Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Improving the Performance of Constructed Wetlands Using Biochar
2.1. Performance Enhancement through Biochar and Immobilizated Microorganisms
2.2. Combined Enhancement through Biochar and Oxygen Supply
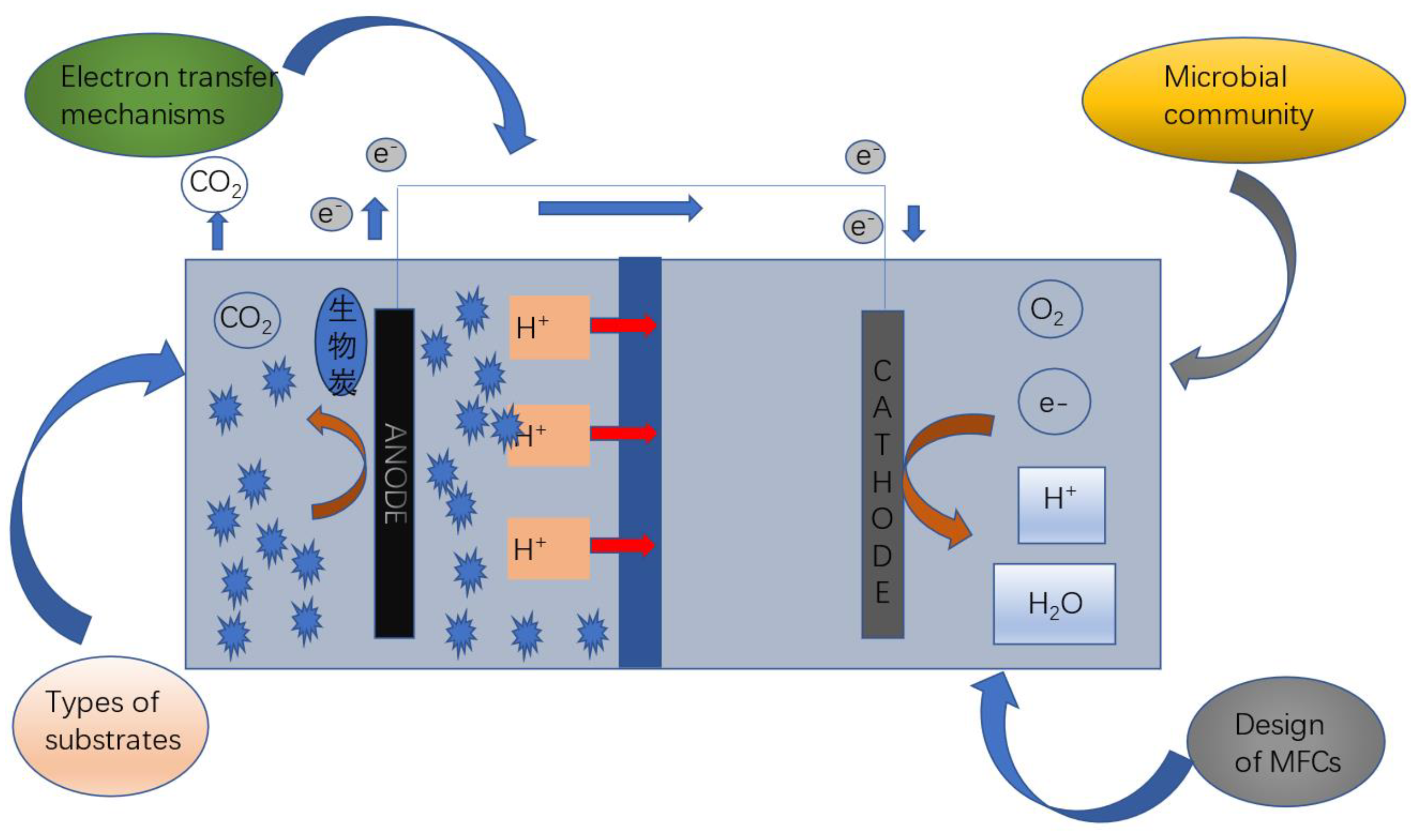
2.3. Performance Enhancement through Biochar Coupling Electrochemical
2.4. Performance Enhancement through Biochar Modification
3. Effects of Biochar on Microbial Communities in Constructed Wetlands
3.1. Effects of Biochar on the Composition and Structure of EPS
3.2. Effect of Biochar on Enzyme Activity
3.3. Effects of Biochar on Functional Genes
3.4. Biochar on Microbial Alpha Diversity
3.5. Effects of Biochar on Microbial Community Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji B, Chen J, Mei J, et al. Roles of biochar media and oxygen supply strategies in treatment performance, greenhouse gas emissions, and bacterial community features of subsurface-flow constructed wetlands [J]. Bioresource Technology, 2020, 302: 122890. [CrossRef]
- Zhang Y, Li M, Dong L, et al. Effects of biochar dosage on treatment performance, enzyme activity and microbial community in aerated constructed wetlands for treating low C/N domestic sewage [J]. Environmental Technology & Innovation, 2021, 24: 101919. [CrossRef]
- Parde D, Patwa A, Shukla A, et al. A review of constructed wetland on type, treatment and technology of wastewater [J]. Environmental Technology & Innovation, 2021, 21: 101261. [CrossRef]
- Feng L K, Wang R G, Jia L X, et al. Can biochar application improve nitrogen removal in constructed wetlands for treating anaerobically-digested swine wastewater [J]. Chmical Engineering Journal, 2020, 379. [CrossRef]
- Zhang J, Cheng S, He F, et al. Effects of Cd2+ and Pb2+ on the substrate bioflms in the integrated vertical-flow constructed wetland [J]. Journal of Environmental Sciences, 2008, 20(8): 900-6. [CrossRef]
- Lei Y, Langenhoff A, Bruning H, et al. Sorption of micropollutants on selected constructed wetland support matrices [J]. Chemosphere, 2021, 275. [CrossRef]
- Zhou X, Wang R, Liu H, et al. Nitrogen removal responses to biochar addition in intermittent-aerated subsurface flow constructed wetland microcosms: Enhancing role and mechanism [J]. Ecological Engineering, 2019, 128: 57-65. [CrossRef]
- Deng C, Huang L, Liang Y, et al. Response of microbes to biochar strengthen nitrogen removal in subsurface flow constructed wetlands: Microbial community structure and metabolite characteristics [J]. Science of The Total Environment, 2019, 694: 133687. [CrossRef]
- Liu Y, Liu S, Yang Z, et al. Synergetic effects of biochars and denitrifier on nitrate removal [J]. Bioresource Technology, 2021, 335: 125245. [CrossRef]
- Zhong L, Yang S-S, Ding J, et al. Enhanced nitrogen removal in an electrochemically coupled biochar-amended constructed wetland microcosms: The interactive effects of biochar and electrochemistry [J]. Science of The Total Environment, 2021, 789: 147761. [CrossRef]
- Li J, Fan J, Zhang J, et al. Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands [J]. Environmental Science And Pollution Researchr, 2018, 25(14): 13929-37. [CrossRef]
- Zhuang L-L, Li M, Li Y, et al. The performance and mechanism of biochar-enhanced constructed wetland for wastewater treatment [J]. Journal of Water Process Engineering, 2022, 45: 102522. [CrossRef]
- Deng S, Chen J, Chang J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits [J]. Journal of Cleaner Production, 2021, 293: 126156. [CrossRef]
- Xing C, Xu X, Xu Z, et al. Study on the Decontamination Effect of Biochar-Constructed Wetland under Different Hydraulic Conditions [J]. Water, 2021, 13(7). [CrossRef]
- Feng L, Liu Y, Zhang J, et al. Dynamic variation in nitrogen removal of constructed wetlands modified by biochar for treating secondary livestock effluent under varying oxygen supplying conditions [J]. Journal of Environmental Management, 2020, 260: 110152. [CrossRef]
- Hamada M S, Ibaid Z Z, Shatat M. Performance of citrus charcoal and olivepomace charcoal as natural substrates in the treatment of municipal wastewater by vertical flow subsurface constructed wetlands [J]. Bioresource Technology Reports, 2021, 15: 100801. [CrossRef]
- Xing C J, Xu X X, Xu Z H, et al. Study on the Decontamination Effect of Biochar-Constructed Wetland under Different Hydraulic Conditions [J]. Water, 2021, 13(7). [CrossRef]
- Zhang Z, Solaiman Z M, Meney K, et al. Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil [J]. Journal of Soils And Sediments, 2013, 13(1): 140-51. [CrossRef]
- Liu H, Cheng C, Wu H. Sustainable utilization of wetland biomass for activated carbon production: A review on recent advances in modification and activation methods [J]. Science of The Total Environment, 2021, 790: 148214. [CrossRef]
- Cui X, Hao H, Zhang C, et al. Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars [J]. Science of The Total Environment, 2016, 539: 566-75. [CrossRef]
- Hou W, Wang S, Li Y, et al. Influence of modified biochar supported Fe-Cu/polyvinylpyrrolidone on nitrate removal and high selectivity towards nitrogen in constructed wetlands [J]. Environmental Pollution, 2021, 289. [CrossRef]
- Chen X, Zhu H, Bañuelos G, et al. Biochar reduces nitrous oxide but increases methane emissions in batch wetland mesocosms [J]. Chemical Engineering Journal, 2020, 392: 124842. [CrossRef]
- Guo X F, Cui X Y, Li H S. Effects of fillers combined with biosorbents on nutrient and heavy metal removal from biogas slurry in constructed wetlands [J]. Science of The Total Environment, 2020, 703. [CrossRef]
- Bonetti G, Trevathan-Tackett S M, Hebert N, et al. Microbial community dynamics behind major release of methane in constructed wetlands [J]. Applied Soil Ecology, 2021, 167: 104163. [CrossRef]
- Bolton L, Joseph S, Greenway M, et al. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater [J]. Ecological Engineering, 2019, 142: 100005. [CrossRef]
- Li J, Fan J L, Zhang J, et al. Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands [J]. Environmental Science And Pollution Research, 2018, 25(14): 13929-37. [CrossRef]
- Kizito S, Wu S, Kirui W K, et al. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry [J]. Science of The Total Environmente, 2015, 505: 102-12. [CrossRef]
- Madadi R, Bester K. Fungi and biochar applications in bioremediation of organic micropollutants from aquatic media [J]. Marine Pollution Bulletin, 2021, 166: 112247. [CrossRef]
- Hu B, Hu S S, Vymazal J, et al. Arbuscular mycorrhizal symbiosis in constructed wetlands with different substrates: Effects on the phytoremediation of ibuprofen and diclofenac [J]. Journal of Environmental Management, 2021, 296. [CrossRef]
- Yu G, Peng H, Fu Y, et al. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria [J]. Bioresource Technology, 2019, 280: 337-44. [CrossRef]
- Jia W, Yang Y C, Yang L Y, et al. High-efficient nitrogen removal and its microbiological mechanism of a novel carbon self-sufficient constructed wetland [J]. Science of The Total Environment, 2021, 775. [CrossRef]
- Jia L, Wang R, Feng L, et al. Intensified nitrogen removal in intermittently-aerated vertical flow constructed wetlands with agricultural biomass: Effect of influent C/N ratios [J]. Chemical Engineering Journal, 2018, 345: 22-30. [CrossRef]
- Zhou X, Wang X, Zhang H, et al. Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland [J]. Bioresource Technology, 2017, 241: 269-75. [CrossRef]
- Chand N, Suthar S, Kumar K, et al. Enhanced removal of nutrients and coliforms from domestic wastewater in cattle dung biochar-packed Colocasia esculenta-based vertical subsurface flow constructed wetland [J]. Journal of Water Process Engineering, 2021, 41: 101994. [CrossRef]
- Feng L K, Wu H M, Zhang J, et al. Simultaneous elimination of antibiotics resistance genes and dissolved organic matter in treatment wetlands: Characteristics and associated relationship [J]. Chemical Engineering Journal, 2021, 415. [CrossRef]
- Zhuang L-L, Yang T, Zhang J, et al. The configuration, purification effect and mechanism of intensified constructed wetland for wastewater treatment from the aspect of nitrogen removal: A review [J]. Bioresource Technology, 2019, 293: 122086. [CrossRef]
- Zhou X, Chen Z, Li Z, et al. Impacts of aeration and biochar addition on extracellular polymeric substances and microbial communities in constructed wetlands for low C/N wastewater treatment: Implications for clogging [J]. Chemical Engineering Journal, 2020, 396: 125349. [CrossRef]
- Guo Z Z, Kang Y, Hu Z, et al. Removal pathways of benzofluoranthene in a constructed wetland amended with metallic ions embedded carbon [J]. Bioresource Technology, 2020, 311. [CrossRef]
- Zhou X, Jia L, Liang C, et al. Simultaneous enhancement of nitrogen removal and nitrous oxide reduction by a saturated biochar-based intermittent aeration vertical flow constructed wetland: Effects of influent strength [J]. Chemical Engineering Journal, 2018, 334: 1842-50. [CrossRef]
- Zhou X, Gao L, Zhang H, et al. Determination of the optimal aeration for nitrogen removal in biochar-amended aerated vertical flow constructed wetlands [J]. Bioresource Technology, 2018, 261: 461-4. [CrossRef]
- Yu B, Liu C, Wang S, et al. Applying constructed wetland-microbial electrochemical system to enhance NH4+ removal at low temperature [J]. Science of The Total Environment, 2020, 724: 138017. [CrossRef]
- Zhong L, Yang S S, Ding J, et al. Enhanced nitrogen removal in an electrochemically coupled biochar-amended constructed wetland microcosms: The interactive effects of biochar and electrochemistry [J]. Science of The Total Environment, 2021, 789. [CrossRef]
- Prado A, Berenguer R, Esteve-Núñez A. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer [J]. Carbon, 2019, 146: 597-609. [CrossRef]
- Prathiba S, Kumar P S, Vo D-V N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment [J]. Chemosphere, 2022, 286: 131856. [CrossRef]
- Li Z, Zhang P, Qiu Y, et al. Biosynthetic FeS/BC hybrid particles enhanced the electroactive bacteria enrichment in microbial electrochemical systems [J]. Science of The Total Environment, 2021, 762: 143142. [CrossRef]
- Saeed T, Miah M J, Khan T. Intensified constructed wetlands for the treatment of municipal wastewater: experimental investigation and kinetic modelling [J]. Environmental Science And Pollution Research, 2021, 28(24): 30908-28. [CrossRef]
- Jia W, Sun X, Gao Y, et al. Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland [J]. Science of The Total Environment, 2020, 740: 139534. [CrossRef]
- Wu H, Ma W, Kong Q, et al. Spatial-temporal dynamics of organics and nitrogen removal in surface flow constructed wetlands for secondary effluent treatment under cold temperature [J]. Chemical Engineering Journal, 2018, 350: 445-52. [CrossRef]
- Wang B, Liu S Y, Li F Y, et al. Removal of nitrate from constructed wetland in winter in high-latitude areas with modified hydrophyte biochars [J]. Korean Journal of Chemical Engineering, 2017, 34(3): 717-22. [CrossRef]
- Wang H, Xu J, Sheng L. Preparation of straw biochar and application of constructed wetland in China: A review [J]. Journal of Cleaner Production, 2020, 273: 123131. [CrossRef]
- Sha N Q, Wang G H, Li Y H, et al. Removal of abamectin and conventional pollutants in vertical flow constructed wetlands with Fe-modified biochar [J]. Rsc Advances, 2020, 10(72): 44171-82. [CrossRef]
- Wang B, Liu S-y, Li F-y, et al. Removal of nitrate from constructed wetland in winter in high-latitude areas with modified hydrophyte biochars [J]. Korean Journal of Chemical Engineering, 2017, 34(3): 717-22.
- Sun Y, Zhou P, Zhang N, et al. Effects of matrix modification and bacteria amendment on the treatment efficiency of municipal tailwater pollutants by modified vertical flow constructed wetland [J]. Journal of Environmental Management, 2021, 281: 111920. [CrossRef]
- Guo Z, Zhang J, Kang Y, et al. Rapid and efficient removal of Pb(II) from aqueous solutions using biomass-derived activated carbon with humic acid in-situ modification [J]. Ecotoxicology and Environmental Safety, 2017, 145: 442-8. [CrossRef]
- Min L, Zhongsheng Z, Zhe L, et al. Removal of nitrogen and phosphorus pollutants from water by FeCl3- impregnated biochar [J]. Ecological Engineering, 2020, 149: 105792. [CrossRef]
- Kasak K, Truu J, Ostonen I, et al. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands [J]. Science Of The Total Environment, 2018, 639: 67-74. [CrossRef]
- Xu J, Liu X, Huang J, et al. The contributions and mechanisms of iron-microbes-biochar in constructed wetlands for nitrate removal from low carbon/nitrogen ratio wastewater [J]. Rsc Advances, 2020, 10(39): 23212-20. [CrossRef]
- Sun Y P, Zhou P C, Zhang N, et al. Effects of matrix modification and bacteria amendment on the treatment efficiency of municipal tailwater pollutants by modified vertical flow constructed wetland [J]. Journal of Environmental Management, 2021, 281. [CrossRef]
- Easton Z M, Rogers M, Davis M, et al. Mitigation of sulfate reduction and nitrous oxide emission in denitrifying environments with amorphous iron oxide and biochar [J]. Ecological Engineering, 2015, 82: 605-13. [CrossRef]
- Jia L, Wu W, Zhang J, et al. Insight into heavy metals (Cr and Pb) complexation by dissolved organic matters from biochar: Impact of zero-valent iron [J]. Science of The Total Environment, 2021, 793: 148469. [CrossRef]
- Zhou X, Chen Z H, Li Z R, et al. Impacts of aeration and biochar addition on extracellular polymeric substances and microbial communities in constructed wetlands for low C/N wastewater treatment: Implications for clogging [J]. Chemical Engineering Journal, 2020, 396. [CrossRef]
- Tang S, Liao Y, Xu Y, et al. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review [J]. Bioresource Technology, 2020, 314: 123759. [CrossRef]
- Feng L K, Liu Y, Zhang J Y, et al. Dynamic variation in nitrogen removal of constructed wetlands modified by biochar for treating secondary livestock effluent under varying oxygen supplying conditions [J]. Journal Of Environmental Management, 2020, 260. [CrossRef]
- Jia W, Sun X, Gao Y, et al. Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland [J]. Science of The Total Environment, 2020, 740. [CrossRef]
- Zhang Y, Li Y, Wang J, et al. Interactions of chlorpyrifos degradation and Cd removal in iron-carbon-based constructed wetlands for treating synthetic farmland wastewater [J]. Journal of Environmental Management, 2021, 299: 113559. [CrossRef]
- Hou W, Wang S, Li Y, et al. Influence of modified biochar supported Fe–Cu/polyvinylpyrrolidone on nitrate removal and high selectivity towards nitrogen in constructed wetlands [J]. Environmental Pollution, 2021, 289: 117812. [CrossRef]
- Zheng C, Zhang X, Gan L, et al. Effects of biochar on the growth of Vallisneria natans in surface flow constructed wetland [J]. Environment Science And Pollution Research. [CrossRef]
- Meng F C, Feng L J, Yin H J, et al. Assessment of nutrient removal and microbial population dynamics in a non-aerated vertical baffled flow constructed wetland for contaminated water treatment with composite biochar addition [J]. Journal of Environment Management, 2019, 246: 355-61. [CrossRef]
- Liang Y, Wang Q, Huang L, et al. Insight into the mechanisms of biochar addition on pollutant removal enhancement and nitrous oxide emission reduction in subsurface flow constructed wetlands: Microbial community structure, functional genes and enzyme activity [J]. Bioresource Technology, 2020, 307: 123249. [CrossRef]
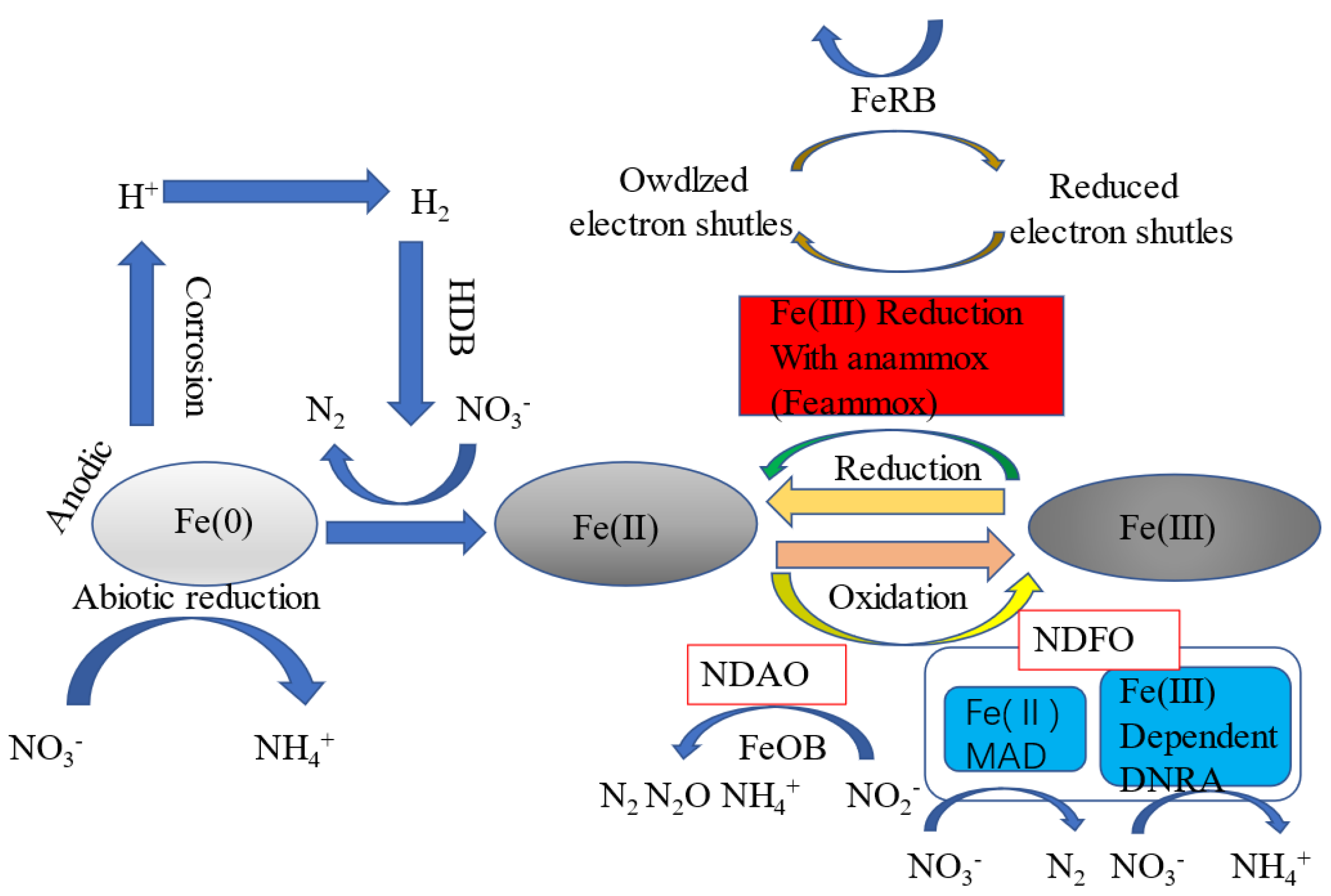
- Peng Y, He S, Wu F. Biochemical processes mediated by iron-based materials in water treatement: Enhancing nitrogen and phosphorus removal in low C/N ratio wastewater [J]. Science of The Total Environment, 2021, 775: 145137. [CrossRef]
- Jia L, Li C, Zhang Y, et al. Microbial community responses to agricultural biomass addition in aerated constructed wetlands treating low carbon wastewater [J]. Journal of Environmental Management, 2020, 270: 110912. [CrossRef]
- Shen X T, Zhang J, Xie H J, et al. Electron shuttles enhance phenanthrene removal in constructed wetlands filled with manganese oxides-coated sands [J]. Chemical Engineering Journal, 2021, 426. [CrossRef]
- Saeed T, Haque I, Khan T. Organic matter and nutrients removal in hybrid constructed wetlands: Influence of saturation [J]. Chemical Engineering Journal, 2019, 371: 154-65. [CrossRef]

| CW techniques |
oxygen supply |
Biochar | COD/N | Wastewater type | Removal efficiency (%) | N2O emission flux (μg⋅m−2⋅h−1) |
References | |||
| source | COD | NH4+-N | N03--N | TN | ||||||
| SSFCWs | Intermittent aeration | Bamboo | <7 | Synthetic wastewaters | 89%-99% | 97%-99% | - | 46%-98% | [7] | |
| VFCWs | Intermittent aeration | Oenanthe javanica |
low C/N | Synthetic wastewaters | 95%-97% | 63%-98% | 63%-82% | 271–884 μg m−2 h−1 | [40] | |
| VFCWs | Intermittent aeration | Oenanthe javanica |
low C/N | Synthetic wastewaters | 94.90% | 99.10% | 52.70% | 60.54 μg m−2 h−1 | [33] | |
| VFCWs | Intermittent aeration | low C/N | Synthetic wastewaters | >90% | >99% | >67% | [2] | |||
| VFCWs | Intermittent aeration | 0.5 | Synthetic wastewaters | 97% | 99% | 96% | [32] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




