Introduction
Since the electronic era began, electronic equipment has never been as popular as in recent years. For many years Sn-Pb solders were used due to its low melting temperature, excellent mechanical and thermal properties. Although tin lead alloys have many excellent properties, lead is toxic to the human body, thus causing the restriction of its use. After the European RoHS (Reduction of Hazardous Substances) announced a ban on the use of lead solders, to develop lead-free solders is necessary. Subsequently, the operational to develop innovative lead-free solder alloy continues to grasp the attention of researchers [
1,
2]. The researchers were mainly focuses on Tin-based alloys, various compositions were studied, and the most common were Sn-Ag, Sn-Cu, Sn-Zn and Sn-Bi. However, no new lead-free solder that can completely replace Sn-Pb solder was found, some third or fourth elements are added to increase the performance. The performance of the solders used in electronic surface mounted technology (SMT) affects directly the reliability of the electronic products. Alloy elements affect the behavior of the solder. Requests of products reliability and environment harmony during their whole service life lead to the research of the green soldering worldwide. The Sn-Ag solders are expensive due to the presence of silver and it also presents a relatively high melting point, what causes the limited use of Sn-Ag solders [
3]. Sn-Cu solders are economical and have good mechanical properties; however, similar to Sn-Ag solders, Sn-Cu also had a high melting point. The problem of high temperature is that many electronic components are sensitive and cannot withstand high reflow temperatures. Besides, due to their high melting points, they are easy to cause warpage and deformation of the substrate and soldering defects in the high degree of integration and thinness of chip packaging, which cannot meet the needs of the new generation. And low-temperature lead-free solder has been presented as one of the main measures to solve above problems. Sn-Zn solders had low price and good mechanical properties; however, it presents a poor wettability and Zn is susceptible to oxidation and corrosion [
4,
5]. Sn-Bi had been more investigated lately due to its reliability and melting temperature, but due to the presence of Bi creates brittleness and poor fatigue resistance, which could affect the reliability of solders joints.
For these reasons, currently, in order to further improve the properties of the Sn-Bi solder alloy, many researchers have modified it by adding some third or fourth elements. Xin F. Tan et al [
6] added Sb to the Sn-37Bi solder alloy. The results showed that the addition of Sb does not refine the microstructure of Sn-Bi solder alloy, this was related to the fact that Sb preferentially dissolves in the primary Sn phase, and only dissolves in Bi and forms SnSb when the concentration is above the solubility limit in Sn [
6]. Yuki Hirata et al [
7] added Zn and In to Sn-Bi to prepare Sn-Bi-Zn and Sn-Bi-Zn-In solders, the investigations found that the addition of In can potentially help reduce the brittleness of Sn–Bi alloys, this was related to the suppressed microstructural coarsening of SBZ during thermal aging. Wenchao Yang et al [
8] studied the effect of a small amount of Al on the microstructure and properties of non-eutectic Sn-20Bi solder alloy, and found that Al reduced the wettability of solder alloys, increased the micro-hardness and the corrosion resistance. Tianqi Yang et al [
9] found that the addition of Ag to the Sn-Bi eutectic solder, increased the solidification temperature and the tensile strength, and reduced the fusing latent heat of alloy, but had no influence on the melting point of the alloy.
Recently, several research groups had focused on manufacturing new alloys doped with rare earths (RE) elements. Rare earth elements are divided in two groups, known as
light RE or Ce group of elements and heavy RE or Y group of elements. Results reported [
10,
11,
12] that rare earths elements can refine the solder microstructure, increase significantly the wettability, and increase the mechanicals properties. Until now, most of the studies only reported the effect of light RE on the lead-free solders such as Ce, La, Pr, Nd etc. Although the two groups have similar
physical-chemical properties, the effect of Y group on improving properties of lead-free solder are still to be proven. Based on the effect of light rare elements addition in Sn alloys, in the present work, heavy rare earth Y was selected to serve as the alloy element, and the influence of different Y content on the properties of the solder was explored. Therefore, in this study, to improve the properties and modify the microstructure of the alloys, Sn-20Bi alloy with low Bi content has been used as the research object to study the effects on microstructure and properties by adding rare earth element Y. The effects of Y concentration on the microstructure, wettability and mechanical properties of the Sn-Bi -Al solder alloy was investigated to produce a viable solder alloy.
Materials and Methods
The alloy samples were prepared from Tin granule (purity of 99.99%), Bismuth granule (purity of 99.99%), Aluminum wire (purity of 99.99%, 1mm diameter) and Yttrium powder (purity of 99.9%) raw materials.
Table 1 showed the nominal chemical compositions of solders alloy used in this study. The following steps were followed to prepare the samples: (1) Heat the furnace to 600 degrees. (2) A crucible was used as a container of the alloy’s mixture, due to the differences between the melting point of the elements, to prevent the damage caused by burning, tin was added first and after 15 minutes the rest of elements was added. (3) The raw materials were melted for 60 minutes and agitate every 10 minutes. (4) Finally, the melted alloys were poured into mold and then cooled to room temperature, the obtained melted alloy surface is smooth and clean, without inclusions, after cutting, the internal structure is uniform, dense and without shrinkage cavities.
Microstructure Observation
The alloy sample for microstructure observation had a rectangle shape with a size of 5mm×5mm×2mm. Metallographic sample mosaic machine (XQ-2B) was used. Samples were mounted using hot mounting resin. The mounted specimens were grinded and polished, then the samples were etched using a solution of () + (HCL)+(). The microstructure was analyzed using the optical microscope. The elemental composition of solder alloy was determined using Energy dispersive spectroscopy (EDS). The phases of the alloy were observed using an X-Ray diffraction (XRD, 18KW/D/max2550VB/PC) using a Copper Target operating at 18KW (450mA). When the test is completed, the obtained data was imported into Jade software for phase matching, and then import XRD measured data into Origin software for graphic analysis.
Wettability Test
Spreading method was used to evaluate the wettability of Sn-20Bi-0.3Al-xY solder alloys. The Cu substrate with a size of 30 mm × 30 mm × 1 mm was first polished and then cleaned by acetone and alcohol respectively; alloy sample with a weight of 0.3g were prepared, polished and cleaned. In this study, a solder alloy was selected for welding at a temperature of 270ºC. Firstly, we preheat the furnace to achieve the required welding temperature. The alloy was placed on Cu substrate with resin flux and then inserted in a furnace with a holding time of 5 minutes. Each alloy was conducted 3 times and then obtain the average area. The spreading area on the copper substrates was measured using image processing software.
Micro-Hardness Test
The micro-hardness test id essential a small load Vickers hardness test. The samples were cut in a size of 5 mm × 5 mm × 2 mm, embedded on a mounting machine and then the surface was ground and polished. Followed by hardness measurement on an HXD-1000TMC/LCD micro hardness machine at room temperature. The loading conditions were: load 25 g, saturation time 15 s, observe at a magnification of 40 × 15. Measure each sample for three times and then take the average value.
Tensile Test
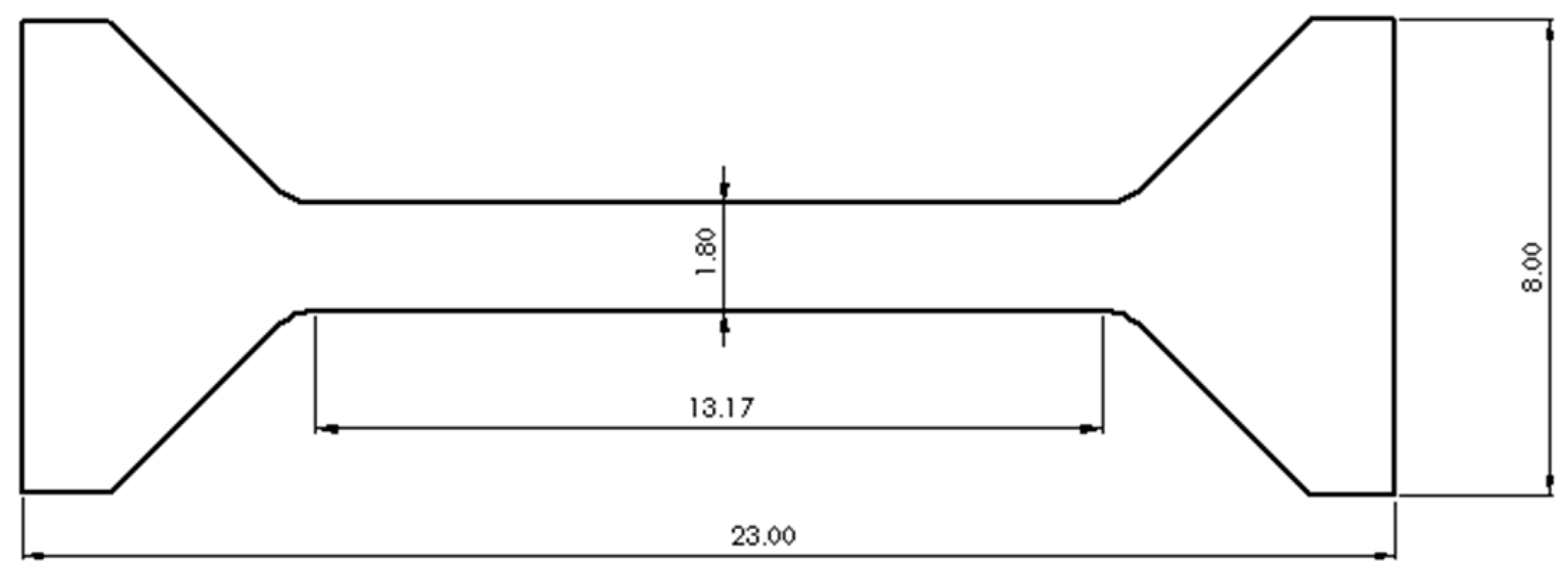
The ultimate tensile strength (UTS) and ductility of solder specimens were obtained from tensile testing. The schematic illustration of tensile testing of specimens are shown in
Figure 1, the specimen thickness is 1mm. The specimen was tested on a universal testing machine (HY-0350,Shanghai, China) at room temperature and a constant strain rate of 0.8 mm/min. After performing the tensile tests, the fractured surfaces of the samples were examined by using S3400N s
canning Electron Microscope (SEM).
Results and Discussions
Microstructure Analysis
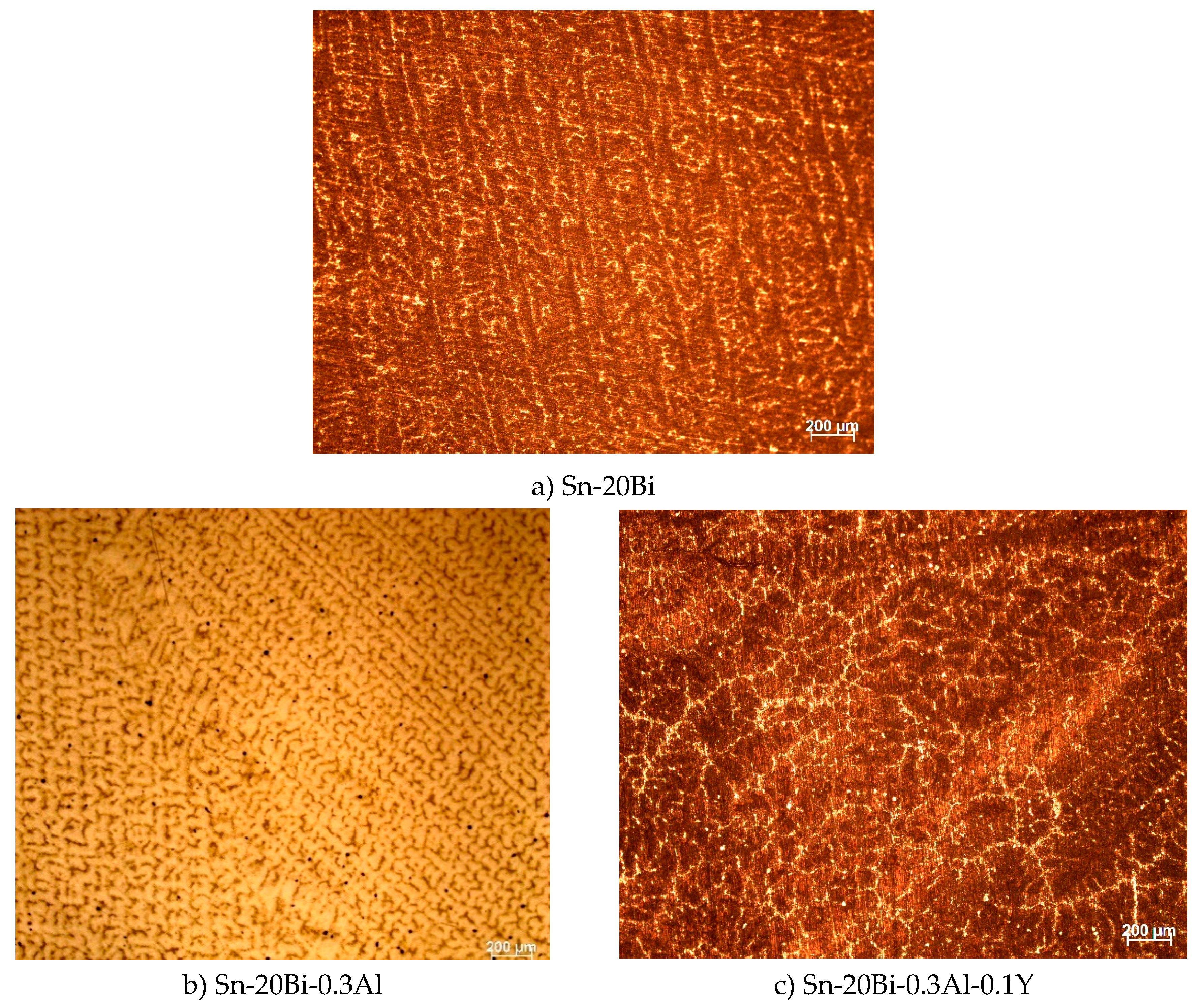
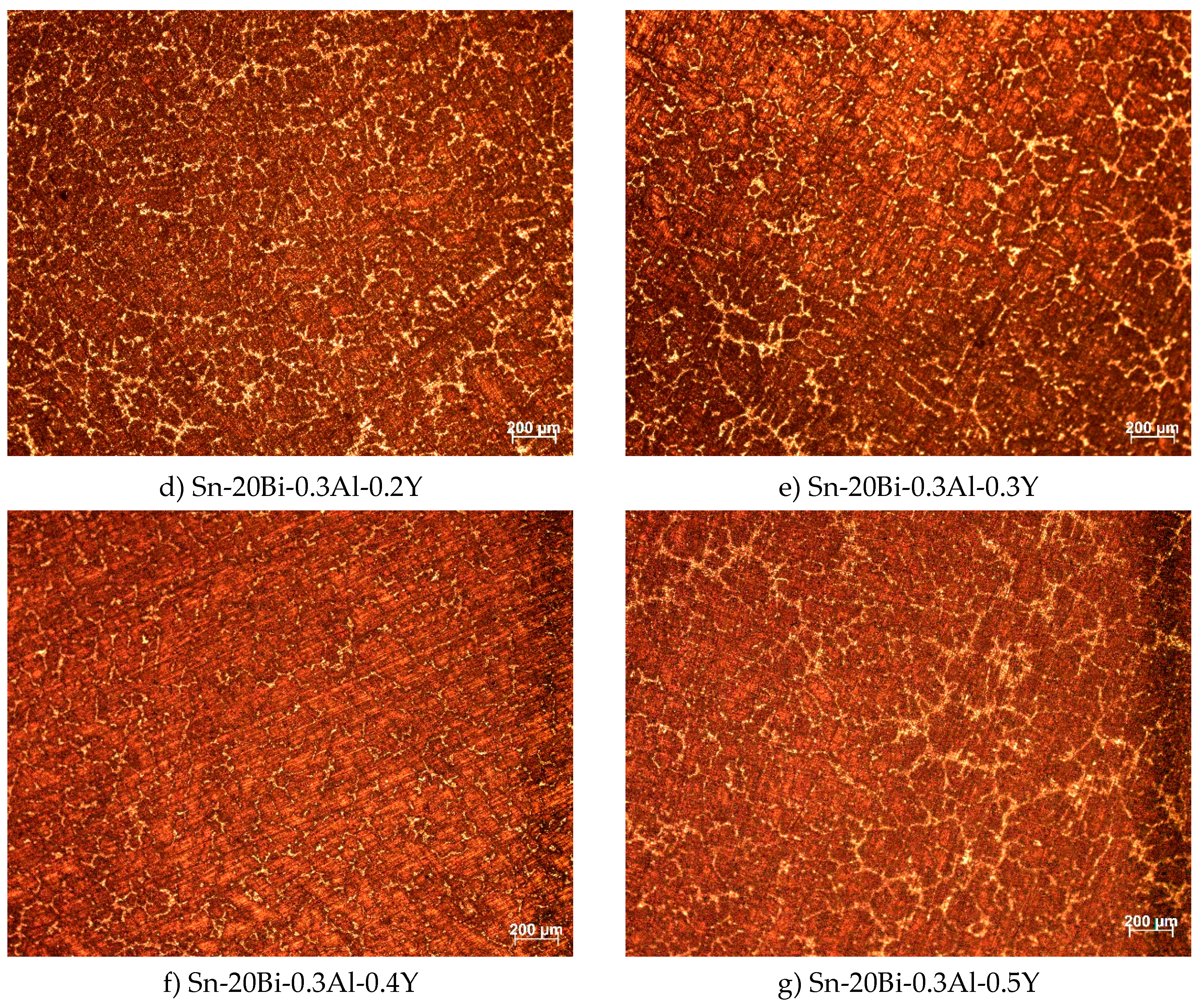
The microstructures of the alloys captured by optical microscope are shown in
Figure 2. All seven alloys present a similar microstructure, which consist in a bright granule phase that is distributed in a primary phase. However, the appearance of the alloy changes according to the fraction of Y. As we can see in
Figure 2b, before the addition of Y, beside the bright granule phase and the primary phase, there is a few black particles distributed in β-Sn phase. Wenchao Yang et al [
8] find that the black particle shape is Al-rich phase. Adding Y into Sn-20Bi-0.3Al solder alloy, the bright phase gradually refines and present a uniform distribution; when the content of Y was 0.3wt%, the microstructure dispersion and level of refinement was the best. When the content of Y continues to increase, a few bright granule phases in the microstructure start to transform into large pieces, which result in a coarser microstructure. As shown in
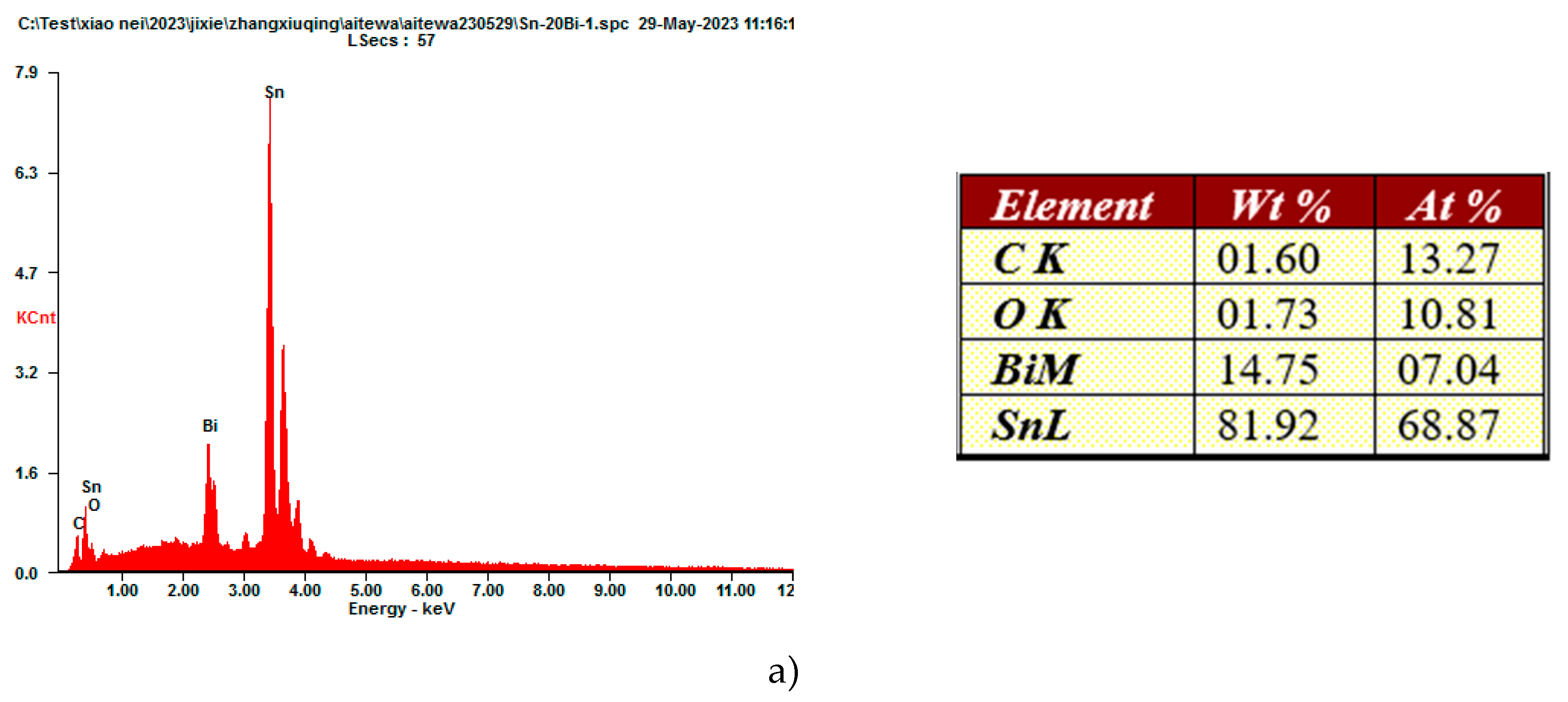
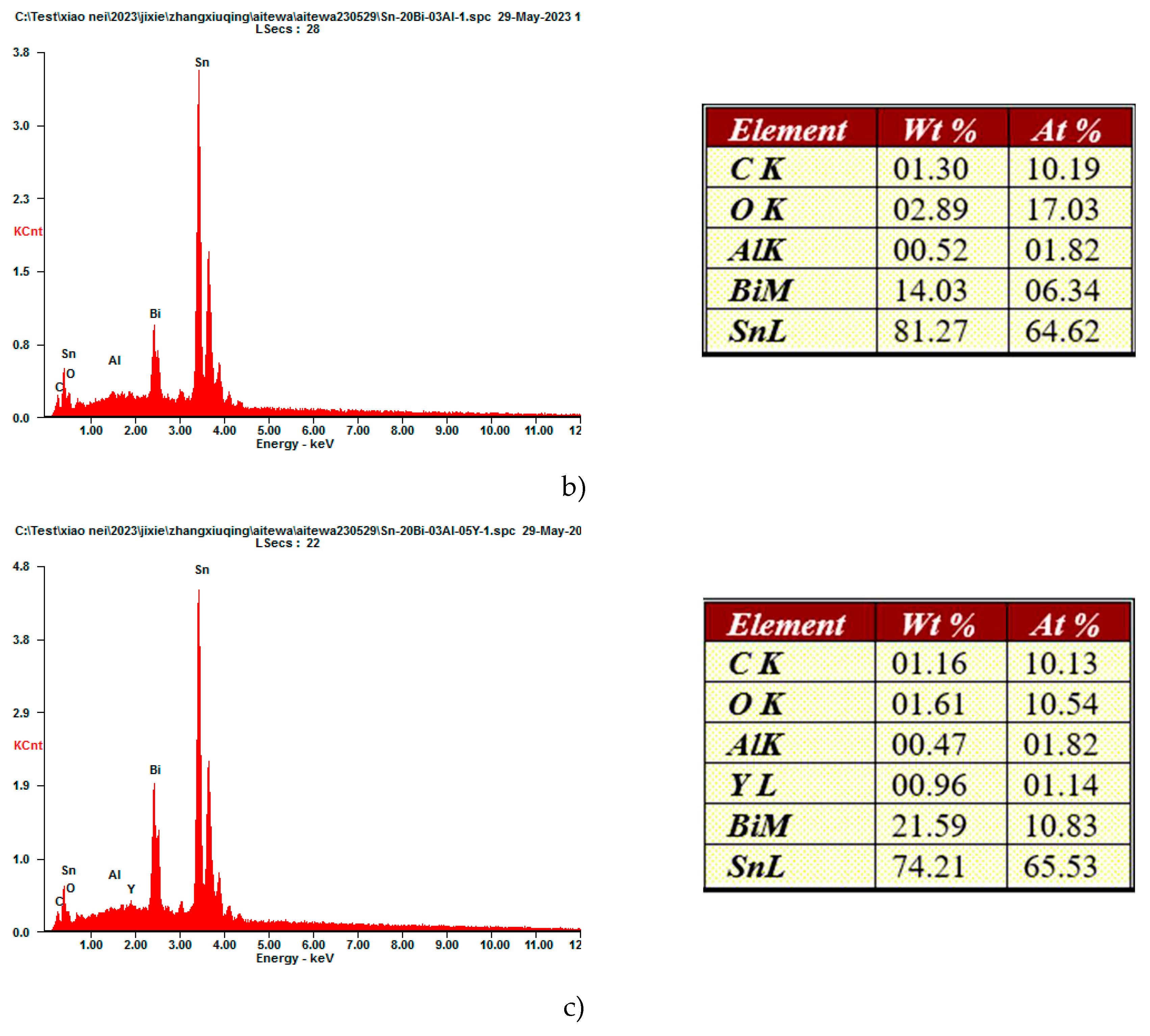
Figure 2g when the content of Y was 0.5wt%, it is clear how the microstructure become more coarsened, by increasing the volume of the bright phase. Due to the unique effect of metamorphism, rare earth Y, act as a grain refiner which can promote nucleation and increase the volume fraction of the second phase, which resulted in a refining microstructure of the solders. To understand how the elements are distributed on the microstructure, Sn-20Bi, Sn-20Bi-0.3Al and Sn-20Bi-0.3Al-0.5Y alloys were submitted to an EDS analysis, results are shown in
Figure 3, alloys were submitted to a point scan analysis,
Figure 3c confirm the presence of Y as simple phase, revealing that the atom percentage is about 1.14%.
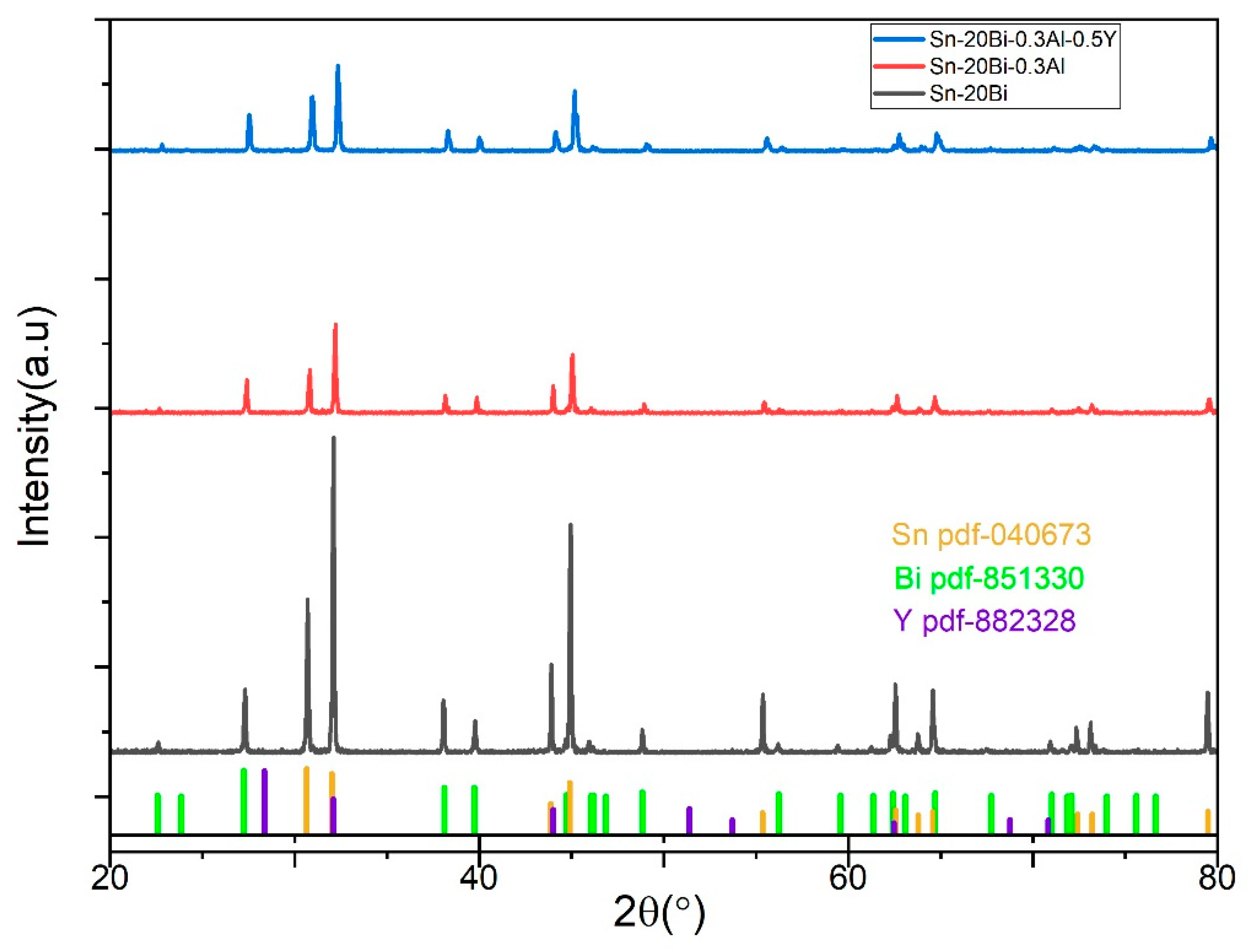
Figure 4 shows the XRD patterns of Sn-20Bi, Sn-20Bi-0.3Al and Sn-20Bi-0.3Al-0.5Y alloys.
XRD analysis was achieved to evaluate and identify the existence of the phase’s structures of IMCs in the solder alloys. The XRD diffraction reveals that, the principal peaks are β-Sn and Bi phases, but also has trace Y phase, as expected and predicted by the EDS results, indicating that the addition of Y did not lead to the formation of any
intermetallic compound new phases.
Wettability
A solder alloy is required to have an acceptable wettability, which is the ability to produce a reliable connection.
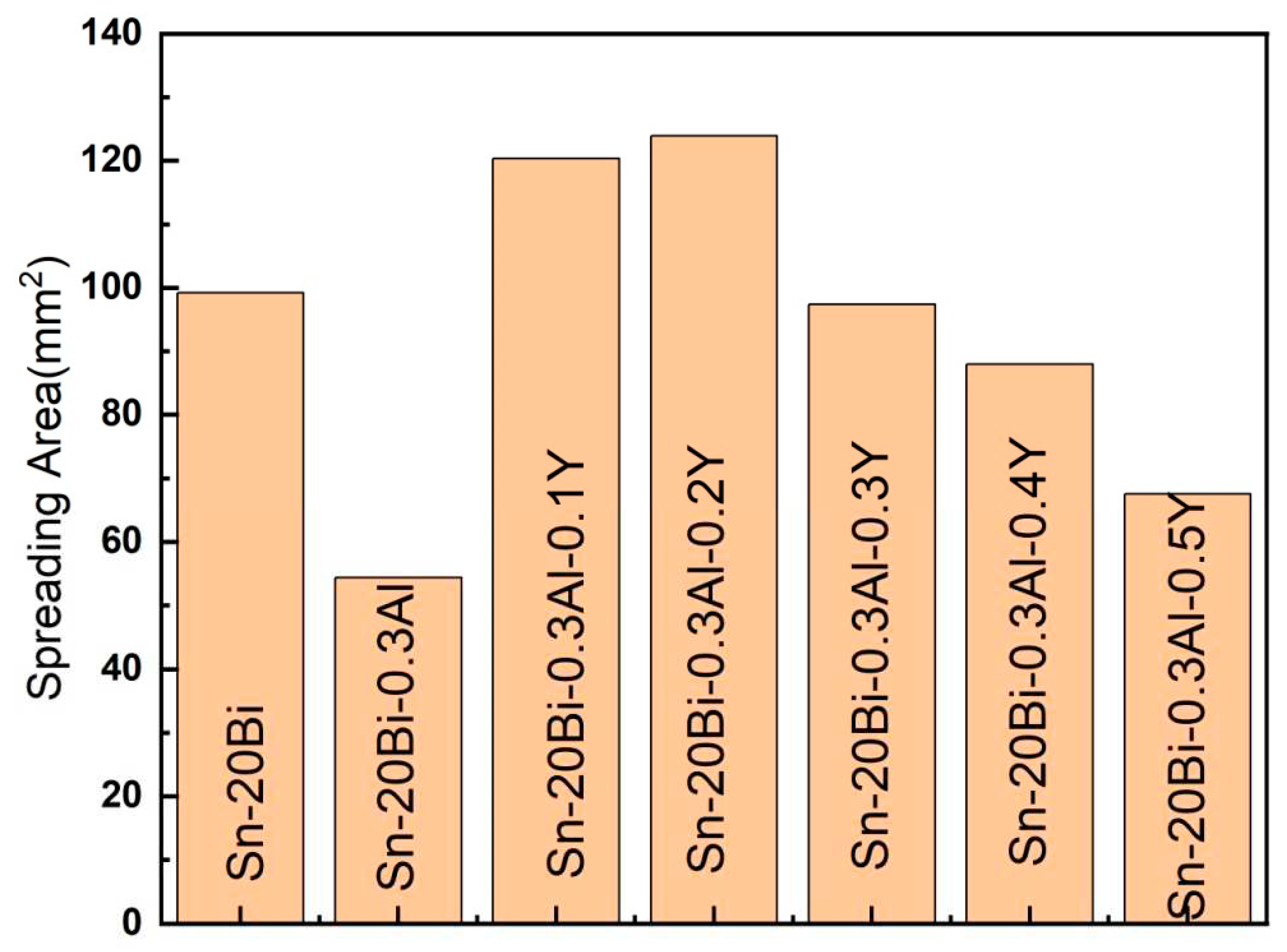
Figure 5 shows the results obtained from the spreading test of Sn-20Bi-0.3Al-xY solder alloys on Cu substrate. According to the results, the wettability of Sn-20Bi-0.3Al-xY solder alloy had improve with the increase of the Y fraction. When Y is not added, the spreading area of the alloy on the copper plate is
. When the amount of Y added to solder alloy is 0.2wt%, the spreading area of the solder is the largest. As Y gradually increases, the spread area of Sn-20Bi-0.3Al-xY solder first increases and then decreases. When content of Y increase to 0.5wt%, the spreading area decreased to
, but still high than that without Y, indicating that the addition of Y in the solder alloy can significantly improve the wetting performance of the solder.
Rare earth is known as a surface-active element, which can decrease the surface tension of liquid solder and enhance the wetting of the solder on the substrate [
16]
. Previous studies [
13,
14,
15] shows that the enhancement in wettability by adding light RE element was only observed when the content of rare earth element was low and without a formation of intermetallic, indicate that the variability in wettability is attributed to a formation of discrete rare earth-tin intermetallic compound and a discrepancy in a microstructure. Thus, the decrease in wettability is explained by the formation of oxide residue during the soldering process, the increase in rare earth content will make the alloy more liable to oxidation, which reduce the wettability on the solder. It is noted that in this study, the same trend for the effect of heavy RE Y addition on the wetting area was observed.
Micro-Hardness Analysis
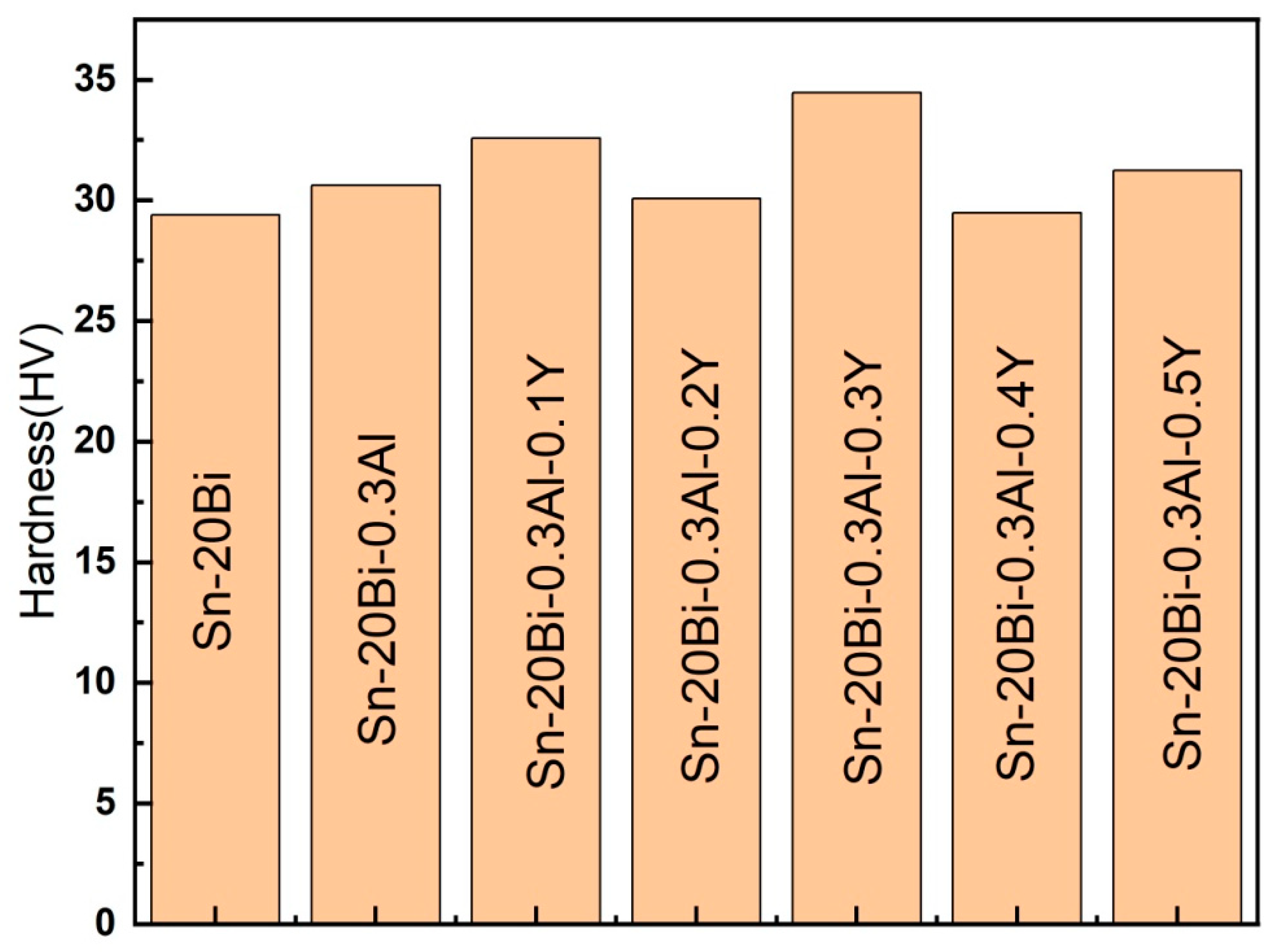
The micro-hardness test results of Sn-20Bi-0.3Al-xY solder alloy are shown in
Figure 5. It could be seen that the hardness of Sn-20Bi-0.3Al-xY had significantly improved with the addition of Y. The results shows that when the fraction of Y was 0.3%, the hardness of the solder alloy was the highest (34.467HV), and when the fraction of Y was 0.4% shows the lowest hardness (29.467HV), but still present a slight improve compared to Sn-20Bi alloy, indicating that the Y-addition is beneficial to improve the micro-hardness properties of solder alloys. When the content of Y is 0.2wt% and 0.4wt%, the hardness of the two groups presents the lowest value after the addition of rare earth Y. This is because after the addition of Y, the Sn-Bi-Al ternary alloy becomes a relatively complex quaternary alloy, and the hardness value of the alloy is influenced by a group of elements such as microstructure shape, phase, grain size etc. Thus, the researchers attributed the increase in the micro-hardness of the alloys containing Y element to the fact that rare earth elements can cause grain refining strengthening and dispersion strengthening mechanism of solders [
13], which means the number of hard particles has increased and are homogenously distributed in the solder matrix. However, when the fraction of Y continues to increase can easily form oxides and compounds, causing inclusions and loosening, leading to a slight decrease in the hardness of the alloy.
Figure 6.
Micro-hardness of Sn-20Bi-0.3Al-xY solder alloy.
Figure 6.
Micro-hardness of Sn-20Bi-0.3Al-xY solder alloy.
Tensile Properties
To determine the mechanical properties of the solder, tensile tests were conducted on the alloy.
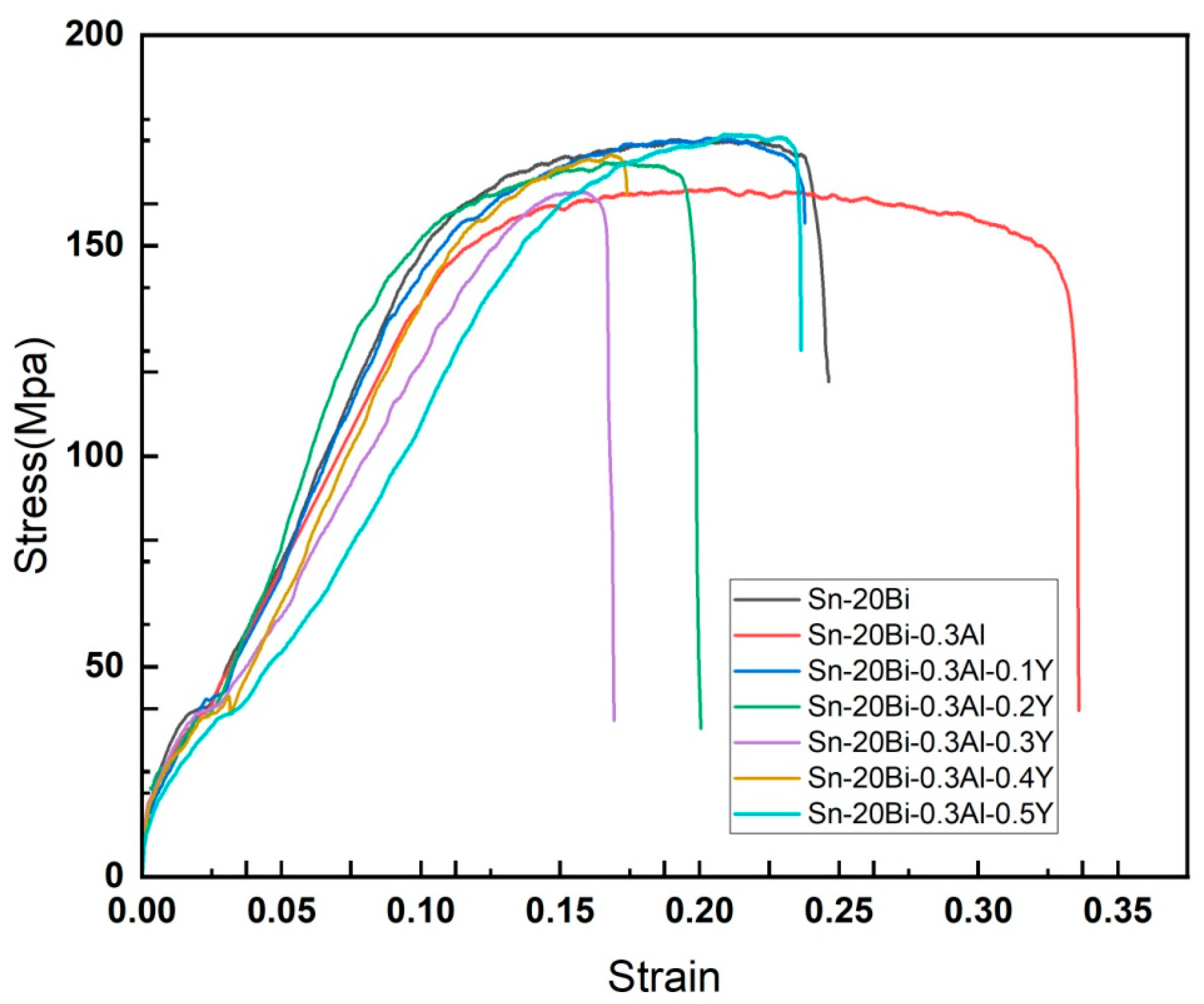
Figure 7 shows the tensile strength of Sn-20Bi-0.3Al solder and Sn-20Bi-0.3Al solder alloy containing different amount of rare earth Y stress-strain curves. As shown in
Table 2, adding rare earth element Y to the alloy can enhance the tensile strength of the solder to a certain extent. However, there is a decrease in the elongation. This is because the presence of hard Y particles forms local agglomeration of the Y-bearing phase. Comparatively, it can be seen that the tensile strength of the Sn-Bi-Al alloy without Y added is 163.68 MPa. When Y is added, the tensile strength of the solder alloy has increased to a certain extent, and as the amount of Y added increases, the tensile strength of the alloy also increases. When the Y content is at 0.3% the UTS decrease by a rate of 0.5% compared to Sn-20Bi-0.3Al. The UTS of the Sn-20Bi-0.3Al alloy was 163.68 MPa. With 0.1wt% Y addition, the UTS increased to 175.62 MPa. When Y was further increased to 0.5wt%, the UTS was further increased to 176.6 MPa. However, continuing to add Y elements to the solder alloy showed a slight decrease in the tensile strength of the alloy, but overall, the tensile strength was still greater than that of the matrix alloy without Y added. The UTS and elongation of alloy are summarized in
Table 2. The addition of Y resulted in finer microstructures, and Bi precipitates in the solder matrix [
15]. The results of those modifications are strengthening mechanisms.
Fracture Analysis
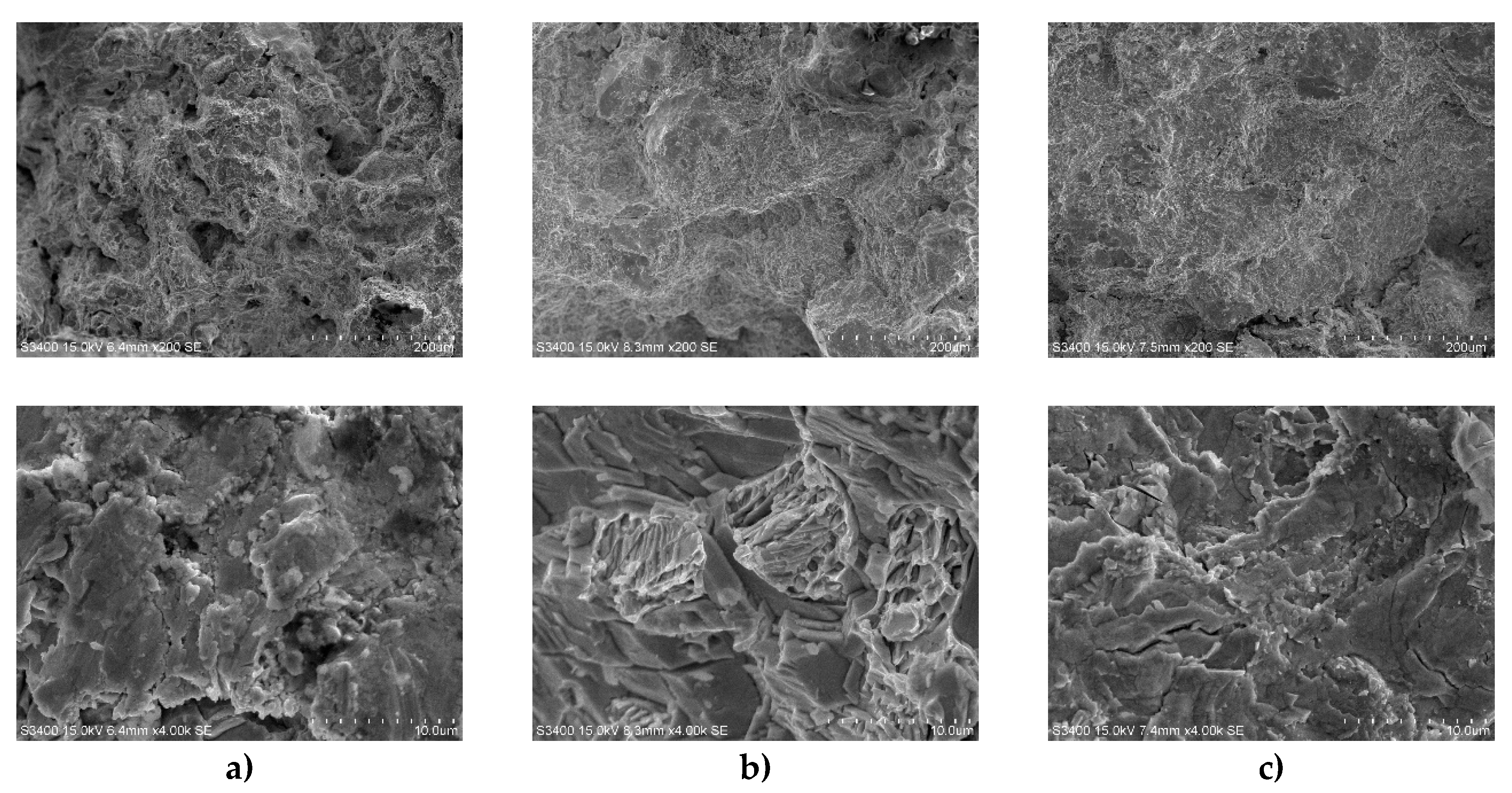
Ultimate tensile strength of solder alloys with different Y contents was measured according to experiments. In order to understand the tensile fracture mode and the relationship between fracture mode and microstructure, in this study fracture surface morphology was analyzed using the SEM.
Figure 8 shows the tensile fracture morphology and enlarged view of Sn-20Bi-0.3Al-xY solder alloy. From the enlarged view, we can see that the fracture mode of the solder alloy was shown to be a mixed fracture mode, combining ductile fracture and brittle fracture. As shown in Figure 8a, some brittle fracture characteristics of cleavage can be seen as well as some ductile fracture characteristics of dimples. Figure 8b, with the addition of 0.1wt% Y, a smooth plane appears at the fracture location, with obvious cleavage steps and surface and the dimples at the fracture gradually decreased, indicating that the sample have a greater brittleness compared to Sn-20Bi-0.3Al, which is consistent with the results in Table 2. As the content of Y increase to 0.5wt% in Figure 8c, from the enlarged image, it can be observed that compared with the sample in Figure 8b, the number of cleavage planes in the fracture surface of Sn-20Bi-0.3Al-0.5Y sample reduced, indicating partial improvement in the brittle fracture characteristics, and there were many pull-out morphologies.
Conclusions
The addition of Y significantly changes the alloy microstructure, by refining the bright granule phase. In Sn-20Bi-0.3Al-Xy (x=0.1, 0.2, 0.3, 0.4, 0.5), Sn-20Bi-0.3Al-0.3Y has the best refinement. With the increase of Y fraction, the microstructure became coarser. XRD results shows that no compounds were formed and mainly exist β-Sn phase and Bi phase.
As Y gradually increases, the spread area of Sn-20Bi-0.3Al-xY solder first increases and then decreases.
As Y gradually increases, the hardness of Sn-20Bi-0.3Al-xY had improved significantly. The maximum and minimum hardness values recorded were 34.467HV and 29.467HV respectively, when the added content of Y was 0.3wt% and 0.4wt% respectively.
The ultimate tensile strength of the Sn-20Bi-0.3Al-Xy solders was increased by strengthening mechanisms such as grain boundary strengthening, and precipitation strengthening.
Funding
This study was funded by Department of mechanical engineering.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Osvaldo Fornaro, "Directional Solidification of Sn-Cu6Sn5 In Situ Composites", Advances in Materials Science and Engineering. 2019, 2019, 9210713. [CrossRef]
- Directive 2002/95/Ec on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. European Parliament and the Council, Official Journal of the European Union 2003, 46.
- J. Wu, S. Xue, J. Wang, M. Wu, J. Wang, Effects of a-Al2O3 nano particles doped on microstructure and properties of Sn–0.3 Ag–0.7 Cu low-Ag solder, J. [CrossRef]
- Mater. Sci.: Mater. Electron. 2018, 29, 7372–7387.
- L.R. Garcia, W.R. Osorio, ´ L.C. Peixoto, A. Garcia, Mechanical properties of Sn–Zn lead-free solder alloys based on the microstructure array, Mater. Charact. 2010, 61, 212–220. [CrossRef]
- D. Huang, J. Zhou, P.P. Li, Corrosion performance of Pb-free Sn-Zn solders in salt spray, in: IEEE International Conference on Electronic Packaging Technology & High Density Packaging ICEPT-HDP, 2008, pp. 1–4.
- Xin, F. Tan, QichaoHao, QinfenGu, Stuart D. McDonald, Keith Sweatman, Michael Bermingham, Kazuhiro Nogita, The effects of Sb on the lattice and microstructure characteristics of hypo-eutectic Sn-Bi alloys, Materials Characterization 2023, 201, 112934. [Google Scholar] [CrossRef]
- Yuki Hirata, Chih-han Yang, Shih-kang Lin, Hiroshi Nishikawa, Improvements in mechanical properties of Sn–Bi alloys with addition of Zn and In, Materials Science and Engineering: A. 2021, 813, 141131. [CrossRef]
- Wenchao Yang, Jidong Li,Yitai Li et al. Effect of Aluminum Addition on the Microstructure and Properties of Non-Eutectic Sn-20Bi Solder Alloys. Materials 2019, 12, 1194. [CrossRef] [PubMed]
- Tianqi Yang, Xiuchen Zhao, Zishan Xiong, Wei Tan, Yuhang Wei, Chengwen Tan, Xiaodong Yu, Yingchun Wang, Improvement of microstructure and tensile properties of Sn–Bi–Ag alloy by heterogeneous nucleation of β-Sn on Ag3Sn, Materials Science and Engineering: A. 2020, 785, 139372. [CrossRef]
- Wu J, Xue S B, Wang J W, et al. Effect of Pr addition on properties and Sn whisker growth of Sn-0.3Ag-0.7Culow-Ag solder for electronic packaging[J]. Journal of Materials Science: Materials in Electronics. 2017, 28, 10230–10244. [CrossRef]
- Xu J C, Xue S B, Xue P, et al. Study on microstructure and properties of Sn-0.3Ag-0.7Cu solder bearing Nd. Journal of Materials Science: Materials in Electronics. 2016, 27, 8771–8777. [CrossRef]
- Choi H, Kaplan W D, Choe H. Effect of Yttrium on fracture strength of the Sn-1.0Ag-0.5Cu solder joint. Journal of Electronic materials. 2016, 45, 3260–3262. [CrossRef]
- Dong Wenxing,Shi Yaowu,Xia Zhidong,et al. Effects of Trace amounts of Rare Earth Additions on Microstructure and Properties of Sn-Bi-Based Solder Alloy. Journal of Electronic Materials. 2008, 37, 982–991. [CrossRef]
- Xiao W., Shi Y., Lei Y., Xia Z., Guo F. AUTHOR FULL NAMES: Xiao, Weimin; Shi, Yaowu ; Lei, Yongping; Xia, Zhidong; Guo, Fu . Comparative study of microstructures and properties of three valuable SnAgCuRE lead-free solder alloys. Journal of Electronic Materials. 2006, 35, 1095–1103. [CrossRef]
- Suchart Chantaramanee, Phairote Sungkhaphaitoon, Combined effects of Bi and Sb elements on microstructure, thermal and mechanical properties of Sn-0.7Ag-0.5Cu solder alloys, Transactions of Nonferrous Metals Society of China. 2022, 32, 3301–3311. [CrossRef]
- Li, Hui. “Effects of Small Amount Addition of Rare Earth Y on Microstructure and Property of Sn3.0Ag0.5Cu Solder.” Key Engineering Materials, vol. 584, Trans Tech Publications, Ltd., Sept. 2013, pp. 3–8.
- Y.K. Tang, E.J. Lin, J.Y. Wang, Y.X. Lin, C.Y. Wu, C.Y. Chiu, C.H. Lee, C.P. Wang, C.Y. Liu, Effect of eutectic reaction between depositing atoms and substrate elements on morphological evolution of Sn–Bi–Sn multilayer deposition, Materials Chemistry and Physics. 2020, 250, 122960. [CrossRef]
- Yuki Hirata, Chih-han Yang, Shih-kang Lin, Hiroshi Nishikawa, Improvements in mechanical properties of Sn–Bi alloys with addition of Zn and In, Materials Science and Engineering: A. 2021, 813, 141131. [CrossRef]
- Georg Siroky, Elke Kraker, Dietmar Kieslinger, Ernst Kozeschnik, Werner Ecker, Simulation and experimental characterization of microporosity during solidification in Sn-Bi alloys, Materials & Design. 2021, 212, 110258. [CrossRef]
- Zhuangzhuang Hou, Xiuchen Zhao, Yue Gu, Chengwen Tan, Yongjun Huo, Hong li, Sujun Shi, Ying Liu, Enhancement mechanism of Te doping on microstructure, wettability and mechanical properties of Sn–Bi-based solder, Materials Science and Engineering: A. 2022, 848, 143445. [CrossRef]
- Xin F. Tan, Qichao Hao, Qinfen Gu, Stuart D. McDonald, Keith Sweatman, Michael Bermingham, Kazuhiro Nogita, The effects of Sb on the lattice and microstructure characteristics of hypo-eutectic Sn-Bi alloys, Materials Characterization. 2023, 201, 112934. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).