Submitted:
09 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Design
2.1. Participants and Selection Criteria
2.2. Ethics and dissemination
2.3. Interventions
2.4. Treatments
2.5. Primary Outcome
2.5.1. Electrical taste perception
2.6. Secondary Outcomes
2.6.1. Chemical taste perception
2.6.2. Smell perception
2.6.3. Nutritional status
2.6.4. Morphofunctional Assessment
2.6.4.1. Anthropometric parameters
2.6.4.2. Electrical bioimpedance
2.6.4.3. Dynamometry
2.6.4.4. Nutritional Ultrasound
2.6.4.5. Functionality test
2.6.5. Diet
2.6.6. Physical Activity
2.6.7. Quality of life
2.6.8. Tolerance and adverse events
2.6.9. General biochemical parameters
2.6.10. Specialized biochemical parameters
2.6.10.1. Essential fatty acids and polyunsaturated fatty acid status
2.6.10.2. Biomarkers of inflammation (plasma cytokines)
2.6.10.3. Oxidative stress and antioxidant defense system (ADS)
2.6.10.4. Plasma metabolomics
2.6.10.5. Oral and intestinal microbiota and metagenomics
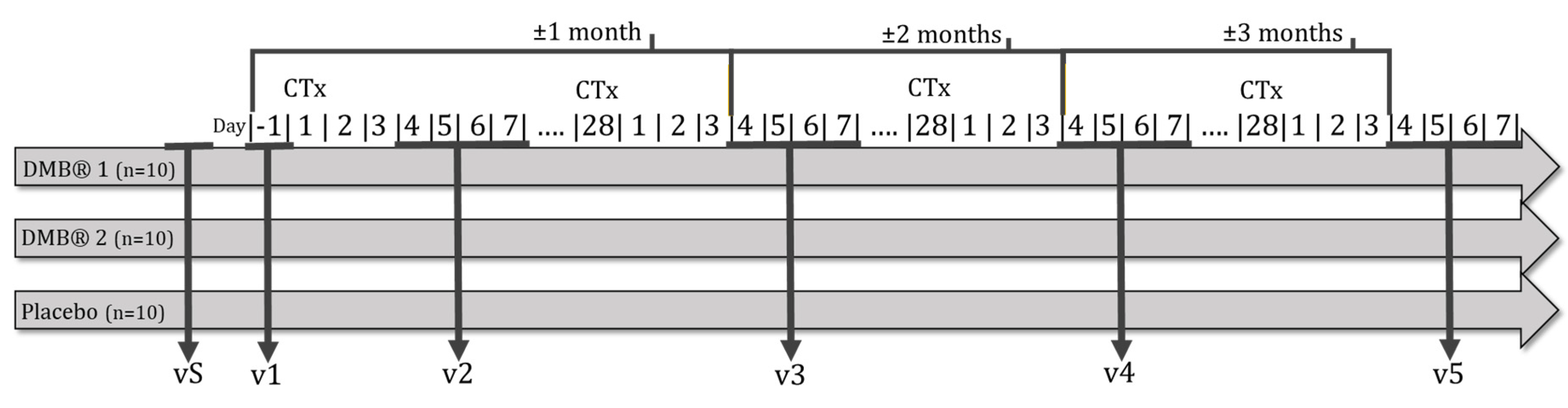
2.7. Recruitment and timeline
2.8. Sample size
2.9. Assignment of interventions
2.10. Data collection and management
2.11. Statistical methods
2.12. Monitoring
3. Materials and Equipment
3.1. Olfactory-gustatory tests
3.1.1. Electrogustometry
3.1.2. Taste strips test
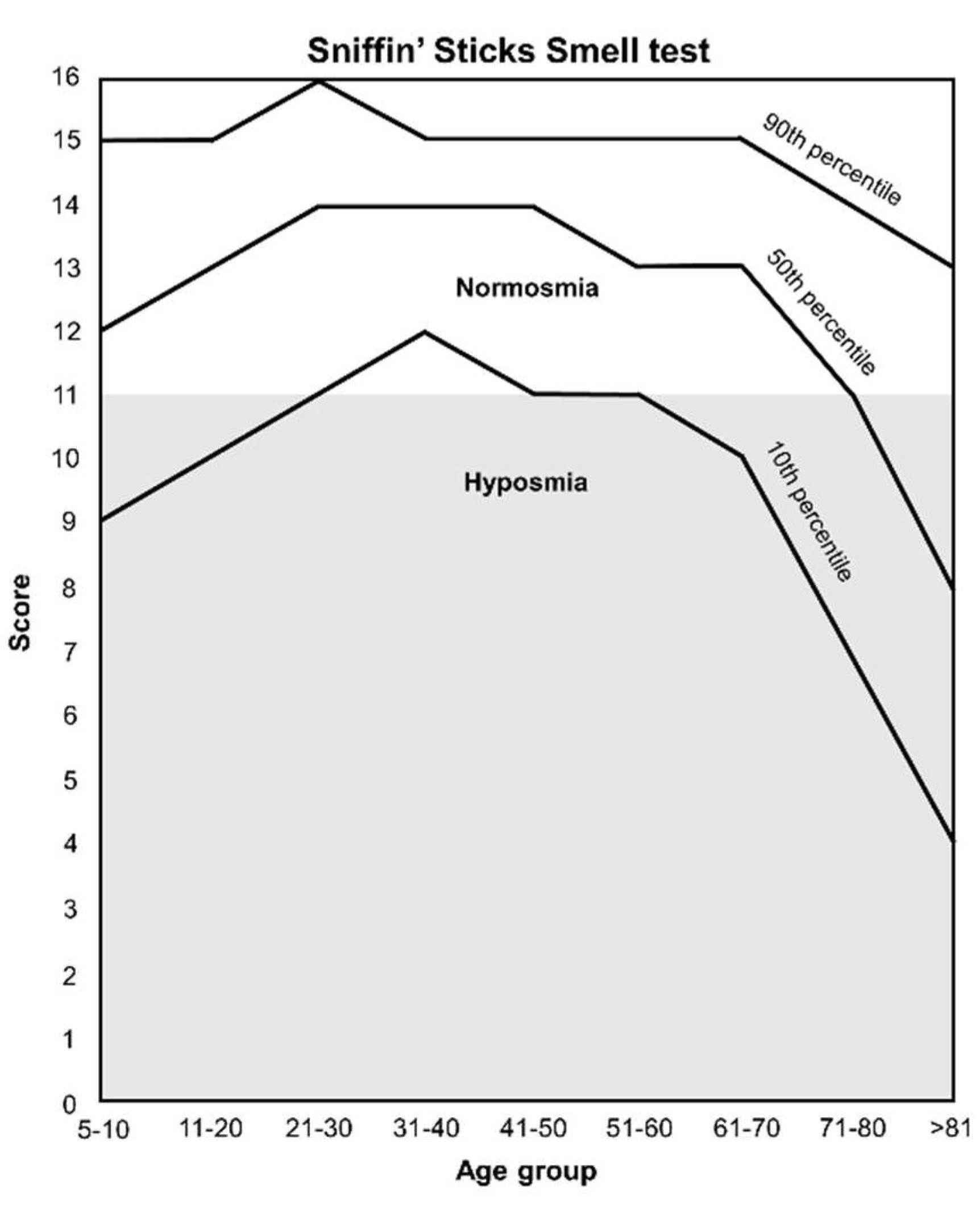
3.1.3. Sniffin’ Sticks Smell test
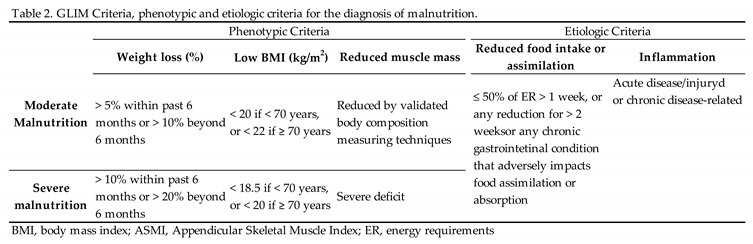
3.2. Malnutrition Criteria
3.3. Morphofunctional Studies
3.3.1. Anthropometric parameters
3.3.2. Bioelectrical Impedance Analysis
3.3.3. Dinamometry
3.3.4. Nutritional ultrasound
3.3.5. Timed up and Go Test
3.4. Surveys
3.4.1. Taste and Smell Survey
3.4.2. Food Daily Record
3.4.3. Food Frequency Questionnaire
3.4.4. Physical Activity
3.4.5. Quality of life
3.4.6. Miraculin-based food supplement perception efficacy
3.4.7. Tolerance and Adverse Events
3.4.8. Blood pressure and heart rate
3.5. Blood Parameters
3.5.1. General biochemical analysis.
3.5.2. Specialized biochemical analysis
3.5.2.1. Plasma and erythrocyte collection
3.5.2.2. Urine collection
3.5.2.3. Fatty acid profile of erythrocytes
3.5.2.4. Evaluation of corporal oxidative stress
3.5.2.5. Evaluation of the antioxidant defense system Plasma total antioxidant capacity (TAC)
3.5.2.6. Plasma cytokines
3.5.2.7. Plasma metabolomics analysis.
3.6. Saliva and stool microbiota
3.6.1. Saliva sampling
3.6.2. Stool sampling
3.6.3. Flow cytometry
3.6.4. Fecal DNA amplification and sequencing
3.6.5. Saliva and stool metagenomics
4. Detailed Procedure
4.1. Selection Phase
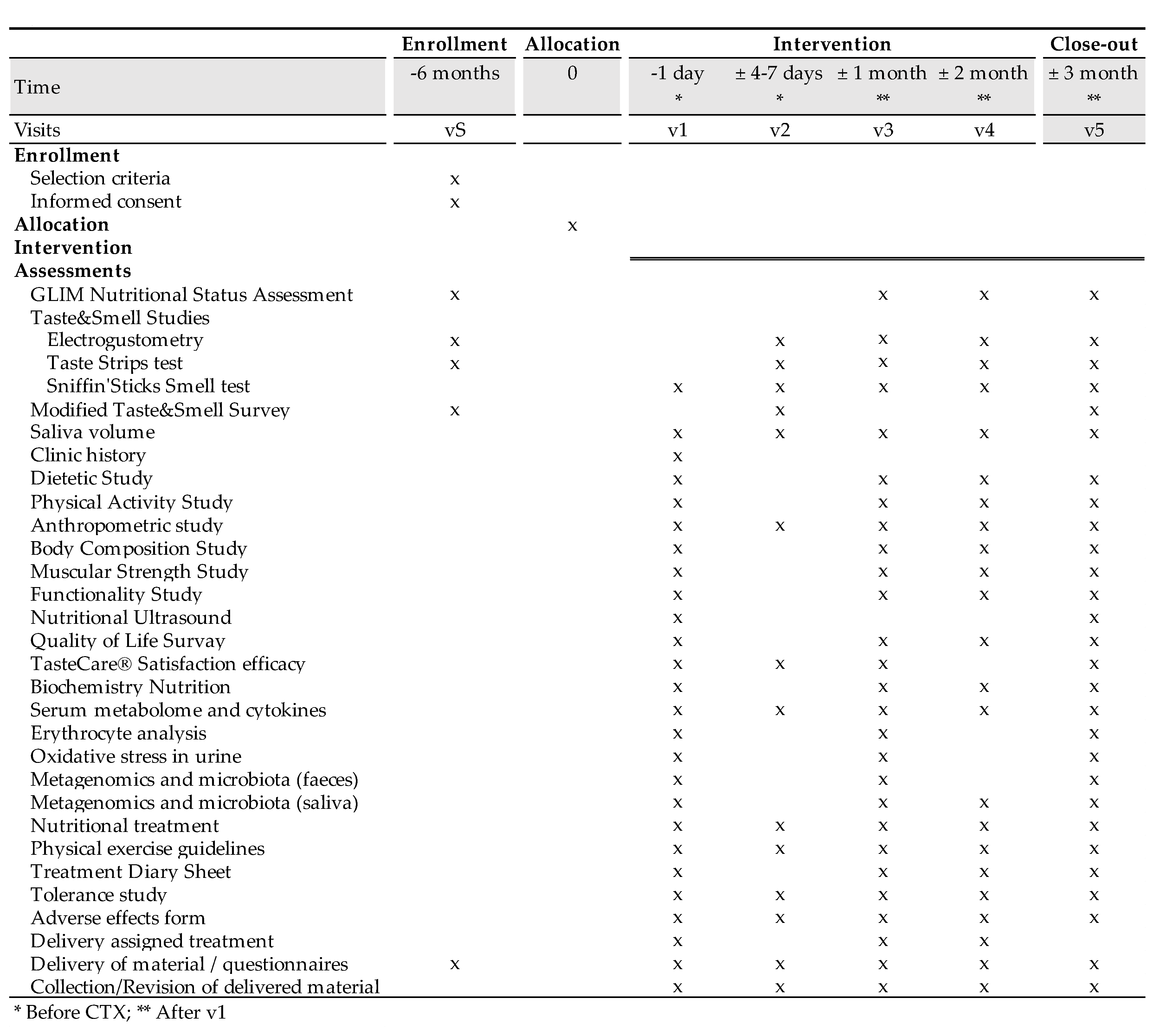
4.1.1. Visit 0 (v0, selection visit).
- Nutritional status assessment (GLIM Criteria)
- Electrogustometry
- Taste strips test
- Taste and Smell Survey Modified
- Food Daily Record (3 days per holiday)
- Food Frequency Questionnaire
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Blood sample extraction appointment
- Stool container for microbiota and metagenome analysis
- Urine container for 8-iso-PGF2α determination
4.2. Experimental Phase
4.2.1. Visit 1 (v1)
- Health study (blood pressure and heart rate)
-
Morphofunctional assessment:
- ▪
- Anthropometric measurements
- ▪
- Electrical bioimpedance
- ▪
- Dynamometry
- ▪
- Nutritional ultrasound
- ▪
- Up and Go Test
- Sniffin’ Stick Smell Test
- Collection and measurement of saliva volume
-
Blood sample extraction coinciding with the prechemotherapy analysis:
- ▪
- Biochemical parameters
- ▪
- Plasma metabolomic analysis
- ▪
- Plasma cytokine profile
- ▪
- Fatty acids from the erythrocyte membrane
- ▪
- Enzymatic antioxidant defense system in erythrocytes
- ▪
- Body oxidative stress in urine
- ▪
- Fecal microbiota and metagenome
- ▪
- Saliva microbiota and metagenome
- Food Daily Record (3 days per holiday)
- Food consumption frequency
- International Physical Activity Questionnaire
- Quality of life questionnaire
- Feces sample
- Urine sample
- Nutritional treatment. If an oral nutritional supplement is needed, a polymeric, hypercaloric, and hyperproteic formula enriched in omega-3 fatty acids is prescribed depending on their energy requirements.
- Healthy eating guidelines for cancer patients using the book “Cooking with Science Against Cancer” as a support are given to the patient.
- Physical exercise guidelines
- Product efficacy satisfaction questionnaire (prechemotherapy)
- Next face-to-face visit date.
- The following documentation is also delivered to bring on the third visit (v3):
- Product consumption control daily sheet
- Product consumption tolerance record sheet
- Record sheet of adverse effects
4.2.1. Visit 2 (v2)
- Anthropometric measurements
- Electrogustometry
-
Smell and taste tests:
- ▪
- Taste Strips Test
- ▪
- Sniffin ‘Sticks Smell Test
- Collection and measurement of saliva volume
- Taste and Smell Modified Survey
- Product Efficacy Satisfaction Questionnaire (Post Chemotherapy)
- Nutritional treatment and physical activity
- Consumption and registration of the assigned treatment.
- Tolerance and adverse effects registry
- Food Daily Record (3 days per holiday)
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Blood sample extraction appointment
- Feces container
- Urine container
4.2.1. Visit 3 (v3)
- Nutritional status assessment
- Health study (blood pressure and heart rate)
-
Morphofunctional assessment:
- ▪
- Anthropometric measurements
- ▪
- Electrical bioimpedance
- ▪
- Dynamometry
- ▪
- Up and Go Test
- Electrogustometry
-
Smell and taste tests:
- ▪
- Taste Strips Test
- ▪
- Sniffin ‘Sticks Smell Test
- Collection and measurement of saliva volume
-
Blood sample extraction coinciding with the prechemotherapy analysis:
- ▪
- Biochemical parameters
- ▪
- Plasma metabolomic analysis
- ▪
- Plasma cytokine profile
- ▪
- Fatty acids from the erythrocyte membrane
- ▪
- Enzymatic antioxidant defense system in erythrocytes
- ▪
- Body oxidative stress in urine
- ▪
- Fecal microbiota and metagenome
- ▪
- Saliva microbiota and metagenome
- Product efficacy satisfaction questionnaire
- Food Daily Record (3 days per holiday)
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Product consumption control daily sheet
- Product consumption tolerance record sheet
- Record sheet of adverse effects
- Feces sample
- Urine sample
- Nutritional treatment and physical activity
- Consumption and registration of the assigned treatment.
- Tolerance and adverse effects registry
- Next face-to-face visit date
- Treatment product based on randomization
- Blood sample extraction appointment
- Food Daily Record (3 days per holiday)
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Product consumption control daily sheet
- Product consumption tolerance record sheet
- Record sheet of adverse effects
4.2.1. Visit 4 (v4)
- Nutritional status assessment
- Health study (blood pressure and heart rate)
-
Morphofunctional assessment:
- ▪
- Anthropometric measurements
- ▪
- Electrical bioimpedance
- ▪
- Dynamometry
- ▪
- Up and Go Test
- Electrogustometry
-
Smell and taste tests:
- ▪
- Taste Strips Test
- ▪
- Sniffin’ Sticks Smell Test
- Collection and measurement of saliva volume
-
Blood sample extraction coinciding with the prechemotherapy analysis:
- ▪
- Biochemical parameters
- ▪
- Plasma metabolomic analysis
- ▪
- Plasma cytokine profile
- ▪
- Fatty acids from the erythrocyte membrane
- ▪
- Enzymatic antioxidant defense system in erythrocytes
- ▪
- Body oxidative stress in urine
- ▪
- Fecal microbiota and metagenome
- ▪
- Saliva microbiota and metagenome
- Food Daily Record (3 days, one holiday)
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Product consumption control daily sheet
- Product consumption tolerance record sheet
- Record sheet of adverse effects
- Nutritional treatment and physical activity
- Consumption and registration of the assigned treatment.
- Tolerance and adverse effects registry
- Next face-to-face visit date
- Treatment product based on randomization
- Food Daily Record (3 days per holiday)
- Food consumption frequency
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Product consumption control daily sheet
- Product consumption tolerance record sheet
- Record sheet of adverse effects
- Blood sample extraction appointment
- Feces sample
- Urine sample
4.2.1. Visit 5 (v5)
- Nutritional status assessment
- Health study (blood pressure and heart rate)
-
Morphofunctional assessment:
- ▪
- Anthropometric measurements
- ▪
- Electrical bioimpedance
- ▪
- Dynamometry
- ▪
- Nutritional ultrasound
- ▪
- Up and Go Test
- Electrogustometry
- Taste and Smell Survey Modified
-
Smell and taste tests:
- ▪
- Taste Strips Test
- ▪
- Sniffin’ Sticks Smell Test
- Collection and measurement of saliva volume
-
Blood sample extraction coinciding with the prechemotherapy analysis:
- ▪
- Biochemical parameters
- ▪
- Plasma metabolomic analysis
- ▪
- Plasma cytokine profile
- ▪
- Fatty acids from the erythrocyte membrane
- ▪
- Enzymatic antioxidant defense system in erythrocytes
- ▪
- Body oxidative stress in urine
- ▪
- Fecal microbiota and metagenome
- ▪
- Saliva microbiota and metagenome
- Product efficacy satisfaction questionnaire
- Food Daily Record (3 days, one holiday)
- Food consumption frequency
- International Physical Activity Questionnaire
- Quality of Life Questionnaire
- Product consumption control daily sheet
- Product consumption tolerance record sheet
- Record sheet of adverse effects
- Feces sample
- Urine sample
- Nutritional treatment and physical activity
5. Expected Results
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix: Informed consent form
References
- Hovan, A.J.; Williams, P.M.; Stevenson-moore, P.; Wahlin, Y.B. A Systematic Review of Dysgeusia Induced by Cancer Therapies. Support Care Cancer 2010, 18, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Erkurt, E.; Erkişi, M.; Tunali, C. Supportive Treatment in Weight-Losing Cancer Patients Due to the Additive Adverse Effects of Radiation Treatment and/or Chemotherapy. J. Exp. Clin. Cancer Res. 2000. [Google Scholar]
- Amézaga, J.; Alfaro, B.; Ríos, Y.; Larraioz, A.; Ugartemendia, G.; Urruticoechea, A.; Tueros, I. Assessing Taste and Smell Alterations in Cancer Patients Undergoing Chemotherapy According to Treatment. Support. Care Cancer 2018, 26, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Gamper, E.M.; Zabernigg, A.; Wintner, L.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Sperner-Unterweger, B.; Holzner, B. Coming to Your Senses: Detecting Taste and Smell Alterations in Chemotherapy Patients. A Systematic Review. J. Pain Symptom Manage. 2012, 44, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Berteretche, M.V.; Dalix, A.M.; D’Ornano, A.M.C.; Bellisle, F.; Khayat, D.; Faurion, A. Decreased Taste Sensitivity in Cancer Patients under Chemotherapy. Support. Care Cancer 2004, 12, 571–576. [Google Scholar] [CrossRef]
- Ponticelli, E.; Clari, M.; Frigerio, S.; De Clemente, A.; Bergese, I.; Scavino, E.; Bernardini, A.; Sacerdote, C. Dysgeusia and Health-Related Quality of Life of Cancer Patients Receiving Chemotherapy: A Cross-Sectional Study. Eur. J. Cancer Care (Engl). 2017, 26. [Google Scholar] [CrossRef]
- Henkin, R.I.; Schecter, P.J.; Friedewald, W.T.; Demets, D.L.; Raff, M. A Double Blind Study of the Effects of Zinc Sulfate on Taste and Smell Dysfunction. Am. J. Med. Sci. 1976, 272, 285–299. [Google Scholar] [CrossRef]
- Ripamonti, C.; Zecca, E.; Brunelli, C.; Fulfaro, F.; Villa, S.; Balzarini, A.; Bombardieri, E.; De Conno, F. A Randomized, Controlled Clinical Trial to Evaluate the Effects of Zinc Sulfate on Cancer Patients with Taste Alterations Caused by Head and Neck Irradiation. Cancer 1998, 82, 1938–1945. [Google Scholar] [CrossRef]
- Lyckholm, L.; Heddinger, S.P.; Parker, G.; Coyne, P.J.; Ramakrishnan, V.; Smith, T.J.; Henkin, R.I. A Randomized, Placebo Controlled Trial of Oral Zinc for Chemotherapy-Related Taste and Smell Disorders. J. Pain Palliat. Care Pharmacother. 2012, 26, 111–114. [Google Scholar] [CrossRef]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III Randomized Trial of Amifostine as a Radioprotector in Head and Neck Cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef]
- Büntzel, J.; Schuth, J.; Küttner, K.; Glatzel, M. Radiochemotherapy with Amifostine Cytoprotection for Head and Neck Cancer. Support. Care Cancer 1998, 6, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Komaki, R.; Lee, J.S.; Milas, L.; Lee, H.K.; Fossella, F.V.; Herbst, R.S.; Allen, P.K.; Liao, Z.; Stevens, C.W.; Lu, C.; et al. Effects of Amifostine on Acute Toxicity from Concurrent Chemotherapy and Radiotherapy for Inoperable Non-Small-Cell Lung Cancer: Report of a Randomized Comparative Trial. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Merlo, F.D.; Agnello, E.; Monge, T.; Devecchi, A.; Casalone, V.; Montemurro, F.; Ghigo, E.; Sapino, A.; Bo, S. Dysgeusia in Patients with Breast Cancer Treated with Chemotherapy-A Narrative Review. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, S.M.; Habiger, S.; Wichmann, M.G., H. T. Zinc Gluconate in the Treatment of Dysgeusia: A Double Blinded Controlled Study. Chem. Senses. 2033, 28, E24. [Google Scholar]
- Hsieh, J.W.; Daskalou, D.; Macario, S.; Voruz, F.; Landis, B.N. How to Manage Taste Disorders. Curr. Otorhinolaryngol. Rep. 2022, 10, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Beidler, L.M. Taste-Modifying Protein from Miracle Fruit. Science 1968, 161, 1241–1243. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F. and F.A. (NDA); Turck, D.; Castenmiller, J.; Henauw, S. De; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Dried Fruits of Synsepalum Dulcificum as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6600. [Google Scholar] [CrossRef]
- Soares, H.P.; Cusnir, M.; Schwartz, M.A.; Pizzolato, J.F.; Lutzky, J.; Campbell, R.J.; Beaumont, J.L.; Eton, D.; Stonick, S.; Lilenbaum, R. Treatment of Taste Alterations in Chemotherapy Patients Using the “Miracle Fruit”: Preliminary Analysis of a Pilot Study. J. Clin. Oncol. 2010, 28, e19523–e19523. [Google Scholar] [CrossRef]
- Wilken, M.K.; Satiroff, B.A. Pilot Study of “Miracle Fruit” to Improve Food Palatability for Patients Receiving Chemotherapy. Clin. J. Oncol. Nurs. 2012, 16. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition - A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Frank, M.E.; Smith, D.V. Electrogustometry: A Simple Way to Test Taste. In Smell and Taste in Health and Disease; Getchell, T.V., Doty, R.L., Vartoshuk, L.M., Snow, J.B., Eds.; New York, 1991; pp. 503–5014. [Google Scholar]
- Barry, M.A.; Gatenby, J.C.; Zeiger, J.D.; Gore, J.C. Hemispheric Dominance of Cortical Activity Evoked by Focal Electrogustatory Stimuli. Chem. Senses 2001, 26, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Hichami, A.; Khan, A.S.; Ghiringhelli, F.; Khan, N.A. Alteration in Taste Perception in Cancer: Causes and Strategies of Treatment. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Bromley, S.M. Smell and Taste Disorders: A Primary Care Approach. Am. Fam. Physician 2000, 61, 427–436. [Google Scholar]
- Özkan, İ.; Taylan, S.; Eroğlu, N.; Kolaç, N. The Relationship between Malnutrition and Subjective Taste Change Experienced by Patients with Cancer Receiving Outpatient Chemotherapy Treatment. Nutr. Cancer 2022, 74, 1670–1679. [Google Scholar] [CrossRef]
- De Melo Silva, F.R.; De Oliveira, M.G.O.A.; Souza, A.S.R.; Figueroa, J.N.; Santos, C.S. Factors Associated with Malnutrition in Hospitalized Cancer Patients: A Croos-Sectional Study. Nutr. J. 2015, 14, 1–8. [Google Scholar] [CrossRef]
- Mueller, C. , Kallert, S. , Renner, B., Stiassny, K., Temmel, A.F., Hummel, T., Kobal, G. Quantitative Assessment of Gustatory Function in a Clinical Context Using Impregnated “Taste Strips.” Rhinology 2003, 41, 2–6. [Google Scholar]
- Sevryugin, O.; Kasvis, P.; Vigano, M.L.; Vigano, A. Taste and Smell Disturbances in Cancer Patients: A Scoping Review of Available Treatments. Support. Care Cancer 2021, 29, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Okada, N.; Nakamura, S.; Kagawa, K.; Fujii, S.; Miki, H.; Ishizawa, K.; Abe, M.; Sato, Y. A Genome-Wide Association Study Predicts the Onset of Dysgeusia Due to Anti-Cancer Drug Treatment. Biol. Pharm. Bull. 2022, 45, 114–117. [Google Scholar] [CrossRef]
- Cohen, J.; E. Wakefield, C.; G. Laing, D. Smell and Taste Disorders Resulting from Cancer and Chemotherapy. Curr. Pharm. Des. 2016, 22, 2253–2263. [Google Scholar] [CrossRef]
- Uí Dhuibhir, P.; Barrett, M.; O’Donoghue, N.; Gillham, C.; El Beltagi, N.; Walsh, D. Self-Reported and Objective Taste and Smell Evaluation in Treatment-Naive Solid Tumour Patients. Support. Care Cancer 2020, 28, 2389–2396. [Google Scholar] [CrossRef]
- Rozin, P. “Taste-Smell Confusions” and the Duality of the Olfactory Sense. Percept. Psychophys. 1982, 31, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Podlesek, D. Clinical Assessment of Olfactory Function. Chem. Senses 2021, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Frasnelli, J.; Reden, J.; Lacroix, J.S.; Hummel, T. Differences between Orthonasal and Retronasal Olfactory Functions in Patients with Loss of the Sense of Smell. Arch. Otolaryngol. Head. Neck Surg. 2005, 131, 977–981. [Google Scholar] [CrossRef]
- Deems, D.A.; Doty, R.L.; Settle, R.G.; Moore-Gillon, V.; Shaman, P.; Mester, A.F.; Kimmelman, C.P.; Brightman, V.J.; Snow, J.B. Smell and Taste Disorders, a Study of 750 Patients from the University of Pennsylvania Smell and Taste Center. Arch. Otolaryngol. Head. Neck Surg. 1991, 117, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, B.B.; Leopold, D.A. Clinical Assessment of Patients with Smell and Taste Disorders. Otolaryngol. Clin. North Am. 2004, 37, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, L. Basics in Clinical Nutrition, Galen, Ed., 4th ed.; 2012. [Google Scholar]
- Pirlich, M.; Schütz, T.; Kemps, M.; Luhman, N.; Minko, N.; Lübke, H.J.; Rossnagel, K.; Willich, S.N.; Lochs, H. Social Risk Factors for Hospital Malnutrition. Nutrition 2005, 21, 295–300. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Almeida, J.M.G.; García, C.G.; Aguilar, I.M.V.; Castañeda, V.B.; Guerrero, D.B. Morphofunctional Assessment of Patient’s Nutritional Status: A Global Approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [CrossRef]
- García de Lorenzo, A.; Álvarez Hernández, J.; Planas, M.; Burgos, R.; Araujo, K. ; Spain, multidisciplinary consensus work-team on the approach to hospital malnutrition in Multidisciplinary Consensus on the Approach to Hospital Malnutrition in Spain. Nutr. Hosp. 2011, 26. [Google Scholar] [CrossRef]
- Madden, A.M.; Smith, S. Body Composition and Morphological Assessment of Nutritional Status in Adults: A Review of Anthropometric Variables. J. Hum. Nutr. Diet. 2016, 29, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Mili, N.; Paschou, S.A.; Goulis, D.G.; Dimopoulos, M.A.; Lambrinoudaki, I.; Psaltopoulou, T. Obesity, Metabolic Syndrome, and Cancer: Pathophysiological and Therapeutic Associations. Endocrine 2021, 74, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-Height Ratio Is a Better Screening Tool than Waist Circumference and BMI for Adult Cardiometabolic Risk Factors: Systematic Review and Meta-Analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic Criteria for Malnutrition - An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.A.; Ballinger, T.J.; Bhandari, R.; Dieli-Cornwright, C.M.; Guertin, K.A.; Hibler, E.A.; Kalam, F.; Lohmann, A.E.; Ippolito, J.E. Imaging Modalities for Measuring Body Composition in Patients with Cancer: Opportunities and Challenges. J. Natl. Cancer Inst. Monogr. 2023, 2023, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Chesworth, B.; Madill, J. Is Phase Angle an Appropriate Indicator of Malnutrition in Different Disease States? A Systematic Review. Clin. Nutr. ESPEN 2019, 29, 1–14. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical Phase Angle and Impedance Vector Analysis – Clinical Relevance and Applicability of Impedance Parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Arab, A.; Karimi, E.; Vingrys, K.; Shirani, F. Is Phase Angle a Valuable Prognostic Tool in Cancer Patients’ Survival? A Systematic Review and Meta-Analysis of Available Literature. Clin. Nutr. 2021, 40, 3182–3190. [Google Scholar] [CrossRef]
- García-García, C.; Vegas-Aguilar, I.M.; Rioja-Vázquez, R.; Cornejo-Pareja, I.; Tinahones, F.J.; García-Almeida, J.M. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Paiva, S.I.; Borges, L.R.; Halpern-Silveira, D.; Assunção, M.C.F.; Barros, A.J.D.; Gonzalez, M.C. Standardized Phase Angle from Bioelectrical Impedance Analysis as Prognostic Factor for Survival in Patients with Cancer. Support. Care Cancer 2010, 19, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, J.; Cheng, S.; Liang, B. The Role of Standardized Phase Angle in the Assessment of Nutritional Status and Clinical Outcomes in Cancer Patients: A Systematic Review of the Literature. Nutrients 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand Grip Strength: Outcome Predictor and Marker of Nutritional Status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Budziareck, M.B.; Pureza Duarte, R.R.; Barbosa-Silva, M.C.G. Reference Values and Determinants for Handgrip Strength in Healthy Subjects. Clin. Nutr. 2008, 27, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Gariballa, S.; Alessa, A. Impact of Poor Muscle Strength on Clinical and Service Outcomes of Older People during Both Acute Illness and after Recovery. BMC Geriatr. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Torralvo, F.J.; Porras, N.; Abuín Fernández, J.; García Torres, F.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Rojo Martínez, G.; Olveira, G. Normative Reference Values for Hand Grip Dynamometry in Spain. Association with Lean Mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip Strength across the Life Course: Normative Data from Twelve British Studies. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional Ultrasound®: Conceptualisation, Technical Considerations and Standardisation. Endocrinol. diabetes y Nutr. 2023, 70 Suppl 1, 74–84. [Google Scholar] [CrossRef]
- Berger, J.; Bunout, D.; Barrera, G.; de la Maza, M.P.; Henriquez, S.; Leiva, L.; Hirsch, S. Rectus Femoris (RF) Ultrasound for the Assessment of Muscle Mass in Older People. Arch. Gerontol. Geriatr. 2015, 61, 33–38. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Bergland, A.; Jørgensen, L.; Emaus, N.; Strand, B.H. Mobility as a Predictor of All-Cause Mortality in Older Men and Women: 11.8 Year Follow-up in the Tromsø Study. BMC Health Serv. Res. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Stähelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a Cut-off Point for Normal Mobility: A Comparison of the Timed “up and Go” Test in Community-Dwelling and Institutionalised Elderly Women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef]
- FD, O. Definition of Standardized Nutritional Assessment and Interventional Pathways in Oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14. [CrossRef]
- Serra Majem, L. Objetivos Nutricionales Para La Población Española: Consenso de La Sociedad Española de Nutrición Comunitaria 2011. Rev. española Nutr. comunitaria = Spanish J. community Nutr. 2011, 17, 178–199. [Google Scholar]
- López García, E.; Bretón Lesmes, I.; Díaz Perales, A.; Moreno Arribas, V.; Portillo Baquedano, M.; Rivas Velasco, A.; Fresán Salvo, U.; Tejedor Romero, L.; Ortega Porcel, B.; Lizalde Gil, M.; et al. Informe Del Comité Científico de La Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) Sobre Recomendaciones Dietéticas Sostenibles y Recomendaciones de Actividad Física Para La Población Española. Rev. científica la AESAN 2022, 36, 11–70. [Google Scholar]
- SENC Guía Alimentaria 2016 de La SENC. Nutr. Hosp. 2016, 33, 60.
- Gibney, E.; Elia, M.; Jebb, S.A.; Murgatroyd, P.; Jennings, G. Total Energy Expenditure in Patients with Small-Cell Lung Cancer: Results of a Validated Study Using the Bicarbonate-Urea Method. Metabolism 1997, 46, 1412–1417. [Google Scholar] [CrossRef]
- Moses, A.W.G.; Slater, C.; Preston, T.; Barber, M.D.; Fearon, K.C.H. Reduced Total Energy Expenditure and Physical Activity in Cachectic Patients with Pancreatic Cancer Can Be Modulated by an Energy and Protein Dense Oral Supplement Enriched with N-3 Fatty Acids. Br. J. Cancer 2004, 90, 996–1002. [Google Scholar] [CrossRef]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Medicine, I. of Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Diet. Ref. Intakes Energy, Carbohydrate, Fiber, Fat, Fat. Acids, Cholesterol, Protein, Amin. Acids, 2002; 1–1331. [Google Scholar] [CrossRef]
- Booth, M. Assessment of Physical Activity: An International Perspective. Res. Quartely Excercise Sport 2000, 71, s114–120. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.E.; Zandbergen, M.C.; Eerenstein, S.E.J.; van Tulder, M.W.; Leemans, C.R.; Kramer, M.H.H.; Weijs, P.J.M. Effect of Nutritional Interventions on Nutritional Status, Quality of Life and Mortality in Patients with Head and Neck Cancer Receiving (Chemo)Radiotherapy: A Systematic Review. Clin. Nutr. 2013, 32, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722–e732. [Google Scholar] [CrossRef] [PubMed]
- Boltong, A.; Keast, R.; Aranda, S. Experiences and Consequences of Altered Taste, Flavour and Food Hedonics during Chemotherapy Treatment. Support. Care Cancer 2012, 20, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Common Terminology Criteria for Adverse Events. 2009. Available from: Https://Evs.Nci.Nih.Gov/Ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.Pdf. 2009, 1–78.
- Kobayashi, Y.; Inose, H.; Ushio, S.; Yuasa, M.; Hirai, T.; Yoshii, T.; Okawa, A. Body Mass Index and Modified Glasgow Prognostic Score Are Useful Predictors of Surgical Site Infection After Spinal Instrumentation Surgery: A Consecutive Series. Spine (Phila. Pa. 1976). 2020, 45, E148–E154. [Google Scholar] [CrossRef]
- Beck, F.K.; Rosenthal, T.C. Prealbumin: A Marker for Nutritional Evaluation. Am. Fam. Physician 2002, 65, 1575–1579. [Google Scholar] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Kano, H.; Midorikawa, Y.; Song, P.; Nakayama, H.; Moriguchi, M.; Higaki, T.; Tsuji, S.; Takayama, T. High C-Reactive Protein/Albumin Ratio Associated with Reduced Survival Due to Advanced Stage of Intrahepatic Cholangiocarcinoma. Biosci. Trends 2020, 14, 304–309. [Google Scholar] [CrossRef]
- Li, L.; Dai, L.; Wang, X.; Wang, Y.; Zhou, L.; Chen, M.; Wang, H. Predictive Value of the C-Reactive Protein-to-Prealbumin Ratio in Medical ICU Patients. Biomark. Med. 2017, 11, 329–337. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 Polyunsaturated Fatty Acids, Inflammation, and Inflammatory Diseases. Am. J. Clin. Nutr. 2006, 83. [Google Scholar] [CrossRef] [PubMed]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic Acid (EPA, an Omega-3 Fatty Acid from Fish Oils) for the Treatment of Cancer Cachexia. Cochrane database Syst. Rev. 2007, 2007. [Google Scholar] [CrossRef]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 Fatty Acid-Derived Mediators That Control Inflammation and Tissue Homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Devarshi, P.P.; Ekimura, S.; Marshall, K.; Mitmesser, S.H. Long-Chain Omega-3 Fatty Acid Serum Concentrations across Life Stages in the USA: An Analysis of NHANES 2011-2012. BMJ Open 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, J.; Wang, J.; Lin, J.; Chen, L.; Lin, L. song; Pan, L. zhen; Shi, B.; Qiu, Y.; Zheng, X. yan; et al. Erythrocyte ω-3 Polyunsaturated Fatty Acids Are Inversely Associated with the Risk of Oral Cancer: A Case-Control Study. Nutr. Diabetes 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.W.; DeFina, L.F.; Tintle, N.L.; Leonard, D.; Cooper, K.H.; Barlow, C.E.; Haskell, W.L.; Pavlovic, A.; Harris, W.S. Association of the Omega-3 Index with Incident Prostate Cancer with Updated Meta-Analysis: The Cooper Center Longitudinal Study. Nutrients 2021, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Westheim, A.J.F.; Stoffels, L.M.; Dubois, L.J.; van Bergenhenegouwen, J.; van Helvoort, A.; Langen, R.C.J.; Shiri-Sverdlov, R.; Theys, J. The Modulatory Effects of Fatty Acids on Cancer Progression. Biomedicines 2023, 11. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-Associated Cachexia - Understanding the Tumour Macroenvironment and Microenvironment to Improve Management. Nat. Rev. Clin. Oncol. 2023, 20. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Pimentel, G.D.; Costa, F.O.; Carvalheira, J.B.C. Molecular and Neuroendocrine Mechanisms of Cancer Cachexia. J. Endocrinol. 2015, 226, R29–R43. [Google Scholar] [CrossRef]
- Tisdale, M.J. Are Tumoral Factors Responsible for Host Tissue Wasting in Cancer Cachexia? Future Oncol. 2010, 6, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L.; Petschow, B.W.; Shaw, A.L.; Weaver, E. Gut Barrier Dysfunction and Microbial Translocation in Cancer Cachexia: A New Therapeutic Target. Curr. Opin. Support. Palliat. Care 2013, 7, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial Reactive Oxygen Species and Cancer. Cancer Metab. 2014, 2, 99–116. [Google Scholar] [CrossRef]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive Oxygen Species in Cancer: A Dance with the Devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef]
- Shagieva, G.; Domnina, L.; Makarevich, O.; Chernyak, B.; Skulachev, V.; Dugina, V. Depletion of Mitochondrial Reactive Oxygen Species Downregulates Epithelial-to-Mesenchymal Transition in Cervical Cancer Cells. Oncotarget 2017, 8, 4901–4913. [Google Scholar] [CrossRef]
- Brooks, S.A.; Lomax-Browne, H.J.; Carter, T.M.; Kinch, C.E.; Hall, D.M.S. Molecular Interactions in Cancer Cell Metastasis. Acta Histochem. 2010, 112, 3–25. [Google Scholar] [CrossRef]
- Karwowski, B.T. FapydG in the Shadow of OXOdG-A Theoretical Study of Clustered DNA Lesions. Int. J. Mol. Sci. 2023, 24, 5361. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative Stress, DNA Methylation and Carcinogenesis. Cancer Lett. 2008, 266, 6–11. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways That Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Ziob, M.; Adamek, B.; Kasperczyk, J.; Romuk, E.; Hudziec, E.; Chwalińska, E.; Dobija-Kubica, K.; Rogoziński, P.; Bruliński, K. Activity of Antioxidant Enzymes in the Tumor and Adjacent Noncancerous Tissues of Non-Small-Cell Lung Cancer. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology. CA. Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Cui, P.; Li, X.; Huang, C.; Li, Q.; Lin, D. Metabolomics and Its Applications in Cancer Cachexia. Front. Mol. Biosci. 2022, 9. [Google Scholar] [CrossRef]
- Boguszewicz; Bieleń, A. ; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Wygoda, A.; Kotylak, A.; Składowski, K.; Sokół, M. NMR-Based Metabolomics in Real-Time Monitoring of Treatment Induced Toxicity and Cachexia in Head and Neck Cancer: A Method for Early Detection of High Risk Patients. Metabolomics 2019, 15. [Google Scholar] [CrossRef]
- Yang, Q.J.; Zhao, J.R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.L.; Wan, L.L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and Urine Metabolomics Study Reveals a Distinct Diagnostic Model for Cancer Cachexia. J. Cachexia. Sarcopenia Muscle 2018, 9, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.R. The Oral Microbiome.Methods and Protocols; Adami, G.R., Ed.; Methods in Molecular Biology; Springer US: New York, NY, 2021; Vol. 2327; ISBN 978-1-0716-1517-1. [Google Scholar]
- Schwartz, J.L.; Peña, N.; Kawar, N.; Zhang, A.; Callahan, N.; Robles, S.J.; Griebel, A.; Adami, G.R. Old Age and Other Factors Associated with Salivary Microbiome Variation. BMC Oral Health 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Jabaz, M.F.; Yassir, A.D.; Jebur, A.N. Biochemical Changes of Saliva in Patients with Radiotherapy in Comparison to Healthy Subjects. Res. J. Pharm. Technol. 2023, 16, 184–186. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; dos Santos, C.M. Supragingival and Subgingival Microbiota from Patients with Poor Oral Hygiene Submitted to Radiotherapy for Head and Neck Cancer Treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers (Basel). 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Feng, R.; Chang, E.T.; Debelius, J.W.; Yin, L.; Xu, M.; Huang, T.; Zhou, X.; Xiao, X.; Li, Y.; et al. Influence of Pre-Treatment Saliva Microbial Diversity and Composition on Nasopharyngeal Carcinoma Prognosis. Front. Cell. Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Yang, S.F.; Huang, H. Da; Fan, W.L.; Jong, Y.J.; Chen, M.K.; Huang, C.N.; Chuang, C.Y.; Kuo, Y.L.; Chung, W.H.; Su, S.C. Compositional and Functional Variations of Oral Microbiota Associated with the Mutational Changes in Oral Cancer. Oral Oncol. 2018, 77, 1–8. [Google Scholar] [CrossRef]
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodríguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.A.; Alashkar Alhamwe, B.; Alves, R.J.; Carrato, A.; et al. A Faecal Microbiota Signature with High Specificity for Pancreatic Cancer. Gut 2022, 71. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; del Valle Cano, A.; Fernández, M.F.; Fontana, L. Gut Microbiota and Breast Cancer: The Dual Role of Microbes. Cancers (Basel). 2023, 15. [Google Scholar] [CrossRef]
- Li, Z.; Ke, X.; Zuo, D.; Wang, Z.; Fang, F.; Li, B. New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Boutriq, S.; Plaza-Andrades, I.; Peralta-Linero, J.; Alba, E.; González-González, A.; Queipo-Ortuño, M.I. A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers (Basel). 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.A.; Galisteo, F.; Rueda, F. Cocina Con Ciencia Contra El Cáncer; Editorial Almuzara, S.L., 2018; ISBN 978-84-17558-57-4.
- Lin, J.Y.; Lu, Y. Establishing a Data Monitoring Committee for Clinical Trials. Shanghai Arch. psychiatry 2014, 26, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Arbeitsgemeinschaft OuG, S. AWMF: Aktuelle Leitlinien; 1996. [Google Scholar]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips” - a Rapid, Lateralized, Gustatory Bedside Identification Test Based on Impregnated Filter Papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks Normative Data Based on an Extended Sample of 9139 Subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO/UNICEF Methodology of Nutritional Surveillance. Report of a Joint FAO/UNICEF/WHO Expert Committee.; World Health Organ Tech Rep Ser. 593, 1976; ISBN 9241205938.
- Norton, K.; Whittingham, N.; Carter, L.; Kerr, C.; Goere, C.; Marfell-Jones, M. Measurement Techniques in Anthropometry; Editorial: Sydney, 1966. [Google Scholar]
- International Society for the Advancement of Kinathropometry (ISAK) International Standard for Anthropometric Assessment; International Society for the Advancement of Kinathropometry (ISAK): New Zealand, 2001.
- Lukaski, H.C. Methods for the Assessment of Human Body Composition: Traditional and New. Am. J. Clin. Nutr. 1987, 46, 537–556. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Volland, G.; Kashman, N. Reliability and Validity of Hand Strength Evaluations. J. Hand Surg. Am. 1984, 9, 222–226. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Togers, S. Grip and Pinch Strength: Normative Data for Adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the Measurement of Muscle Mass: A Need for a Reference Standard. J. Cachexia. Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Hamagawa, K.; Matsumura, Y.; Kubo, T.; Hayato, K.; Okawa, M.; Tanioka, K.; Yamasaki, N.; Kitaoka, H.; Yabe, T.; Nishinaga, M.; et al. Abdominal Visceral Fat Thickness Measured by Ultrasonography Predicts the Presence and Severity of Coronary Artery Disease. Ultrasound Med. Biol. 2010, 36, 1769–1775. [Google Scholar] [CrossRef]
- McGettigan, N.; Dhuibhir, P.U.; Barrett, M.; Sui, J.; Balding, L.; Higgins, S.; O’Leary, N.; Kennedy, A.; Walsh, D. Subjective and Objective Assessment of Taste and Smell Sensation in Advanced Cancer. Am. J. Hosp. Palliat. Care 2019, 36, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Soeda, H.; Komine, K.; Otsuka, K.; Shibata, H. Preliminary Estimation of the Prevalence of Chemotherapy-Induced Dysgeusia in Japanese Patients with Cancer. BMC Palliat. Care 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, B.M.; Olson, K.; Baracos, V.E.; Wismer, W.V. Reframing Eating during Chemotherapy in Cancer Patients with Chemosensory Alterations. Eur. J. Oncol. Nurs. 2012, 16, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary Assessment Methods: Dietary Records. Nutr. Hosp. 2015, 31 Suppl 3, 38–45. [Google Scholar] [CrossRef]
- Ruíz, M.; Martínez, E.; Gil, A. Fotográfica de Porciones de Alimentos Consumidos En España; Fundación; España: Granada, 2019; ISBN 978-84-09-08860-7. [Google Scholar]
- Martin-moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and Validation of a Food Frequency Questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Arraras, J.I.; Arias, F.; Tejedor, M.; Pruja, E.; Marcos, M.; Martínez, E.; Valerdi, J. The EORTC QLQ-C30 (Version 3.0) Quality of Life Questionnaire: Validation Study for Spain with Head and Neck Cancer Patients. Psychooncology. 2002, 11, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens. (Dallas, Tex. 1979) 2018, 71, E13–E115. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Specific Methylation of Plasma Nonesterified Fatty Acids in a One-Step Reaction. J Lipid Res 1988, 29, 227–235. [Google Scholar] [CrossRef]
- de la Torre-Aguilar, M.J.; Gomez-Fernandez, A.; Flores-Rojas, K.; Martin-Borreguero, P.; Mesa, M.D.; Perez-Navero, J.L.; Olivares, M.; Gil, A.; Gil-Campos, M. Docosahexaenoic and Eicosapentaenoic Intervention Modifies Plasma and Erythrocyte Omega-3 Fatty Acid Profiles But Not the Clinical Course of Children With Autism Spectrum Disorder: A Randomized Control Trial. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef]
- Miles, E.A.; Noakes, P.S.; Kremmyda, L.S.; Vlachava, M.; Diaper, N.D.; Rosenlund, G.; Urwin, H.; Yaqoob, P.; Rossary, A.; Farges, M.C.; et al. The Salmon in Pregnancy Study: Study Design, Subject Characteristics, Maternal Fish and Marine n-3 Fatty Acid Intake, and Marine n-3 Fatty Acid Status in Maternal and Umbilical Cord Blood. Am. J. Clin. Nutr. 2011, 94. [Google Scholar] [CrossRef]
- García-Rodríguez, C.E.; Mesa, M.D.; Olza, J.; Vlachava, M.; Kremmyda, L.S.; Diaper, N.D.; Noakes, P.S.; Miles, E.A.; Ramírez-Tortosa, M.C.; Liaset, B.; et al. Does Consumption of Two Portions of Salmon per Week Enhance the Antioxidant Defense System in Pregnant Women? Antioxid. Redox Signal. 2012, 16, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, C.E.; Helmersson-Karlqvist, J.; Dolores Mesa, M.; Miles, E.A.; Noakes, P.S.; Vlachava, M.; Kremmyda, L.S.; Diaper, N.D.; Godfrey, K.M.; Calder, P.C.; et al. Does Increased Intake of Salmon Increase Markers of Oxidative Stress in Pregnant Women? The Salmon in Pregnancy Study. Antioxid. Redox Signal. 2011, 15, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, C.E.; Olza, J.; Mesa, M.D.; Aguilera, C.M.; Miles, E.A.; Noakes, P.S.; Vlachava, M.; Kremmyda, L.S.; Diaper, N.D.; Godfrey, K.M.; et al. Fatty Acid Status and Antioxidant Defense System in Mothers and Their Newborns after Salmon Intake during Late Pregnancy. Nutrition 2017, 33, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione Reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Leone, L.; Bompadre, S. High-Performance Liquid Chromatography-EC Assay of Mitochondrial Coenzyme Q9, Coenzyme Q9H2, Coenzyme Q10, Coenzyme Q10H2, and Vitamin E with a Simplified On-Line Solid-Phase Extraction. Methods Enzymol. 2004, 378, 156–162. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, M.D.; Gil-Campos, M.; Flores-Rojas, K.; Muñoz-Villanueva, M.C.; Mesa, M.D.; de la Torre-Aguilar, M.J.; Gil, Á.; Pérez-Navero, J.L. Impaired Antioxidant Defence Status Is Associated With Metabolic-Inflammatory Risk Factors in Preterm Children With Extrauterine Growth Restriction: The BIORICA Cohort Study. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are We Close to Defining a Metabolomic Signature of Human Obesity? A Systematic Review of Metabolomics Studies. Metabolomics 2019, 15. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gil, A. Nutrimetabolomics: An Update on Analytical Approaches to Investigate the Role of Plant-Based Foods and Their Bioactive Compounds in Non-Communicable Chronic Diseases. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gomez-Fernández, A.; de la Torre-Aguilar, M.J.; Gil, A.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Gil-Campos, M. Metabolic Profiling in Children with Autism Spectrum Disorder with and without Mental Regression: Preliminary Results from a Cross-Sectional Case-Control Study. Metabolomics 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Kathagen, G.; D’Hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative Microbiome Profiling Links Gut Community Variation to Microbial Load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; Kötzsch, S.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Monitoring Microbiological Changes in Drinking Water Systems Using a Fast and Reproducible Flow Cytometric Method. Water Res. 2013, 47, 7131–7142. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, A.B.; Tunsjø, H.S.; Meisal, R.; Charnock, C. A Preliminary Study on the Potential of Nanopore MinION and Illumina MiSeq 16S RRNA Gene Sequencing to Characterize Building-Dust Microbiomes. Sci. Rep. 2020, 10, 3209. [Google Scholar] [CrossRef]
- Matsuo, Y.; Komiya, S.; Yasumizu, Y.; Yasuoka, Y.; Mizushima, K.; Takagi, T.; Kryukov, K.; Fukuda, A.; Morimoto, Y.; Naito, Y.; et al. Full-Length 16S RRNA Gene Amplicon Analysis of Human Gut Microbiota Using MinIONTM Nanopore Sequencing Confers Species-Level Resolution. BMC Microbiol. 2021, 21. [Google Scholar] [CrossRef]
- de Siqueira, G.M.V.; Pereira-dos-Santos, F.M.; Silva-Rocha, R.; Guazzaroni, M.E. Nanopore Sequencing Provides Rapid and Reliable Insight Into Microbial Profiles of Intensive Care Units. Front. public Heal. 2021, 9. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Manzano, M.; Ruiz-Ojeda, F.J.; Giron, M.D.; Salto, R.; López-Pedrosa, J.M.; Santos-Fandila, A.; Garcia-Corcoles, M.T.; Rueda, R.; Gil, Á. Intake of Slow-Digesting Carbohydrates Is Related to Changes in the Microbiome and Its Functional Pathways in Growing Rats with Obesity Induced by Diet. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef]
- Brouwer, J.N.; Glaser, D.; af Segerstad, C.H.; Hellekant, G.; Ninomiya, Y.; van der Wel, H. The Sweetness-Inducing Effect of Miraculin; Behavioural and Neurophysiological Experiments in the Rhesus Monkey Macaca Mulatta. J. Physiol. 1983, 337, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Comeau, T.B.; Epstein, J.B.; Migas, C. Taste and Smell Dysfunction in Patients Receiving Chemotherapy: A Review of Current Knowledge. Support. Care Cancer 2001, 9, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Benoist, S.; Brouquet, A. Nutritional Assessment and Screening for Malnutrition. J. Visc. Surg. 2015, 152 Suppl 1, S3–S7. [Google Scholar] [CrossRef]
- Pavlidis, P.; Schittek, G.A.; Saratziotis, A.; Ferfeli, M.; Kekes, G.; Gouveris, H. Electrogustometry: Normative Data for Stimulus Duration, Tongue Site and Age Decline. Clin. Otolaryngol. 2021, 46, 767–774. [Google Scholar] [CrossRef]
- Obiefuna, S.; Donohoe, C. Neuroanatomy, Nucleus Gustatory. StatPearls 2023. [Google Scholar]
- Buttiron Webber, T.; Briata, I.M.; DeCensi, A.; Cevasco, I.; Paleari, L. Taste and Smell Disorders in Cancer Treatment: Results from an Integrative Rapid Systematic Review. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki Otani; Amano, K. ; Morita, T.; Miura, T.; Mori, N.; Tatara, R.; Kessoku, T.; Matsuda, Y.; Tagami, K.; Mori, M.; et al. Impact of Taste/Smell Disturbances on Dietary Intakes and Cachexia-Related Quality of Life in Patients with Advanced Cancer. Support. Care Cancer 2023, 31. [Google Scholar] [CrossRef]
- Turcott, J.G.; Juárez-Hernández, E.; De La Torre-Vallejo, M.; Sánchez-Lara, K.; Luvian-Morales, J.; Arrieta, O. Value: Changes in the Detection and Recognition Thresholds of Three Basic Tastes in Lung Cancer Patients Receiving Cisplatin and Paclitaxel and Its Association with Nutritional and Quality of Life Parameters. Nutr. Cancer 2016, 68, 241–249. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D. Contents of Phenolics and Flavonoids and Antioxidant Activities in Skin, Pulp, and Seeds of Miracle Fruit. J. Food Sci. 2011, 76, C479–C482. [Google Scholar] [CrossRef]
- Swamy, K.B.; Hadi, S.A. b.; Sekaran, M.; Pichika, M.R. a. The Clinical Effects of Synsepalum Dulcificum: A Review. J. Med. Food 2014, 17, 1165–1169. [Google Scholar] [CrossRef]
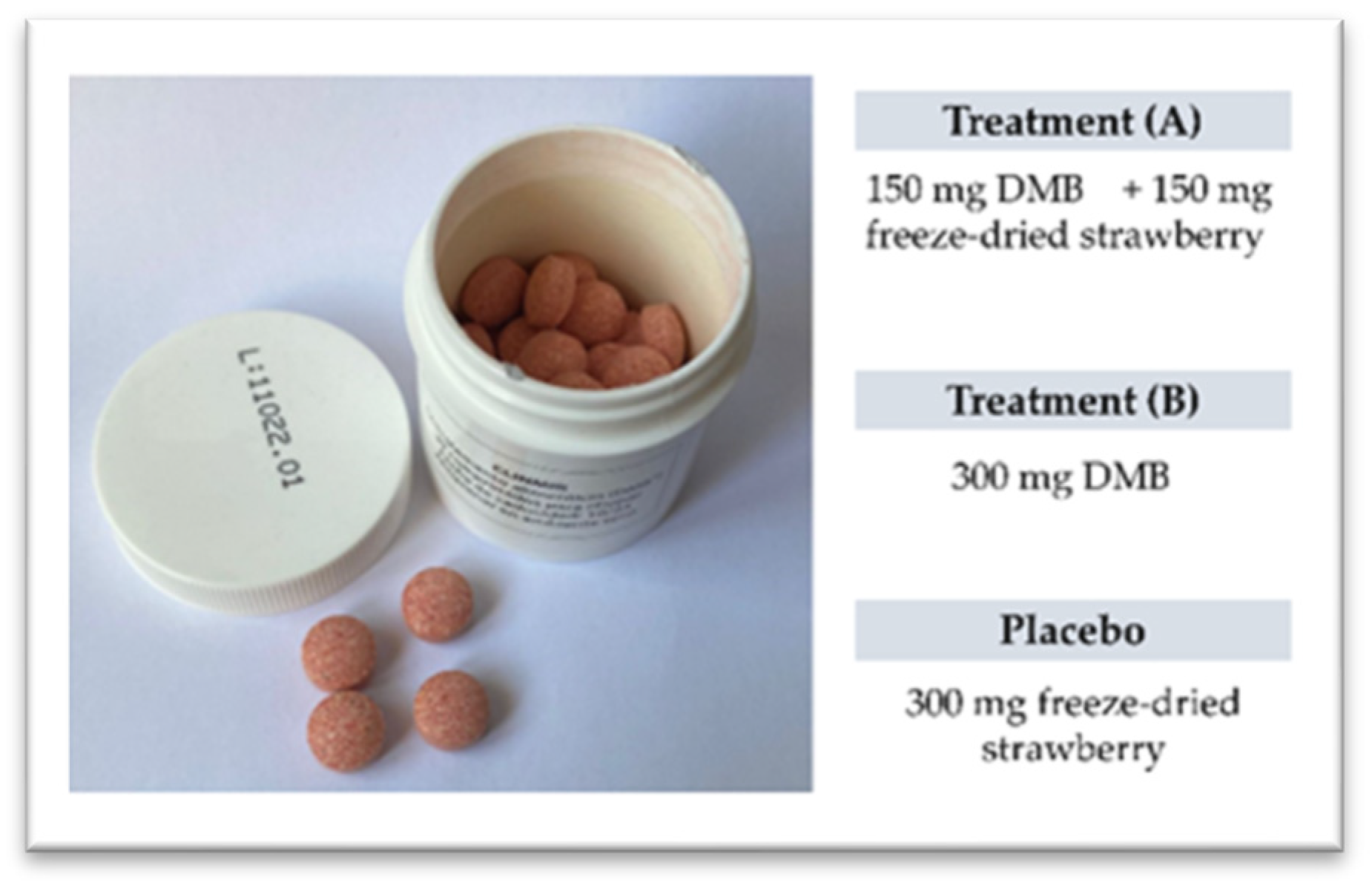
| 150 mg DMB + 150 mg strawberry freeze-dried | 300 mg DMB | Placebo (300 mg strawberry freeze-dried) | ||
|---|---|---|---|---|
| Energy | kcal | 0.99 | 1 | 0.97 |
| Carbohydrates | mg | 194 | 234 | 154 |
| Sugars | mg | 156 | 162 | 150 |
| Fiber | mg | 26 | 6 | 46 |
| Proteins | mg | 20 | 15 | 24 |
| Lipids | mg | 9 | 5 | 12 |
| Saturated fatty acids | mg | 2 | 2 | 1 |
| Sodium chloride | mg | 0.1 | 0.1 | 0.03 |
| Humidity | mg | 4 | 4 | 5 |
| Ash | mg | 12 | 14 | 15 |
| Miraculin | mg | 2.8 | 5.5 | 0 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





