Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
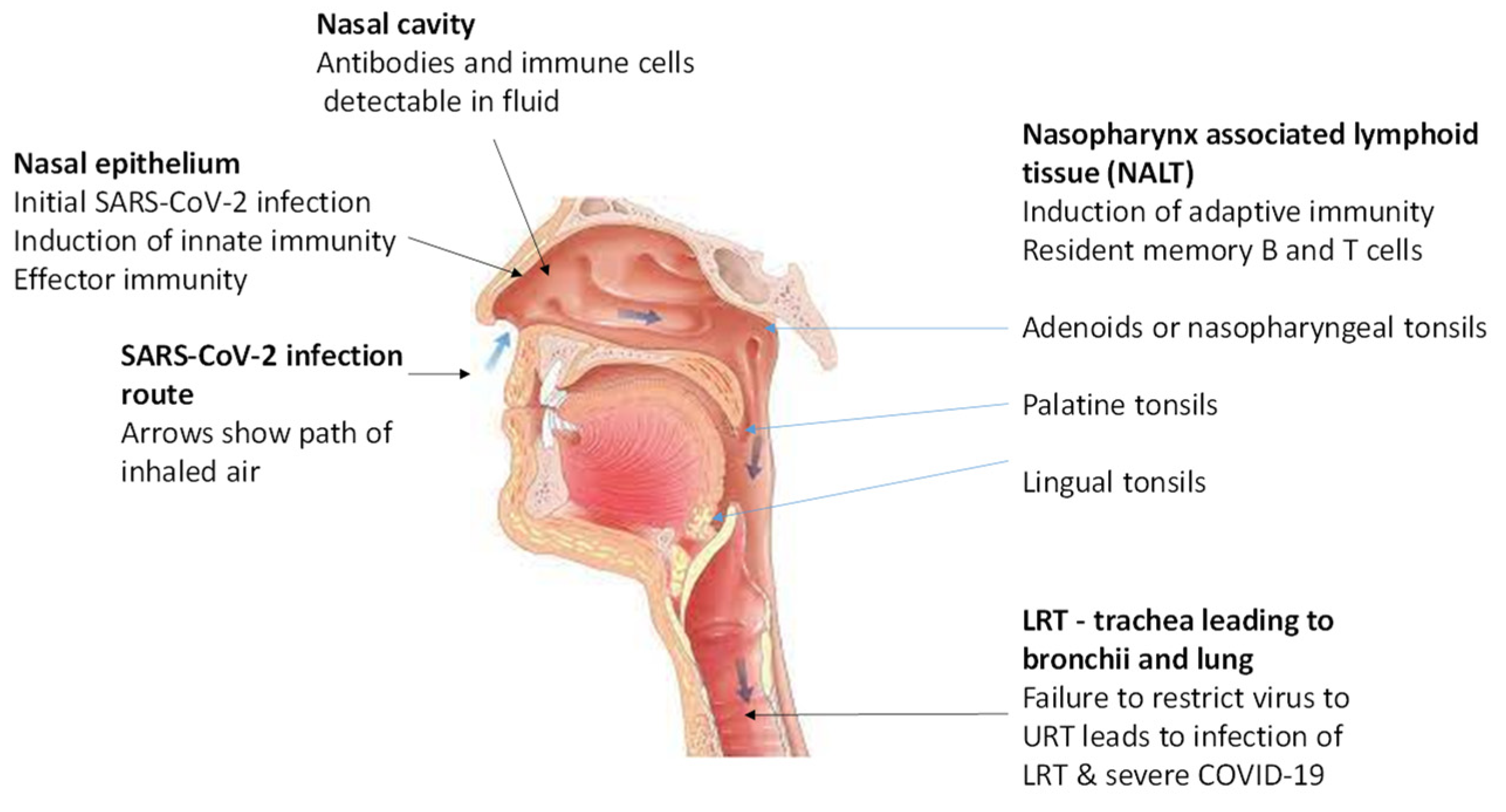
2. Correlates of Protective Immunity in the URT
2.1. Innate immunity in the URT
2.2. Adaptive immunity in the URT
2.2.1. After infection with SARS-CoV-2 or other human coronaviruses
2.2.2. After intramuscular vaccination of infection-naive persons
2.2.3. After intranasal vaccination of infection-naïve persons
3. Mucosal (nasal and oral) vaccines for COVID-19
3.1. Lessons from influenza vaccines for developing nasal COVID-19 vaccines
3.2. General considerations for developing nasally-administered COVID-19 vaccines
3.2.1. Possible limitations of intranasal vaccination
3.3. Studies of intranasal COVID-19 vaccines in animal models
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization. Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 4 October 2023).
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Yamana, T.K.; Galanti, M.; Pei, S.; Di Fusco, M.; Angulo, F.J.; Moran, M.M.; Khan, F.; Swerdlow, D.L.; Shaman, J. The impact of COVID-19 vaccination in the US: Averted burden of SARS-COV-2-related cases, hospitalizations and deaths. PLoS. ONE 2023, 18, e0275699. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; McLaughlin, J.M.; Khan, F.; Angulo, F.J.; Anis, E.; Lipsitch, M.; Singer, S.R.; Mircus, G.; Brooks, N.; Smaja, M.; et,al. Infections, hospitalizations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: A retrospective surveillance study. Lancet Infect. Dis. 2022, 22, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Zasada, A.A.; Darlinska, A.; Wiatrzyk, A.; Woznica, K.; Forminska, K.; Czajka, U.; Główka, M.; Lis, K.; Górska, P. COVID-19 vaccines over three years after the outbreak of the COVID-19 epidemic. Viruses 2023, 15, 1786. [Google Scholar] [CrossRef]
- Knisely, J. M.; Buyon, L. E.; Mandt, R.; Farkas, R.; Balasingam, S.; Bok, K.; Buchholz, U. J.; D'Souza, M. P.; Gordon, J. L.; King, D. F. L.; et al. Mucosal vaccines for SARS-CoV-2: Scientific gaps and opportunities-workshop report. NPJ. Vaccines 2023, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Nasal conditioning of inspired air, innate immunity in the respiratory tract and SARS-CoV-2 infectivity. Open Sci. Forum 2020. Available online: https://osf.io/4j95b/ (accessed on 7 October 2023).
- Ramasamy, R. Perspective of the relationship between the susceptibility to initial SARS-CoV-2 infectivity and optimal nasal conditioning of inhaled air. Int. J. Mol. Sci. 2021, 22, 7919. [Google Scholar] [CrossRef]
- Ramasamy, R. Innate and adaptive immune responses in the upper respiratory tract and the infectivity of SARS-CoV-2. Viruses 2022, 14, 933. [Google Scholar] [CrossRef]
- Otter, C.J. Fausto, A.; Tan, L.H.; Khosla, A.S.; Cohen, N.A.; Weiss, S. R. Infection of primary nasal epithelial cells differentiates among lethal and seasonal human coronaviruses. PNAS. 2023, 120, e2218083120. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- De Neck, S.; Penrice-Randal, R.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Mega, D.F.; Han, X.; Owen, A.; Hiscox, J.A.; et al. The stereotypic response of the pulmonary vasculature to respiratory viral infections: Findings in mouse models of SARS-CoV-2, influenza A and gamma herpesvirus infections. Viruses 2023, 15, 1637. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R. Overview of immunological and virological factors driving the evolution and global spread of SARS-CoV-2 variants. Indian J. Med. Res. 2023, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D. S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Fröberg, J.; Gillard, J.; Philipsen, R.; Lanke, K.; Rust, J.; van Tuijl, D.; Teelen, K.; Bousema, T.; Simonetti, E.; van der Gaast-de Jongh, C.E.; et al. SARS-CoV-2, mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat. Commun. 2021, 12, 5621. [Google Scholar] [CrossRef]
- Fröberg, J.; Koomen, V.J.C.H.; van der Gaast-de Jongh, C.E.; Philipsen, R.; GeurtsvanKessel, C.H.; de Vries, R.D.; Baas, M.C.; van der Molen, R.G.; de Jonge, M.I.; Hilbrands, L.B.; et al. Primary exposure to SARS-CoV-2 via infection or vaccination determines mucosal antibody-dependent ACE2 binding inhibition. J. Infect. Dis. 2023, jiad385. [Google Scholar] [CrossRef]
- Netea, M.G.; Ziogas, A.; Benn, C.S.; Giamarellos-Bourboulis, E.J.; Joosten, L.A.B.; Arditi, M.; Chumakov, K.; van Crevel, R.; Gallo, R.; Aaby, P.; et al. The role of trained immunity in COVID-19: Lessons for the next pandemic. Cell Host Microbe 2023, 31, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef]
- Butler, S.E.; Crowley, A.R.; Natarajan, H.; Xu, S.; Weiner, J.A.; Bobak, C.A.; Mattox, D.E.; Lee, J.; Wieland-Alter, W.; Connor, R.I.; et al. Distinct features and functions of systemic and mucosal humoral immunity among SARS-CoV-2 convalescent individuals. Front. Immunol. 2021, 11, 618685. [Google Scholar] [CrossRef]
- Chan, R.W.Y.; Chan, K.C.C.; Lui, G.C.Y.; Tsun, J.G.S.; Chan, K.Y.Y.; Yip, J.S.K.; Liu, S.; Yu, M.W.L.; Ng, R.W.Y.; Chong, K.K.L.; et al. Mucosal antibody response to SARS-CoV-2 in paediatric and adult patients: A longitudinal study. Pathogens 2022, 11, 397. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Dowell, A. C., Tut, G., Begum, J., Bruton, R., Bentley, C., Butler, M., Uwenedi, G., Zuo, J., Powell, A. A., Brent, A. J.; et al. Nasal mucosal IgA levels against SARS-CoV-2 and seasonal coronaviruses are low in children but boosted by reinfection. J. Infect. 2023, S0163-4453(23)00465-6. [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef]
- Roukens, A.H.E.; Pothast, C.R.; König, M.; Huisman, W.; Dalebout, T.; Tak, T.; Azimi, S.; Kruize, Y.; Hagedoorn, R.S.; Zlei, M.; et al. Prolonged activation of nasal immune cell populations and development of tissue-resident SARS-CoV-2-specific CD8+ T cell responses following COVID-19. Nature Immunol. 2022, 23, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Milanez-Almeida, P.; Martins, A.J.; Radtke, A.J.; Hoehn, K.B.; Oguz, C.; Chen, J.; Liu, C.; Tang, J.; Grubbs, G.; et al. Adaptive immune responses to SARS-CoV-2 persist in the pharyngeal lymphoid tissue of children. Nature Immunol. 2023, 24, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Augusto, D.G.; Murdolo, L.D.; Chatzileontiadou, D.S.M.; Sabatino, J.J.Jr.; Yusufali, T.; Peyser, N.D.; Butcher, X.; Kizer, K.; Guthrie, K.; Murray, V.W.; et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection. Nature, 2023, 620, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Aksyuk, A.A.; Bansal, H.; Wilkins, D.; Stanley, A.M.; Sproule, S.; Maaske, J.; Sanikommui, S.; Hartman, W.R.; Sobieszczyk, M.E.; Falsey, A.R.; et al. AZD1222-induced nasal antibody responses are shaped by prior SARS-CoV-2 infection and correlate with virologic outcomes in breakthrough infection. Cell Rep. Med. 2023, 4, 100882. [Google Scholar] [CrossRef] [PubMed]
- Ketas, T.J.; Chaturbhuj, D.; Portillo, V.M.C.; Francomano, E.; Golden, E.; Chandrasekhar, S.; Debnath, G.; Díaz-Tapia, R.; Yasmeen, A.; Kramer, K.D.; et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog. Immun. 2021, 6, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Mades, A.; Chellamathu, P.; Kojima, N.; Lopez, L.; MacMullan, M. A.; Denny, N.; Angel, A. N.; Santacruz, M.; Casian, J. G.; Brobeck, M.; et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. Sci. Rep. 2021, 11, 24448. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.Y.; Liu, S.; Cheung, J.Y.; Tsun, J.G.S.; Chan, K.C.; Chan, K.Y.Y.; Fung, G.P.G.; Li, A.M.; Lam, H.S. The mucosal and serological immune responses to the novel coronavirus (SARS-CoV-2) vaccines. Front. Immunol. 2021, 12, 744887. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zeng, C.; Cox, T.M.; Li, C.; Son, Y.M.; Cheon, I.S.; Wu, Y.; Behl, S.; Taylor, J.J.; Chakaraborty, R.; et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 2022, 7, eadd4853. [Google Scholar] [CrossRef] [PubMed]
- Ssemaganda, A.; Nguyen, H.M.; Nuhu, F.; Jahan, N.; Card, C.M.; Kiazyk, S.; Severini, G.; Keynan, Y.; Su, R.C.; Ji, H.; et al. Expansion of cytotoxic tissue-resident CD8+ T cells and CCR6+CD161+ CD4+ T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat. Commun. 2022, 13, 3357. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.E.; Tan, A.T.; Le Bert, N.; Hang, S.K.; Low, J.G.H.; Bertoletti, A. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J. Exp. Med. 2022, 219, e20220780. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.M.L.; Rybkina, K.; Kato, Y.; Kubota, M.; Matsumoto, R.; Bloom, N.I.; Zhang, Z.; Hastie, K.M.; Grifoni, A.; Weiskopf, D.; et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021, 6, eabl9105. [Google Scholar] [CrossRef] [PubMed]
- Rotrosen, E.; Kupper, T.S. Assessing the generation of tissue resident memory T cells by vaccines. Nat. Rev. Immunol. 2023, 23, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Allie, S.R.; Randall, T.D. Resident memory B cells. Viral Immunol. 2020, 33, 282–293. [Google Scholar] [CrossRef]
- Painter, M.M.; Johnston, T.S.; Lundgreen, K.A.; Santos, J.J.S.; Qin, J.S.; Goel, R.R.; Apostolidis, S.A.; Mathew, D.; Fulmer, B.; Williams, J.C.; et al. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat. Immunol. 2023, 24, 1711–1724. [Google Scholar] [CrossRef]
- Andersson, N.W.; Thiesson, E.M.; Baum, U.; Pihlström, N.; Starrfelt, J.; Faksová, K.; Poukka, E.; Meijerink, H.; Ljung, R.; Hviid, A. Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: Nationwide cohort study. BMJ Clinical research ed. 2023, 382, e075286. [Google Scholar] [CrossRef]
- Ramasamy, R. Surface antigens on haemoparasites and their relevance to protective immunity. Biochem. Soc. Trans. 1981, 9, 535–536. [Google Scholar] [CrossRef]
- Iwasaki, A. Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 2016, 34, 575–608. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. China and India approve nasal COVID vaccines - are they a game changer? Nature 2022, 609, 450. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin®). NPJ vaccines 2023, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Purushotham, J.N.; Schulz, J.E.; Holbrook, M.G.; Bushmaker, T.; Carmody, A.; Port, J.R.; Yinda, C.K.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021, 13, eabh0755. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A. O. , Feldmann, F., Zhao, H., Curiel, D. T., Okumura, A., Tang-Huau, T. L., Case, J. B., Meade-White, K., Callison, J., Chen, R. E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yasawardena, S.; Zomer, A.; Venema, G.; Kok, J.; Leenhouts, K. Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunizations. Vaccine 2006, 24, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, G.; Ramasamy, R. Mucosal immunization of mice with malaria protein on lactic acid bacterial cell walls. Vaccine 2007, 25, 3636–3645. [Google Scholar] [CrossRef]
- Moorthy, S.A.; Yasawardena, S.G.; Ramasamy, R. Age-dependent systemic antibody responses and immunization-associated changes in mice orally and nasally immunized with Lactococcus lactis expressing a malaria parasite protein. Vaccine 2009, 27, 4947–4952. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Hao, J.; Han, J.; Fu, T.; Bai, J.; Tian, M.; Jin, N.; Zhu, G.; Li, C. Mucosal IgA response elicited by intranasal immunization of Lactobacillus plantarum expressing surface-displayed RBD protein of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 190, 409–416. [Google Scholar] [CrossRef]
- Saveria, T.; Parthiban, C.; Seilie, A.M.; Brady, C.; Martinez, A.; Manocha, R.; Afreen, E.; Zhao, H.; Krzeszowski, A.; Ferrara, J.; et al. Needle-free, spirulina-produced Plasmodium falciparum circumsporozoite vaccination provides sterile protection against pre-erythrocytic malaria in mice. NPJ Vaccines 2022, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.R.; Martinez, C.I.; Dora, E.G.; Showalter, L.J.; Mercedes, A.R.; Tucker, S.N. Mucosal immunization with Ad5-based vaccines protects Syrian hamsters from challenge with omicron and delta variants of SARS-CoV-2. Front. Immunol. 2023, 14, 1086035. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250, Erratum in Nat. Rev. Immunol. 2022, 22, 266. [Google Scholar] [CrossRef]
- Ramasamy, R. Immunity to human influenza A—An overview. Brunei Darussalam J. Health 2010, 4, 1–8. [Google Scholar]
- Sridhar, S.; Brokstad, K.A.; Cox, R. J. Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Denney, L.; Ho, L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018, 41, 218–233, Tamura, S.; Kurata, T. Defence mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect. Dis. 2004, 57, 236–247.. [Google Scholar] [CrossRef]
- Nguyen, T.H.O.; Koutsakos, M.; van de Sandt, C.E.; Crawford, J.C.; Loh, L.; Sant, S.; Grzelak, L.; Allen, E.K.; Brahm, T.; Clemens, E.B.; et al. Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients. Nat. Commun. 2021, 12, 2691. [Google Scholar] [CrossRef]
- Pizzolla, A.; Nguyen, T.; Smith, J.M.; Brooks, A.G.; Kedzieska, K.; Heath,W. R.; Reading, P.C.; Wakim, L.M. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2017, 2, eaam6970. [Google Scholar] [CrossRef]
- Mettelman, R.C.; Souquette, A.; Van de Velde, L.A.; Vegesana, K.; Allen, E.K.; Kackos, C.M.; Trifkovic, S.; DeBeauchamp, J.; Wilson, T.L.; St James, D.G.; et al. Baseline innate and T cell populations are correlates of protection against symptomatic influenza virus infection independent of serology. Nat. Immunol. 2023, 24, 1511–1526. [Google Scholar] [CrossRef]
- Sridhar, S.; Brokstad, K.A.; Cox, R. J. Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Paul, W.E. Fundamental Immunology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 1–1603. [Google Scholar]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Kohl, K.S.; Gidudu, J.; Amato, A.; Bakshi, N.; Baxter, R.; Burwen, D.R.; Cornblath, D.R.; Cleerbout, J.; Edwards, K. M.; et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011, 29, 599–612. [Google Scholar] [CrossRef]
- Lecomte, E.; Laureys, G.; Verbeke, F.; Domingo Carrasco, C.; Van Esbroeck, M.; Huits, R. A clinician's perspective on yellow fever vaccine-associated neurotropic disease. J. Travel. Med. 2020, 27, taaa172. [Google Scholar] [CrossRef]
- Lapuente, D.; Fuchs, J.; Willar, J.; Vieira Antão, A.; Eberlein, V.; Uhlig, N.; Issmail, L.; Schmidt, A.; Oltmanns, F.; Peter, A.S; et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat. Commun. 2021, 12, 6871. [Google Scholar] [CrossRef]
- Tokunoh, N.; Tamiya, S.; Watanabe, M.; Okamoto, T.; Anindita, J.; Tanaka, H.; Ono, C.; Hirai, T.; Akita, H.; Matsuura, Y.; et al. A nasal vaccine with inactivated whole-virion elicits protective mucosal immunity against SARS-CoV-2 in mice. Front. Immunol. 2023, 14, 1224634. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.; Temperton, N.; Mayora-Neto, M.; Da Costa, K.; Cantoni, D.; Horlacher, R.; Günther, A.; Brosig, A.; Morath, J.; Jakobs, B.; et al. Novel intranasal vaccine targeting SARS-CoV-2 receptor binding domain to mucosal microfold cells and adjuvanted with TLR3 agonist Riboxxim™ elicits strong antibody and T-cell responses in mice. Sci. Rep. 2023, 13, 4648. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Matsuoka, Y.; Luongo, C.; Yang, L.; Santos, C.; Liu, X.; Ahlers, L.R.H.; Moore, I.N.; Afroz, S.; Johnson, R.F.; et al. Intranasal immunization with avian paramyxovirus type 3 expressing SARS-CoV-2 spike protein protects hamsters against SARS-CoV-2. NPJ Vaccines 2022, 7, 72. [Google Scholar] [CrossRef]
- Le Nouën, C.; Nelson, C.E.; Liu, X.; Park, H.S. Matsuoka, Y.; Luongo, C.; Santos, C.; Yang, L.; Herbert, R.; Castens, A.; et al. Intranasal pediatric parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in monkeys. Cell 2022, 185, 4811–4825.e17. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Israelow, B.; Peña-Hernández, M.A.; Suberi, A.; Zhou, L.; Luyten, S.; Reschke, M.; Dong, H.; Homer, R.J.; Saltzman, W.M.; et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 2022, 378, eabo2523. [Google Scholar] [CrossRef] [PubMed]
- Bonhoeffer, J.; Imoukhuede, E.B.; Aldrovandi, G.; Bachtiar, N.S.; Chan, E.S. Chang, S.; Chen, R.T. Fernandopulle, R.; Goldenthal, K.L.; Heffelfinger, J.D.; et al. Template protocol for clinical trials investigating vaccines--focus on safety elements. Vaccine 2013, 31, 5602–5620. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Immune response | Reference |
|---|---|---|
| 1. ChAdOx1 nCoV-19 (AZD1222) – replication deficient simian adenovirus expressing S | Anti-S IgG antibodies in nasal fluid, probably translocated from plasma by neonatal Fc receptors, persisting for a year after boost. | [31] |
| 2. Pfizer/BioNTech BNT162b2 and Moderna mRNA1273 mRNA vaccines expressing S |
Anti-S IgG and IgA antibodies in saliva and nasal fluid | [19,20,32,33,34,35] |
| Possible Limitation | Reference |
|---|---|
| Type 1 hypersensitivity reaction to antigenic molecules reaching lungs | [65] |
| Exacerabation or development of airway diseases e.g., asthma and rhinitis | [65] |
| Adverse neurological events | [66,67] |
| Weaker immune responses in the elderly and very young | [53] |
| Possible disseminated infections with attenuated vectors or viruses in immunocompromised persons | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





