Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental section
2.1. Materials and Methods
2.2. Preparations of the materials
2.3. Catalytic studies
3. Results and discusion
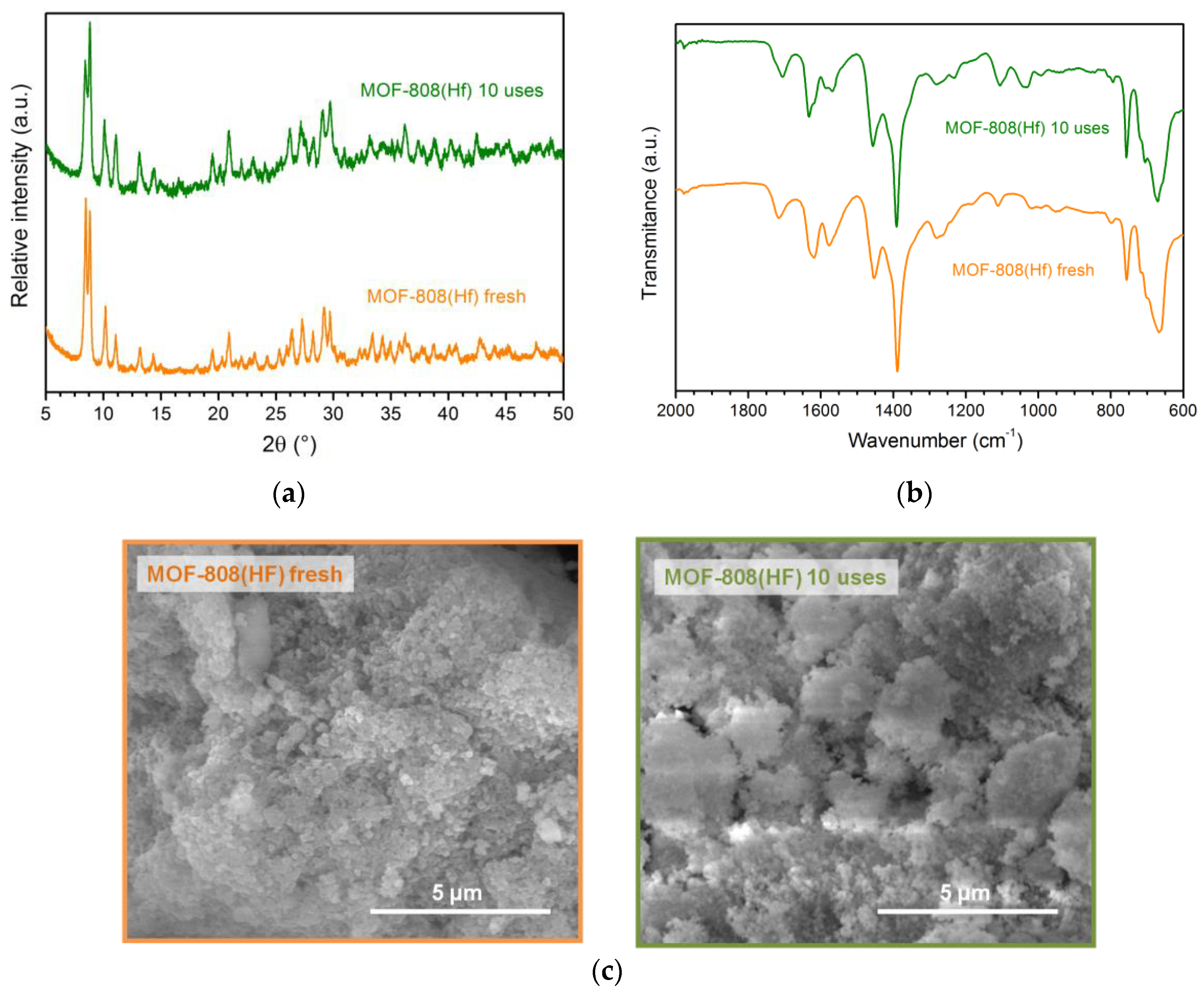
3.1. Catalysts characterization
3.2. Acidity characterization
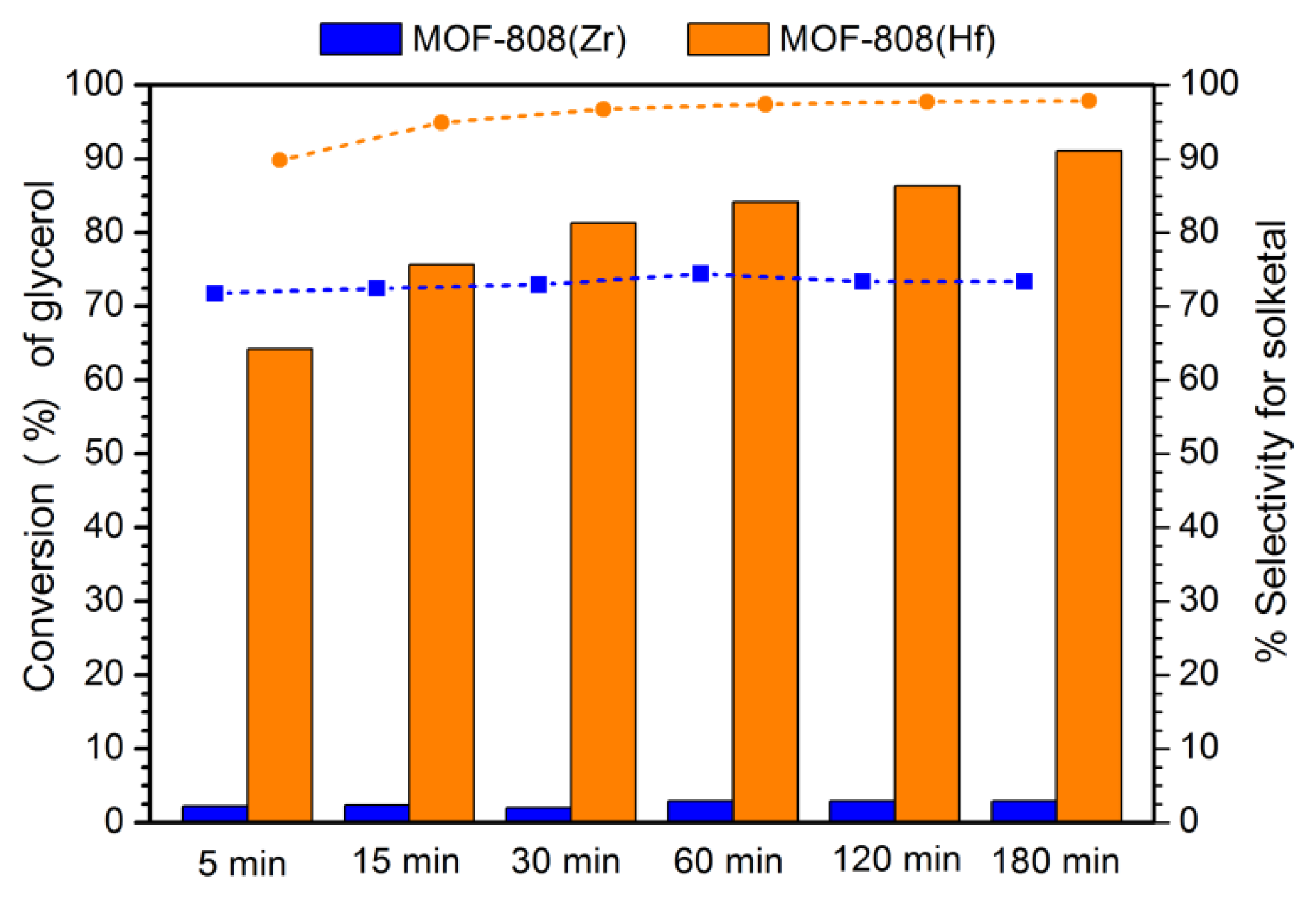
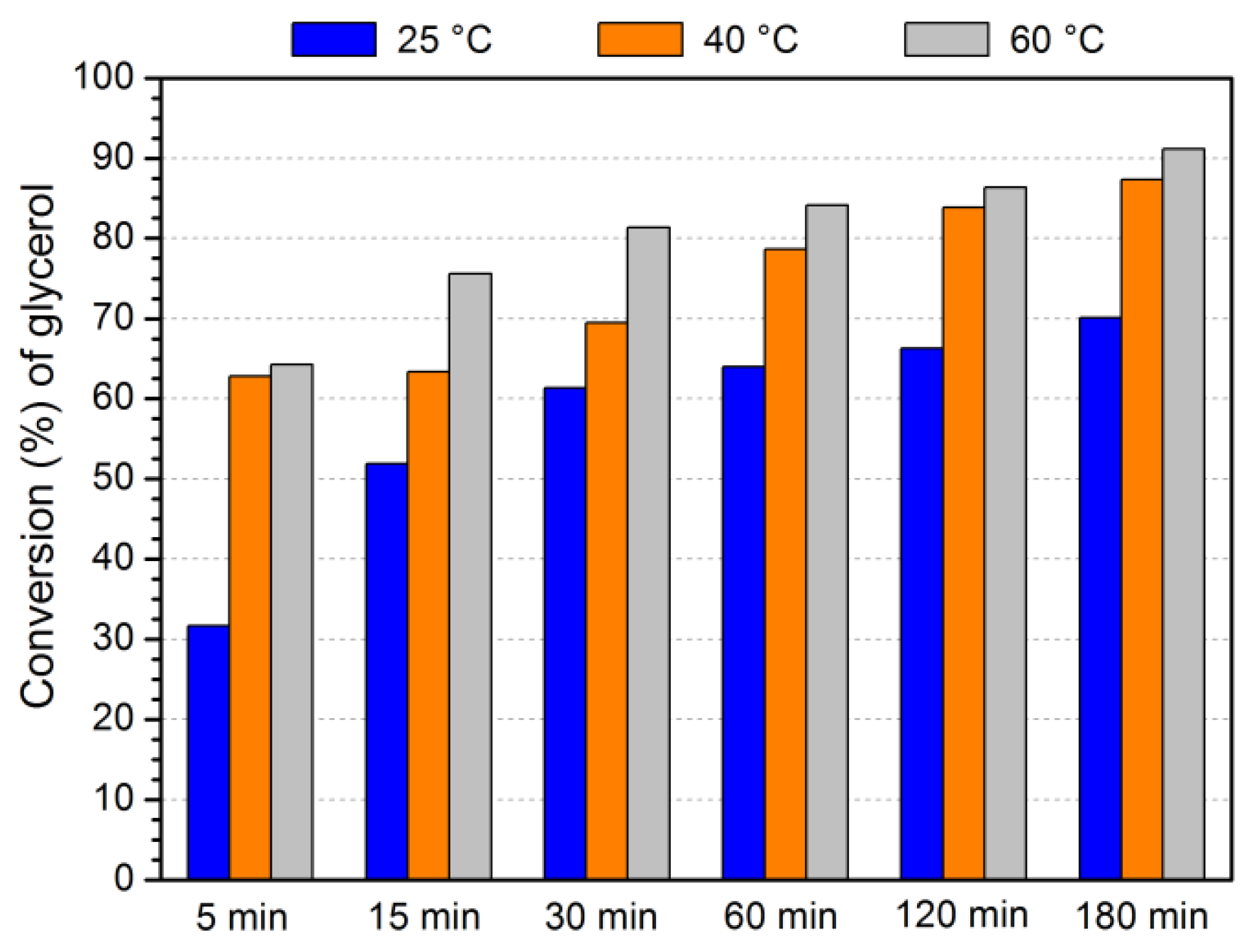
3.3. Evaluation of catalytic activity
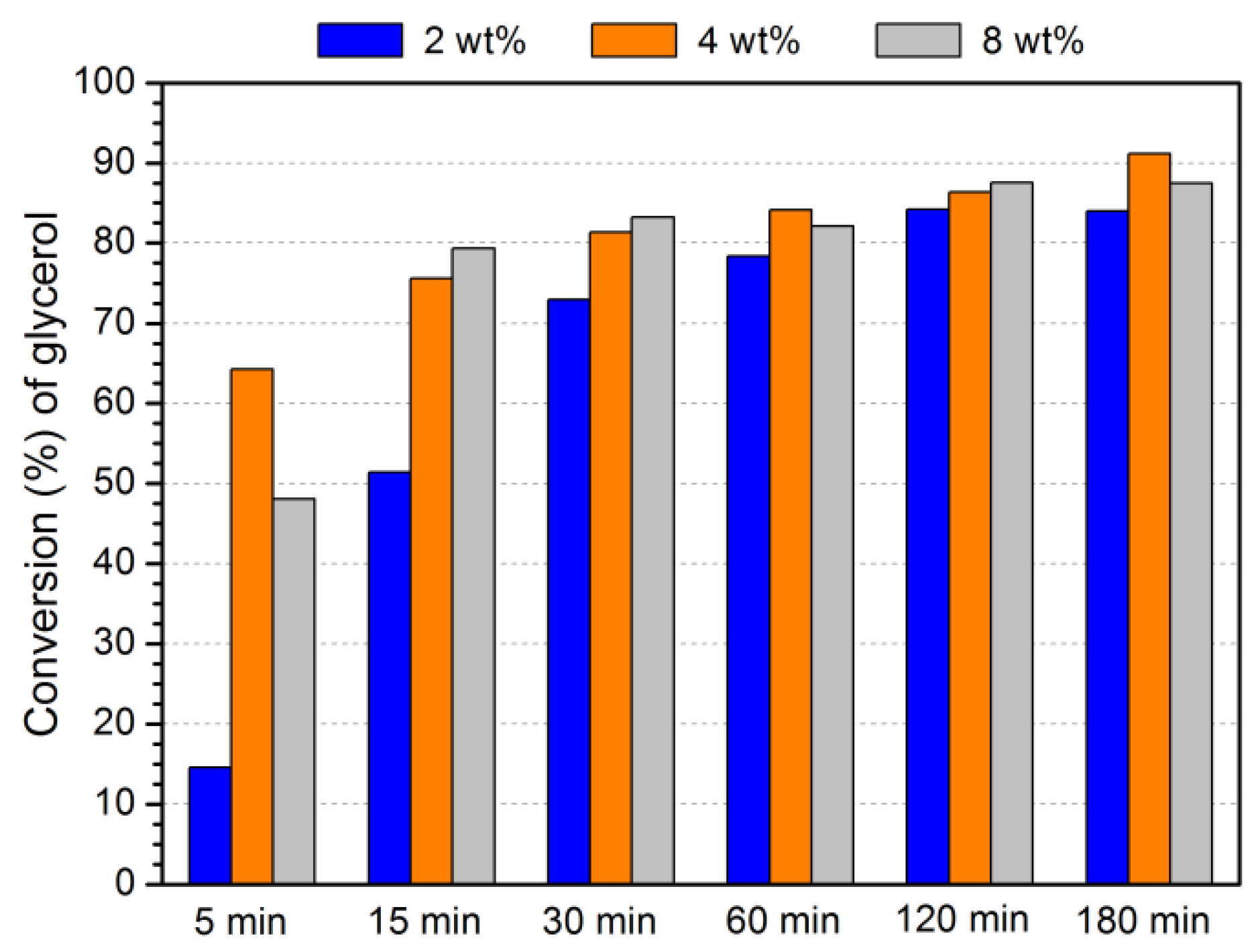
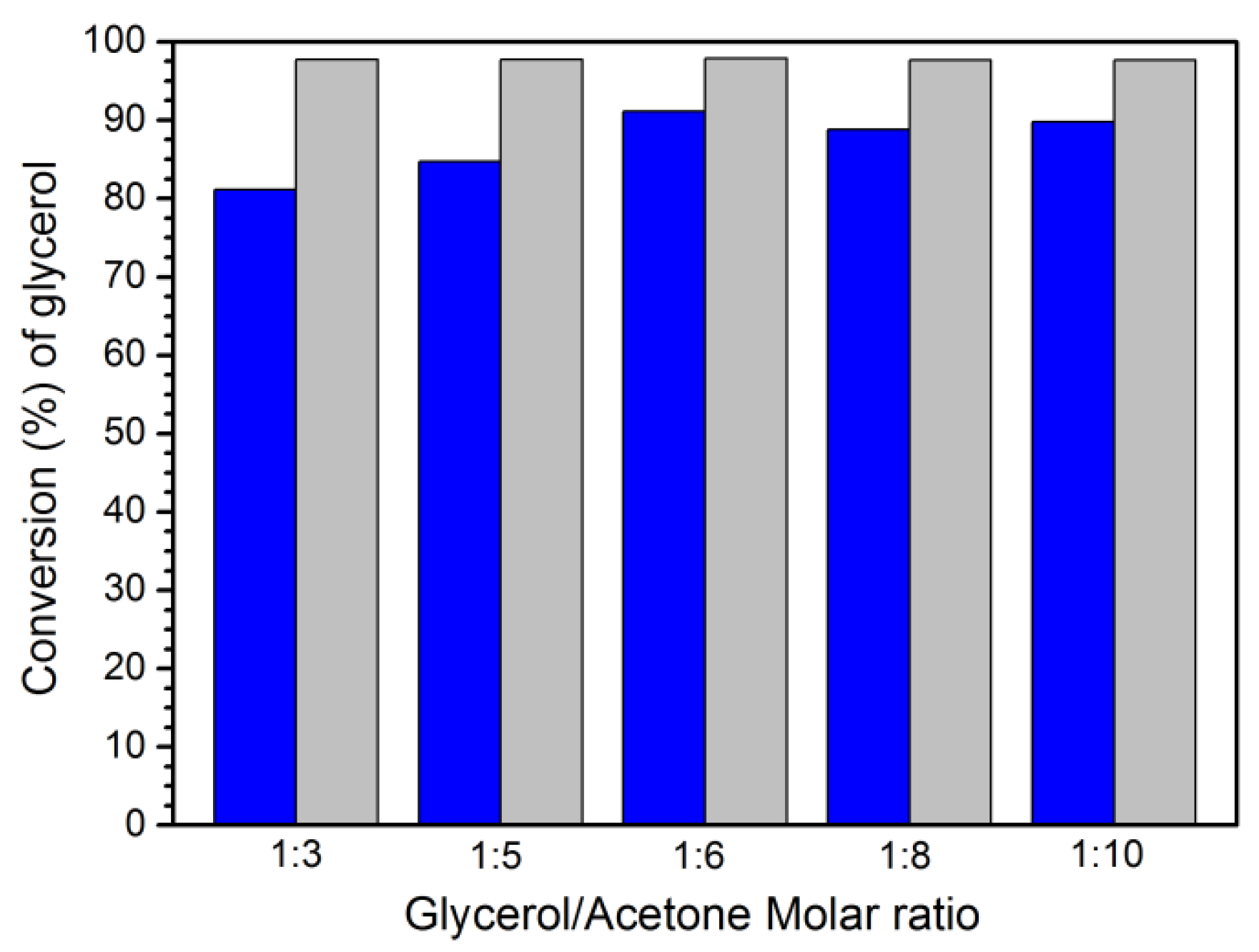
3.4. Optimization of acetalization reaction
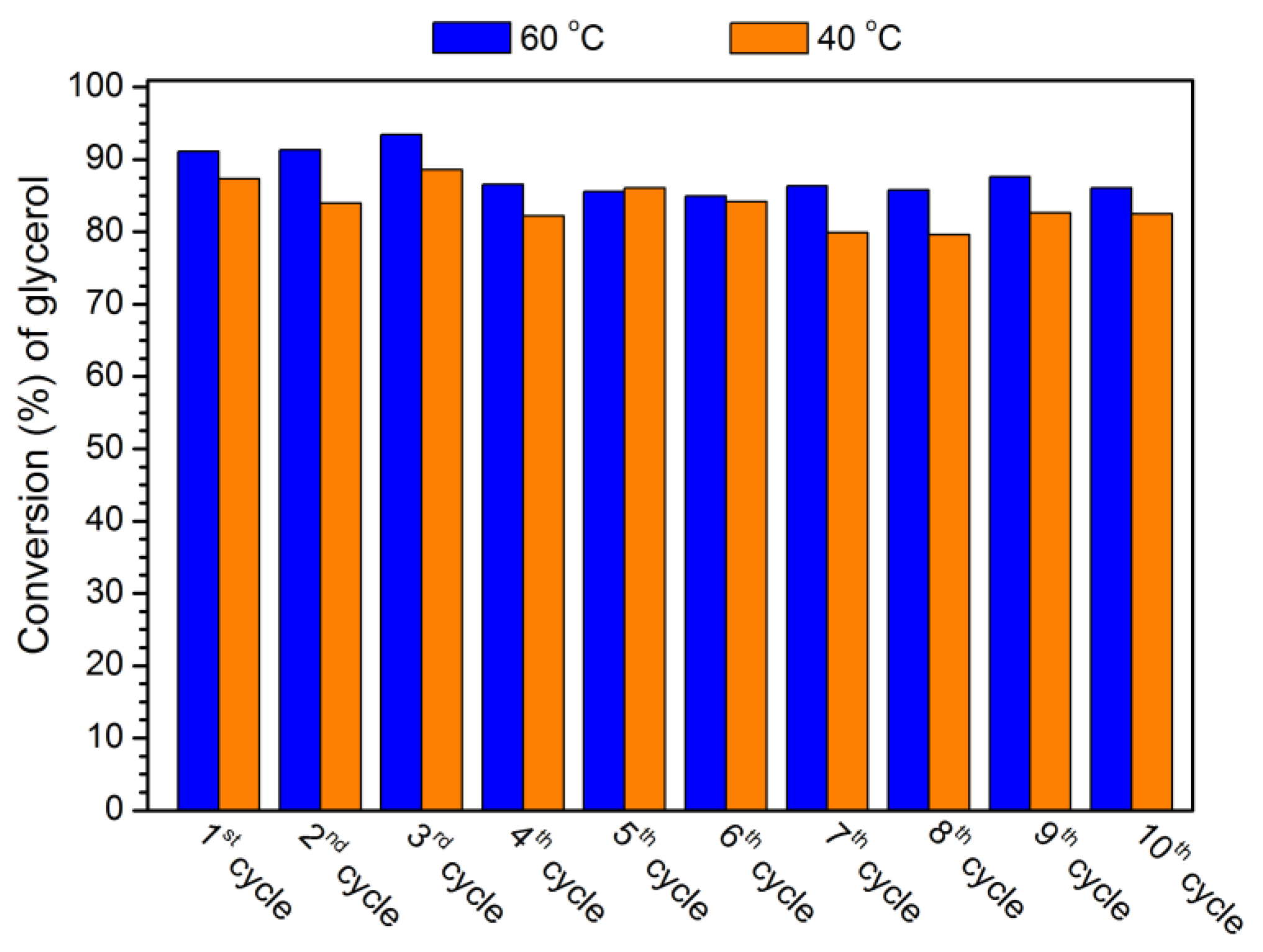
3.5. Reutilization of MOF-808(Hf) catalyst
3.6. Comparison with repotred related catalysts
3.7. Catalyst stability
5. Concluding remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yue, X.-L.; Gao, Q.-X. , Contributions of natural systems and human activity to greenhouse gas emissions. Advances in Climate Change Research 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Gollakota, A. R. K.; Kishore, N.; Gu, S. , A review on hydrothermal liquefaction of biomass. Renewable and Sustainable Energy Reviews 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- IEA Renewables 2019; 2019.
- Moreira, M. N.; Corrêa, I.; Ribeiro, A. M.; Rodrigues, A. E.; Faria, R. P. V. , Solketal Production in a Fixed Bed Adsorptive Reactor through the Ketalization of Glycerol. Industrial & Engineering Chemistry Research 2020, 59, 2805–2816. [Google Scholar]
- Research, M. Glycerol Market Size, Share & Trends Analysis Report By Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), By Type (Crude, Refined) By End Use (Food & Beverage, Pharmaceutical), By Region, And Segment Forecasts, 2020 - 2027; San Francisco, CA, USA,, 2020; p 148.
- Nda-Umar, U. I.; Ramli, I.; Taufiq-Yap, Y. H.; Muhamad, E. N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Catalysts 2019, 9((1)). [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. , Green Chemistry: Principles and Practice. Chemical Society Reviews 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, I.; Faria, R. P. V.; Rodrigues, A. E. , Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustainable Chemistry 2021, 2((2)). [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. , Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renewable and Sustainable Energy Reviews 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Al-Saadi, L. S.; Eze, V. C.; Harvey, A. P. , Techno-economic analysis of glycerol valorization via catalytic applications of sulphonic acid-functionalized copolymer beads. Frontiers in chemistry 2020, 7, 882. [Google Scholar] [CrossRef]
- Nanda, M. R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H. S.; Xu, C. , Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renewable and Sustainable Energy Reviews 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Menezes, F. D. L.; Guimaraes, M. D. O.; da Silva, M. J. , Highly Selective SnCl2-Catalyzed Solketal Synthesis at Room Temperature. Industrial & Engineering Chemistry Research 2013, 52, 16709–16713. [Google Scholar]
- Clarkson, J. S.; Walker, A. J.; Wood, M. A. , Continuous reactor technology for ketal formation: An improved synthesis of solketal. Organic Process Research and Development 2001, 5, 630–635. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. , Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. Journal of Catalysis 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I. M.; Ramos, A. M.; Vital, J.; Castanheiro, J. E. , Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Applied Catalysis B: Environmental 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J. A.; Morales, G.; Paniagua, M.; Martín, E. , Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chemistry 2010, 12, 899–907. [Google Scholar] [CrossRef]
- da Silva, C. X. A.; Gonçalves, V. L. C.; Mota, C. J. A. , Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chemistry 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Férey, G. , Hybrid porous solids: past, present, future. Chemical Society Reviews 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Guo, J.; Qin, Y.; Zhu, Y.; Zhang, X.; Long, C.; Zhao, M.; Tang, Z. , Metal–organic frameworks as catalytic selectivity regulators for organic transformations. Chemical Society Reviews 2021, 50, 5366–5396. [Google Scholar] [CrossRef]
- Rui, K.; Zhao, G.; Chen, Y.; Lin, Y.; Zhou, Q.; Chen, J.; Zhu, J.; Sun, W.; Huang, W.; Dou, S. X. , Hybrid 2D Dual-Metal–Organic Frameworks for Enhanced Water Oxidation Catalysis. Advanced Functional Materials 2018, 28, 1801554. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. , Catalysis and photocatalysis by metal organic frameworks. Chemical Society Reviews 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Timofeeva, M. N.; Panchenko, V. N.; Khan, N. A.; Hasan, Z.; Prosvirin, I. P.; Tsybulya, S. V.; Jhung, S. H. , Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Applied Catalysis A: General 2017, 529, 167–174. [Google Scholar] [CrossRef]
- Bakuru, V. R.; Churipard, S. R.; Maradur, S. P.; Kalidindi, S. B. , Exploring the Brønsted acidity of UiO-66 (Zr, Ce, Hf) metal–organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Transactions 2019, 48, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Santos-Vieira, I. C. M. S.; Mendes, R. F.; Almeida Paz, F. A.; Rocha, J.; Simões, M. M. Q. , Solketal Production via Solvent-Free Acetalization of Glycerol over Triphosphonic-Lanthanide Coordination Polymers. Catalysts 2021, 11((5)). [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Ye, B.; Hou, Z. , Acetalization of glycerol over sulfated UiO-66 under mild condition. Journal of Industrial and Engineering Chemistry 2022, 110, 357–366. [Google Scholar] [CrossRef]
- Dashtipour, B.; Dehghanpour, S.; Sharbatdaran, M. , Improvement of the acidic properties of MOF by doped SnO2 quantum dots for the production of solketal. Journal of Chemical Sciences 2022, 134, 106. [Google Scholar] [CrossRef]
- Melchiorre, M.; Lentini, D.; Cucciolito, M. E.; Taddeo, F.; Hmoudah, M.; Di Serio, M.; Ruffo, F.; Russo, V.; Esposito, R. , Sustainable Ketalization of Glycerol with Ethyl Levulinate Catalyzed by the Iron(III)-Based Metal-Organic Framework MIL-88A. Molecules 2022, 27, 7229. [Google Scholar] [CrossRef]
- Plessers, E.; Fu, G.; Tan, C. Y.; De Vos, D. E.; Roeffaers, M. B. J. , Zr-Based MOF-808 as Meerwein–Ponndorf–Verley Reduction Catalyst for Challenging Carbonyl Compounds. Catalysts 2016, 6((7)). [Google Scholar] [CrossRef]
- Liu, Y.; Klet, R. C.; Hupp, J. T.; Farha, O. , Probing the correlations between the defects in metal–organic frameworks and their catalytic activity by an epoxide ring-opening reaction. Chemical Communications 2016, 52, 7806–7809. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Liu, Y.; Hupp, J. T.; Farha, O. K. , Instantaneous Hydrolysis of Nerve-Agent Simulants with a Six-Connected Zirconium-Based Metal–Organic Framework. Angewandte Chemie International Edition 2015, 54, 6795–6799. [Google Scholar] [CrossRef]
- Granadeiro, C. M.; Silva, P.; Saini, V. K.; Paz, F. A. A.; Pires, J.; Cunha-Silva, L.; Balula, S. S. , Novel heterogeneous catalysts based on lanthanopolyoxometalates supported on MIL-101(Cr). Catalysis Today 2013, 218, 35–42. [Google Scholar] [CrossRef]
- Balula, S. S.; Granadeiro, C. M.; Barbosa, A. D. S.; Santos, I.; Cunha-Silva, L. , Multifunctional catalyst based on sandwich-type polyoxotungstate and MIL-101 for liquid phase oxidations. Catalysis Today 2013, 210, 142–148. [Google Scholar] [CrossRef]
- Granadeiro, C. M.; de Castro, B.; Balula, S. S.; Cunha-Silva, L. , Lanthanopolyoxometalates: From the structure of polyanions to the design of functional materials. Polyhedron 2013, 52, 10–24. [Google Scholar] [CrossRef]
- Juliao, D.; Gomes, A. C.; Pillinger, M.; Valenca, R.; Ribeiro, J. C.; de Castro, B.; Goncalves, I. S.; Cunha Silva, L.; Balula, S. S. , Zinc-Substituted Polyoxotungstate@amino-MIL-101(Al) - An Efficient Catalyst for the Sustainable Desulfurization of Model and Real Diesels. European Journal of Inorganic Chemistry 2016, (32), 5114–5122. [Google Scholar] [CrossRef]
- Viana, A. M.; Juliao, D.; Mirante, F.; Faria, R. G.; de Castro, B.; Balula, S. S.; Cunha-Silva, L. , Straightforward activation of metal-organic framework UiO-66 for oxidative desulfurization processes. Catalysis Today 2021, 362, 28–34. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P. H.; Teller, E. , Adsorption of Gases in Multimolecular Layers. Journal of the American Chemical Society 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Thommes, M. , Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distributions. Carbon 2004, 42, 1227–1232. [Google Scholar] [CrossRef]
- Peixoto, A. F.; Soliman, M. M. A.; Pinto, T. V.; Silva, S. M.; Costa, P.; Alegria, E. C. B. A.; Freire, C. , Highly active organosulfonic aryl-silica nanoparticles as efficient catalysts for biomass derived biodiesel and fuel additives. Biomass and Bioenergy 2021, 145, 105936. [Google Scholar] [CrossRef]
- Hu, Z.; Kundu, T.; Wang, Y.; Sun, Y.; Zeng, K.; Zhao, D. , Modulated Hydrothermal Synthesis of Highly Stable MOF-808(Hf) for Methane Storage. ACS Sustainable Chemistry & Engineering 2020, 8, 17042–17053. [Google Scholar]
- Fernandes, S. C.; Viana, A. M.; de Castro, B.; Cunha-Silva, L.; Balula, S. S. , Synergistic combination of the nanoporous system of MOF-808 with a polyoxomolybdate to design an effective catalyst: simultaneous oxidative desulfurization and denitrogenation processes. Sustainable Energy & Fuels 2021, 5, 4032–4040. [Google Scholar]
- Julião, D.; Mirante, F.; Balula, S. S. Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. In Molecules. 2022, Vol. 27. [Google Scholar]
- Smirnov, A. A.; Selishcheva, S. A.; Yakovlev, V. A. , Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8((12)). [Google Scholar] [CrossRef]
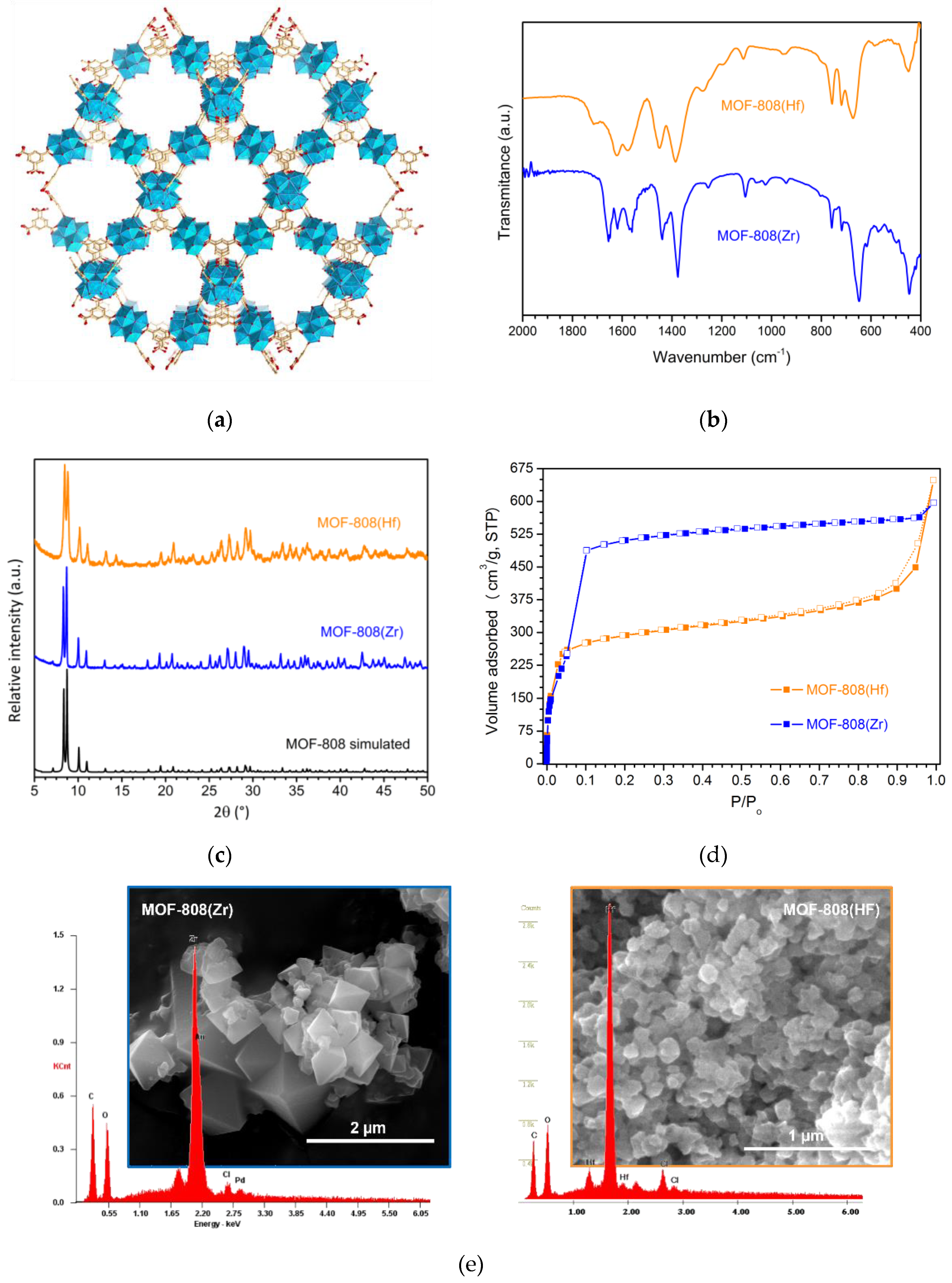
| MOF | pH (before titration) | Acidity (mmol.g-1) |
|---|---|---|
| MOF-808(Hf) | 3.61 | 0.7505 |
| MOF-808(Zr) | 4.35 | 0.4292 |
| Catalyst | Temperature (ºC) | Glycerol / Acetone | Time(h) | Conversion (%) | Reference |
|---|---|---|---|---|---|
| MIL-100(V) | 25 | 1:5 | 1.5 | 83 (98) | [22] |
| MIL-47(V) | 25 | 1:5 | 1.5 | 73 (87( | [22] |
| UiO-66-Zr | r. t. | 1:4 | 1 | 1.5 (73) | [23] |
| UiO-66-Hf | r. t. | 1:4 | 1 | 94 (97) | [23] |
| UiO-66-SO3H | 60 | 1:10 | 1 | 60 (99) | [25] |
| Mil-118-SnO2 | reflux | 1:10 | 4 | 76 (97) | [26] |
| UAV-63 | 55 | 1:10 | 6 | 84(96) | [24] |
| MOF-808(Zr) | 60 | 1:6 | 3 | 6 (100) | This work |
| MOF-808(Hf) | 60 | 1:6 | 3 | 91 (98) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





