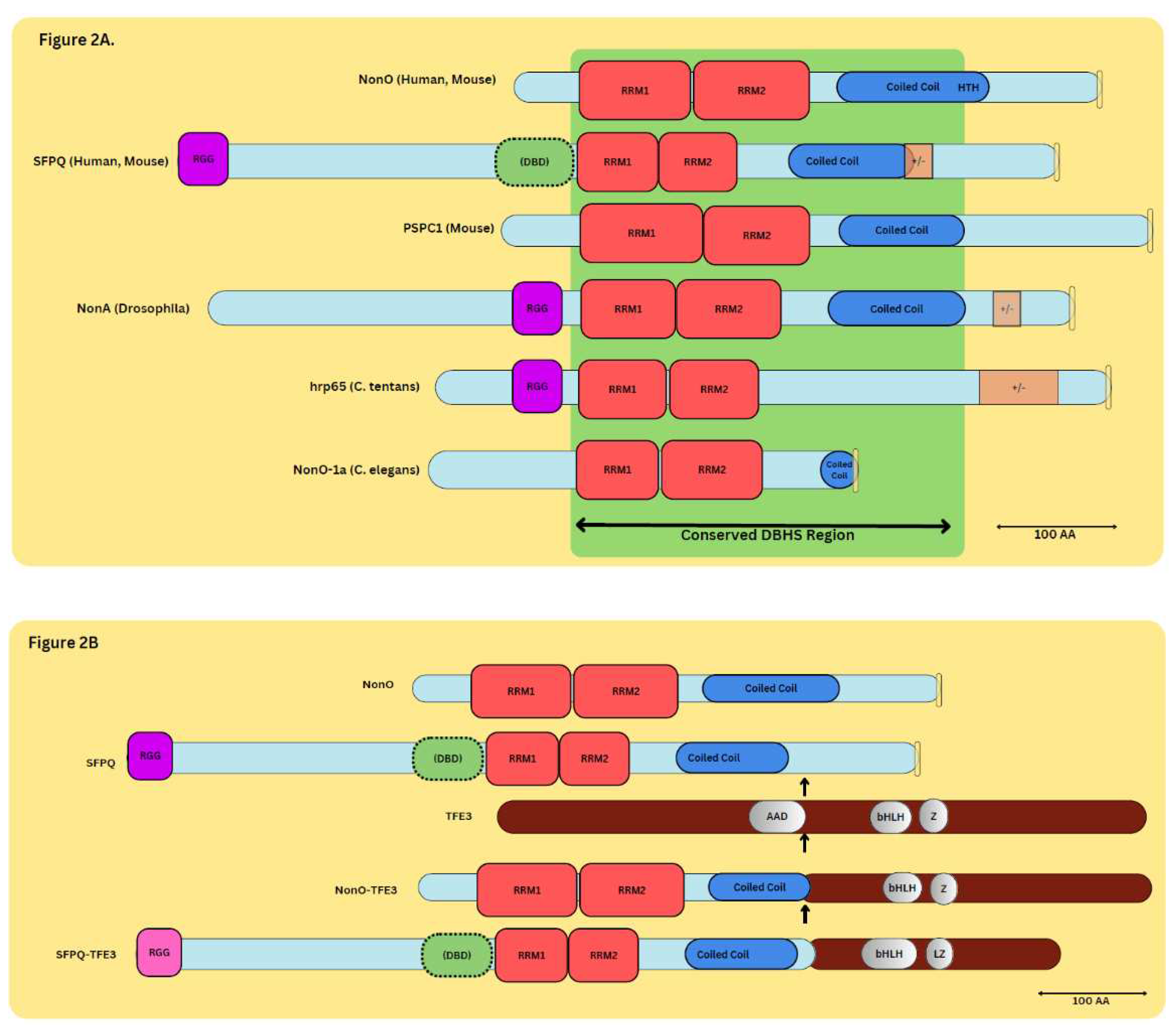
Multifunctional roles of NonO and SFPQ
Focus of this review
NonO and SFPQ in cell cycle and cell cycle arrest
NonO and SFPQ participate in DNA damage repair
NonO and SFPQ participate in Nonhomologous End Joining (NHEJ)
NonO and SFPQ in Homologous Recombination (HR)
NonO and/or SFPQ are associated with DSB repair factors
Posttranscriptional modification of NonO facilitates DNA damage repair
NonO and SFPQ in telomere stability
Summary and Cancer Implications
Acknowledgments
Conflicts of interest
References
- Hanke, J.H.; Landolfi, N.F.; Tucker, P.W.; Capra, J.D. Identification of Murine Nuclear Proteins That Bind to the Conserved Octamer Sequence of the Immunoglobulin Promoter Region. Proc Natl Acad Sci U S A 1988, 85, 3560–3564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Hanke, J.H.; Carayannopoulos, L.; Craft, C.M.; Capra, J.D.; Tucker, P.W. NonO, a Non-POU-Domain-Containing, Octamer-Binding Protein, Is the Mammalian Homolog of Drosophila nonAdiss. Mol Cell Biol 1993, 13, 5593–5603. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Horowitz, D.S.; Kobayashi, R.; Krainer, A.R. Purification and cDNA Cloning of HeLa Cell P54nrb, a Nuclear Protein with Two RNA Recognition Motifs and Extensive Homology to Human Splicing Factor PSF and Drosophila NONA/BJ6. Nucleic Acids Res 1993, 21, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.G.; Mayer, S.A.; Tempst, P.; Nadal-Ginard, B. Characterization and Molecular Cloning of Polypyrimidine Tract-Binding Protein: A Component of a Complex Necessary for Pre-mRNA Splicing. Genes Dev 1991, 5, 1237–1251. [Google Scholar] [CrossRef]
- Patton, J.G.; Porro, E.B.; Galceran, J.; Tempst, P.; Nadal-Ginard, B. Cloning and Characterization of PSF, a Novel Pre-mRNA Splicing Factor. Genes Dev 1993, 7, 393–406. [Google Scholar] [CrossRef]
- Shav-Tal, Y.; Zipori, D. PSF and P54(Nrb)/NonO--Multi-Functional Nuclear Proteins. FEBS Lett 2002, 531, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yarosh, C.A.; Iacona, J.R.; Lutz, C.S.; Lynch, K.W. PSF: Nuclear Busy-Body or Nuclear Facilitator? Wiley Interdiscip Rev RNA 2015, 6, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, Y.; Iino, K.; Takayama, K.-I.; Ikeda, K.; Suzuki, T.; Aogi, K.; Kawabata, H.; Suzuki, Y.; Horie-Inoue, K.; Inoue, S. PSF Promotes ER-Positive Breast Cancer Progression via Posttranscriptional Regulation of ESR1 and SCFD2. Cancer Res 2020, 80, 2230–2242. [Google Scholar] [CrossRef]
- Takayama, K.-I.; Honma, T.; Suzuki, T.; Kondoh, Y.; Osada, H.; Suzuki, Y.; Yoshida, M.; Inoue, S. Targeting Epigenetic and Posttranscriptional Gene Regulation by PSF Impairs Hormone Therapy-Refractory Cancer Growth. Cancer Res 2021, 81, 3495–3508. [Google Scholar] [CrossRef]
- Figueroa, A.; Fujita, Y.; Gorospe, M. Hacking RNA: Hakai Promotes Tumorigenesis by Enhancing the RNA-Binding Function of PSF. Cell Cycle 2009, 8, 3648–3651. [Google Scholar] [CrossRef]
- Stagsted, L.V.W.; O’Leary, E.T.; Ebbesen, K.K.; Hansen, T.B. The RNA-Binding Protein SFPQ Preserves Long-Intron Splicing and Regulates circRNA Biogenesis in Mammals. Elife 2021, 10, e63088. [Google Scholar] [CrossRef] [PubMed]
- Cosker, K.E.; Fenstermacher, S.J.; Pazyra-Murphy, M.F.; Elliott, H.L.; Segal, R.A. The RNA-Binding Protein SFPQ Orchestrates an RNA Regulon to Promote Axon Viability. Nat Neurosci 2016, 19, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, S.; Ikei, A.; Taguchi, Y.; Tabuchi, Y.; Fujimoto, N.; Obinata, M.; Uesugi, S.; Kurihara, Y. PSPC1, NONO, and SFPQ Are Expressed in Mouse Sertoli Cells and May Function as Coregulators of Androgen Receptor-Mediated Transcription. Biol Reprod 2006, 75, 352–359. [Google Scholar] [CrossRef] [PubMed]
- King, H.A.; Cobbold, L.C.; Pichon, X.; Pöyry, T.; Wilson, L.A.; Booden, H.; Jukes-Jones, R.; Cain, K.; Lilley, K.S.; Bushell, M.; et al. Remodelling of a Polypyrimidine Tract-Binding Protein Complex during Apoptosis Activates Cellular IRESs. Cell Death Differ 2014, 21, 161–171. [Google Scholar] [CrossRef]
- Kaneko, S.; Rozenblatt-Rosen, O.; Meyerson, M.; Manley, J.L. The Multifunctional Protein P54nrb/PSF Recruits the Exonuclease XRN2 to Facilitate Pre-mRNA 3’ Processing and Transcription Termination. Genes Dev 2007, 21, 1779–1789. [Google Scholar] [CrossRef]
- Fox, A.H.; Lam, Y.W.; Leung, A.K.L.; Lyon, C.E.; Andersen, J.; Mann, M.; Lamond, A.I. Paraspeckles: A Novel Nuclear Domain. Curr Biol 2002, 12, 13–25. [Google Scholar] [CrossRef]
- Fox, A.H.; Lamond, A.I. Paraspeckles. Cold Spring Harb Perspect Biol 2010, 2, a000687. [Google Scholar] [CrossRef]
- McCluggage, F.; Fox, A.H. Paraspeckle Nuclear Condensates: Global Sensors of Cell Stress? Bioessays 2021, 43, e2000245. [Google Scholar] [CrossRef]
- Bond, C.S.; Fox, A.H. Paraspeckles: Nuclear Bodies Built on Long Noncoding RNA. J Cell Biol 2009, 186, 637–644. [Google Scholar] [CrossRef]
- Takeiwa, T.; Mitobe, Y.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Roles of Splicing Factors in Hormone-Related Cancer Progression. Int J Mol Sci 2020, 21, 1551. [Google Scholar] [CrossRef]
- Knott, G.J.; Bond, C.S.; Fox, A.H. The DBHS Proteins SFPQ, NONO and PSPC1: A Multipurpose Molecular Scaffold. Nucleic Acids Res 2016, 44, 3989–4004. [Google Scholar] [CrossRef]
- Kiesler, E.; Miralles, F.; Ostlund Farrants, A.-K.; Visa, N. The Hrp65 Self-Interaction Is Mediated by an Evolutionarily Conserved Domain and Is Required for Nuclear Import of Hrp65 Isoforms That Lack a Nuclear Localization Signal. J Cell Sci 2003, 116, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Knott, G.J.; Lee, M.; Passon, D.M.; Fox, A.H.; Bond, C.S. Caenorhabditis Elegans NONO-1: Insights into DBHS Protein Structure, Architecture, and Function. Protein Sci 2015, 24, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Rubin, G.M. Molecular Analysis of No-on-Transient A, a Gene Required for Normal Vision in Drosophila. Neuron 1990, 4, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, T.; Palazzolo, L.; Taiana, E.; Saporiti, S.; Ben Mariem, O.; Guerrini, U.; Neri, A.; Eberini, I. Molecular Modelling of NONO and SFPQ Dimerization Process and RNA Recognition Mechanism. Int J Mol Sci 2022, 23, 7626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Zhang, L.X.; Busch, R.K.; Farrés, J.; Busch, H. Purification and Characterization of a DNA-Binding Heterodimer of 52 and 100 kDa from HeLa Cells. Biochem J 1993, 290 Pt 1, 267–272. [Google Scholar] [CrossRef]
- Huang, C.-J.; Das, U.; Xie, W.; Ducasse, M.; Tucker, H.O. Altered Stoichiometry and Nuclear Delocalization of NonO and PSF Promote Cellular Senescence. Aging (Albany NY) 2016, 8, 3356–3374. [Google Scholar] [CrossRef]
- Passon, D.M.; Lee, M.; Rackham, O.; Stanley, W.A.; Sadowska, A.; Filipovska, A.; Fox, A.H.; Bond, C.S. Structure of the Heterodimer of Human NONO and Paraspeckle Protein Component 1 and Analysis of Its Role in Subnuclear Body Formation. Proc Natl Acad Sci U S A 2012, 109, 4846–4850. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Casas Garcia, G.P.; Perugini, M.A.; Fox, A.H.; Bond, C.S.; Lee, M. Crystal Structure of a SFPQ/PSPC1 Heterodimer Provides Insights into Preferential Heterodimerization of Human DBHS Family Proteins. J Biol Chem 2018, 293, 6593–6602. [Google Scholar] [CrossRef]
- Schell, B.; Legrand, P.; Fribourg, S. Crystal Structure of SFPQ-NONO Heterodimer. Biochimie 2022, 198, 1–7. [Google Scholar] [CrossRef]
- Feng, P.; Li, L.; Deng, T.; Liu, Y.; Ling, N.; Qiu, S.; Zhang, L.; Peng, B.; Xiong, W.; Cao, L.; et al. NONO and Tumorigenesis: More than Splicing. J Cell Mol Med 2020, 24, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Dye, B.T.; Pérez, I.; Barnard, D.C.; Thompson, A.B.; Patton, J.G. PSF and P54nrb Bind a Conserved Stem in U5 snRNA. RNA 2002, 8, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-B.; Xiang, J.-F.; Li, X.; Xu, Y.; Xue, W.; Huang, M.; Wong, C.C.; Sagum, C.A.; Bedford, M.T.; Yang, L.; et al. Protein Arginine Methyltransferase CARM1 Attenuates the Paraspeckle-Mediated Nuclear Retention of mRNAs Containing IRAlus. Genes Dev 2015, 29, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Hupalowska, A.; Jedrusik, A.; Zhu, M.; Bedford, M.T.; Glover, D.M.; Zernicka-Goetz, M. CARM1 and Paraspeckles Regulate Pre-Implantation Mouse Embryo Development. Cell 2018, 175, 1902–1916.e13. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Feng, J. RNA Splicing Regulators Play Critical Roles in Neurogenesis. Wiley Interdiscip Rev RNA 2022, 13, e1728. [Google Scholar] [CrossRef] [PubMed]
- Sury, M.D.; McShane, E.; Hernandez-Miranda, L.R.; Birchmeier, C.; Selbach, M. Quantitative Proteomics Reveals Dynamic Interaction of C-Jun N-Terminal Kinase (JNK) with RNA Transport Granule Proteins Splicing Factor Proline- and Glutamine-Rich (Sfpq) and Non-POU Domain-Containing Octamer-Binding Protein (Nono) during Neuronal Differentiation. Mol Cell Proteomics 2015, 14, 50–65. [Google Scholar] [CrossRef]
- Major, A.T.; Hogarth, C.A.; Young, J.C.; Kurihara, Y.; Jans, D.A.; Loveland, K.L. Dynamic Paraspeckle Component Localisation during Spermatogenesis. Reproduction 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Cheng, R.; Zhu, S.; Guo, S.; Min, L.; Xing, J.; Guo, Q.; Li, P.; Zhang, S. Downregulation of NONO Induces Apoptosis, Suppressing Growth and Invasion in Esophageal Squamous Cell Carcinoma. Oncol Rep 2018, 39, 2575–2583. [Google Scholar] [CrossRef]
- Brown, S.A.; Ripperger, J.; Kadener, S.; Fleury-Olela, F.; Vilbois, F.; Rosbash, M.; Schibler, U. PERIOD1-Associated Proteins Modulate the Negative Limb of the Mammalian Circadian Oscillator. Science 2005, 308, 693–696. [Google Scholar] [CrossRef]
- Luo, W.; Guo, F.; McMahon, A.; Couvertier, S.; Jin, H.; Diaz, M.; Fieldsend, A.; Weerapana, E.; Rosbash, M. NonA and CPX Link the Circadian Clockwork to Locomotor Activity in Drosophila. Neuron 2018, 99, 768–780.e3. [Google Scholar] [CrossRef]
- Hu, Z.; Dong, L.; Li, S.; Li, Z.; Qiao, Y.; Li, Y.; Ding, J.; Chen, Z.; Wu, Y.; Wang, Z.; et al. Splicing Regulator P54nrb /Non-POU Domain-Containing Octamer-Binding Protein Enhances Carcinogenesis Through Oncogenic Isoform Switch of MYC Box-Dependent Interacting Protein 1 in Hepatocellular Carcinoma. Hepatology 2020, 72, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, X.; Burley, S.K.; Zheng, X.F.S. mTOR Regulates Aerobic Glycolysis through NEAT1 and Nuclear Paraspeckle-Mediated Mechanism in Hepatocellular Carcinoma. Theranostics 2022, 12, 3518–3533. [Google Scholar] [CrossRef] [PubMed]
- Iino, K.; Mitobe, Y.; Ikeda, K.; Takayama, K.-I.; Suzuki, T.; Kawabata, H.; Suzuki, Y.; Horie-Inoue, K.; Inoue, S. RNA-Binding Protein NONO Promotes Breast Cancer Proliferation by Post-Transcriptional Regulation of SKP2 and E2F8. Cancer Sci 2020, 111, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.-I.; Suzuki, T.; Fujimura, T.; Yamada, Y.; Takahashi, S.; Homma, Y.; Suzuki, Y.; Inoue, S. Dysregulation of Spliceosome Gene Expression in Advanced Prostate Cancer by RNA-Binding Protein PSF. Proc Natl Acad Sci U S A 2017, 114, 10461–10466. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Osawa, T.; Sasaki, Y.; Yamamoto, S.; Anai, M.; Izumi, K.; Matsumura, Y.; Sakai, J.; Aburatani, H.; Mizokami, A.; et al. Overexpression of P54nrb/NONO Induces Differential EPHA6 Splicing and Contributes to Castration-Resistant Prostate Cancer Growth. Oncotarget 2018, 9, 10510–10524. [Google Scholar] [CrossRef]
- Clark, J.; Lu, Y.J.; Sidhar, S.K.; Parker, C.; Gill, S.; Smedley, D.; Hamoudi, R.; Linehan, W.M.; Shipley, J.; Cooper, C.S. Fusion of Splicing Factor Genes PSF and NonO (P54nrb) to the TFE3 Gene in Papillary Renal Cell Carcinoma. Oncogene 1997, 15, 2233–2239. [Google Scholar] [CrossRef]
- Skalsky, Y.M.; Ajuh, P.M.; Parker, C.; Lamond, A.I.; Goodwin, G.; Cooper, C.S. PRCC, the Commonest TFE3 Fusion Partner in Papillary Renal Carcinoma Is Associated with Pre-mRNA Splicing Factors. Oncogene 2001, 20, 178–187. [Google Scholar] [CrossRef]
- Mathur, M.; Das, S.; Samuels, H.H. PSF-TFE3 Oncoprotein in Papillary Renal Cell Carcinoma Inactivates TFE3 and P53 through Cytoplasmic Sequestration. Oncogene 2003, 22, 5031–5044. [Google Scholar] [CrossRef]
- Udayakumar, D.; Bladen, C.L.; Hudson, F.Z.; Dynan, W.S. Distinct Pathways of Nonhomologous End Joining That Are Differentially Regulated by DNA-Dependent Protein Kinase-Mediated Phosphorylation. J Biol Chem 2003, 278, 41631–41635. [Google Scholar] [CrossRef]
- Bladen, C.L.; Udayakumar, D.; Takeda, Y.; Dynan, W.S. Identification of the Polypyrimidine Tract Binding Protein-Associated Splicing Factor.P54(Nrb) Complex as a Candidate DNA Double-Strand Break Rejoining Factor. J Biol Chem 2005, 280, 5205–5210. [Google Scholar] [CrossRef]
- Alfano, L.; Costa, C.; Caporaso, A.; Altieri, A.; Indovina, P.; Macaluso, M.; Giordano, A.; Pentimalli, F. NONO Regulates the Intra-S-Phase Checkpoint in Response to UV Radiation. Oncogene 2016, 35, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Lukong, K.E.; Huot, M.-E.; Richard, S. BRK Phosphorylates PSF Promoting Its Cytoplasmic Localization and Cell Cycle Arrest. Cell Signal 2009, 21, 1415–1422. [Google Scholar] [CrossRef]
- Rajesh, C.; Baker, D.K.; Pierce, A.J.; Pittman, D.L. The Splicing-Factor Related Protein SFPQ/PSF Interacts with RAD51D and Is Necessary for Homology-Directed Repair and Sister Chromatid Cohesion. Nucleic Acids Res 2011, 39, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Lone, B.A.; Siraj, F.; Sharma, I.; Verma, S.; Karna, S.K.L.; Ahmad, F.; Nagar, P.; Sachidanandan, C.; Pokharel, Y.R. Non-POU Domain-Containing Octomer-Binding (NONO) Protein Expression and Stability Promotes the Tumorigenicity and Activation of Akt/MAPK/β-Catenin Pathways in Human Breast Cancer Cells. Cell Commun Signal 2023, 21, 157. [Google Scholar] [CrossRef]
- Roberts, E.C.; Hammond, K.; Traish, A.M.; Resing, K.A.; Ahn, N.G. Identification of G2/M Targets for the MAP Kinase Pathway by Functional Proteomics. Proteomics 2006, 6, 4541–4553. [Google Scholar] [CrossRef] [PubMed]
- Casado, P.; Prado, M.A.; Zuazua-Villar, P.; Del Valle, E.; Artime, N.; Cabal-Hierro, L.; Rupérez, P.; Burlingame, A.L.; Lazo, P.S.; Ramos, S. Microtubule Interfering Agents and KSP Inhibitors Induce the Phosphorylation of the Nuclear Protein P54(Nrb), an Event Linked to G2/M Arrest. J Proteomics 2009, 71, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Shav-Tal, Y.; Cohen, M.; Lapter, S.; Dye, B.; Patton, J.G.; Vandekerckhove, J.; Zipori, D. Nuclear Relocalization of the Pre-mRNA Splicing Factor PSF during Apoptosis Involves Hyperphosphorylation, Masking of Antigenic Epitopes, and Changes in Protein Interactions. Mol Biol Cell 2001, 12, 2328–2340. [Google Scholar] [CrossRef]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA Double-Strand Break Repair-Pathway Choice in Somatic Mammalian Cells. Nat Rev Mol Cell Biol 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Lieber, M.R. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End-Joining Pathway. Annu Rev Biochem 2010, 79, 181–211. [Google Scholar] [CrossRef]
- Li, S.; Kuhne, W.W.; Kulharya, A.; Hudson, F.Z.; Ha, K.; Cao, Z.; Dynan, W.S. Involvement of P54(Nrb), a PSF Partner Protein, in DNA Double-Strand Break Repair and Radioresistance. Nucleic Acids Res 2009, 37, 6746–6753. [Google Scholar] [CrossRef]
- Salton, M.; Lerenthal, Y.; Wang, S.-Y.; Chen, D.J.; Shiloh, Y. Involvement of Matrin 3 and SFPQ/NONO in the DNA Damage Response. Cell Cycle 2010, 9, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, D.; Dynan, W.S. Characterization of DNA Binding and Pairing Activities Associated with the Native SFPQ·NONO DNA Repair Protein Complex. Biochem Biophys Res Commun 2015, 463, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.; Shu, F.-J.; Xiong, H.; Phillips, A.C.; Dynan, W.S. Double-Strand Break Repair Deficiency in NONO Knockout Murine Embryonic Fibroblasts and Compensation by Spontaneous Upregulation of the PSPC1 Paralog. Nucleic Acids Res 2014, 42, 9771–9780. [Google Scholar] [CrossRef] [PubMed]
- Krietsch, J.; Caron, M.-C.; Gagné, J.-P.; Ethier, C.; Vignard, J.; Vincent, M.; Rouleau, M.; Hendzel, M.J.; Poirier, G.G.; Masson, J.-Y. PARP Activation Regulates the RNA-Binding Protein NONO in the DNA Damage Response to DNA Double-Strand Breaks. Nucleic Acids Res 2012, 40, 10287–10301. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Stark, J.M.; Ommundsen, M.; Jasin, M. Rad51 Overexpression Promotes Alternative Double-Strand Break Repair Pathways and Genome Instability. Oncogene 2004, 23, 546–553. [Google Scholar] [CrossRef]
- Bertrand, P.; Lambert, S.; Joubert, C.; Lopez, B.S. Overexpression of Mammalian Rad51 Does Not Stimulate Tumorigenesis While a Dominant-Negative Rad51 Affects Centrosome Fragmentation, Ploidy and Stimulates Tumorigenesis, in P53-Defective CHO Cells. Oncogene 2003, 22, 7587–7592. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, Y.; Takizawa, Y.; Takaku, M.; Kurumizaka, H. Human PSF Binds to RAD51 and Modulates Its Homologous-Pairing and Strand-Exchange Activities. Nucleic Acids Res 2009, 37, 4296–4307. [Google Scholar] [CrossRef]
- Rajesh, C.; Gruver, A.M.; Basrur, V.; Pittman, D.L. The Interaction Profile of Homologous Recombination Repair Proteins RAD51C, RAD51D and XRCC2 as Determined by Proteomic Analysis. Proteomics 2009, 9, 4071–4086. [Google Scholar] [CrossRef]
- Straub, T.; Grue, P.; Uhse, A.; Lisby, M.; Knudsen, B.R.; Tange, T.O.; Westergaard, O.; Boege, F. The RNA-Splicing Factor PSF/P54 Controls DNA-Topoisomerase I Activity by a Direct Interaction. J Biol Chem 1998, 273, 26261–26264. [Google Scholar] [CrossRef]
- Kawano, S.; Miyaji, M.; Ichiyasu, S.; Tsutsui, K.M.; Tsutsui, K. Regulation of DNA Topoisomerase IIbeta through RNA-Dependent Association with Heterogeneous Nuclear Ribonucleoprotein U (hnRNP U). J Biol Chem 2010, 285, 26451–26460. [Google Scholar] [CrossRef]
- Kuhnert, A.; Schmidt, U.; Monajembashi, S.; Franke, C.; Schlott, B.; Grosse, F.; Greulich, K.O.; Saluz, H.-P.; Hänel, F. Proteomic Identification of PSF and P54(Nrb) as TopBP1-Interacting Proteins. J Cell Biochem 2012, 113, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Klaric, J.A.; Wüst, S.; Panier, S. New Faces of Old Friends: Emerging New Roles of RNA-Binding Proteins in the DNA Double-Strand Break Response. Front Mol Biosci 2021, 8, 668821. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, A.M.; Grenon, M.; Gilbert, C.; Toh, G.W.-L.; Green, C.M.; Lowndes, N.F. Multiple Approaches to Study S. Cerevisiae Rad9, a Prototypical Checkpoint Protein. Methods Enzymol 2006, 409, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Furuya, K.; Paderi, F.; Carr, A.M.; Wang, T.S.F. Rad3-Dependent Phosphorylation of the Checkpoint Clamp Regulates Repair-Pathway Choice. Nat Cell Biol 2007, 9, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and Its Associated Nucleases in DNA Damage Response. DNA Repair (Amst) 2019, 81, 102651. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, R.; Kraus, W.L. The PARP Side of the Nucleus: Molecular Actions, Physiological Outcomes, and Clinical Targets. Mol Cell 2010, 39, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, L.; Li, Z.; Li, S.; Dynan, W.S. SFPQ•NONO and XLF Function Separately and Together to Promote DNA Double-Strand Break Repair via Canonical Nonhomologous End Joining. Nucleic Acids Res 2017, 45, 1848–1859. [Google Scholar] [CrossRef]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging Mechanisms and Functions. Nat Rev Mol Cell Biol 2017, 18, 452–465. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, R.; Zhang, X.; Liu, Y.; Hu, Y.; Li, Y.; Liu, X.; Yu, X.; Wu, C. OGA Is Associated with Deglycosylation of NONO and the KU Complex during DNA Damage Repair. Cell Death Dis 2021, 12, 622. [Google Scholar] [CrossRef]
- Sakasai, R.; Tibbetts, R. RNF8-Dependent and RNF8-Independent Regulation of 53BP1 in Response to DNA Damage. J Biol Chem 2008, 283, 13549–13555. [Google Scholar] [CrossRef]
- Deshar, R.; Yoo, W.; Cho, E.-B.; Kim, S.; Yoon, J.-B. RNF8 Mediates NONO Degradation Following UV-Induced DNA Damage to Properly Terminate ATR-CHK1 Checkpoint Signaling. Nucleic Acids Res 2019, 47, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Petti, E.; Buemi, V.; Zappone, A.; Schillaci, O.; Broccia, P.V.; Dinami, R.; Matteoni, S.; Benetti, R.; Schoeftner, S. SFPQ and NONO Suppress RNA:DNA-Hybrid-Related Telomere Instability. Nat Commun 2019, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Lee, Y.; Wischnewski, H.; Brun, C.M.; Schwarz, T.; Azzalin, C.M. RNaseH1 Regulates TERRA-Telomeric DNA Hybrids and Telomere Maintenance in ALT Tumour Cells. Nat Commun 2014, 5, 5220. [Google Scholar] [CrossRef] [PubMed]
- Brieño-Enríquez, M.A.; Moak, S.L.; Abud-Flores, A.; Cohen, P.E. Characterization of Telomeric Repeat-Containing RNA (TERRA) Localization and Protein Interactions in Primordial Germ Cells of the Mouse†. Biol Reprod 2019, 100, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Gigi, V.; Lewis, S.; Shestova, O.; Mijušković, M.; Deriano, L.; Meng, W.; Luning Prak, E.T.; Roth, D.B. RAG2 Mutants Alter DSB Repair Pathway Choice in Vivo and Illuminate the Nature of “Alternative NHEJ. ” Nucleic Acids Res 2014, 42, 6352–6364. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat Rev Mol Cell Biol 2017, 18, 495–506. [Google Scholar] [CrossRef]
- de Silva, H.C.; Lin, M.Z.; Phillips, L.; Martin, J.L.; Baxter, R.C. IGFBP-3 Interacts with NONO and SFPQ in PARP-Dependent DNA Damage Repair in Triple-Negative Breast Cancer. Cell Mol Life Sci 2019, 76, 2015–2030. [Google Scholar] [CrossRef]
- Bi, O.; Anene, C.A.; Nsengimana, J.; Shelton, M.; Roberts, W.; Newton-Bishop, J.; Boyne, J.R. SFPQ Promotes an Oncogenic Transcriptomic State in Melanoma. Oncogene 2021, 40, 5192–5203. [Google Scholar] [CrossRef]
- Liu, P.Y.; Erriquez, D.; Marshall, G.M.; Tee, A.E.; Polly, P.; Wong, M.; Liu, B.; Bell, J.L.; Zhang, X.D.; Milazzo, G.; et al. Effects of a Novel Long Noncoding RNA, lncUSMycN, on N-Myc Expression and Neuroblastoma Progression. J Natl Cancer Inst 2014, 106, dju113. [Google Scholar] [CrossRef]
- Zhang, S.; Cooper, J.A.; Chong, Y.S.; Naveed, A.; Mayoh, C.; Jayatilleke, N.; Liu, T.; Amos, S.; Kobelke, S.; Marshall, A.C.; et al. NONO Enhances mRNA Processing of Super-Enhancer-Associated GATA2 and HAND2 Genes in Neuroblastoma. EMBO Rep 2023, 24, e54977. [Google Scholar] [CrossRef]
- Kameoka, S.; Duque, P.; Konarska, M.M. P54(Nrb) Associates with the 5’ Splice Site within Large Transcription/Splicing Complexes. EMBO J 2004, 23, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




