Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
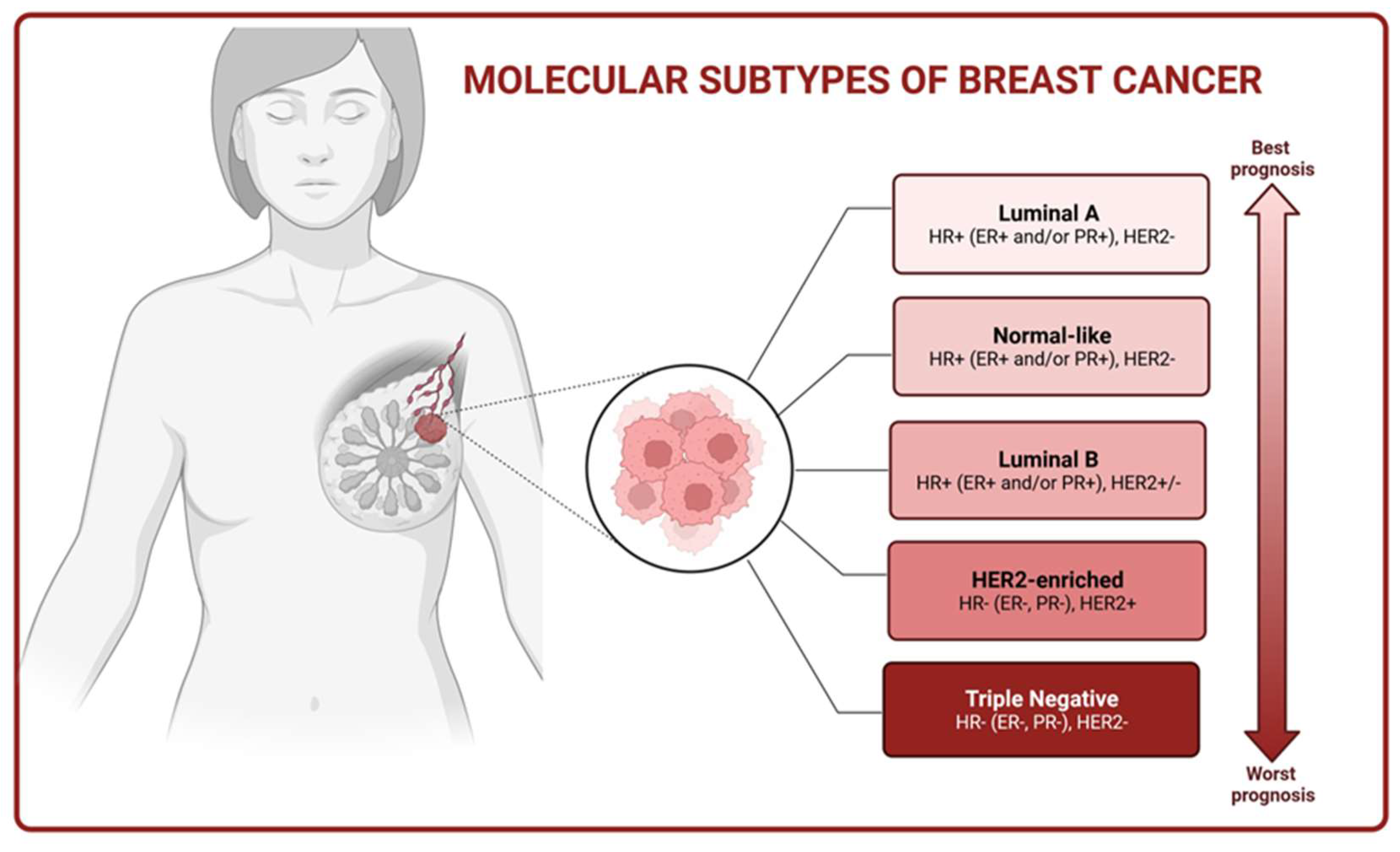
2. Molecular Basis of Breast Cancer
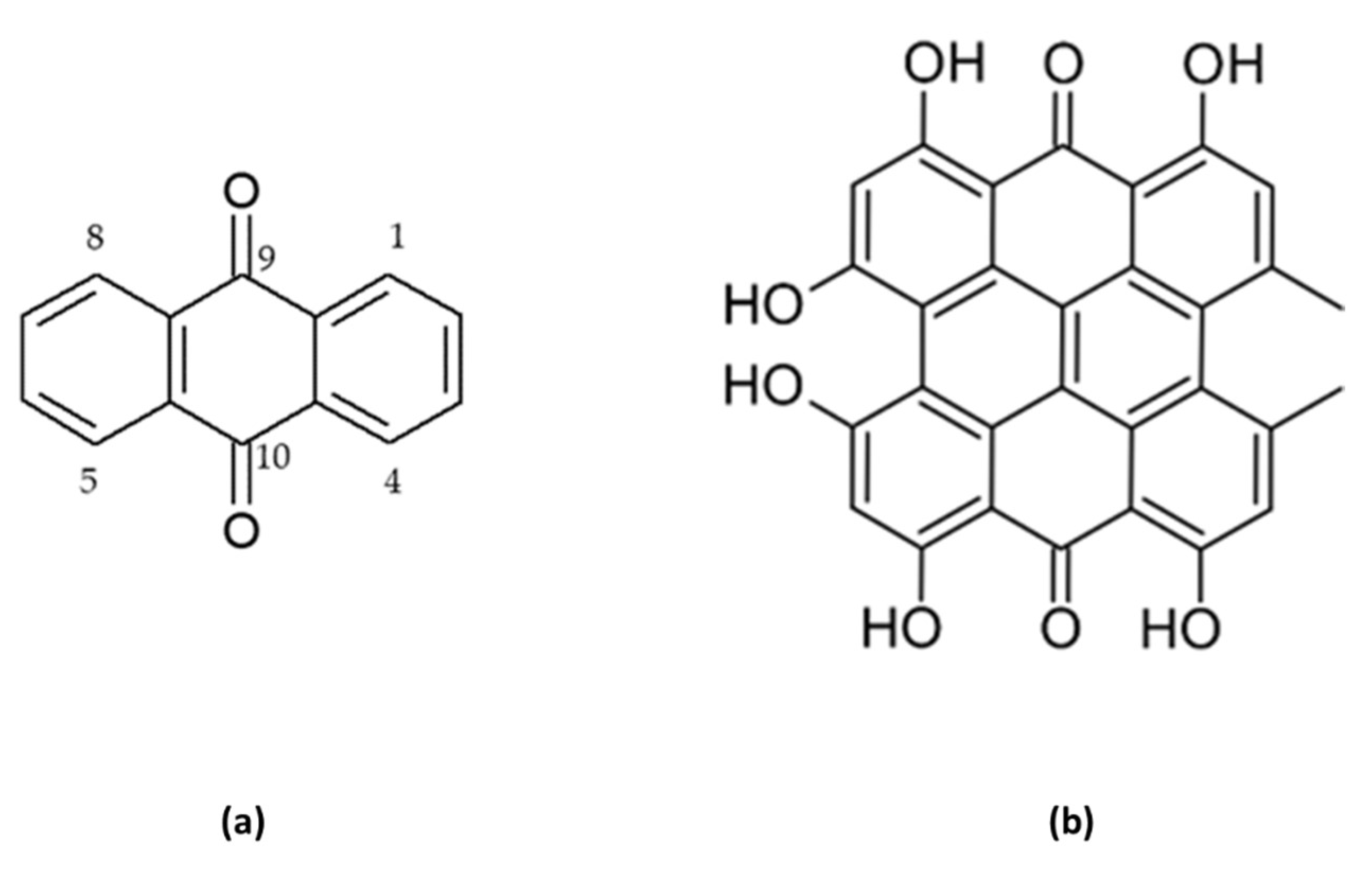
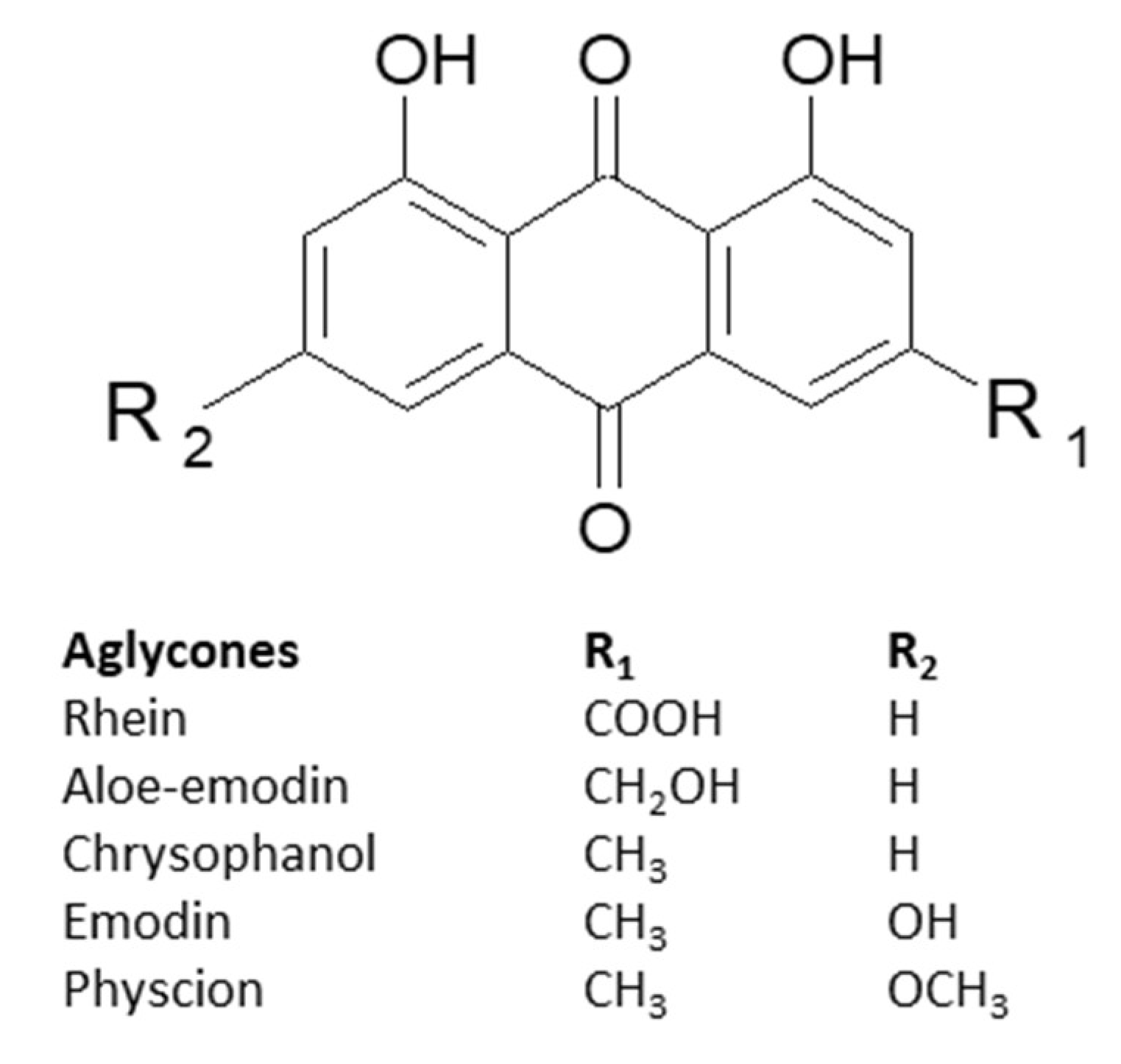
3. Characteristics of Anthracene Derivatives
4. Anticancer Activity of Anthracene Derivatives in Breast Cancer In Vitro Models
4.1. Emodin
4.2. Aloe-emodin
4.3. Aloin A & B
4.4. Physcion
4.4. Rhein
4.5. Chrysophanol
4.6. Hypericin
5. Anticancer Activity of Anthracene Derivatives in Breast Cancer In Vivo Models
6. Sensitizing properties of 1,8-dihydroxyanthraquinones towards known drugs
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef]
- Fabiani, R. Antitumoral Properties of Natural Products. Molecules 2020, 25, 650. [Google Scholar] [CrossRef] [PubMed]
- Arrousse, N.; Harras, M.F.; El Kadiri, S.; Haldhar, R.; Ichou, H.; Bousta, D.; Grafov, A.; Rais, Z.; Taleb, M. New anthraquinone drugs and their anticancer activities: Cytotoxicity, DFT, docking and ADMET properties. Results Chem. 2023, 6, 100996. [Google Scholar] [CrossRef]
- Baviera, G.S.; Donate, P.M. Recent advances in the syntheses of anthracene derivatives. Beilstein J. Org. Chem. 17131 2021, 17, 2028–2050. [Google Scholar] [CrossRef] [PubMed]
- Şeker Karatoprak, G.; Küpeli Akkol, E.; Yücel, Ç.; Bahadir Acikara, Ö.; Sobarzo-Sánchez, E. Advances in Understanding the Role of Aloe Emodin and Targeted Drug Delivery Systems in Cancer. Oxid. Med. Cell. Longev. 2022, 7928200. [Google Scholar] [CrossRef] [PubMed]
- Montazeri Aliabadi, H.; Manda, A.; Sidgal, R.; Chung, C. Targeting Breast Cancer: The Familiar, the Emerging, and the Uncharted Territories. Biomolecules 2023, 13, 1306. [Google Scholar] [CrossRef] [PubMed]
- Azadnajafabad, S.; Saeedi Moghaddam, S.; Mohammadi, E.; Delazar, S.; Rashedi, S.; Baradaran, H.R.; Mansourian, M. Patterns of better breast cancer care in countries with higher human development index and healthcare expenditure: Insights from GLOBOCAN 2020. Front. Public Heal. 2023, 11, 1137286. [Google Scholar] [CrossRef]
- Wawruszak, A.; Halasa, M.; Okon, E.; Kukula-Koch, W.; Stepulak, A. Valproic Acid and Breast Cancer: State of the Art in 2021. Cancers 2021, Vol. 13, Page 3409 2021, 13, 3409. [Google Scholar] [CrossRef]
- Uchida, N.; Suda, T.; Ishiguro, K. Effect of Chemotherapy for Luminal A Breast Cancer. Yonago Acta Med. 2013, 56, 51. [Google Scholar]
- Creighton, C.J. The molecular profile of luminal B breast cancer. Biol. Targets Ther. 2012, 6, 289–297. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. Breast Cancer 2022, 31–42. [Google Scholar]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022 151 2022, 15, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid. Based. Complement. Alternat. Med. 2018, 8324696. [Google Scholar] [CrossRef]
- Thomson, R. Naturally Occurring Quinones. Available online: https://books.google.pl/books?hl=pl&lr=&id=iwFs2rpKOFgC&oi=fnd&pg=PP1&ots=E10gXwxkcL&sig=MxN3Epb0yXu_kfr-mF5weiKnjwg&redir_esc=y#v=onepage&q&f=false.
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crops Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Farmacognosia Da Planta Ao Medicamento | PDF | Biodiversidade | Biologia de Conservação . Available online: https://www.scribd.com/document/377628211/Farmacognosia-Da-Planta-Ao-Medicamento (accessed on 5 October 2023).
- Han, Y.S.; Van Der Heijden, R.; Verpoorte, R. Improved anthraquinone accumulation in cell cultures of Cinchona “Robusta” by feeding of biosynthetic precursors and inhibitors. Biotechnol. Lett. 2002, 24, 705–710. [Google Scholar] [CrossRef]
- Derksen, G.C.H.; Naayer, M.; van Beek, T.A.; Capelle, A.; Haaksman, I.K.; van Doren, H.A.; de Groot, Æ. Chemical and enzymatic hydrolysis of anthraquinone glycosides from madder roots. Phytochem. Anal. 2003, 14, 137–144. [Google Scholar] [CrossRef]
- de Morais, F.A.P.; Balbinot, R.B.; Bakoshi, A.B.K.; Lazarin-Bidoia, D.; da Silva Souza Campanholi, K.; da Silva Junior, R.C.; Gonçalves, R.S.; Ueda-Nakamura, T.; de Oliveira Silva, S.; Caetano, W.; et al. Advanced theranostic nanoplatforms for hypericin delivery in the cancer treatment. J. Photochem. Photobiol. B. 2023, 247, 112782. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, G.; Shi, P. Emodin-induced apoptosis in human breast cancer BCap-37 cells through the mitochondrial signaling pathway. Arch. Pharm. Res. 2008, 31, 742–748. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef]
- Yan, Y.; Su, X.; Liang, Y.; Zhang, J.; Shi, C.; Lu, Y.; Gu, L.; Fu, L. Emodin azide methyl anthraquinone derivative triggers mitochondrial-dependent cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol. Cancer Ther. 2008, 7, 1688–1697. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2015, 33, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mun, G.H.; Choi, B.; Aseh, A.; Mildred, L.; Patel, A.; Zhang, Q.; Price, J.E.; Chang, D.; Robb, G.; et al. Repair and reconstruction of a resected tumor defect using a composite of tissue flap-nanotherapeutic-silk fibroin and chitosan scaffold. Ann. Biomed. Eng. 2011, 39, 2374–2387. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, G.; Shi, P. Effects of emodin on the gene expression profiling of human breast carcinoma cells. Cancer Detect. Prev. 2009, 32, 286–291. [Google Scholar] [CrossRef]
- Zu, C.; Zhang, M.; Xue, H.; Cai, X.; Zhao, L.; He, A.; Qin, G.; Yang, C.; Zheng, X. Emodin induces apoptosis of human breast cancer cells by modulating the expression of apoptosis-related genes. Oncol. Lett. 2015, 10, 2919. [Google Scholar] [CrossRef]
- Li, W.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Emodin induces cytotoxic effect in human breast carcinoma MCF-7 cell through modulating the expression of apoptosis-related genes. Pharm. Biol. 2013, 51, 1175–1181. [Google Scholar] [CrossRef]
- Zhang, L.; Lau, Y.K.; Xi, L.; Hong, R.L.; Kim, D.S.H.L.; Chen, C.F.; Hortobagyi, G.N.; Chang, C.J.; Hung, M.C. Tyrosine kinase inhibitors, emodin and its derivative repress HER-2/neu-induced cellular transformation and metastasis-associated properties. Oncogene 1998, 16, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Tyrosine Kinase Inhibitor Emodin Suppresses Growth of HER-2/neu-overexpressing Breast Cancer Cells in Athymic Mice and Sensitizes These Cells to the Inhibitory Effect of Paclitaxel1 | Clinical Cancer Research | American Association for Cancer Research . Available online: https://aacrjournals.org/clincancerres/article/5/2/343/199283/Tyrosine-Kinase-Inhibitor-Emodin-Suppresses-Growth (accessed on 5 October 2023).
- Shao-Chun, W.; Lisha, Z.; Hortobagyi, G.N.; Mien-Chie, H. Targeting HER2: Recent developments and future directions for breast cancer patients. Semin. Oncol. 2001, 28, 21–29. [Google Scholar] [CrossRef]
- N, U.; N, K.; M, H. Growth suppression of low HER-2/neu-expressing breast cancer cell line MDA-MB-435 by tyrosine kinase inhibitor emodin. Oncol. Rep. 1996, 3, 509–511. [Google Scholar] [CrossRef]
- Li, F.; Song, X.; Zhou, X.; Chen, L.; Zheng, J. Emodin attenuates high lipid-induced liver metastasis through the AKT and ERK pathways in vitro in breast cancer cells and in a mouse xenograft model. Heliyon 2023, 9, e17052. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Sheng, A.; Huang, S.; Tang, Y.; Ma, S.; Hong, G. Emodin Inhibits the Proliferation of MCF-7 Human Breast Cancer Cells Through Activation of Aryl Hydrocarbon Receptor (AhR). Front. Pharmacol. 2020, 11, 622046. [Google Scholar] [CrossRef]
- Sui, J.Q.; Xie, K.P.; Zou, W.; Xie, M.J. Emodin inhibits breast cancer cell proliferation through the ERα-MAPK/Akt-cyclin D1/Bcl-2 signaling pathway. Asian Pac. J. Cancer Prev. 2014, 15, 6247–6251. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Huang, C.Y.; Chen, M.C.; Lee, Y.T.; Yue, C.H.; Wang, H.Y.; Lin, H. Emodin and Aloe-Emodin Suppress Breast Cancer Cell Proliferation through ERα Inhibition. Evid. Based. Complement. Alternat. Med. 2013, 2013, 376123. [Google Scholar] [CrossRef]
- Song, X.; Zhou, X.; Qin, Y.; Yang, J.; Wang, Y.; Sun, Z.; Yu, K.; Zhang, S.; Liu, S. Emodin inhibits epithelial-mesenchymal transition and metastasis of triple negative breast cancer via antagonism of CC-chemokine ligand 5 secreted from adipocytes. Int. J. Mol. Med. 2018, 42, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Zheng, L.S.; Zhang, X.; Chen, L.K.; Singh, S.; Wang, F.; Zhang, J.Y.; Liang, Y.J.; Dai, C.L.; Gu, L.Q.; et al. Blockade of Her2/neu binding to Hsp90 by emodin azide methyl anthraquinone derivative induces proteasomal degradation of Her2/neu. Mol. Pharm. 2011, 8, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Jelassi, B.; Anchelin, M.; Chamouton, J.; Cayuela, M.L.; Clarysse, L.; Li, J.; Goré, J.; Jiang, L.H.; Roger, S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2 × 7 receptors. Carcinogenesis 2013, 34, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Lara, R.; Adinolfi, E.; Harwood, C.A.; Philpott, M.; Barden, J.A.; Di Virgilio, F.; McNulty, S. P2 × 7 in Cancer: From Molecular Mechanisms to Therapeutics. Front. Pharmacol. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Iwanowycz, S.; Wang, J.; Altomare, D.; Hui, Y.; Fan, D. Emodin Bidirectionally Modulates Macrophage Polarization and Epigenetically Regulates Macrophage Memory. J. Biol. Chem. 2016, 291, 11491. [Google Scholar] [CrossRef]
- Iwanowycz, S.; Wang, J.; Hodge, J.; Wang, Y.; Yu, F.; Fan, D. Emodin inhibits breast cancer growth by blocking the tumor-promoting feedforward loop between cancer cells and macrophages. Mol. Cancer Ther. 2016, 15, 1931. [Google Scholar] [CrossRef]
- Jia, X.; Yu, F.; Wang, J.; Iwanowycz, S.; Saaoud, F.; Wang, Y.; Hu, J.; Wang, Q.; Fan, D. Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res. Treat. 2014, 148, 291–302. [Google Scholar] [CrossRef]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef]
- Hsu, H.C.; Liu, L.C.; Wang, H.Y.; Hung, C.M.; Lin, Y.C.; Ho, C.T.; Way, T. Der Stromal Fibroblasts from the Interface Zone of Triple Negative Breast Carcinomas Induced Epithelial-Mesenchymal Transition and its Inhibition by Emodin. PLoS One 2017, 12, e0164661. [Google Scholar] [CrossRef]
- Kwak, H.J.; Park, M.J.; Park, C.M.; Moon, S.I.; Yoo, D.H.; Lee, H.C.; Lee, S.H.; Kim, M.S.; Lee, H.W.; Shin, W.S.; et al. Emodin inhibits vascular endothelial growth factor-A-induced angiogenesis by blocking receptor-2 (KDR/Flk-1) phosphorylation. Int. J. Cancer 2006, 118, 2711–2720. [Google Scholar] [CrossRef]
- Ma, J.; Lu, H.; Wang, S.; Chen, B.; Liu, Z.; Ke, X.; Liu, T.; Fu, J. The anthraquinone derivative Emodin inhibits angiogenesis and metastasis through downregulating Runx2 activity in breast cancer. Int. J. Oncol. 2015, 46, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer metastasis: building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. min; Zhou, J.; Shi, J.; Xie, J. sheng; Huang, L.; Yip, A.Y.S.; Loo, W.T.Y.; Chow, L.W.C.; Ng, E.L.Y. Emodin affects ERCC1 expression in breast cancer cells. J. Transl. Med. 2012, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.; Qin, G.; Yang, C.; Liu, N.; He, A.; Zhang, M.; Zheng, X. Low dose Emodin induces tumor senescence for boosting breast cancer chemotherapy via silencing NRARP. Biochem. Biophys. Res. Commun. 2018, 505, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Christowitz, C.; Davis, T.; Isaacs, A.; Van Niekerk, G.; Hattingh, S.; Engelbrecht, A.M. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhuang, Y.; Wang, P.; Zou, T.; Lan, M.; Li, L.; Liu, F.; Cai, T.; Cai, Y. Polymeric Lipid Hybrid Nanoparticles as a Delivery System Enhance the Antitumor Effect of Emodin in Vitro and in Vivo. J. Pharm. Sci. 2021, 110, 2986–2996. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Upadhyay, P.; Sarker, S.; Basu, A.; Das, S.; Ghosh, A.; Ghosh, S.; Adhikary, A. Combinatorial therapy of Thymoquinone and Emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim. Biophys. Acta - Gen. Subj. 2020, 1864, 129695. [Google Scholar] [CrossRef]
- Fu, M.; Tang, W.; Liu, J.J.; Gong, X.Q.; Kong, L.; Yao, X.M.; Jing, M.; Cai, F.Y.; Li, X.T.; Ju, R.J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2020, 28, 245–258. [Google Scholar] [CrossRef]
- Guo, J.; Li, W.; Shi, H.; Xie, X.; Li, L.; Tang, H.; Wu, M.; Kong, Y.; Yang, L.; Gao, J.; et al. Synergistic effects of curcumin with emodin against the proliferation and invasion of breast cancer cells through upregulation of miR-34a. Mol. Cell. Biochem. 2013, 382, 103–111. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Kothandan, G.; Manoharan, R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim. Biophys. Acta - Mol. Basis Dis. 2020, 1866, 165897. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, S.J.; Choi, S.; Kim, S.H.; Kang, K.S. In Vitro Estrogenic and Breast Cancer Inhibitory Activities of Chemical Constituents Isolated from Rheum undulatum L. Mol. 2018, Vol. 23, Page 1215 2018, 23, 1215. [Google Scholar] [CrossRef] [PubMed]
- Effect of Aloe emodin on invasion and metastasis of high metastatic breast cancer MDA-MB-231 cells . Available online: https://pubmed.ncbi.nlm.nih.gov/24620697/ (accessed on 6 October 2023).
- Majumder, R.; Parida, P.; Paul, S.; Basak, P. In vitro and in silico study of Aloe vera leaf extract against human breast cancer. Nat. Prod. Res. 2020, 34, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Freag, M.S.; Elnaggar, Y.S.R.; Abdelmonsif, D.A.; Abdallah, O.Y. Stealth, biocompatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: in vitro and in vivo studies. Int. J. Nanomedicine 2016, 11, 4799–4818. [Google Scholar] [CrossRef]
- Chen, R.; Wang, S.; Zhang, J.; Chen, M.; Wang, Y. Aloe-emodin loaded solid lipid nanoparticles: formulation design and in vitro anti-cancer study. Drug Deliv. 2015, 22, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Das, C.K.; Banerjee, I.; Chandra Jena, B.; Mandal, A.; Das, P.; Pradhan, A.K.; Das, S.; Basak, P.; Das, S.K.; et al. Screening of the Prime bioactive compounds from Aloe vera as potential anti-proliferative agents targeting DNA. Comput. Biol. Med. 2022, 141, 105052. [Google Scholar] [CrossRef]
- Esmat, A.Y.; El-Gerzawy, S.M.; Rafaat, A. DNA ploidy and S phase fraction of breast and ovarian tumor cells treated with a natural anthracycline analog (aloin). Cancer Biol. Ther. 2005, 4, 115–119. [Google Scholar] [CrossRef]
- Beerman, H.; Kluin, P.M.; Hermans, J.; Van de Velde, C.J.H.; Cornelisse, C.J. Prognostic significance of DNA-ploidy in a series of 690 primary breast cancer patients. Int. J. cancer 1990, 45, 34–39. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Tanner, M.; Rantanen, V.; Bärlund, M.; Borg, Å.; Grénman, S.; Isola, J. Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am. J. Pathol. 2000, 156, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Esmat, A.Y.; Tomasetto, C.; Rio, M.C. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2: topoisomerase IIalpha coamplification. Cancer Biol. Ther. 2006, 5, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Identification of natural products and FDA-approved drugs for targeting cancer stem cell (CSC) propagation. Aging (Albany. NY). 2022, 14, 9466–9483. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, R.; Wang, Y.; Wang, L.; Zhou, T.; Jia, D.; Meng, Z. The anti-breast cancer property of physcion via oxidative stress-mediated mitochondrial apoptosis and immune response. Pharm. Biol. 2021, 59, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Zhao, S.S.; Liu, D.Y.; Wang, Z.; Niu, L.L.; Hou, L.H.; Wang, C.L. ROS-Ca(2+) is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis. Chem. Biol. Interact. 2011, 190, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.J.F.; Low, I.C.C.; Pervaiz, S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 2014, 19, 39–48. [Google Scholar] [CrossRef]
- Kim, B.M.; Chung, H.W. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem. Biophys. Res. Commun. 2007, 363, 745–750. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Chung, H.-J.; Bae, S.Y.; Trung, T.N.; Bae, K.; Lee, S.K. Induction of Cell Cycle Arrest and Apoptosis by Physcion, an Anthraquinone Isolated From Rhubarb (Rhizomes of Rheum tanguticum), in MDA-MB-231 Human Breast Cancer Cells. J. cancer Prev. 2014, 19, 273–278. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhao, Y.; Tian, W.; Zhai, L.; Pang, H.; Kang, J.; Hou, H.; Chen, Y.; Li, D. Rhein Derivative 4F Inhibits the Malignant Phenotype of Breast Cancer by Downregulating Rac1 Protein. Front. Pharmacol. 2020, 11, 754. [Google Scholar] [CrossRef]
- Tian, W.; Li, J.; Su, Z.; Lan, F.; Li, Z.; Liang, D.; Wang, C.; Li, D.; Hou, H. Novel Anthraquinone Compounds Induce Cancer Cell Death through Paraptosis. ACS Med. Chem. Lett. 2019, 10, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Naidu, V.G.M.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules 2020, 25, 2278. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Song, G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways. Toxicol. Appl. Pharmacol. 2018, 360, 201–211. [Google Scholar] [CrossRef]
- Ni, C.H.; Chen, P.Y.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Wu, S.H.; Ip, S.W.; Wu, C.T.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced necrotic-like cell death through an impaired mitochondrial ATP synthesis in Hep3B human liver cancer cells. Arch. Pharm. Res. 2012, 35, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, J.; Gao, Y.; Luo, Y.; Yang, D.; Wang, P. The pharmacological properties of chrysophanol, the recent advances. Biomed. Pharmacother. 2020, 125, 110002. [Google Scholar] [CrossRef]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376–4382. [Google Scholar] [CrossRef]
- de Souza, M.V.F.; Shinobu-Mesquita, C.S.; Meirelles, L.E.F.; Mari, N.L.; César, G.B.; Gonçalves, R.S.; Caetano, W.; Damke, E.; Silva, V.R.S.; Damke, G.M.Z.F.; et al. Effects of hypericin encapsulated on Pluronic F127 photodynamic therapy against triple negative breast cancer. Asian Pac. J. Cancer Prev. 2022, 23, 1741–1751. [Google Scholar] [CrossRef]
- Damke, G.M.Z.F.; Damke, E.; de Souza Bonfim-Mendonça, P.; Ratti, B.A.; de Freitas Meirelles, L.E.; da Silva, V.R.S.; Gonçalves, R.S.; César, G.B.; de Oliveira Silva, S.; Caetano, W.; et al. Selective photodynamic effects on cervical cancer cells provided by P123 Pluronic®-based nanoparticles modulating hypericin delivery. Life Sci. 2020, 255, 117858. [Google Scholar] [CrossRef]
- Diwu, Z.; William Lown, J. Photosensitization with anticancer agents 17. EPR studies of photodynamic action of hypericin: Formation of semiquinone radical and activated oxygen species on illumination. Free Radic. Biol. Med. 1993, 14, 209–215. [Google Scholar] [CrossRef]
- Solár, P.; Ferenc, P.; Koval, J.; Mikeš, J.; Solárova, Z.; Hrčková, G.; Fulton, B.L.; Fedoročko, P. Photoactivated hypericin induces downregulation of HER2 gene expression. Radiat. Res. 2011, 175, 51–56. [Google Scholar] [CrossRef]
- Kimáková, P.; Solár, P.; Fecková, B.; Sačková, V.; Solárová, Z.; Ilkovičová, L.; Kello, M. Photoactivated hypericin increases the expression of SOD-2 and makes MCF-7 cells resistant to photodynamic therapy. Biomed. Pharmacother. 2017, 85, 749–755. [Google Scholar] [CrossRef]
- de Andrade, G.P.; Manieri, T.M.; Nunes, E.A.; Viana, G.M.; Cerchiaro, G.; Ribeiro, A.O. Comparative in vitro study of photodynamic activity of hypericin and hypericinates in MCF-7 cells. J. Photochem. Photobiol. B Biol. 2017, 175, 89–98. [Google Scholar] [CrossRef]
- Freitas, V.M.; do Amaral, J.B.; Silva, T.A.; Santos, E.S.; Mangone, F.R.; Pinheiro, J. de J.; Jaeger, R.G.; Nagai, M.A.; Machado-Santelli, G.M. Decreased expression of ADAMTS-1 in human breast tumors stimulates migration and invasion. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- The effects of hypericin on ADAMTS and p53 gene expression in MCF-7 breast cancer cells . Available online: https://pubmed.ncbi.nlm.nih.gov/25261644/ (accessed on 6 October 2023).
- Stojanovic, G.; Dordevic, A.; Smelcerovic, A. Do other Hypericum species have medical potential as St. John’s wort (Hypericum perforatum)? Curr. Med. Chem. 2013, 20, 2273–2295. [Google Scholar] [CrossRef] [PubMed]
- Shemanko, C.S.; Cong, Y.; Forsyth, A. What Is Breast in the Bone? Int. J. Mol. Sci. 2016, 17, 1764. [Google Scholar] [CrossRef]
- Ouyang, Z.; Guo, X.; Chen, X.; Liu, B.; Zhang, Q.; Yin, Z.; Zhai, Z.; Qu, X.; Liu, X.; Peng, D.; et al. Hypericin targets osteoclast and prevents breast cancer-induced bone metastasis via NFATc1 signaling pathway. Oncotarget 2018, 9, 1868. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Zhai, Z.; Li, H.; Liu, X.; Qu, X.; Li, X.; Fan, Q.; Tang, T.; Qin, A.; Dai, K. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem. Pharmacol. 2014, 90, 276–287. [Google Scholar] [CrossRef]
- Ma, Y.S.; Weng, S.W.; Lin, M.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S.; Lai, K.C.; Lin, J.P.; Tang, N.Y.; Lin, J.G.; et al. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol. 2012, 50, 1271–1278. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y. ping; Huang, X. zhi; He, M.; Chen, Y. ying; Shi, G. ying; Li, H.; Yi, J.; Wang, J. Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem. Pharmacol. 2010, 79, 1134–1140. [Google Scholar] [CrossRef]
- Chen, H.; Wei, W.; Guo, Y.; Liu, A.; Tong, H.; Wang, Z.; Tan, W.; Liu, J.; Lin, S. Enhanced effect of gemcitabine by emodin against pancreatic cancer in vivo via cytochrome C-regulated apoptosis. Oncol. Rep. 2011, 25, 1253–1261. [Google Scholar] [CrossRef]
- Lin, S.Z.; Wei, W.T.; Chen, H.; Chen, K.J.; Tong, H.F.; Wang, Z.H.; Ni, Z.L.; Liu, H. Bin; Guo, H.C.; Liu, D.L. Antitumor Activity of Emodin against Pancreatic Cancer Depends on Its Dual Role: Promotion of Apoptosis and Suppression of Angiogenesis. PLoS One 2012, 7, e42146. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, H.; Wei, W.; Ye, S.; Liao, W.; Gong, J.; Jiang, Z.; Wang, L.; Lin, S. Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer. Oncol. Rep. 2011, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Chun-Guang, W.; Jun-Qing, Y.; Bei-Zhong, L.; Dan-Ting, J.; Chong, W.; Liang, Z.; Dan, Z.; Yan, W. Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur. J. Pharmacol. 2010, 627, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, X.; Zhou, X.; Chen, L.; Zheng, J. Emodin attenuates high lipid-induced liver metastasis through the AKT and ERK pathways in vitro in breast cancer cells and in a mouse xenograft model. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, J.W.; Ree, J.; Lim, J.S.; Lee, J.; Kim, J. Il; Thapa, S.B.; Sohng, J.K.; Park, Y. Il Aloe emodin 3-O-glucoside inhibits cell growth and migration and induces apoptosis of non-small-cell lung cancer cells via suppressing MEK/ERK and Akt signalling pathways. Life Sci. 2022, 300, 120495. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zheng, Z.; Chen, S.; Fang, L.; Feng, R.; Zhang, L.; Tang, Q.; Liu, X. Sensitization of Non-Small Cell Lung Cancer Cells to Gefitinib and Reversal of Epithelial–Mesenchymal Transition by Aloe-Emodin Via PI3K/Akt/TWIS1 Signal Blockage. Front. Oncol. 2022, 12, 908031. [Google Scholar] [CrossRef]
- Deng, M.; Xue, Y.; Xu, L.; Wang, Q.; Wei, J.; Ke, X.; Wang, J.; Chen, X. Chrysophanol exhibits inhibitory activities against colorectal cancer by targeting decorin. Cell Biochem. Funct. 2020, 38, 47–57. [Google Scholar] [CrossRef]
- Lu, L.; Li, K.; Mao, Y.H.; Qu, H.; Yao, B.; Zhong, W.W.; Ma, B.; Wang, Z.Y. Gold-chrysophanol nanoparticles suppress human prostate cancer progression through inactivating AKT expression and inducing apoptosis and ROS generation in vitro and in vivo. Int. J. Oncol. 2017, 51, 1089–1103. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Wei, L.; Zhang, J.; Li, X.; Cui, W.; Zhang, S. Physcion-8-O-β-d-glucoside interferes with the nuclear factor-κB pathway and downregulates P-glycoprotein expression to reduce paclitaxel resistance in ovarian cancer cells. J. Pharm. Pharmacol. 2021, 73, 545–552. [Google Scholar] [CrossRef]
- Pan, X. ping; Wang, C.; Li, Y.; Huang, L. hua Physcion induces apoptosis through triggering endoplasmic reticulum stress in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 99, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.J.; Yang, Z.; Zhang, X.L.; Liu, Z.F.; Fan, J.; Zhang, H.Y. Physcion, a naturally occurring anthraquinone derivative, induces apoptosis and autophagy in human nasopharyngeal carcinoma. Acta Pharmacol. Sin. 2016 3712 2016, 37, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Liu, H.N.; Li, X.X.; Wang, G.Y. Antitumor activity of physcion 8-o-β-glucopyranoside against cervical cancer by induction of apoptosis. Trop. J. Pharm. Res. 2016, 15, 1145–1150. [Google Scholar] [CrossRef]
- Yang, L.; Lin, S.; Kang, Y.; Xiang, Y.; Xu, L.; Li, J.; Dai, X.; Liang, G.; Huang, X.; Zhao, C. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef]
- Shen, Z.; Shen, Z.; Li, J.; Qin, L. Rhein Augments Antiproliferative Effects of Atezolizumab Based on Breast Cancer (4T1) Regression. Planta Med. 2019, 85, 1143–1149. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Shao, T.; Song, P.; Kong, Q.; Hua, H.; Luo, T.; Jiang, Y. The natural agent rhein induces β-catenin degradation and tumour growth arrest. J. Cell. Mol. Med. 2018, 22, 589–599. [Google Scholar] [CrossRef]
- Li, X.X.; Dong, Y.; Wang, W.; Wang, H.L.; Chen, Y.Y.; Shi, G.Y.; Yi, J.; Wang, J. Emodin as an effective agent in targeting cancer stem-like side population cells of gallbladder carcinoma. Stem Cells Dev. 2013, 22, 554–566. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Q. Inhibiting 6-phosphogluconate dehydrogenase reverses doxorubicin resistance in anaplastic thyroid cancer via inhibiting NADPH-dependent metabolic reprogramming. Biochem. Biophys. Res. Commun. 2018, 498, 912–917. [Google Scholar] [CrossRef]
- Li, B.; Zhao, X.; Zhang, L.; Cheng, W. Emodin Interferes With AKT1-Mediated DNA Damage and Decreases Resistance of Breast Cancer Cells to Doxorubicin. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef]
- Guo, H.; Xiang, Z.; Zhang, Y.; Sun, D. Inhibiting 6-phosphogluconate dehydrogenase enhances chemotherapy efficacy in cervical cancer via AMPK-independent inhibition of RhoA and Rac1. Clin. Transl. Oncol. 2019, 21, 404–411. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Bao, L.; Yin, T.; Lei, D.; Yu, J.; Tong, X. 6PGD inhibition sensitizes hepatocellular carcinoma to chemotherapy via AMPK activation and metabolic reprogramming. Biomed. Pharmacother. 2019, 111, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Fenig, E.; Nordenberg, J.; Beery, E.; Sulkes, J.; Wasserman, L. Combined effect of aloe-emodin and chemotherapeutic agents on the proliferation of an adherent variant cell line of Merkel cell carcinoma. Oncol. Rep. 2004, 11, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Savenkova, D. V.; Havrysh, V.; Skripova, V.S.; Ionova, N.E.; Nurgalieva, A.K.; Minigulova, L.F.; Bogdanov, M. V.; Kiyamova, R.G. Physcion Enhances Sensitivity of Pancreatic Adenocarcinoma and Lung Carcinoma Cell Lines to Cisplatin. Bionanoscience 2020, 10, 549–553. [Google Scholar] [CrossRef]
| Botanical family | Gender name | Selected species |
|---|---|---|
| Rhamnaceae |
Rhamnus, Ventilago |
Rhamnus purshiana, Rhamnus frangula, Rhamnus catartica, Ventilago maderaspatana |
| Fabaceae | Cassia | Cassia angustifolia, Cassia senna, Cassia obtusifolia |
| Polygonaceae |
Rheum, Rumex |
Rheum palmatum, Rheum officinale, Rheum rabarbarum Rumex conglumeratus, Rumex pulcher, Rumex alpinus, Rumex maritimus, Rumex obtusifolius |
| Liliaceae |
Aloe, Bulbine |
Aloe ferox, Aloe vera, Bulbine capitate |
| Guttiferae | Hypericum | Hypericum perforatum |
| Rubiaceae |
Morinda, Rubia, Galium, Heterophyllea |
Morinda citrifolia, Morinda officinalis Rubia tinctorum, Galium sinaicum, Galium verum, Heterophyllea pustulata |
| Compound | Cancer type | Model | Dosing | Effect | References |
|---|---|---|---|---|---|
| Emodin | colon cancer LS1034 cells |
6-8-weeks male BALB/c nu/nu athymic mice | 40 mg/kg i.p., once every three days for 39 days | G0/G1 phase cell cycle arrest + apoptosis - tumour size (weight and volume) |
[94] |
| Emodin | multidrug resistant gallbladder carcinoma SGC996 cells |
6-weeks BALB/c nu/nu mice | 50 mg/kg i.p. for 15 days | - tumor size + apoptosis - overexpression of MRP1 Sensitizing cisplatin, oxaliplatin and carboplatin |
[95] |
| Emodin | gallbladder cancer SGC-996 GBC-SD cells |
6-weeks BALB/c nu/nu mice | emodin (50 mg/kg i.p.), cisplatin (2 mg/kg i.p.) and emodin/cisplatin every 2 days for 8 days | sensitising effect of emodin to cisplatin - mRNA level of ABCG2 |
[112] |
| Emodin | pancreatic cancer SW1990 cells | 4-6 weeks female BALB/c mice | 40 mg/kg emodin, 126 mg/kg gemcitabine or 40 mg/kg emodin+80 mg/kg gemcitabine every 6 days for 37 days | - tumour size (weight and volume) + tumor integrity + apoptosis -Bcl-Bax ratio + caspase-3 and CytC no effect on VEGFR - MMPs and VEGFR2 receptor expression |
[46] |
| Emodin | pancreatic cancer | 4-6 weeks BALB/c nu/nu mice | 20 and 40 mg/kg p.o. for 8 weeks | - tumour size (weight and volume) - peritoneal dissemination + apoptosis + TUNEL-positive cells - Ki-67, survivin, MMP-9 expression |
[98] |
| Emodin | Blood system cancer K562 cells |
4–6 weeks male BALB/c nude mice | 25, 50, and 100 mg/kg i.p. daily for 12 days | + apoptosis No toxicity - Ki-67 |
[99] |
| Emodin | breast cancer MDA-MB-231 cells |
nude BALB/c nu/nu mice fed a high-fat diet | 400 mg/kg, p. o., q. d. for 4 weeks | Regulation of body weight - cholesterol and fatty acids synthesis - fatty acids oxidation - Fasn, Sed1, Gpat1 genes |
[32] |
| Aloe-emodin 3-O-glucoside | non-small-cell lung cancer A549 cells |
5-6 weeks female BALB/c nude mice | 13 and 26 mg/kg/day for 14 days i.p. | - tumour size (weight and volume) - cell nuclei size + the apoptic DNA fragmentation + caspase 9 and cleaved PARP |
[101] |
| Aloe-emodin | non-small cell lung cancer PC9-GR cells | 4-5 weeks female BALB/c nude mice | 30 mg/kg aloe-emodin, 30 mg/kg gefitinib and their combination | Sensitising gefitinib + E-cadherin -vimetin, Slug and Twist1 proteins |
[102] |
| Chrysophanol | prostate cancer LNCap cells |
4-6 weeks female BALB/c athymic nude mice (nu/nu) | 25 and 50 mg/kg i.p. a day in the form of nanoparticlesfor 28 days | + TUNEL positive cells - tumour size (weight and volume) |
[103] |
| Physcion 8-O-β-D-glucoside | ovarian cancer SK-OV-3/PTX cells |
4-6 weeks male nude mice BALB/c-n | 60 mg/kg, orally in combination with paclitaxel (20 mg/kg, i.p.), twice a week for 21 days | In combination: - P-gp positive tumor cells - tumour size (weight and volume) |
[105] |
| Physcion | breast cancer | 8 weeks male BALB/c nude mice | 30 mg/kg/day i.p. for 2 weeks | + Bax and cleaved caspase-3, - Bcl-xL, Bcl-2, Nrf2, HO-1, SOD-1 and SOD-2 proteins expression in tumor tissue Immunomodulatory action |
[106] |
| Physcion | hepatocellular carcinoma Huh7 cells |
4-6 weeks male BALB/c nude mice | 20 and 40 mg/kg/day i.p. | + TUNEL positive cells - tumour size (weight and volume) + cleaved caspase-3, + caspase-12, overexpression of CHOP - Ki-67 |
[106] |
| Rhein | pancreatic cancer | 5 weeks female athymic BALB/c mice | 60 mg/kg of rhein i.p., 10 mg/kg i.p. of erlotinib | With erlotinib + apoptosis - EGFR and STAT3 pathways - Bcl-2 level + Bax level |
[109] |
| Rhein | breast cancer 4T1 cells |
6–8 weeks female BALB/c mice | 10 mg/kg i.p. rhein every 3 days for a total of three times alone or with 10 mg/kg i.p. atezolizumab once every 2 days for a total of three times | With and without atezolizumab - tumour size (weight and volume) + CD8+ T cells production in the tumor and in the spleen + serum levels of IL-6 and TNF-α |
[110] |
| Rhein | hepatocellular carcinoma HepG2 cells |
5 weeks female nude mice BALB/c-nu | 100 mg/kg/0.2 ml, once a day, i.p. for 3 weeks | - the expression of β-catenin | [111] |
| Type of cancer | Anthraquinone | Chemotherapeutic | Effect | Ref. |
|---|---|---|---|---|
| Anaplastic thyroid cancer | Physcion 20 µM |
Doxorubicin 0.5 µM |
The inhibition of 6PGD by physcion in combination with doxorubicin sensitizes ATC cells to the treatment | [113] |
| Breast cancer | Rhein 10 mg/kg i.p. every 3 days, 3 times |
Atezolizumab 10 mg/kg i.p. every 2 days, 3 times |
- tumour size + CD8+ T cells production in the tumor and in the spleen + serum levels of IL-6 and TNF-α |
[110] |
| Breast cancer | Emodin 110 µM |
Doxorubicin 5µM |
Sensitisation to doxorubicin + apoptosis + DNA damage in cancer cells + γH2Ax expression - drug resistance to doxorubicin (PI3K-AKT pathway) |
[114] |
| Breast cancer | Emodin 20μM |
5-Fluorouracyl 40 μM |
Sensitisation to 5-fluorouracyl +apoptosis + ROS generation +cyclin dependant kinase inhibitors expression - E2F1expression - notch-regulated ankyrin repeat protein expression |
[49] |
| Breast cancer | Physcion 5 mg/kg, i.p. |
Paclitaxel 5 mg/kg, i.p. |
Inhibition of 6PGD by physcion that results in the sensitization of breast cancer cells to paclitaxelpaclitaxel | [113] |
| Cervical cancer | Physcion 10 mg/kg, i.p. |
Paclitaxel 1 mg/kg, i.p. |
Physcion sensitizes paclitaxel-treated cervical cancer by the inhibition of 6PGD | [115] |
| Gallbladder cancer | Emodin 50 mg/kg i.p., every 2 days for 8 days |
Cisplatin (2 mg/kg i.p. every 2 days for 8 days |
sensitising effect of emodin to cisplatin - mRNA level of ABCG2 no toxicity |
[112] |
| Gallbladder cancer | Emodin 50 µM |
cisplatin 2 µM |
Downregulation of MRP1 protein expression Glutathione depletion Enhanced chemosensitivity + apoptosis |
[95] |
| Hepatocellular carcinoma | Physcion 20 and 40 µM |
Cisplatin 100 nM Paclitaxel 500 nM Doxorubicin 10 nM |
Inhibited growth, induced apoptosis and annexin V level in the combination of physcion with other chemotherapeutics | [116] |
| Merkel cell carcinoma | Aloe-emodin From 5 µM |
Abiplatin > 0.25 µg/mL Adriablastin > 0.025 µg/mL 5-Fluorouracyl > 0.1 µg/mL |
Additive effect with abiplatin, adriablastin, 5-fluorouracyl, - cell proliferation No toxicity |
[117] |
| Non-small cell lung cancer | Aloe-emodin 30 mg/kg i.p. |
Gefitinib 30 mg/kg i.p. |
Sensitising gefitinib + E-adherin - vimetin, Slug and Twist1 proteins |
[102] |
| Lung cancer | Physcion 5-15 µM |
Cisplatin 0-128 µM |
+ ROS level Decreased IC50 of cisplatin by 1.6-2.2 times |
[118] |
| Ovarian cancer | Physcion 8-O-β-D-glucoside 60 mg/kg, orally twice a week for 21 days |
Paclitaxel 20 mg/kg, i.p., twice a week for 21 days |
In combination: - P-gp positive tumor cells - tumour size (weight and volume) |
[105] |
| Pancreatic cancer | Rhein 60 mg/kg i.p. |
Erlotinib 10 mg/kg i.p. |
+ apoptosis - EGFR and STAT3 pathways - Bcl-2 level + Bax level |
[109] |
| Pancreatic cancer | Emodin 40 mg/kg emodin every 6 days for 37 days |
Gemcitabine 80 mg/kg every 6 days for 37 days |
- tumour size (weight and volume) + tumor integrity + apoptosis no toxicity -Bcl/Bax ratio + caspase-3 and CytC expression no effect on VEGFR - MMPs and VEGFR2 receptor expression |
[96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





