Introduction
Syncephalastrum species belong to the
Mucorales order [
1]. These species are mostly found in the environment, in tropical and subtropical areas in both the air and soil [
2,
3,
4]. These are generally seen as clinical contaminants, with low pathogenicity and are rarely known to cause human diseases [
5,
6]. However, in recent years, case reports of human infections due to
Syncephalastrum genus have increased significantly, especially in immunocompromised hosts with diabetes [
7,
8], chronic hepatorenal disease [
9], corneal infections, or who have been the recipients of organ transplantations [
4,
10]. These human infections are usually related to the skin, nails, lungs, and central nervous system [
11], and can have fatal outcomes, resulting in a highly invasive diseases [
12,
13]. They can also cause chronic and acute infections in immunocompetent hosts [
14,
15].
Mucormycosis, which is a rare opportunistic infection caused by mucorales fungi (
Lichtheimia (40%),
Rhizopus (30%),
Syncephalastrum (20%) and
Rhizomucor (10%)) [
16], has become the third most common and fatal fungal infection after candidiasis and aspergillosis [
10,
17]. The most common species in the
Syncephalastrum genus is
S. racemosum [
18,
19,
20]. The first report of a human infection caused by
Syncephalastrum sp. was a cutaneous infection in an immunocompromised patient in India [
20]. According to the Index Fungorum (
www.indexfungorum.org), the
Syncephalastrum genus is composed of two species:
S. racemosum and
S. monosporum (composed of three varieties,
S. monosporum var.
monosporum,
cristatum and
pluriproliferum). Recently, a third species,
S. contaminatum was described [
21].
An accurate species morphological differentiation is considerably difficult by usual mycological techniques in clinical laboratories. Different
Mucorales genera, such as
Rhizomucor,
Lichtheimia and
Mucor spp, have a laborious phenotypic identification and characterisation due to their similar morphology. Moreover, correct identification can only be achieved based on specific fungal structures. For example,
Rhizopus spp. identification depends on the presence of rhizoids, while the lack of rhizoids is the only way to distinguish
Mucor spp. from other
Mucorales fungi. In practice, however, adequate fungal identification based on morphological criteria is hampered by a number of exceptions. Among the
Mucorales, the
Cunninghamella and
Syncephalastrum genera can be easily recognized.
Syncephalastraceae fungi are typified by the production of cylindrical merosporangia on the surface of fertile vesicles [
22].
The high inter- and intra-species phylogenetic diversity of the
Mucorales [
15,
23], is a real challenge for species identification and taxon delimitation. Numerous studies based on antifungal susceptibility tests and, more recently, on the use of molecular taxonomic methods, in particular sequencing of the Internal Transcript Spaces (ITS) 1 and 2 and the D1/D2 domains of the large subunit (LSU) of the rRNA gene, displayed more efficiency for the identification of
Mucorales fungi than morphology-based identification [
22,
24]. The reliable identification of these rare infection agents is the key to further strengthening our understanding of species associated with epidemiology, pathogenicity, and outcome. This study aimed to analyse the phylogenetic status and describe the phenotypic characteristics of two new species of
Syncephalastrum genus, relying on multilocus sequence analysis and chemical and physiological characterisation.
Methods
Fungal strains
Two novel Syncephalastrum strains were isolated from clinical samples from distinct patients at the University Hospital Mycology laboratory in Marseille, France. Syncephalastrum massiliense PMMF0073 was isolated from a sputum sample from a 53-year-old patient diagnosed with HIV in 1988 and with syphilis in 2015. This patient had recently fractured and dislocated his elbow and had a radial head prosthesis. He had suffered from headaches, intermittent fever, and generalized rashes with scratching lesions for a few months. Syncephalastrum timoneanum PMMF0107 was isolated from the nails of a 38-year-old patient with a history of bronchiectasis, with no exacerbation of the disease.
MALDI-TOF MS identification
The strains were inoculated on Petri dishes with Sabouraud Dextrose Agar (SDA) supplemented with Gentamycin and Chloramphenicol (GC) at 25°C between four to seven days. After growth, we proceeded to identification using Matrix-assisted Laser Desorption/ Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS), following the protein extraction protocol described by Cassagne et al. (2016) [
25]. The Microflex LT
TM instrument and the MALDI Biotyper
TM system (Bruker Daltonics GmbH, Bremen, Germany), and both the manufacturers’ databases as well as an in house reference spectra database were used, as described in Normand et al. (2017) [
26]. Moreover, MALDI-TOF MS spectra of the two isolates were collected, in addition to other spectra of reference strains from DSMZ and CBS collections;
S. racemosum DSM 859,
S. monosporum Var.
monosporum CBS 567.91,
S. monosporum Var.
cristatum CBS 568.91 and
S. monosporum Var.
pluriproliferum CBS 569.91. All the spectra were used to construct a dendrogram based on protein expression intensity with the MALDI-TOF Biotyper Compass Explorer (Bruker Daltonics).
DNA amplification and sequencing
Four genes were targeted, the Internal Transcribed Spacers 1 and 2 (ITS1/ITS2) in the rRNA small-subunit (SSU), a fragment of the β-tubulin gene (TUB2), a fragment of the Translation Elongation Factor 1-alpha gene (TEF-1-α), and the D1/D2 domains of the rRNA large-subunit (LSU) (
Table 1).
For each gene, the PCR Mix was prepared as followed: 5 μl of DNA extract was added to 20 μl of Mix (12.5 μl ATG (Ampli Taq Gold
TM 360 Master Mix, Applied Biosystems
TM)/ 6 μl sterile water DNase/RNase free/ 0.75 μl Forward/Reverse primer) for achieving to a total volume of 25 μl per well. Particularly for the ITS gene, in order to ensure the entire sequence length, three PCR mixes were prepared for each sample with the ITS1/2, ITS3/4, and ITS1/4 amplifying the ITS1, ITS2, and ITS1-5.8S-ITS2 regions, respectively. The PCR programme for all fungal gene amplifications was constituted by an initial denaturation step at 95 °C for 15 minutes, followed by 39 cycles of 95 °C for one minute denaturation, 56 °C for 30 seconds annealing, 72 °C for one minute extension step, and a final extension at 72 °C for five minutes. PCR amplicons were revealed on a 2% agarose gel with the addition of Sybr SafeTM DNA gel stain (Invitrogen). The gel was visualised using the Safe Imager 2.0 Blue-Light Transilluminator
TM (Invitrogen). 4 μl of the purified DNA was added to the BigDye
TM mix (terminator cycle sequencing kit (Applied Biosystems)), (1 μl BD/ 1.5 μl TP/ 3 μl sterile water DNase/RNase free/ 0.5 μl Forward/Reverse primer) for achieving to a total volume of 10 μl per well. The sequencing reactions for all genomic regions, consisting of 96 °C for one minute, followed by 25 cycles of 96 °C for 10 seconds, 50 °C for five seconds, 60 °C for three minutes, were processed on a 3500 Genetic Analyzer
TM (Applied Biosystems, Inc.). The sequences obtained were assembled and corrected using ChromasPro 2.0. All sequences were deposited in GenBank and the accession numbers are presented in
Table 2.
Phylogenetic analysis
In addition to the sequences of the six strains, we added eight other reference strain sequences obtained from the GenBank database (Accession numbers are presented in
Table 2). Two phylogenetic trees were constructed using the Maximum Parsimony (MP) method, with the MEGA (Molecular Evolutionary Genetics Analysis) software version 11 [
31] by using the default settings, and 1,000 bootstrap replications to assess branch robustness. The first tree was based on the concatenated ITS and D1/D2 sequences of all the strains. The second tree was only based on the concatenated ITS, TUB2, TEF-1-α, and D1/D2 sequences of the six following strains:
Syncephalastrum massiliense PMMF0073,
Syncephalastrum timoneanum PMMF0107,
S. racemosum DSM 859,
S. monosporum var. monosporum CBS 567.91,
S. monosporum var. cristatum CBS 568.91 and
S. monosporum var. pluriproliferum CBS 569.91.
Microsporum canis CBS 496.86 was used as an outgroup. A Bayesian phylogenetic inference was also achieved. Two other multi locus phylogenetic trees were constructed with MrBayes software (3.2.7a) and Figtree (V.1.4.4).
Macroscopic characterisation
To study their growth temperature profiles and macroscopic characters such as time of growth, colony morphology, surface and reverse colours, the six strains were cultivated on SDA GC plates for seven days. They were then subcultured on other SDA GC plates, which were incubated at different temperatures: 4 °C, 25 °C, 30 °C, 37 °C, 40 °C, and 45 °C; and on a dehydrated medium (peptone: 5 g/l; glucose: 10 g/l; potassium dihydrogen phosphate: 1 g/l; magnesium sulfate: 0.5 g/l; dichloran: 0.002 g/l; chloramphenicol: 50mg/l; agar: 15g/l; pH: 5.6 ± 0.2) at 30 °C.
Microscopic characterisation
To compare the microscopic features of the different fungal structures (hyphae, spores, and vesicles), fresh cultures of the six strains on SDA GC were first of all examined by optical microscopy. Slides were prepared by gently dabbing the surface of the fungal colony with adhesive tape. The tape was then mounted with one drop of lactophenol cotton blue between the slide and the slip cover. Photographs were taken with DM 2500 (Leica Camera SARL, Paris, France).
Scanning Electron Microscopy (SEM) was performed with the TM4000 Plus (Hitachi High-Technologies, Tokyo, Japan) microscope using the 15KeV lens mode 4 with a Back-Scattered Electron detector. A fungal colony sample was cut from the petri dish and placed on a microscopy slide. A volume of 400 μl of 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer was poured over the fungal cut for fixation and stored at 30 °C until completely dry. Standardised fungal structures (hyphae, vesicle, sporangiola, merosporangium and the number of sporangiospores within the merosporangial sack) were measured using a specific tool for distance measurement included in the TM4000 Plus microscope. The results are represented in a Principal Component Analysis (PCA) computed with the XLSTAT (Addinsoft, Paris, France) software.
Physiological analysis
1. EDX (Energy-Dispersive X-ray Spectroscopy)
Fresh colonies of the six strains were fixed for at least one hour with glutaraldehyde 2.5% in 0.1M sodium cacodylate buffer. Cytospin was performed using a volume of 200 μl from the fixed solution, followed by a centrifugation at 800 rpm for eight minutes. EDX was carried out with an INCA X-Stream-2 detector (Oxford Instruments) linked to the TM4000 Plus SEM and AztecOne software (Oxford instruments). The slide chemical mapping was performed blindly, and all chemical elements were taken into account. Weight and atomic percentages were subjected to a PCA computed with the XLSTAT (Addinsoft) software.
2. BiologTM phenotypic analysis
The phenotypic analysis was achieved using Biolog
TM advanced phenotypic technology as previously used for yeasts characterisation by Kabtani et al. (2022) [
32]. This system aims to characterise microorganisms by using a patented Redox tetrazolium dye that changes colour in response to cellular respiration in 96 micro well plates that confers a metabolic fingerprint. We used the FF (Filamentous Fungi) MicroPlates (Gen III), for carbon sources utilisation. The carbon sources were selected for their high discrimination between fungal phenotype profiles [
33]. All wells contained the substrate and the dye, with the exception of the control well that only contained the dye. The strains were first cultivated on Malt Extract Agar (MEA) 2% medium, prepared with 20 g/l malt extract and 18 g/l agar in distilled water [
34]. The fungal incubation time depended on its specific growth rate. The
Syncephalastrum is a fast-growing genus that reaches its maximal growth after five to seven days. After colonies had developed and the hyphae colour had turned from white to dark brown, the fungal suspension was prepared in the FF inoculating fluid (Biolog part number 72106) by swabbing the surface of the colony. Transmittance levels were adjusted between 75% and 80% using a Biolog
TM Turbidimeter [
33]. The assay was performed in triplicate, 100 μl of the suspension were poured into each well of the FF MicroPlates (Biolog part number 1006), that were incubated at 26 °C for seven days, and read using the Biolog MicroStation™ Reader. The results were represented as a heat map, performed using the XLSTAT
TM (Addinsoft) software.
Antifungal Susceptibility Testing (AFST)
We determined the in vitro activity of ten antifungal drugs, namely amphotericin B, voriconazole, posaconazole, itraconazole, isavuconazole, fluconazole, micafungin, anidulafungin, flucytosine, and caspofungin, against the two clinical isolates and the four type strains of
Syncephalastrum genus. The minimal inhibitory concentration of each antifungal was determined using E-test
TM (bioMérieux, Craponne, France) concentration gradient agar diffusion assay, as described in Kondori et al. (2011) [
34].
Results
MALDI-TOF MS identification
The MALDI-TOF MS identification of the new isolates,
Syncephalastrum massiliense PMMF0073 and
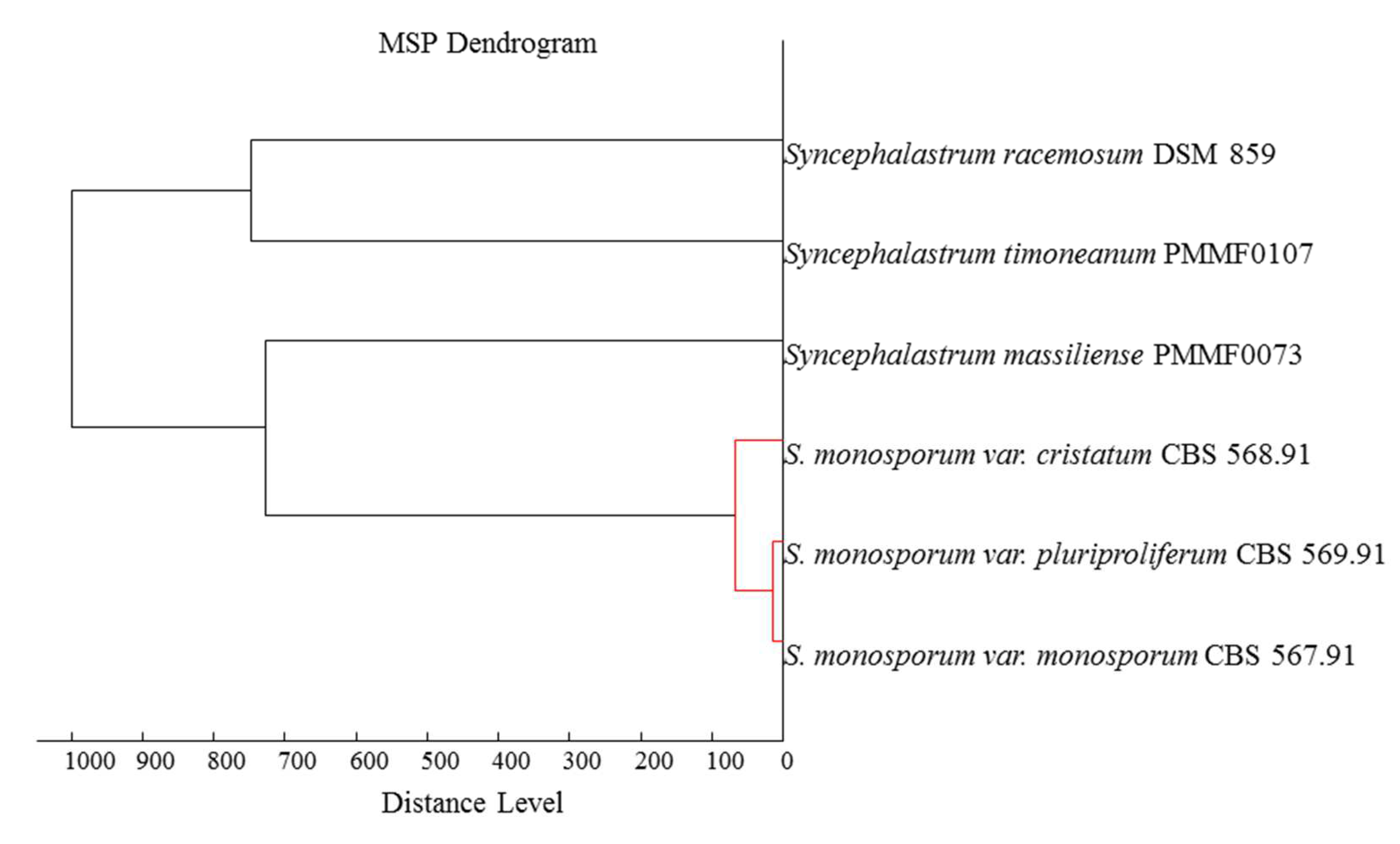
Syncephalastrum timoneanum PMMF0107, did not match with any spectrum present in our laboratory database. The strains spectra did, however, reveal pertinent information about protein expression profiles that was interesting for strain differentiation. The dendrogram (
Figure 1) revealed the similarity of each isolate with a distinct
Syncephalastrum species.
Syncephalastrum timoneanum PMMF0107 clustered with
S. racemosum DSM 859 and
Syncephalastrum massiliense PMMF0073 clustered with the
S. monosporum clade.
DNA sequencing and phylogenetic analysis
The ITS region is recognised as the most precise and distinct marker in
Mucorales (Ramesh et al. 2010). However, the two isolate sequences (accession numbers provided in
Table 2) queried using the search tool (BLAST/NCBI) (
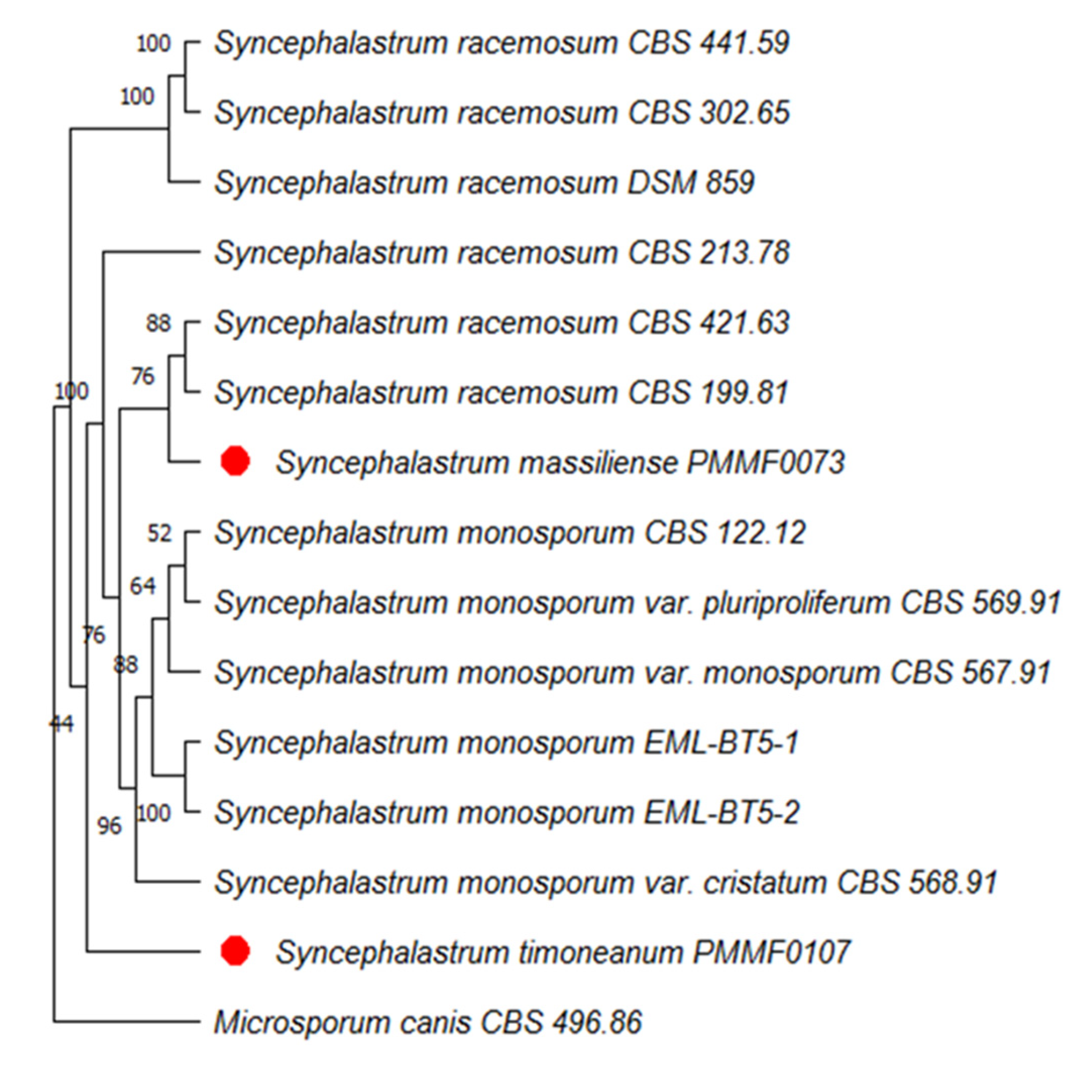
http://blast.ncbi.nlm.nih.gov/blast) against the NCBI nucleotide database showed less than 98% identity with available nucleotide sequences, which was below the usual species identification threshold. Four dendrograms were built. The first were based on the concatenation of the ITS and D1/D2 sequences of 14 strains. The second were based on the concatenation of four loci (ITS, TEF1, TUB2 and D1/D2) of six strains. In the first trees (
Figure 2 and
Figure 4), each new isolates clustered in a distinct clade.
Syncephalastrum massiliense PMMF0073 appeared closely related to
S. racemosum, while
Syncephalastrum timoneanum PMMF0107 appeared relatively distant from both
S. racemosum and
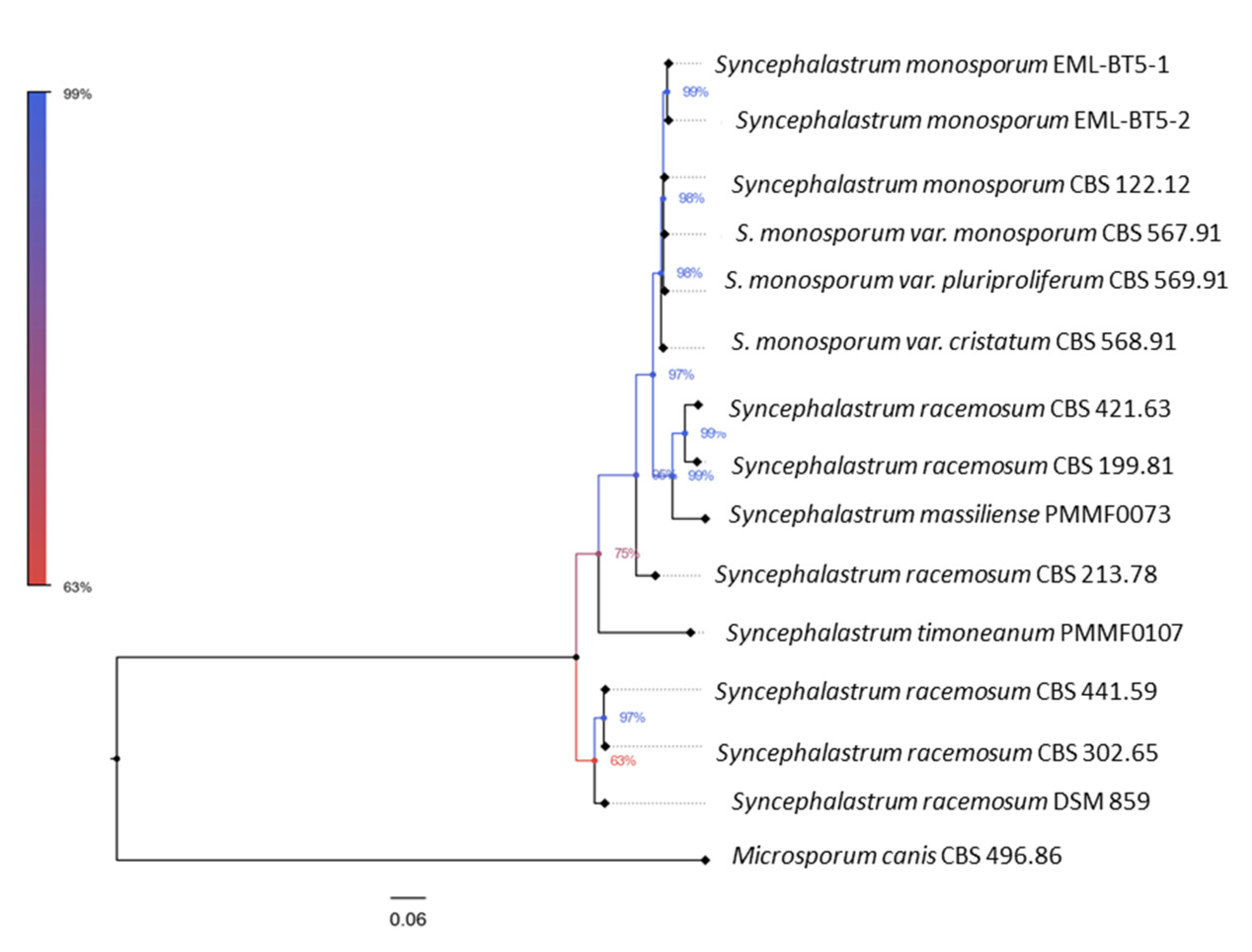
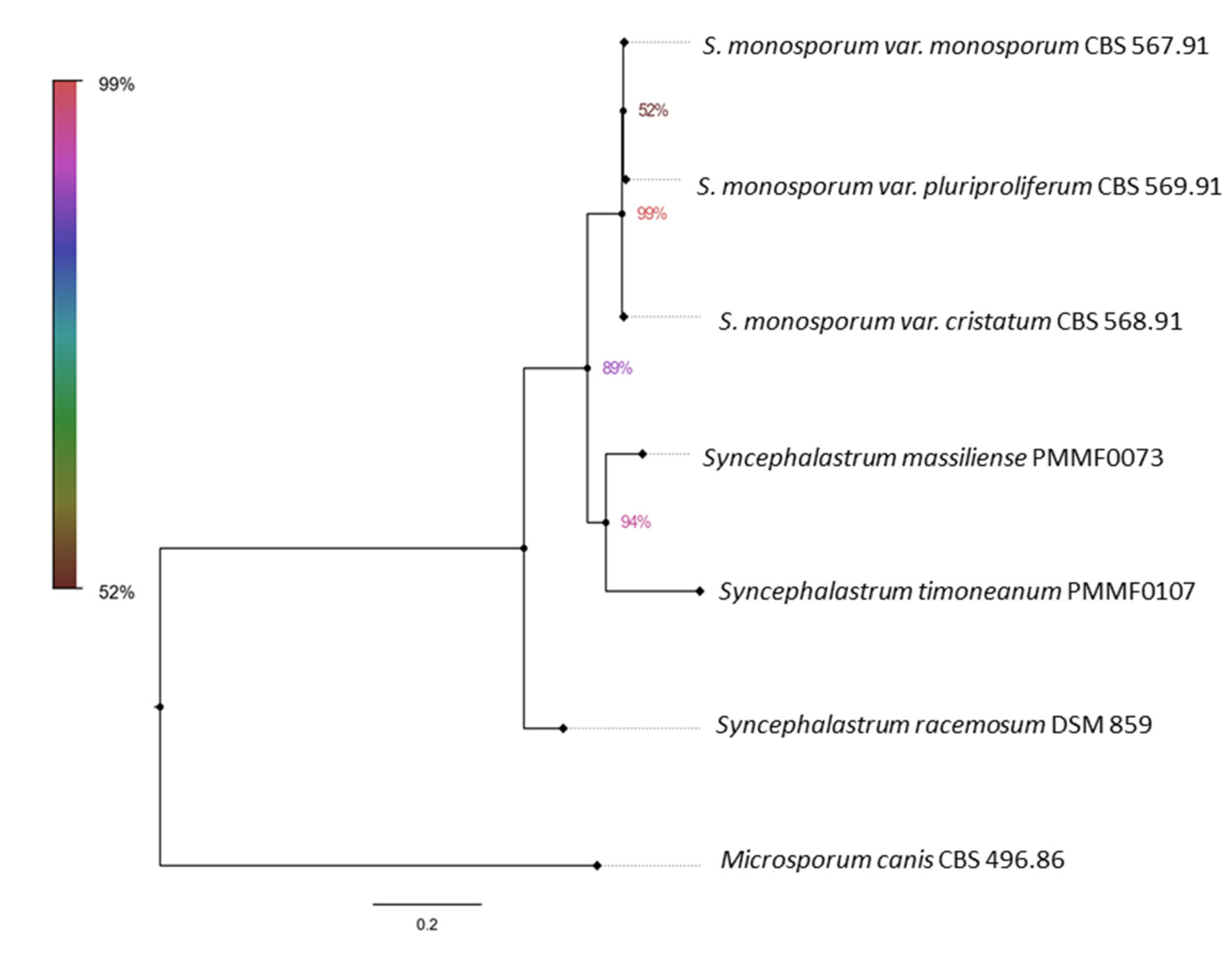
S. monosporum. Furthermore, the second trees (
Figure 3, 5) illustrated the distinct genomic features of the two novel species, which were relatively distant from one another, each clustering with a distinct
Syncephalastrum species:
S. timoneanum PMMF0107 with
S. racemosum, and
S. massiliense PMMF0073 with
S. monosporum.
Macroscopic characterisation
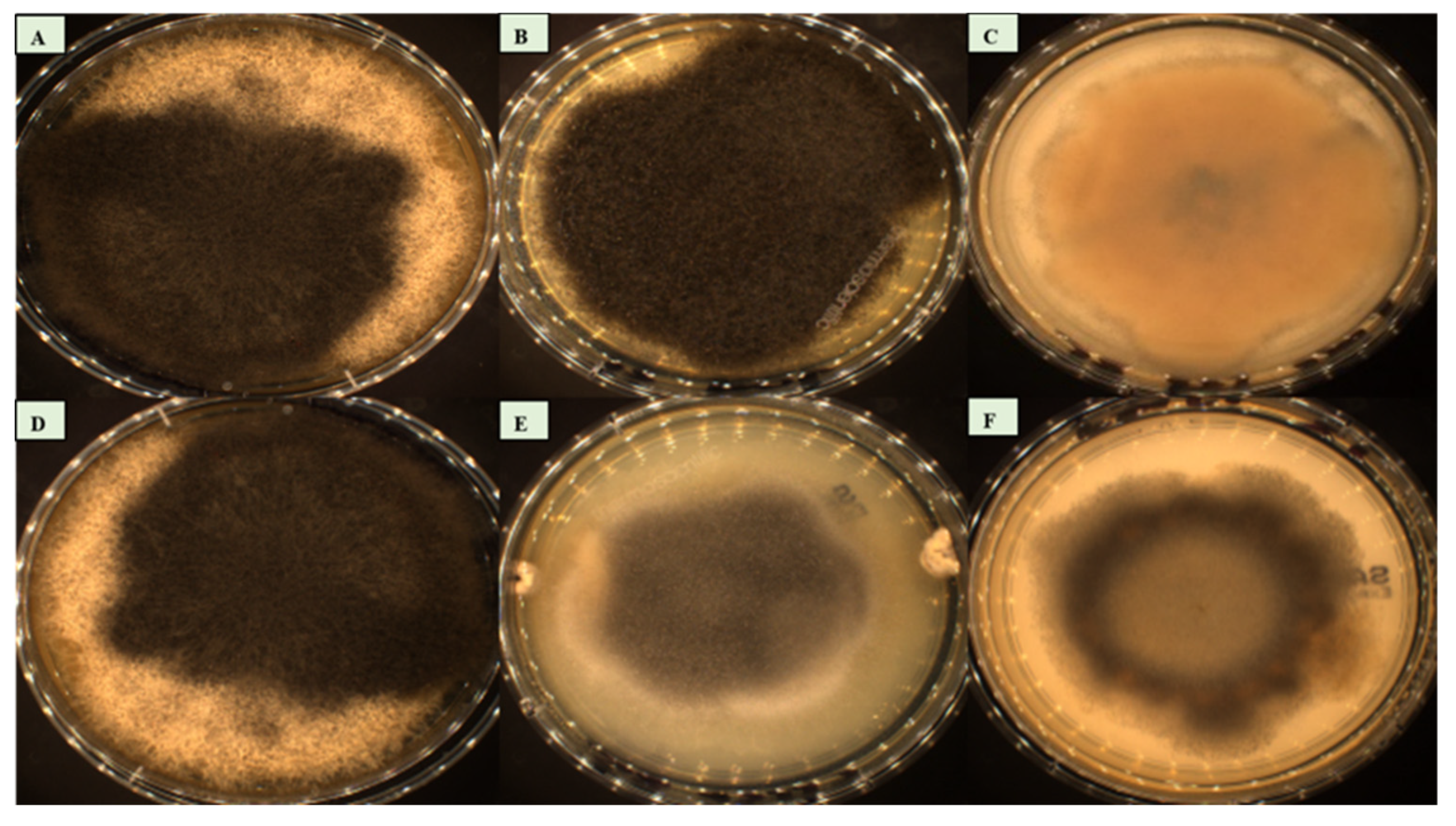
The macroscopic morphological features of the six strains were a rapid time of growth on SDA GC medium, with an optimal growth temperature of 25°C. Colonies with a fluffy and cottony aspect appeared after two to three days of incubation. The colour of the Mycelium was white after 48 hours, then became darker after 72 hours, and reached a high level of sporulation around day five. The mycelium of
Syncephalastrum massiliense PMMF0073,
Syncephalastrum timoneanum PMMF0107, and
S. monosporum var. monosporum CBS 567.91 was dark in colour, while it was grey for
S. monosporum var. cristatum CBS 568.91 and
S. monosporum var. pluriproliferum CBS 569.91.
S. racemosum DSM 859 displayed a lighter colour. All isolates were xerotolerant, as they grew on dehydrated medium. None of them grew at 4°C, 40°C, or 45°C (
Figure 6).
Microscopic characterisation
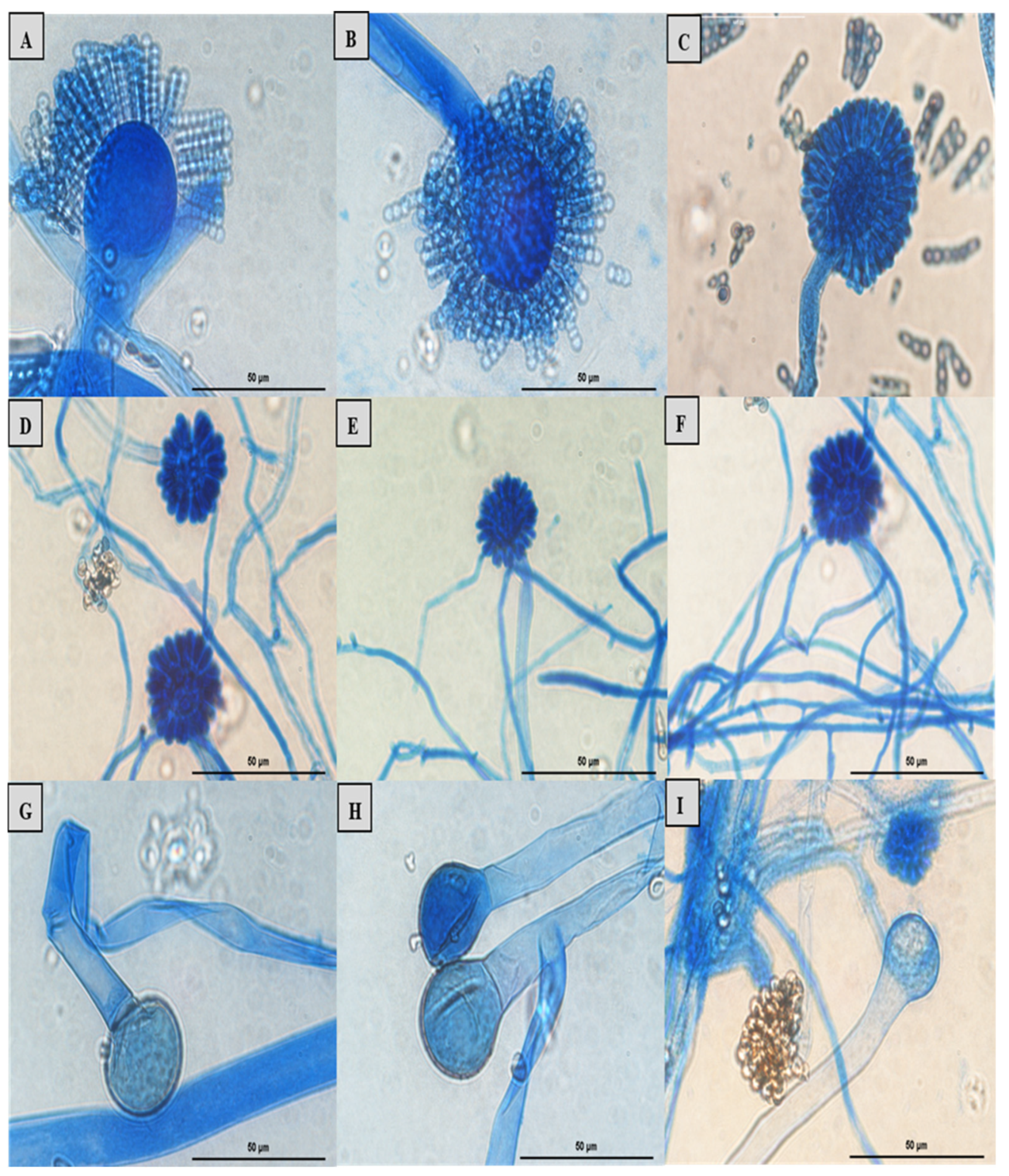
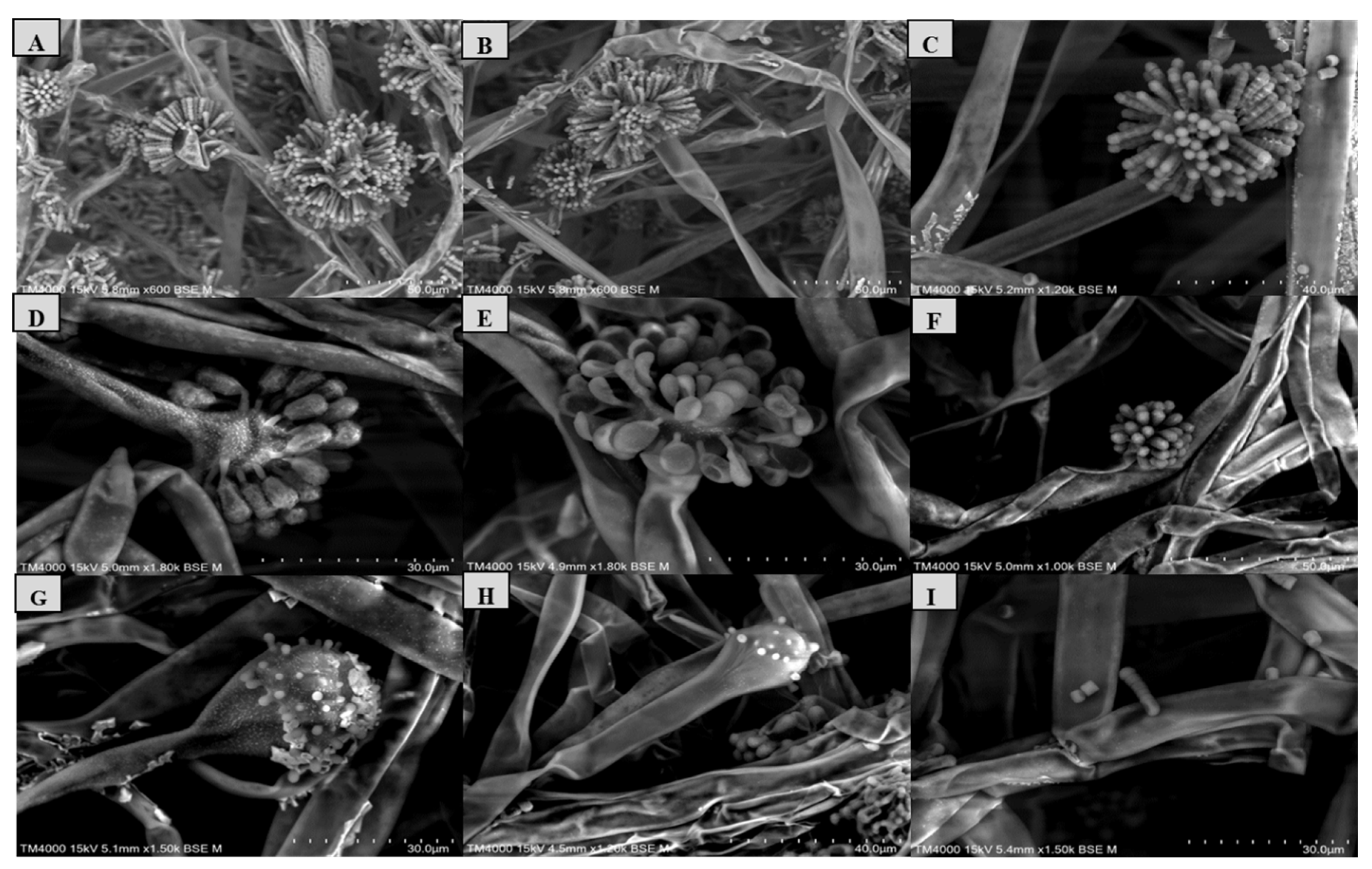
Microscopic observation revealed irregular branched wide hyphae (sporangiophores), aseptate with a ribbon-like aspect, in addition to an ovoid and globose terminal vesicle at the apices for all the strains, with different lengths. Depending on the species, the terminal vesicle generates cylindrical merosporangia over the whole surface, containing several spores in a single row.
S. monosporum species presented the largest hyphae (13-17
μm) and smallest vesicle (15-28
μm) in comparison with
Syncephalastrum massiliense PMMF0073,
Syncephalastrum timoneanum PMMF0107 and
S. racemosum DSMZ 859 which, in contrast, displayed smaller hyphae (7-11
μm) and larger vesicle (29-31
μm). The surface of
S. monosporum vesicle was entirely covered by sporangiola (4-7
μm). However, the vesicle surfaces of
Syncephalastrum massiliense PMMF0073,
Syncephalastrum timoneanum PMMF0107 and
S. racemosum DSMZ 859 were all surrounded by grey cylindrical merosporangia (15-16
μm). Each merosporangial sack contained six or seven light-grey merospores, smooth-walled and spherical to ovoid (3-6
μm). Rhizoids were occasionally observed (
Figure 7 and
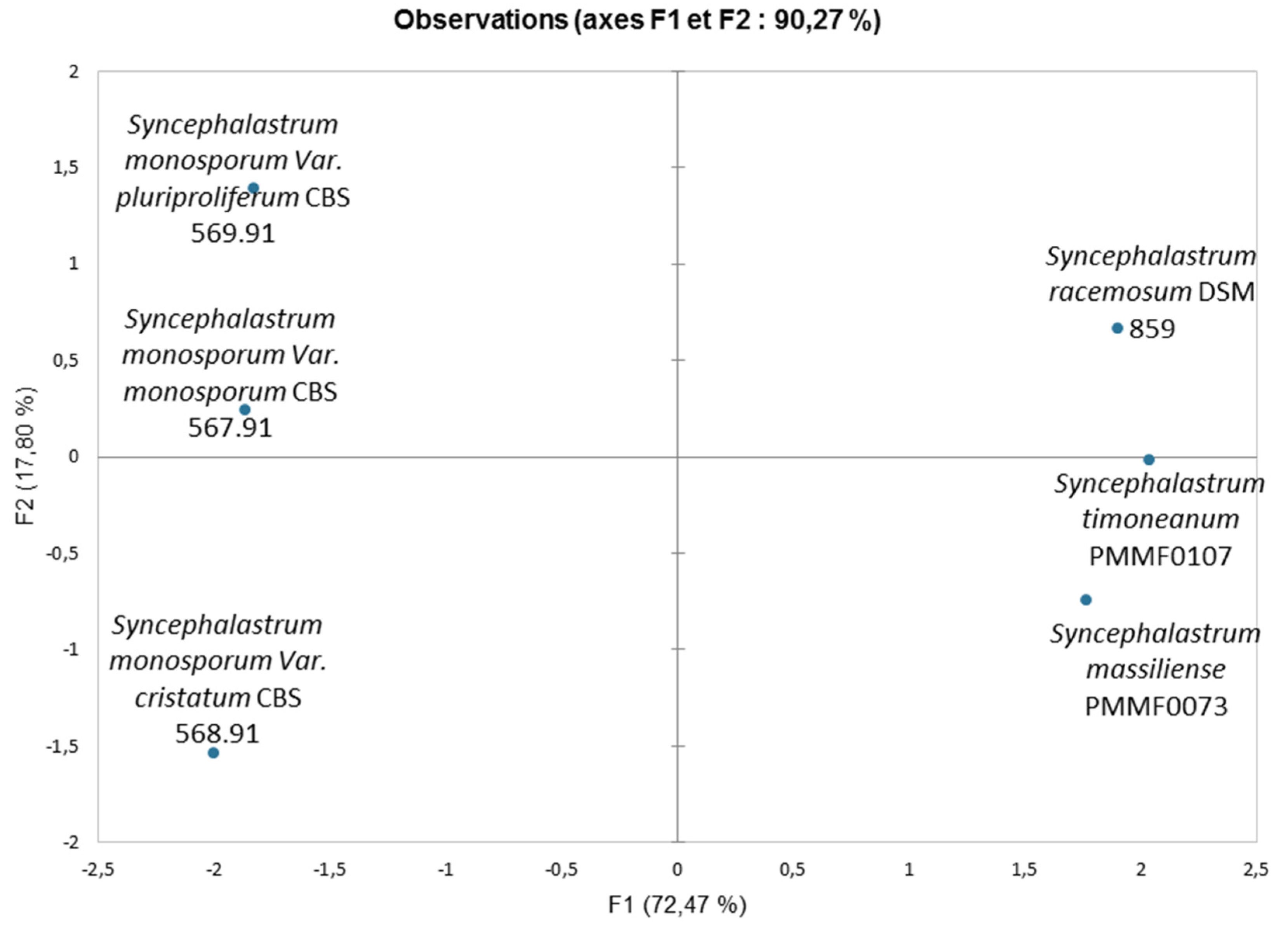
Figure 8). A PCA based on fungal structures measures showed that the microscopic features of the two new strains were relatively similar to
S. racemosum (
Figure 9).
Physiological analysis
1. EDX (Energy-Dispersive X-ray Spectroscopy)
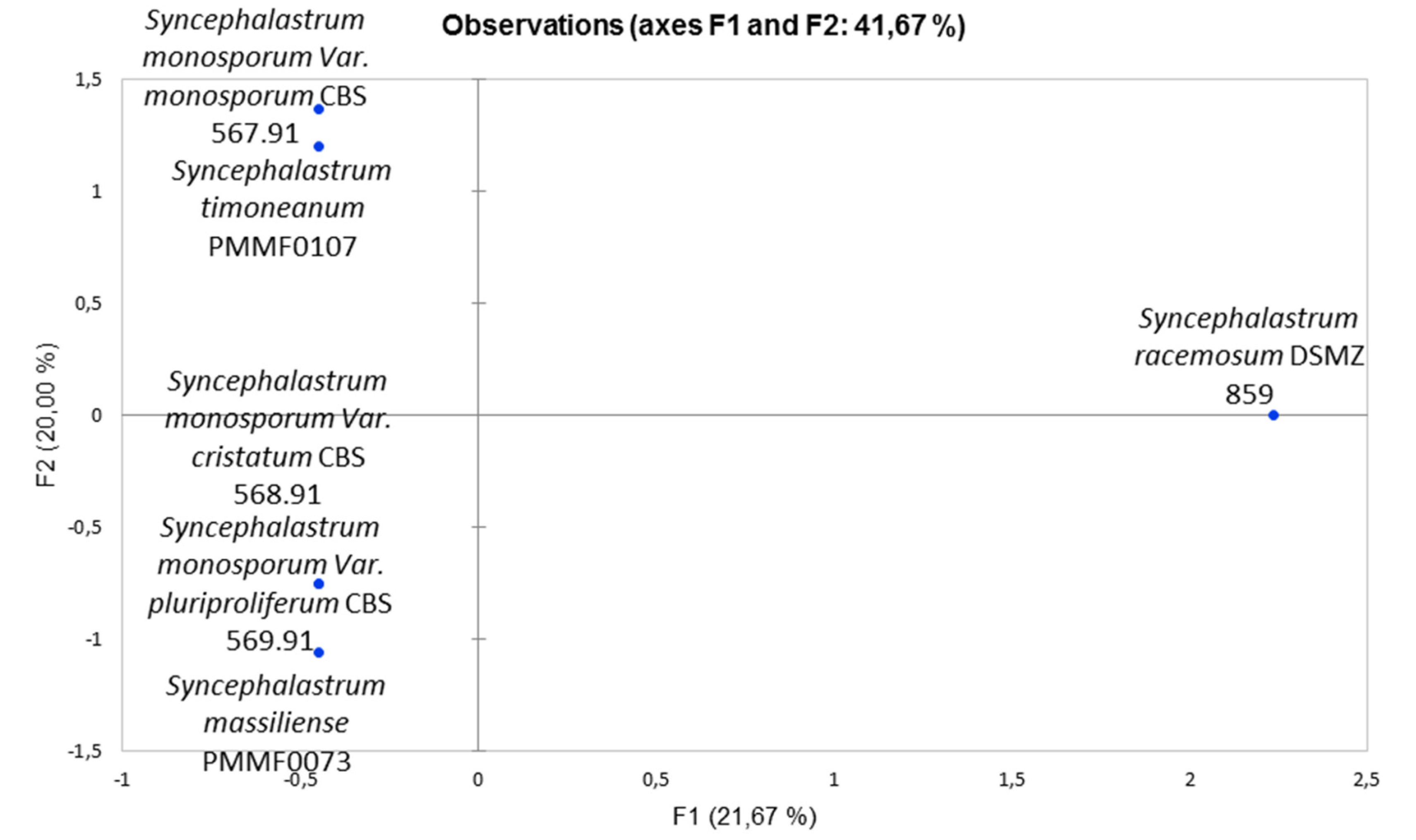
The results are represented as a PCA (
Figure 10), showing that the chemical mapping profiles of the two new species differed from those of
S. racemosum DSM 859.
Syncephalastrum timoneanum PMMF0107 clustered with
S. monosporum var. monosporum CBS 567.91
, while
Syncephalastrum massiliense PMMF0073 clustered with both
S. monosporum var. cristatum CBS 568.91 and
S. monosporum var. pluriproliferum CBS 569.91.
2. BiologTM system
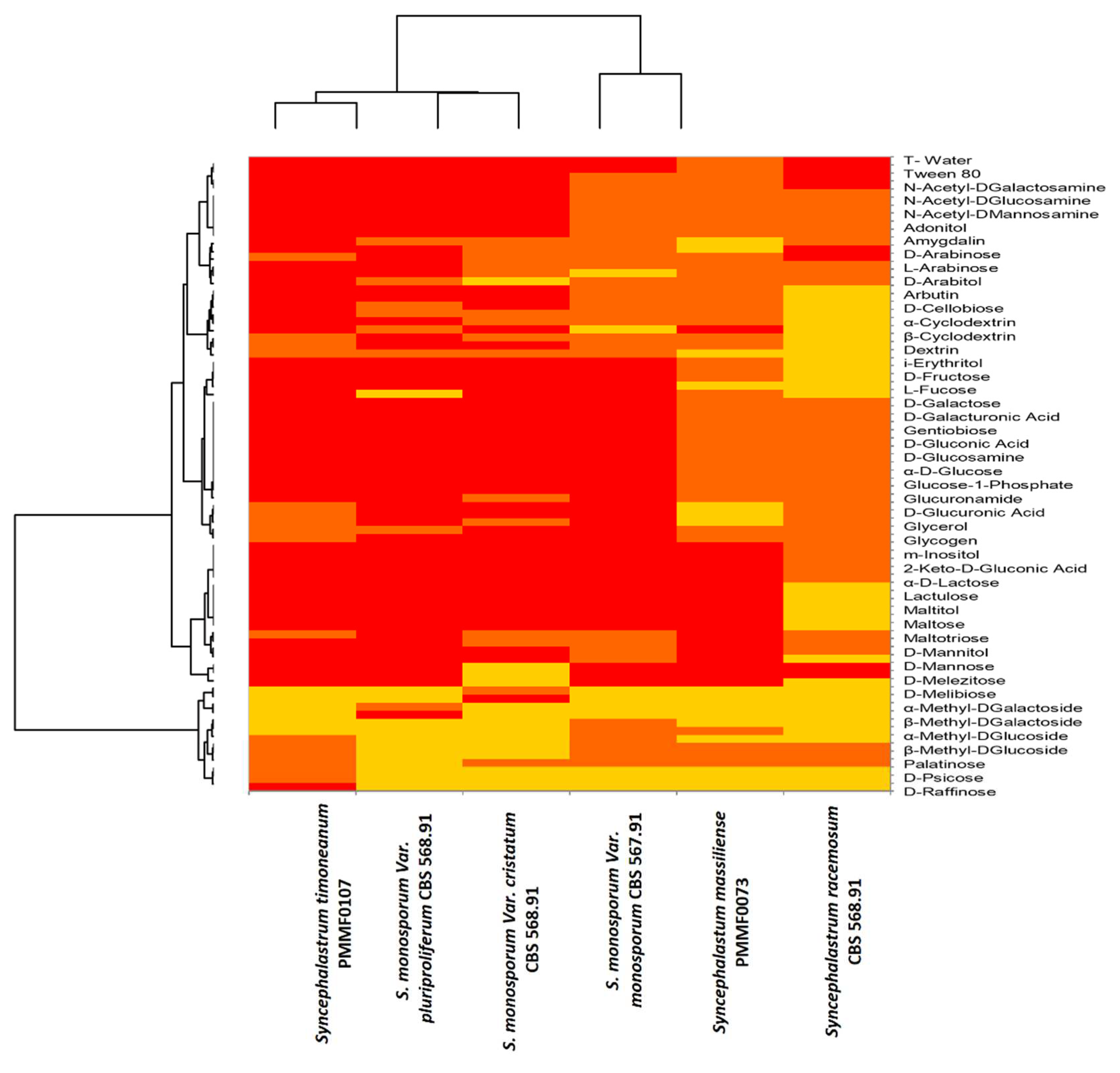
The Biolog
TM phenotypic technology provides valuable information about strain properties using a specific and precise microbial phenotypic characterisation. When reduction occurs in FF plates, the dye colour changes to purple. The Biolog Omnilog equipment analyses images taken over time with a colour camera to quantify the reduced dye (Bochner et al. 2001). The results are represented as a heat map (
Figure 11), which appears fairly heterogeneous, showing that each new species has a substrate assimilation profile close to a distinct
Syncephalastrum species. Most of the substrates were assimilated. The few non assimilated substrates were instrumental for species discrimination.
Syncephalastrum timoneanum PMMF0107 appeared relatively close to
S. monosporum. In contrast,
Syncephalastrum massiliense PMMF0073 appeared closer to
S. racemosum.
Antifungal Susceptibility Testing (AFST)
The minimal inhibitory concentrations (MICs) of the ten antifungal drugs evaluated are displayed in (
Table 3). All strains exhibited high micafungin, anidulafungin, caspofungin, flucytosine, fluconazole, voriconazole, and isavuconazole MICs. Amphotericin B and itraconazole MICs were relatively low against
S. massiliense PMMF0073,
S. timoneanum PMMF0107 and
S. racemosum DSM 859. Whereas, posaconazole MICs were only low against
S. timoneanum PMMF0107 and
S. racemosum DSM 859. Noteworthy, itraconazole and posaconazole MICs were lower against
S. timoneanum PMMF0107 and
S. racemosum DSM 859 than against
S. massiliense PMMF0073.
Taxonomy
Syncephalastrum massiliense Kabtani J. and Ranque S. sp. nov.
MycoBank: MB843858
(Fig 7-A, Fig 8-A)
Etymology: Named after Marseille, the city where it was isolated.
Diagnosis: Syncephalastrum massiliense PMMF0073 is closely similar to S. racemosum DSMZ 859, based on microscopic characters. Both species present small hyphae (7-11 μm) and large vesicle (29-31 μm), in contrast with S. monosporum that present larger hyphae (13-17 μm) and smaller vesicle (15-28 μm). Moreover, S. monosporum vesicle surface is entirely covered by sporangiola (4-7 μm). Meanwhile, both S. massiliense PMMF0073 and S. racemosum DSMZ 859 vesicle surfaces are surrounded by merosporangia (15-16 μm). Each merosporangial sack contains six or seven merospores.
Type: France: Marseille. Human sputum, 11 September 2019. (PMMF0073 – holotype; IHEM 28561 - isotype). GenBank: OL699905 (ITS), ON149883 (Btub2), OM362516 (TEF-1a), OM417069 (D1/D2).
Description: Macroscopic features showed that Syncephalastrum massiliense has a rapid growth time on SDA GC medium, with an optimal temperature of growth at 25 °C. The strain was xerotolerant, and growth was inhibited at temperatures ≤4 °C or ≥40 °C. Colonies with fluffy and cottony aspect were seen from two to three days post-inoculation. The mycelium was white at 48 hours then became darker at 72 hours and reached a high sporulation level around day five. Microscopic observation revealed wide hyphae (sporangiophores), aseptate with a ribbon-like aspect, and an ovoid globose terminal vesicle at the apices. Syncephalastrum massiliense presented small hyphae (7–11 μm) and larger vesicle (29–31 μm). The vesicle surface was entirely surrounded by merosporangia (15-16 μm). Each merosporangial sack contained six or seven sporangiospores (merospores). A few rhizoids were observed.
The BiologTM phenotypic technology provided information on the assimilation capacity of the fungus carbon sources. S. massiliense PMMF0073 was the only Syncephalastrum tested that assimilated the least substrates, including adonitol, alpha-methyl-D-glucoside, trehalose, turanose, succinic acid mono-methyl ester, and alaninamide. It was noteworthy that D-tagatose was only assimilated by S. massiliense PMMF0073 and S. monosporum var. monosporum CBS 567.91. S. massiliense PMMF0073 displayed a carbon source assimilation profile relatively similar to S. racemosum DSM 859.
Host: Human
Syncephalastrum timoneanum Kabtani J. and Ranque S. sp. nov.
MycoBank: MB843870
(Fig 7-B, Fig 8-B)
Etymology: Named after La Timone, the hospital where it was isolated in Marseille, France.
Diagnosis: Syncephalastrum timoneanum PMMF0107 is closely related to S. racemosum DSMZ 859, relying on microscopic characteristics. The two species present small hyphae (7-11 μm) and large vesicle (29-31 μm), in contrast to S. monosporum species which has larger hyphae (13-17 μm) and smaller vesicle (15-28 μm). In addition, while the vesicle surface of S. monosporum is fully covered by sporangiola (4-7 μm), the vesicle surface of both S. timoneanum PMMF0107 and S. racemosum DSMZ 859 was surrounded by merosporangia (15-16 μm). Each merosporangial sack contained six or seven sporangiospores (merospores).
Type: France: Marseille. Human nails, 02 March 2020. (PMMF0107 – holotype; IHEM 28562 - isotype). GenBank: OL699906 (ITS), ON149884 (Btub2), OM362517 (TEF-1a), OM417070 (D1/D2).
Description: Macroscopic features revealed that Syncephalastrum timoneanum PMMF0107 has a rapid growth time on SDA GC medium, with an optimal temperature of growth at 25 °C. The strain can grow on dehydrated medium, demonstrating that is xerotolerant. However, no growth was observed at 4 °C, 40 °C, and 45°C. Colonies with a fluffy and cottony aspect were seen from two to three days. The colour of the mycelium was white in the first 48 hours, then became darker at 72 hours and reached a high level of sporulation around day five. Microscopic observation showed wide hyphae (sporangiophores) and aseptate with a ribbon-like aspect, in addition to an ovoid and globose terminal vesicle at the apices. Syncephalastrum timoneanum PMMF0107 exhibited small hyphae (7–11 μm) and large vesicle (29–31 μm). The surface of its vesicle was entirely covered by merosporangia (15–16 μm). Each merosporangial sack contained about six or seven sporangiospores (merospores). Some rhizoids were observed.
The BiologTM advanced phenotypic technology provided information on the assimilation capacity of the fungus carbon sources. S. timoneanum PMMF0107 was the only species of Syncephalastrum genus that assimilated most of substrates. However, there was some exceptional substrates that were not assimilated (D-tagatose, D-psicose, N-acetyl-D-mannosamine, L-fucose, glucuronamide, sedoheptulosan). Based on this carbon source assimilation, S. timoneanum PMMF0107 displays a relatively similar profile to S. monosporum species.
Host: Human
Additional specimen examined (1): Type: Country of origin unknown. Before 24 January 1977. (DSM 859 - holotype; ATCC 18192 - isotype). GenBank: OL699907 (ITS), ON149885 (Btub2), OM362518 (TEF-1a), OM417071 (D1/D2).
Additional specimen examined (2): Type: China: Zhejiang Prov., Wuxing. Soil, 21 October 1960. (CBS 567.91 - holotype). GenBank: OL699908 (ITS), ON149886 (Btub2), OM362519 (TEF-1a), OM417072 (D1/D2).
Additional specimen examined (3): Type: China: Jiangsu Prov., Nanjing. Soil, 13 October 1960. (CBS 568.91 - holotype). GenBank: OL699909 (ITS), ON149887 (Btub2), OM362520 (TEF-1a), OM417073 (D1/D2).
Additional specimen examined (4): Type: China: Zhejiang Prov., Hangzhou. Pit mud, 19 October 1960. (CBS 569.91 - holotype). GenBank: OL6999010 (ITS), ON149888 (Btub2), OM362521 (TEF-1a), OM417074 (D1/D2).
Discussion
In this study, we propose that Syncephalastrum massiliense PMMF0073, isolated from human sputum, and Syncephalastrum timoneanum PMMF0107, isolated from human nails, are two novel species in the Syncephalastrum genus based on their comprehensive phenotypic and genotypic analysis. The phenotypic analysis highlighted the distinct protein expression profiles of these two isolates assessed using MALDI-TOF MS. Each one appeared closer to a different species of the Syncephalastrum genus. Syncephalastrum timoneanum PMMF0107 seemed closer to S. racemosum DSM 859 and Syncephalastrum massiliense PMMF0073 was closer to S. monosporum clade. The phylogenetic tree constructed using the four loci was congruent with the MALDI-TOF MS dendrogram and showed the same species clustering.
All strains shared the following macroscopic features: colony texture, time and temperature of growth as previously described [
8,
36]. Moreover, the mycelium colour of the new isolates was akin to
S. monosporum. All the strains did not grow ≥40 °C. In contrast to our observations, some authors have described
S. racemosum and
S. monosporum as hydrophilic and thermotolerant moulds [
12], or have declared that
Syncephalastrum species were able to grow above 40 °C [
6,
10].
S. racemosum can be misidentified and confused with some black
Aspergillus species, such as
Aspergillus niger [
5].The hyphal morphology and the merosporangial sack enclosing sporangiospores are key to differentiating these two fungi, but also to distinguishing between
Syncephalastrum species. According to Hoffman et al. (2013) [
37],
Syncephalastrum is the only genus in the
Mucorales which produces merosporangia with the merospores arranged in linear chains. Benjamin RK (1959) [
22] also reported that
S. racemosum produces sporangiospores in deciduous, tubular merosporangial sacks developed across the entire surface of an apical, spherical swelling of the sporangiophore. Indeed, microscopic features are very useful for these fungi classifications. In fact, the two new isolated strains share many
S. racemosum morphological features, mainly relying on the number of sporangiospores contained in each merosporangial sack.
S. racemosum merosporangium contains about six or seven sporangiospores, while
S. monosporum contains only one sporangiola. In all the strains, hyphae are large, aseptate with a ribbon-like aspect as described in Gomes et al. (2011) [
10]. Several comprehensive studies [
38,
39,
40], based on
Mucorales antifungal susceptibility testing reported significant variation between genera, species and strains within the
zygomycetes class. The three species of
S. massiliense PMMF0073,
S. timoneanum PMMF0107 and
S. racemosum DSM 859 were uniformly susceptible to Amphotericin B and itraconazole. However, only
S. timoneanum PMMF0107 and
S. racemosum DSM 859 were susceptible to posaconazole. The susceptibility displayed by
S. racemosum against the three antifungal drugs has already been reported by Vitale et al. 2012 [41]. In line with the microscopic analyses, the antifungal susceptibility profiles of the two novel species,
S. timoneanum PMMF0107 and
S. massiliense PMMF0073, were relatively closer to
S. racemosum than to
S. monosporum.
While molecular methods and phenotypic methods such as MALDI-TOF MS and morphological analysis supply no information about strain properties, the BiologTM system provides information on the assimilation capacity of fungus carbon sources. Whereas the majority of the substrates were assimilated by all strains, some relevant differences were helpful in discriminating between the two isolates. S. massiliense PMMF0073 was the strain that assimilated the least substrates. Among the substrates assimilated by all except S. massiliense PMMF0073 are adonitol, α-methyl-D-glucoside, trehalose, turanose, succinic acid mono-methyl ester, and alaninamide. One exception was D-tagatose, which was assimilated by S. massiliense PMMF0073, and not by S. timoneanum PMMF0107. In contrast, S. timoneanum PMMF0107 was the only species that assimilated almost all substrates, except D-tagatose, D-psicose, N-acetyl-D-mannosamine, L-fucose, glucuronamide, and sedoheptulosan. On the basis of these carbon source assimilation profiles, S. timoneanum PMMF0107 was close to S. monosporum species, and S. massiliense PMMF0073 was close to S. racemosum DSM 859. Besides, relying on the EDX chemical mapping, the new strains were fairly similar to the S. monosporum species. Finally, the morphological features, antifungal susceptibility tests, and the ITS and D1D2 tree highlighted the similarities of both S. massiliense and S. timoneanum with S. racemosum.
Conclusion
The two novel species in the genus Syncephalastrum, S. massiliense PMMF0073 and S. timoneanum PMMF0107 have a similar morphology to S. racemosum but each displays distinct phenotypic and genotypic features. The polyphasic approach, combining the results of complementary assays, including the BiologTM system, MALDI-TOF MS, and EDX, was instrumental in describing and characterising these two new Syncephalastrum species.
Author Contributions
Conceptualisation, JK and SR; Methodology, SR, JK, MM; Formal analysis, JK, MM, FB, SR; Writing original draft, JK; Writing-review and editing, SR, FB, MM. All authors have read and agreed to the final version of the manuscript.
Funding
This research was funded by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection and by the French Government under the “Investissements d’avenir” (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research), (reference: Méditerranée Infection 10-IAHU-03) and the Région Provence-Alpes-Côte d’Azur, European ERDF funding (European regional development fund) and PRIMMI ((Plateformes de Recherche et d’Innovation Mutualisées Méditerranée Infection).
Acknowledgments
The authors acknowledge the technical support of Anthony Fontanini for electron microscopy analyses. We sincerely thank Takashi Irie, Kyoko Imai, Shigeki Matsubara, Taku Sakazume, Toshihide Agemura and the Hitachi team in Japan for the collaborative study conducted together with IHU Méditerranée Infection, and the installation of TM4000 Plus microscopes at the IHU Méditerranée Infection facility.
Availability of data and materials
The Syncephalastrum massiliense holotype is available at the IHU MI (No PMMF0073) and IHEM (No 28561) strain collections. The nucleotide sequences are available on GenBank (Accession Numbers: OL699905, ON149883, OM362516 and OM417069). The datasets analysed during the current study are available from the corresponding author on reasonable request. The Syncephalastrum timoneanum holotype is available at the IHU MI (No PMMF0107) and IHEM (No 28562) strain collections. The nucleotide sequences are available on GenBank (Accession Numbers: OL699906, ON149884, OM362517 and OM417070).
Competing interests
The authors declare that they have no competing interests.
List of Abbreviations
| DNA |
deoxyribonucleic acid |
| GC |
Gentamycin and Chloramphenicol |
| HIV |
Human Immunodeficiency Virus |
| IHU Méditerranée Infection |
Institut Hospitalo-Universitaire Méditerranée Infection |
| ITS |
rRNA Internal Transcribed Spacers |
| SDA |
Sabouraud Dextrose Agar |
| LSU |
large-subunit of the rRNA |
| MALDI-TOF MS |
Matrix-assisted Laser Desorption/ Ionization Time of Flight Mass Spectrometry |
| MP |
Maximum Parsimony |
| NA |
Not available |
| PCA |
Principal Component Analysis |
| rRNA |
ribosomal ribonucleic acid |
| SSU |
small-subunit of the rRNA |
| TEF-1-α |
partial Translation Elongation Factor 1-alpha gene |
| TUB2 |
partial β-tubulin gene |
References
- Amatya, R. ; Khanal, B,; Rijal, A. Syncephalastrum Species Producing Mycetoma-like Lesions. Indian Journal of Dermatology, Venereology, and Leprology. 2010, 3, 76–284. [Google Scholar] [CrossRef]
- Horner, W.E.; Worthan, A.G.; Morey, P.R. Air- and Dustborne Mycoflora in Houses Free of Water Damage and Fungal Growth. Applied and Environmental Microbiology. 2004, 11, 6394–6400. [Google Scholar] [CrossRef]
- Ogunlana, E.O. Fungal Air Spora at Ibadan, Nigeria. Applied Microbiology. 1975, 4, 63–458. [Google Scholar] [CrossRef]
- Rao, C.Y.; Kurukularatne, C.; Garcia-Diaz, J.B.; Kemmerly, S.A.; Reed, D.; Fridkin, S.K.; Morgan, J. Implications of Detecting the Mold Syncephalastrum in Clinical Specimens of New Orleans Residents After Hurricanes Katrina and Rita. Journal of Occupational & Environmental Medicine. 2007, 4, 16–411. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in Human Disease. Clinical Microbiology Reviews. 2000, 2, 236–301. [Google Scholar] [CrossRef]
- Mangaraj, S.G.S.; Patro, M.K.; Padhi, S. A Rare Case of Subcutaneous Mucormycosis Due to Syncephalastrum Racemosum: Case Report and Review of Literature. Indian Journal of Medical Microbiology. 2014, 4, 51–448. [Google Scholar] [CrossRef]
- Mathuram, A.J.; Mohanraj, P.; Mathews, M.S. ; Rhino-Orbital-Cerebral Infection by Syncephalastrum Racemosusm. The Journal of the Association of Physicians of India. 2013, 5, 40–339. [Google Scholar]
- Baby, S.; Ramya, T.G.; Geetha, R.K. Onychomycosis by Syncephalastrum Racemosum: Case Report from Kerala, India. Dermatology Reports. 2015, 7, 5527. [Google Scholar] [CrossRef]
- Baradkar, V.P.; Mathur, M.; Panda, M.; Kumar, S. Sino-orbital infection by Syncephalastrum racemosum in chronic hepatorenal disease. J Oral Maxillofac Pathol. 2008, 12, 7–45. [Google Scholar] [CrossRef]
- Gomes, M.Z.R.; Lewis, R.E.; Kontoyia, nnis, D. P. Mucormycosis Caused by Unusual Mucormycetes, Non-Rhizopus, -Mucor, and -Lichtheimia Species. Clinical Microbiology Reviews. 2011, 2, 45–411. [Google Scholar] [CrossRef]
- Irshad, M.; Nasir, N.; Hashmi, U.H.; Farooqi, J.; Mahmood, S.F. Invasive Pulmonary Infection by Syncephalastrum Species: Two Case Reports and Review of Literature. IDCases. 2020, 21, 00913. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Carrillo-Casas, E.M.; Arenas, R.; García-Méndez, J.O.; Toussaint, S.; Moreno-Morales, M.E.; Schcolnik-Cabrera, A.A.; Xicohtencatl-Cortes, J.; Hernández-Castro, R. Mucormycosis in a Non-Hodgkin Lymphoma Patient Caused by Syncephalastrum Racemosum: Case Report and Review of Literature. Mycopathologia. 2015, 2, 89–93. [Google Scholar] [CrossRef]
- Ramesh, V.; Ramam, M.; Capoor, M.R.; Sugandhan, S.; Dhawan, J.; Khanna, G. Subcutaneous Zygomycosis: Report of 10 Cases from Two Institutions in North India: Subcutaneous Zygomycosis. Journal of the European Academy of Dermatology and Venereology. 2010, 10, 25–1220. [Google Scholar] [CrossRef]
- Schlebusch, S.; Looke, D.F.M. Intraabdominal Zygomycosis Caused by Syncephalastrum Racemosum Infection Successfully Treated with Partial Surgical Debridement and High-Dose Amphotericin B Lipid Complex. Journal of Clinical Microbiology. 2005, 11, 27–5825. [Google Scholar] [CrossRef]
- Voigt, K.; Cigelnik, E.; O’donnell, K. Phylogeny and PCR Identification of Clinically Important Zygomycetes Based on Nuclear Ribosomal-DNA Sequence Data. Journal of Clinical Microbiology. 1999, 12, 64–3957. [Google Scholar] [CrossRef]
- Zaki, S.M.; Elkholy, I.M.; Elkady, N.A.; Abdel-Ghany, K. Mucormycosis in Cairo, Egypt: Review of 10 Reported Cases. Medical Mycology. 2013, 1–8. [Google Scholar] [CrossRef]
- Vander, Straten, R. M.; Balkis, M.M.; Ghannoum, A.M. The role of nondermatophyte molds in onychomycosis: diagnosis and treatment. Dermatologic Therapy. 2002, 15, 89–98. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ryder, J.E.; Baran, R.; Summerbell, R.C. Non Dermatophyte Onychomycosis. Dermatologic Clinics. 2003, 2, 68–257. [Google Scholar] [CrossRef]
- Schroter, J. ; Kryptogamen-Flora, von Schlesien: Pilze. Breslau: J.U. Kern. 1908.
- Urquhart, A.S.; Idnurm, A. Syncephalastrum Contaminatum, a New Species in the Mucorales from Australia. Mycoscience. 2020, 3, 15–111. [Google Scholar] [CrossRef]
- Benjamin, R.K. The merosporangiferous Mucorales. Aliso. 1959, 4, 321–433. [Google Scholar] [CrossRef]
- O’Donnell K, Lutzoni F. M.; Ward, T.J.; Benny, G.L. Evolutionary relationships among mucoralean fungi (Zygomycota): evidence for family polyphyly on a large scale. Mycologia. 2001, 93, 286–296. [Google Scholar] [CrossRef]
- Schwarz, P.; Bretagne, S.; Gantier, J.C.; Garcia-Hermoso, D.; Lortholary, O.; Dromer, F.; Dannaoui, E. Molecular identification of zygomycetes from culture and experimentally infected tissue. J. Clin. Microbiol. 2006, 44, 340–349. [Google Scholar] [CrossRef]
- Cassagne, C.; Normand, A.C.; L’Ollivier, C.; Ranque, S.; Piarroux, R. Performance of MALDI-TOF MS Platforms for Fungal Identification. Mycoses. 2016, 59, 90–678. [Google Scholar] [CrossRef]
- Normand, A.C.; Cassagne, C.; Gautier, M.; Becker, P.; Ranque, S.; Hendrickx, M.; Piarroux, R. Decision criteria for MALDI-TOF MS-based identification of filamentous fungi using commercial and in-house reference databases. BMC Microbiol. 2017, 1, 25. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. 1990, 18, 22–315. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Applied and Environmental Microbiology. 1995, 61, 30–1323. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A Method for Designing Primer Sets for Speciation Studies in Filamentous Ascomycetes. Mycologia, 1999; 91, 553–556. [Google Scholar]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi, 2nd ed.; Universitat Rovira i Virgili Reus: Tarragona, Spain, Ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2000. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Édité par Fabia Ursula Battistuzzi. Molecular Biology and Evolution. 2021, 7, 27–3022. [Google Scholar] [CrossRef]
- Kabtani, J.; Militello, M.; Ranque, S. Coniochaeta massiliensis sp. nov. Isolated from a Clinical Sample. J. Fungi. 2022, 8, 999. [Google Scholar] [CrossRef]
- Bochner, B.R. Phenotype MicroArrays for High-Throughput Phenotypic Testing and Assay of Gene Function. Genome Research. 2001, 11, 55–1246. [Google Scholar] [CrossRef]
- Pinzari, F.; Nadir, Abu-Samra, A. C.; Canfora, L.; Maggi, O.; Persiani, A. Phenotype MicroArrayTM System in the Study of Fungal Functional Diversity and Catabolic Versatility. Research in Microbiology. 2016, 9, 22–710. [Google Scholar] [CrossRef]
- Kondori, N.; Svensson, E.; Mattsby-Baltzer, I. In Vitro Susceptibility of Filamentous Fungi to Itraconazole, Voriconazole and Posaconazole by Clinical and Laboratory Standards Institute Reference Method and E-Test: In Vitro Susceptibility of Filamentous Fungi. Mycoses. 2011, 5, 22–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lee, J.H.; Kim, Y.K.; Ki, C.S.; Huh, H.J.; Lee, N.Y. Identification of Mucorales From Clinical Specimens: A 4-Year Experience in a Single Institution. Annals of Laboratory Medicine. 2016, 1, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Pawłowska, J.; Walther, G.; Wrzosek, M.; de Hoog, G.S.; Benny, G.L.; Kirk, P.M.; Voigt, K. The Family Structure of the Mucorales: A Synoptic Revision Based on Comprehensive Multigene-Genealogies. Persoonia - Molecular Phylogeny and Evolution of Fungi. 2013, 30, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Verweij, P.E. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 2003, 51, 45–52. [Google Scholar] [CrossRef]
- Torres-Narbona, M.; Guinea, J.; Martinez-Alarcon, J.; Pelaez, T.; Bouza, E. In vitro activities of amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole against 45 clinical isolates of zygomycetes: comparison of CLSI M38-A, Sensititre YeastOne, and the Etest. Antimicrob. Agents Chemother. 2007, 51, 1126–1129. [Google Scholar] [CrossRef]
- Almyroudis, N.G.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Kusne, S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 2007, 51, 2587–2590. [Google Scholar] [CrossRef]
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K. Antifungal Susceptibility and Phylogeny of Opportunistic Members of the Order Mucorales. Journal of Clinical Microbiology. 2012, 50, 66–75. [Google Scholar] [CrossRef]
Figure 1.
MALDI-TOF MS dendrogram based on protein expression intensity profile for 4 reference strains and 2 new species of Syncephalastrum, generated by the MALDI-TOF Biotyper Compass Explorer software (Bruker Daltonics).
Figure 1.
MALDI-TOF MS dendrogram based on protein expression intensity profile for 4 reference strains and 2 new species of Syncephalastrum, generated by the MALDI-TOF Biotyper Compass Explorer software (Bruker Daltonics).
Figure 2.
Maximum parsimony dendrogram based on the concatenated ITS and D1/D2 sequences, including Syncephalastrum massiliense PMMF0073, Syncephalastrum timoneanum PMMF0107, and 8 other Syncephalastrum spp. reference strains. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with the maximum parsimony method using MEGA 11 software. Bootstrap values were estimated at 1000 replications.
Figure 2.
Maximum parsimony dendrogram based on the concatenated ITS and D1/D2 sequences, including Syncephalastrum massiliense PMMF0073, Syncephalastrum timoneanum PMMF0107, and 8 other Syncephalastrum spp. reference strains. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with the maximum parsimony method using MEGA 11 software. Bootstrap values were estimated at 1000 replications.
Figure 3.
Maximum parsimony dendrogram based on the concatenated ITS, TUB2, TEF1 and D1/D2 sequences of Syncephalastrum massiliense PMMF0073, and S. timoneanum PMMF0107, and 4 Syncephalastrum spp. type strain. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with the maximum parsimony method using the MEGA 11 software. Bootstrap values were estimated at 1000 replications.
Figure 3.
Maximum parsimony dendrogram based on the concatenated ITS, TUB2, TEF1 and D1/D2 sequences of Syncephalastrum massiliense PMMF0073, and S. timoneanum PMMF0107, and 4 Syncephalastrum spp. type strain. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with the maximum parsimony method using the MEGA 11 software. Bootstrap values were estimated at 1000 replications.
Figure 4.
Bayesian phylogenetic tree based on the concatenated ITS and D1/D2 sequences of Syncephalastrum massiliense PMMF0073, and S. timoneanum PMMF0107, and 4 Syncephalastrum spp. type strain. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with MrBayes software (3.2.7a) and Figtree (V.1.4.4).
Figure 4.
Bayesian phylogenetic tree based on the concatenated ITS and D1/D2 sequences of Syncephalastrum massiliense PMMF0073, and S. timoneanum PMMF0107, and 4 Syncephalastrum spp. type strain. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with MrBayes software (3.2.7a) and Figtree (V.1.4.4).
Figure 5.
Figure 5. Bayesian phylogenetic tree based on the concatenated ITS, TUB2, TEF1 and D1/D2 sequences of Syncephalastrum massiliense PMMF0073, and S. timoneanum PMMF0107, and 4 Syncephalastrum spp. type strain. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with MrBayes software (3.2.7a) and Figtree (V.1.4.4).
Figure 5.
Figure 5. Bayesian phylogenetic tree based on the concatenated ITS, TUB2, TEF1 and D1/D2 sequences of Syncephalastrum massiliense PMMF0073, and S. timoneanum PMMF0107, and 4 Syncephalastrum spp. type strain. Microsporum canis CBS 496.86 was used as outgroup. The tree was constructed with MrBayes software (3.2.7a) and Figtree (V.1.4.4).
Figure 6.
Culture growth on SDA GC at 25°C. A: Syncephalastrum massiliense PMMF0073. B: Syncephalastrum timoneanum PMMF0107. C: S. racemosum DSM 859. D: S. monosporum var. monosporum CBS 567.91. E: S. monosporum var. cristatum CBS 568.91. F: S. monosporum var. pluriproliferum CBS 569.91.
Figure 6.
Culture growth on SDA GC at 25°C. A: Syncephalastrum massiliense PMMF0073. B: Syncephalastrum timoneanum PMMF0107. C: S. racemosum DSM 859. D: S. monosporum var. monosporum CBS 567.91. E: S. monosporum var. cristatum CBS 568.91. F: S. monosporum var. pluriproliferum CBS 569.91.
Figure 7.
Lactophenol cotton blue mount of Syncephalastrum spp. A, B, C: sporangiophore with apical vesicle and merosporangial sacks enclosing merospores of Syncephalastrum massiliense PMMF0073, Syncephalastrum timoneanum PMMF0107 and S. racemosum DSM 859. D, E, F: sporangiophore with apical vesicle and sporangiola of S. monosporum var. monosporum CBS 567.91, S. monosporum var. cristatum CBS 568.91 and S. monosporum var. pluriproliferum CBS 569.91. G: columella and hyphae ribbon like aspect of Syncephalastrum timoneanum PMMF0107. H, I: columella of S. racemosum and S. monosporum. Optical microscopy (Magnification x1000). Scale bars: 50 μm.
Figure 7.
Lactophenol cotton blue mount of Syncephalastrum spp. A, B, C: sporangiophore with apical vesicle and merosporangial sacks enclosing merospores of Syncephalastrum massiliense PMMF0073, Syncephalastrum timoneanum PMMF0107 and S. racemosum DSM 859. D, E, F: sporangiophore with apical vesicle and sporangiola of S. monosporum var. monosporum CBS 567.91, S. monosporum var. cristatum CBS 568.91 and S. monosporum var. pluriproliferum CBS 569.91. G: columella and hyphae ribbon like aspect of Syncephalastrum timoneanum PMMF0107. H, I: columella of S. racemosum and S. monosporum. Optical microscopy (Magnification x1000). Scale bars: 50 μm.
Figure 8.
Morphology of Syncephalastrum spp. A, B, C: sporangiophore with apical vesicle and merosporangial sacks of Syncephalastrum massiliense PMMF0073, Syncephalastrum timoneanum PMMF0107 and S. racemosum DSM 859. D, E, F: sporangiophore with apical vesicle and sporangiola of S. monosporum var. monosporum CBS 567.91, S. monosporum var. cristatum CBS 568.91 and S. monosporum var. pluriproliferum CBS 569.91. G, H: columella of S. racemosum and S. monosporum. I: merosporangium sack with 6 merospores of S. racemosum. Scanning Electron Microscopy TM 4000Plus (15KeV lens mode 4). Scale bars: A, B, F = 50μm, C, H = 40 μm, D, E, G, I =30 μm.
Figure 8.
Morphology of Syncephalastrum spp. A, B, C: sporangiophore with apical vesicle and merosporangial sacks of Syncephalastrum massiliense PMMF0073, Syncephalastrum timoneanum PMMF0107 and S. racemosum DSM 859. D, E, F: sporangiophore with apical vesicle and sporangiola of S. monosporum var. monosporum CBS 567.91, S. monosporum var. cristatum CBS 568.91 and S. monosporum var. pluriproliferum CBS 569.91. G, H: columella of S. racemosum and S. monosporum. I: merosporangium sack with 6 merospores of S. racemosum. Scanning Electron Microscopy TM 4000Plus (15KeV lens mode 4). Scale bars: A, B, F = 50μm, C, H = 40 μm, D, E, G, I =30 μm.
Figure 9.
Principal Component Analysis (PCA) of different structures measurements (hyphae, columella, sporangiola, merosporangium and sporangiospores number within the merosporangial sack) using TM4000 Plus microscope (SEM) for 4 reference strains and two new species of Syncephalastrum. In this analysis, computed with the XLSTAT software, the principal components F1 and F2 explained 90.3% of the fungi structures variance.
Figure 9.
Principal Component Analysis (PCA) of different structures measurements (hyphae, columella, sporangiola, merosporangium and sporangiospores number within the merosporangial sack) using TM4000 Plus microscope (SEM) for 4 reference strains and two new species of Syncephalastrum. In this analysis, computed with the XLSTAT software, the principal components F1 and F2 explained 90.3% of the fungi structures variance.
Figure 10.
Principal Component Analysis of the EDX (Energy-Dispersive X-ray Spectroscopy) chemical mapping profile of 4 reference strains and the 2 novel Syncephalastrum species, In this analysis, computed with the XLSTAT software, the principal components F1 and F2 explained 41.7% of the EDX data variance.
Figure 10.
Principal Component Analysis of the EDX (Energy-Dispersive X-ray Spectroscopy) chemical mapping profile of 4 reference strains and the 2 novel Syncephalastrum species, In this analysis, computed with the XLSTAT software, the principal components F1 and F2 explained 41.7% of the EDX data variance.
Figure 11.
Heat Map of carbon sources assimilation assessed by BiologTM system, for 4 reference strains and two species of Syncephalastrum, computed with the XLSTAT software. Color gradient interpretation: the most assimilated substrate are in red, and the least assimilated substrate in yellow.
Figure 11.
Heat Map of carbon sources assimilation assessed by BiologTM system, for 4 reference strains and two species of Syncephalastrum, computed with the XLSTAT software. Color gradient interpretation: the most assimilated substrate are in red, and the least assimilated substrate in yellow.
Table 1.
Panel of primers used for amplifying ITS, TUB2, TEF-1 alpha, and D1/D2 genetic regions.
Table 1.
Panel of primers used for amplifying ITS, TUB2, TEF-1 alpha, and D1/D2 genetic regions.
| Primers |
Sequences |
Targeted regions |
References |
| ITS1 |
TCCGTAGGTGAACCTGCGG |
18S-5.8S |
[27] |
| ITS2 |
GCTGCGTTCTTCATCGATGC |
18S-5.8S |
[27] |
| ITS3 |
GCATCGATGAAGAACGCAGC |
5.8S-28S |
[27] |
| ITS4 |
TCCTCCGCTTATTGATATGC |
5.8S-28S |
[27] |
| ITS1 |
TCCGTAGGTGAACCTGCGG |
18S-5.8S, 5.8S-28S |
[27] |
| ITS4 |
TCCTCCGCTTATTGATATGC |
18S-5.8S, 5.8S-28S |
[27] |
| Bt-2a |
GGTAACCAAATCGGTGCTGCTTTC |
TUB2 |
[28] |
| Bt-2b |
ACCCTCAGTGTAGTGACCCTTGGC |
TUB2 |
[28] |
| EF1-728F |
CATCGAGAAGTTCGAGAAGG |
TEF1 |
[29] |
| EF1-986R |
TACTTGAAGGAACCCTTACC |
TEF1 |
[29] |
| D1 |
AACTTAAGCATATCAATAAGCGGAGGA |
28S |
[30] |
| D2 |
GGT CCG TGT TTC AAG ACG G |
28S |
[30] |
Table 2.
Fungal collection strain ID and GenBank accession numbers of the nucleotide sequences used in the phylogenetic analysis.
Table 2.
Fungal collection strain ID and GenBank accession numbers of the nucleotide sequences used in the phylogenetic analysis.
| Species |
Strain ID |
GenBank accession numbers |
| ITS |
TUB2 |
D1/D2 |
TEF1 |
Syncephalastrum massiliense
|
PMMF0073 |
OL699905.1 |
ON149883 |
OM417069.1 |
OM362516.1 |
| Syncephalastrum timoneanum |
PMMF0107 |
OL699906.1 |
ON149884 |
OM417070.1 |
OM362517.1 |
| Syncephalastrum racemosum |
DSM 859 |
OL699907.1 |
ON149885 |
OM417071.1 |
OM362518.1 |
| S. monosporum var. monosporum |
CBS 567.91 |
OL699908.1 |
ON149886 |
OM417072.1 |
OM362519.1 |
| S. monosporum var. cristatum |
CBS 568.91 |
OL699909.1 |
ON149887 |
OM417073.1 |
OM362520.1 |
| S. monosporum var. pluriproliferum |
CBS 569.91 |
OL699910.1 |
ON149888 |
OM417074.1 |
OM362521.1 |
| Syncephalastrum racemosum |
CBS 441.59 |
HM999985.1 |
NA* |
MH869451.1 |
NA |
| Syncephalastrum racemosum |
CBS 302.65 |
HM999984.1 |
NA |
MH870214.1 |
NA |
| Syncephalastrum racemosum |
CBS 213.78 |
HM999978.1 |
NA |
MH872886.1 |
NA |
| Syncephalastrum racemosum |
CBS 421.63 |
HM999973.1 |
NA |
MH869932.1 |
NA |
| Syncephalastrum racemosum |
CBS 199.81 |
HM999972.1 |
NA |
HM849718.1 |
NA |
| Syncephalastrum monosporum |
CBS 122.12 |
HM999977.1 |
NA |
JN206575.1 |
NA |
| Syncephalastrum racemosum |
EML-BT5-1 |
KY047152.1 |
NA |
KY047158.1 |
NA |
| Syncephalastrum racemosum |
EML-BT5-2 |
KY047143.1 |
NA |
KY047157.1 |
NA |
| Microsporum canis |
CBS 496.86 |
MH861991.1 |
NA |
NG_069297.1 |
NA |
Table 3.
E-test minimal inhibitory concentrations (MICs) of ten antifungal drugs against six refrence Syncephalastrum strains, including the two novel species: S. massiliense, and S. timoneanum.
Table 3.
E-test minimal inhibitory concentrations (MICs) of ten antifungal drugs against six refrence Syncephalastrum strains, including the two novel species: S. massiliense, and S. timoneanum.
| MIC (mg/l) |
AMB |
AND |
CAS |
MIC |
5-FC |
FL |
ITC |
POS |
VOR |
IS* |
|
Syncephalastrum massiliense PMMF0073 |
0.125 |
>32 |
>32 |
>32 |
>32 |
>256 |
4 |
>32 |
>32 |
>32 |
|
Syncephalastrum timoneanum PMMF0107 |
0.047 |
>32 |
>32 |
>32 |
>32 |
>256 |
1 |
6 |
>32 |
>32 |
|
Syncephalastrum racemosum DSM 859 |
0.25 |
>32 |
>32 |
>32 |
>32 |
>256 |
0.75 |
0.75 |
>32 |
>32 |
|
S. monosporum var. monosporum CBS 567.91 |
>32 |
>32 |
>32 |
>32 |
>32 |
>256 |
>32 |
>32 |
>32 |
>32 |
|
S. monosporum var. cristatum CBS 568.91 |
>32 |
>32 |
>32 |
>32 |
>32 |
>256 |
>32 |
>32 |
>32 |
>32 |
|
S. monosporum var. pluriproliferum CBS 569.91 |
>32 |
>32 |
>32 |
>32 |
>32 |
>256 |
>32 |
>32 |
>32 |
>32 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).