Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
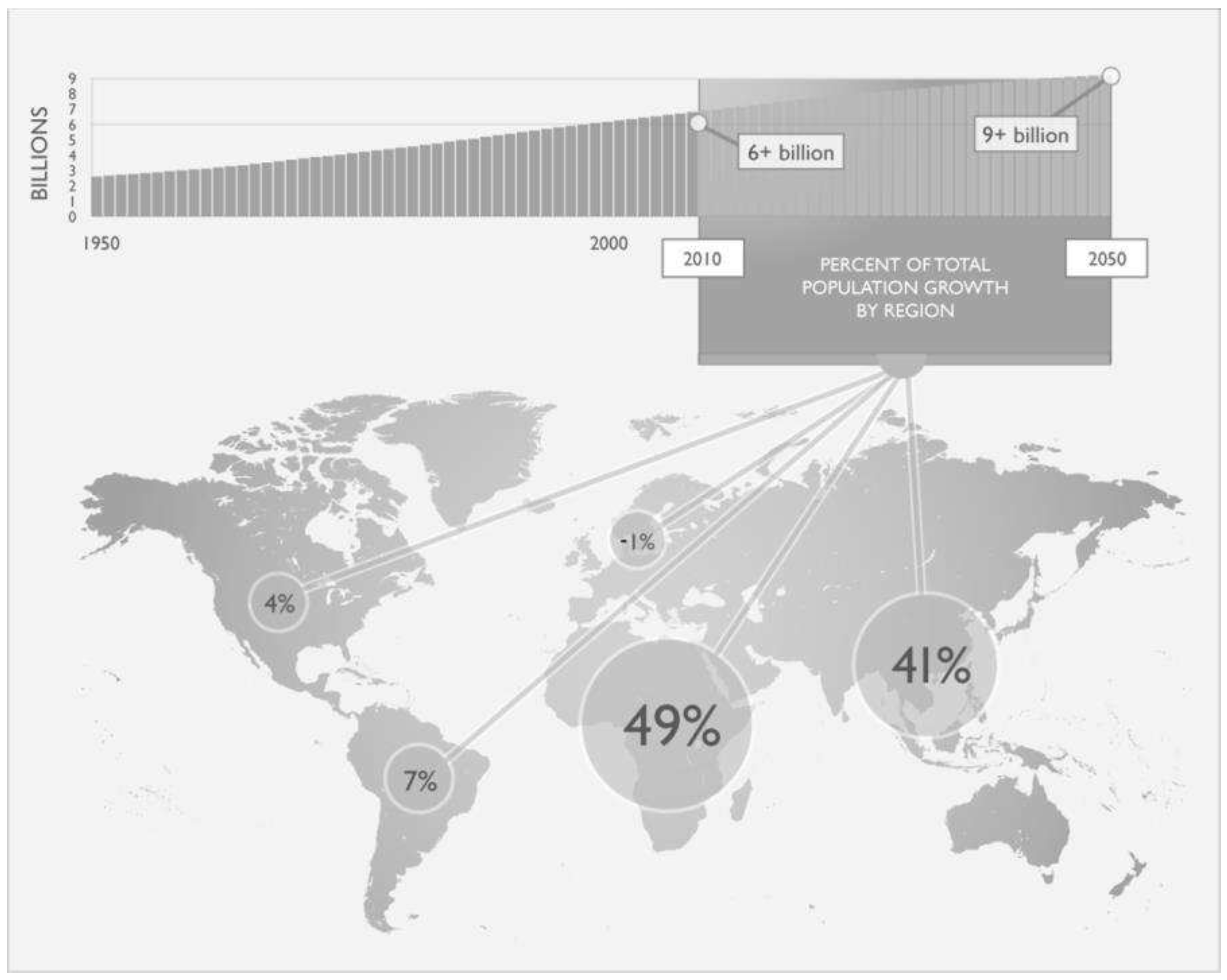
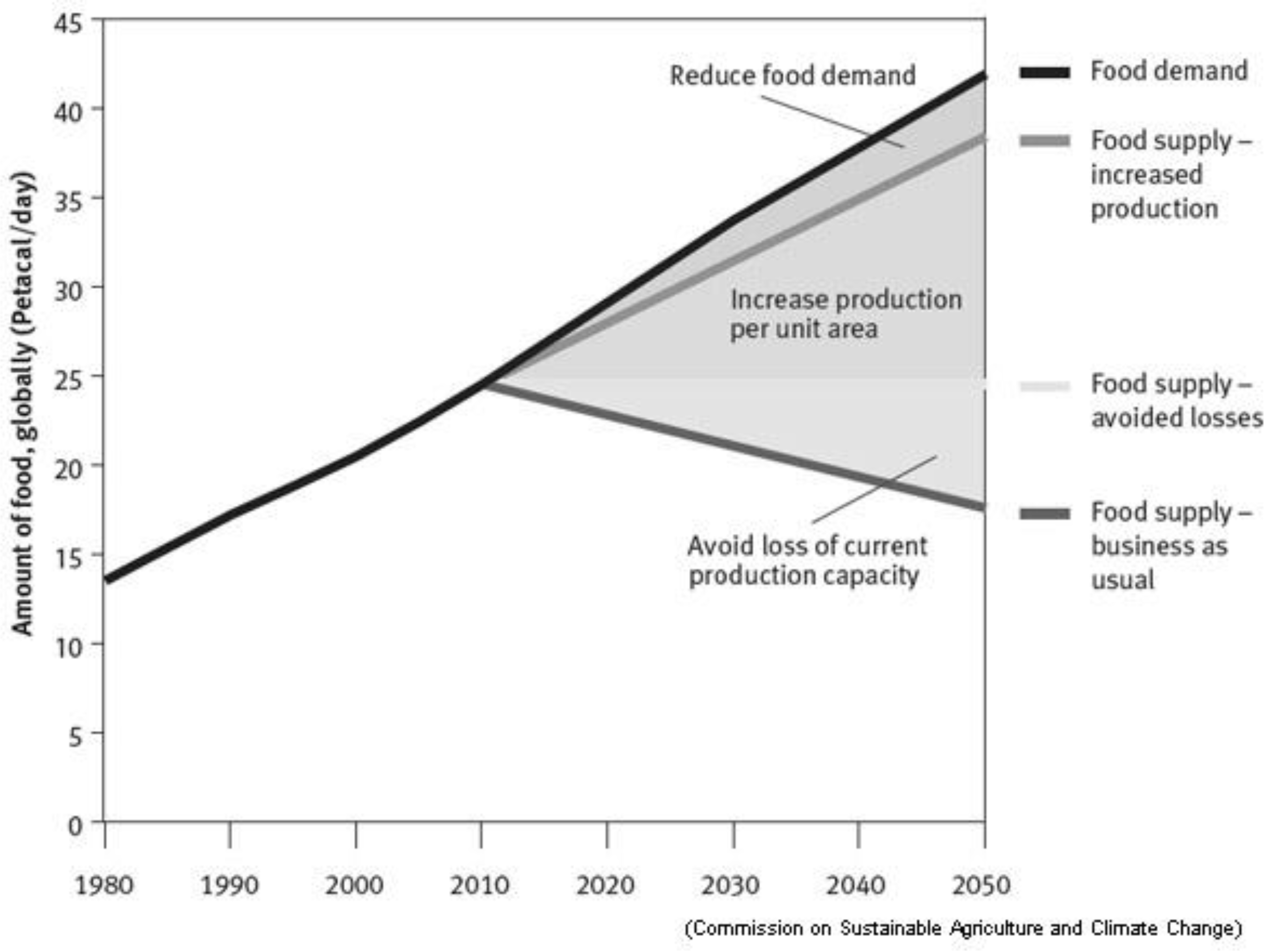
2. General Aspects of Global Agricultural Activity
3. Pesticides
3.1. Agricultural Defensives
3.2. Biological Control
3.3. Agricultural Biodefensives
3.3.1. Formulation of Agricultural Biodefensives
3.3.2. Agricultural Adjuvants as Activators or Enhancers of Agricultural Defensives
3.3.3. Utility Adjuvants
3.3.4. Activator or Enhancer Adjuvants
4. Surfactants
4.1. Chemical Surfactants
4.2. Biobased Surfactants
4.3. Biosurfactants
- Surface activity: Surfactant efficiency is measured by CMC, which ranges from 1 to 2000 mg/L based on molecular structure, as discussed earlier [65]. An optimal biosurfactant can reduce the surface tension of water from 72 to 30-35 mN/m and the interfacial tension of oil and water from 40 to 1 mN/m [90]. Compared to synthetic surfactants, most microbial surfactants have lower surface and interfacial tensions and CMC values, making them more effective.
- Temperature, pH, and ionic strength tolerance: Several biosurfactants remain effective in adverse conditions, such as high temperatures, pH range of 3-12, and up to 10% saline concentration, while synthetic surfactants are inactivated by ≥ 2% NaCl [75].
- Specificity: The high diversity of molecules, each with its own complexity and specific functional groups, confers particular/specific activities to biosurfactants. Like synthetic surfactants, biosurfactants show the ability to self-aggregate and form micelles, which increase their specificity and allow them to have different morphological structures. In addition, their ability to create spherical, rod-shaped, and vesicle-like structures has caught the attention of various industries like food, cosmetics, and pharmaceuticals. They also have the potential in detoxifying pollutants and demulsifying industrial emulsions [75].
- Biocompatibility and digestibility: The composition of biosurfactants makes them more biodegradable and biocompatible than their chemical counterparts under variations in temperature, pH, and degradation time [91].
4.3.1. Application of Biosurfactants in the Agricultural Industry and Trends
4.3.1.1. Soil Quality Enhancement through Soil Amendments
4.3.1.2. Adjuvants for Plant Pathogen Elimination
4.3.1.3. Adjuvants for Seed Germination and Plant Growth
4.3.1.4. Adjuvants for Beneficial Microbe Interactions
4.3.2. Producing Biosurfactant-Based Biopesticides for the Agricultural Industry
| Product | Specifications | Country | Patent ID/year |
|---|---|---|---|
| Biopesticide | Biopesticide compositions and/or biopesticide formulations obtained from Eucalyptus species. The addition of rhamnolipid biosurfactant was cited in the composition of one of the formulations. | Australia | WO2011/013133A3/ 2011 |
| Biocontrol agent | Application of microorganisms as biological control agents, more specifically the Serratia plymuthica strain A30, BCCM Deposit Nº. LMG P-26170, which is capable of degrading acyl-homoserine lactones and producing biosurfactants. | The Netherlands | EP2663659B1/2013 |
| Biopesticides | The invention relates to methods for pest (nematodes) control by a microbial rhamnolipid biosurfactant, implying providing the microbial biosurfactant to pests in such an amount that pests are controlled. | United States | EP1750738B1/ 2007 |
| Insecticide | Obtaining an insecticide that contains biosurfactant in its formulation. Preferably, the biosurfactant is a glycolipid, a glycoside, or their derivatives. | France | EP3122186B1/2017 |
| Additive | A method of producing surfactin, a lipopeptide produced by Bacillus subtilis and its application in aquafeeds to reduce the occurrence of mold contamination. | Taiwan | EP3039968B1/2016 |
| Additive | A rhamnolipid is implemented to replace a chemical surfactant to be adopted as the additive of the pesticide, the fertilizer, and the feed additive so as to ensure significant effects. | China | CN103070167B/ 2010 |
| Biofertilizers, biostimulants, bio dispersants, and other applications | Formulations comprising microbes and/or their growth by-products to be used to improve fertility, salinity, water retention, and other soil characteristics, as well as to control pests and stimulate plant growth. In certain of them, growth by-products are biosurfactants. | United States | WO2021030385A1/2020 |
| Bioremediators of soil | The invention reveals a type of method in which the surfactant repairs the soil contaminated with organochlorine pesticides, removing more than 85% of the pesticides, making the soil reach the environmental safety standard. The operation is simple, economical and efficient and can be applied on a large scale in the repair of soils contaminated with organic pollutants. | China | CN104923558B/2015 |
| Enhancers of fertility and health of soil, pesticides, plant immune modulators, and/or plant growth stimulants | Microbe-based formulations for restoring soil health and controlling pests. They can comprise one or more biosurfactants (glycolipids and/or lipopeptides). | United States | WO2021030385A1/2021 |
| Fruit preservative | The invention belongs to the technical field of food preservation and relates to a sophorolipid fruit preservative and a method for prolonging the preservation life of fruits. By microbiological fermentation technology, a sophorolipid was obtained, which was used in the preparation of a solution (3 mg/mL) sprayed evenly on the fruits to prevent fruit corrosion, maintain freshness and extend the shelf life of fruits at room temperature. | China | CN101886047B/2010 |
| Biofertilizers, biostimulants | Use of sophorolipids to increase the yield of agricultural crops. | Germany | DE102014209346A1/2014 |
| Biopesticide | Sophorolipid agricultural antibiotic and an application thereof to control fungal diseases of crops. | China | CN104178537A/2014 |
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mrabet, R. Sustainable agriculture for food and nutritional security. In Sustainable Agriculture and the Environment; Elsevier, 2023; pp. 25–90. [CrossRef]
- Rebolledo-Leiva, R.; Moreira, M.T.; González-García, S. Progress of social assessment in the framework of bioeconomy under a life cycle perspective. Renew. Sustain. Energy Rev. 2023, 175, 113162. https://doi.org/10.1016/J.RSER.2023.113162. [CrossRef]
- Castro, M.J.L.; Ojeda, C.; Cirelli, A.F. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 2014, 12, 85–95. https://doi.org/10.1007/S10311-013-0432-4/METRICS. [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1–24. [CrossRef]
- Dehnert, G.K.; Freitas, M.B.; Sharma, P.P.; Barry, T.P.; Karasov, W.H. Impacts of subchronic exposure to a commercial 2,4-D herbicide on developmental stages of multiple freshwater fish species. Chemosphere 2021, 263, 127638. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127638. [CrossRef]
- Zhang, C.; Zhou, T.; Xu, Y.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Zhu, L. Ecotoxicology of strobilurin fungicides. Sci. Total Environ. 2020, 742, 140611. https://doi.org/10.1016/J.SCITOTENV.2020.140611. [CrossRef]
- Calista, N.; Haikael, M.D.; Athanasia, M.O.; Neema, K.; Judith, K. Does Pesticide exposure contribute to the growing burden of non - communicable diseases in Tanzania. Sci. African 2022, 17, e01276. https://doi.org/10.1016/J.SCIAF.2022.E01276. [CrossRef]
- Carrança Thaís Agrotóxico mais usado do Brasil está associado a 503 mortes infantis por ano, revela estudo - BBC News Brasil Available online: https://www.bbc.com/portuguese/brasil-57209799 (accessed on May 18, 2023).
- Campanhola, C.; Bettiol, W. Métodos alternativos de controle fitossanitário.; Campanhola, C., BETTIOL, W. (Ed. )., Eds.; Jaguariúna: Embrapa Meio Ambiente, 2003.: Jaguariúna, SP, 2003; ISBN 85-85771-22-4.
- Ward, L.T.; Hladik, M.L.; Guzman, A.; Winsemius, S.; Bautista, A.; Kremen, C.; Mills, N.J. Pesticide exposure of wild bees and honey bees foraging from field border flowers in intensively managed agriculture areas. Sci. Total Environ. 2022, 831, 154697. https://doi.org/10.1016/J.SCITOTENV.2022.154697. [CrossRef]
- Rigotto, R.M.; Vasconcelos, D.P. e; Rocha, M.M. Pesticide use in Brazil and problems for public health. Cad. Saude Publica 2014, 30, 1360–1362. https://doi.org/10.1590/0102-311XPE020714. [CrossRef]
- Jhansi Rani, S.; Usha, R. Transgenic plants: Types, benefits, public concerns and future. J. Pharm. Res. 2013, 6, 879–883. https://doi.org/10.1016/J.JOPR.2013.08.008. [CrossRef]
- Rauchecker, M. The territorial and sectoral dimensions of advocacy – The conflicts about pesticide use in Argentina. Polit. Geogr. 2019, 75, 102067. https://doi.org/10.1016/j.polgeo.2019.102067. [CrossRef]
- Bailey, K.L.; Boyetchko, S.M.; Längle, T. Social and economic drivers shaping the future of biological control: A Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol. Control 2010, 52, 221–229. https://doi.org/10.1016/J.BIOCONTROL.2009.05.003. [CrossRef]
- Cavalcante, B.D. arc. M.; Scapini, T.; Camargo, A.F.; Ulrich, A.; Bonatto, C.; Dalastra, C.; Mossi, A.J.; Fongaro, G.; Di Piero, R.M.; Treichel, H. Orange peels and shrimp shell used in a fermentation process to produce an aqueous extract with bioherbicide potential to weed control. Biocatal. Agric. Biotechnol. 2021, 32, 101947. https://doi.org/10.1016/J.BCAB.2021.101947. [CrossRef]
- Ulrich, A.; Lerin, L.A.; Camargo, A.F.; Scapini, T.; Diering, N.L.; Bonafin, F.; Gasparetto, I.G.; Confortin, T.C.; Sansonovicz, P.F.; Fabian, R.L.; et al. Alternative bioherbicide based on Trichoderma koningiopsis: Enzymatic characterization and its effect on cucumber plants and soil organism. Biocatal. Agric. Biotechnol. 2021, 36, 102127. https://doi.org/10.1016/J.BCAB.2021.102127. [CrossRef]
- Mann, R..; Bidwell, J.. The acute toxicity of agricultural surfactants to the tadpoles of four Australian and two exotic frogs. Environ. Pollut. 2001, 114, 195–205. https://doi.org/10.1016/S0269-7491(00)00216-5. [CrossRef]
- Sangwan, S.; Kaur, H.; Sharma, P.; Sindhu, M.; Wati, L. Routing microbial biosurfactants to agriculture for revitalization of soil and plant growth. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier, 2022; pp. 313–338. [CrossRef]
- Medeiros, A.O.; Da Gloria C. Da Silva, M.; Almeida, D.G.; Meire, H.M.; Da Silva, M.E.P.; Brasileiro, P.P.F.; Sarubbo, L.A. Incorporation of Natural Surfactants in Natural Resin-based Coatings and Analysis of Rheological Behaviour to Obtain Natural Antifouling Agents. Chem. Eng. Trans. 2020, 79, 193–198. https://doi.org/10.3303/CET2079033. [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R. de C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. https://doi.org/10.1016/j.ejbt.2021.02.002. [CrossRef]
- Rocha e Silva, N.M.P.; Meira, H.M.; Almeida, F.C.G.; Soares da Silva, R. de C.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural Surfactants and Their Applications for Heavy Oil Removal in Industry. Sep. Purif. Rev. 2019, 48, 267–281. https://doi.org/10.1080/15422119.2018.1474477. [CrossRef]
- Sivapathasekaran, C.; Sen, R. Origin, properties, production and purification of microbial surfactants as molecules with immense commercial potential. Tenside, Surfactants, Deterg. 2017, 54, 92–104. https://doi.org/10.3139/113.110482. [CrossRef]
- Maldaner, J.; Oliveira, M.N.; De Alexandria Santos, D.; Simote Silva, S.Y.; Da Cruz Silva, S.; Da Costa Lima, T.; Da Silva, M.L.; Lima Silva, H.T.; Siqueira-Silva, D.H.; Pauli kist Steffen, G.; et al. Bioherbicide and anesthetic potential of Aniba canelilla essential oil, a contribution to the demands of the agricultural sector. Biocatal. Agric. Biotechnol. 2022, 42, 102353. https://doi.org/10.1016/j.bcab.2022.102353. [CrossRef]
- Serrano-Carreón, L.; Aranda-Ocampo, S.; Balderas-Ruíz, K.A.; Juárez, A.M.; Leyva, E.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Galindo, E. A case study of a profitable mid-tech greenhouse for the sustainable production of tomato, using a biofertilizer and a biofungicide. Electron. J. Biotechnol. 2022, 59, 13–24. https://doi.org/10.1016/j.ejbt.2022.06.003. [CrossRef]
- Shokouhi, D.; Seifi, A. Organic extracts of seeds of Iranian Moringa peregrina as promising selective biofungicide to control Mycogone perniciosa. Biocatal. Agric. Biotechnol. 2020, 30, 101848. https://doi.org/10.1016/j.bcab.2020.101848. [CrossRef]
- Mertens, M. Genetic engineering: modified crops, more pesticides Available online: https://eu.boell.org/en/PesticideAtlas-genetic-engineering (accessed on May 23, 2023).
- Benbrook, C.M. Impacts of genetically engineered crops on pesticide use in the U.S. -- the first sixteen years. Environ. Sci. Eur. 2012, 24, 24. https://doi.org/10.1186/2190-4715-24-24. [CrossRef]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: the role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. https://doi.org/10.1016/j.tree.2022.09.009. [CrossRef]
- Rondeau, S.; Raine, N.E. Fungicides and bees: a review of exposure and risk. Environ. Int. 2022, 165, 107311. https://doi.org/10.1016/j.envint.2022.107311. [CrossRef]
- Treviño, M.J.S.; Pereira-Coelho, M.; López, A.G.R.; Zarazúa, S.; dos Santos Madureira, L.A.; Majchrzak, T.; Płotka-Wasylka, J. How pesticides affect neonates? - Exposure, health implications and determination of metabolites. Sci. Total Environ. 2023, 856, 158859. https://doi.org/10.1016/j.scitotenv.2022.158859. [CrossRef]
- Roltsch, W.J.; Bürgi, L.P.; Tomic-Carruthers, N.; Rugman-Jones, P.F.; Stouthamer, R.; Mills, N.J. Mortality of light brown apple moth egg masses in coastal California: Impact of resident Trichogramma parasitism and predation. Biol. Control 2021, 152, 104465. https://doi.org/10.1016/j.biocontrol.2020.104465. [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. https://doi.org/10.1017/S0021859605005708. [CrossRef]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. https://doi.org/10.3390/pathogens10101311. [CrossRef]
- Ramírez-Camejo, L.A.; Bayman, P.; Mejía, L.C. Drosophila melanogaster as an emerging model host for entomopathogenic fungi. Fungal Biol. Rev. 2022, 42, 85–97. https://doi.org/10.1016/j.fbr.2022.09.001. [CrossRef]
- dos Santos Gomes, A.C.; da Silva, R.R.; Moreira, S.I.; Vicentini, S.N.C.; Ceresini, P.C. Biofungicides: An eco-friendly approach for plant disease management. Encycl. Mycol. 2021, 641–649. https://doi.org/10.1016/B978-0-12-819990-9.00036-6. [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. https://doi.org/10.1016/J.JIP.2018.01.008. [CrossRef]
- Gouli, V. V.; Marcelino, J.A.P.; Gouli, S.Y. Microbial formulations for control of noxious organisms. In Microbial Pesticides; Elsevier, 2021; pp. 193–247. [CrossRef]
- do Nascimento, J.; Cristina Goncalves, K.; Pinto Dias, N.; Luiz de Oliveira, J.; Bravo, A.; Antonio Polanczyk, R. Adoption of Bacillus thuringiensis-based biopesticides in agricultural systems and new approaches to improve their use in Brazil. Biol. Control 2022, 165, 104792. https://doi.org/10.1016/j.biocontrol.2021.104792. [CrossRef]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.V.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. https://doi.org/10.1016/J.CHEMOSPHERE.2021.131761. [CrossRef]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 74–81. https://doi.org/10.1016/j.jip.2018.10.008. [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation optimization of D-limonene-loaded nanoemulsions as a natural and efficient biopesticide. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 596, 124746. https://doi.org/10.1016/J.COLSURFA.2020.124746. [CrossRef]
- Riyanto; Mulwandari, M.; Asysyafiiyah, L.; Sirajuddin, M.I.; Cahyandaru, N. Direct synthesis of lemongrass (Cymbopogon citratus L.) essential oil-silver nanoparticles (EO-AgNPs) as biopesticides and application for lichen inhibition on stones. Heliyon 2022, 8, e09701. https://doi.org/10.1016/j.heliyon.2022.e09701. [CrossRef]
- Wu, H. qu; Sun, L. li; Liu, F.; Wang, Z. Ying; Cao, C. Wang Preparation of dry flowable formulations of Clonostachys rosea by spray drying and application for Sclerotinia sclerotiorum control. J. Integr. Agric. 2018, 17, 613–620. https://doi.org/10.1016/S2095-3119(17)61811-2. [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Recent advances in downstream processing and formulations of Bacillus thuringiensis based biopesticides. Process Biochem. 2006, 41, 323–342. https://doi.org/10.1016/J.PROCBIO.2005.07.015. [CrossRef]
- Werner, S.J.; DeLiberto, S.T.; Mangan, A.M.; Pettit, S.E.; Ellis, J.W.; Carlson, J.C. Anthraquinone-based repellent for horned larks, great-tailed grackles, American crows and the protection of California’s specialty crops. Crop Prot. 2015, 72, 158–162. https://doi.org/10.1016/J.CROPRO.2015.03.020. [CrossRef]
- Adetunji, C.O.; Jeevanandam, J.; Inobeme, A.; Olaniyan, O.T.; Anani, O.A.; Thangadurai, D.; Islam, S. Application of biosurfactant for the production of adjuvant and their synergetic effects when combined with different agro-pesticides. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier, 2021; pp. 255–277. [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. https://doi.org/10.1007/s00253-012-4641-8. [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H. E.; Bagley, W. E.;. Derksen, R. C; Krause, C. R. Adjuvant Effects on Evaporation Time and Wetted Area of Droplets on Waxy Leaves. Trans. ASABE 2010, 53, 13–20. https://doi.org/10.13031/2013.29495. [CrossRef]
- Gaskin, R.E.; Murray, R.J.; Krishna, H.; Carpenter, A. Effect of adjuvants on the retention of insecticide spray on cucumber and pea foliage. New Zeal. Plant Prot. 2000, 53, 355–359. https://doi.org/10.30843/NZPP.2000.53.3608. [CrossRef]
- Ramsey, R.J.L.; Stephenson, G.R.; Hall, J.C. A review of the effects of humidity, humectants, and surfactant composition on the absorption and efficacy of highly water-soluble herbicides. Pestic. Biochem. Physiol. 2005, 82, 162–175. https://doi.org/10.1016/j.pestbp.2005.02.005. [CrossRef]
- Kudsk, P.; Mathiassen, S.K. Analysis of adjuvant effects and their interactions with variable application parameters. Crop Prot. 2007, 26, 328–334. https://doi.org/10.1016/j.cropro.2005.06.012. [CrossRef]
- Wang, C.J.; Liu, Z.Q. Foliar uptake of pesticides—Present status and future challenge. Pestic. Biochem. Physiol. 2007, 87, 1–8. https://doi.org/10.1016/J.PESTBP.2006.04.004. [CrossRef]
- Stock, D. and G.B. Physicochemical Properties of Adjuvants: Values and Applications. Weed Technol. 2000, 14, 798–806.
- Tu, M.; Hurd, C.; Randall, J.M.; Rice, B. Weed Control Methods Handbook; The Nature Conservancy, 2001; ISBN 5032301221.
- L. Hazen, J. Adjuvants: Terminology, Classification, and Chemistry on JSTOR. In Weed Technology; 2000; Vol. 14, pp. 773–784.
- Yu, Y.; Zhu, H.; Ozkan, H. E.; Derksen, R. C.; Krause C. R. Evaporation and Deposition Coverage Area of Droplets Containing Insecticides and Spray Additives on Hydrophilic, Hydrophobic, and Crabapple Leaf Surfaces. Trans. ASABE 2009, 52, 39–49. https://doi.org/10.13031/2013.25939. [CrossRef]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. https://doi.org/10.1007/BF02286399. [CrossRef]
- Schönherr, J.; Baur, P.; Uhlig, B.A. Rates of cuticular penetration of 1-naphthylacetic acid (NAA) as affected by adjuvants, temperature, humidity and water quality. Plant Growth Regul. 2000, 31, 61–74. https://doi.org/10.1023/A:1006354732358. [CrossRef]
- Mulqueen, P. Recent advances in agrochemical formulation. Adv. Colloid Interface Sci. 2003, 106, 83–107. https://doi.org/10.1016/S0001-8686(03)00106-4. [CrossRef]
- Blackwell, P.S. Management of water repellency in Australia, and risks associated with preferential flow, pesticide concentration and leaching. J. Hydrol. 2000, 231–232, 384–395. https://doi.org/10.1016/S0022-1694(00)00210-9. [CrossRef]
- Petrović, M.; Barceló, D. Analysis and fate of surfactants in sludge and sludge-amended soils. TrAC Trends Anal. Chem. 2004, 23, 762–771. https://doi.org/10.1016/j.trac.2004.07.015. [CrossRef]
- Reddy, S.; Verma, V.; Srivastava, N. Marine Biosurfactants. In Marine Surfactants; Reddy, S., Verma, V., Srivastava, N., Eds.; CRC Press: Boca Raton, 2022; pp. 255–269.
- Almeida, D.G.; da Silva, M. da G.C.; do Nascimento Barbosa, R.; de Souza Pereira Silva, D.; da Silva, R.O.; de Souza Lima, G.M.; de Gusmão, N.B.; de Queiroz Sousa, M. de F.V. Biodegradation of marine fuel MF-380 by microbial consortium isolated from seawater near the petrochemical Suape Port, Brazil. Int. Biodeterior. Biodegradation 2017, 116, 73–82. https://doi.org/10.1016/j.ibiod.2016.09.028. [CrossRef]
- Silva, I.A.; Almeida, F.C.G.; Souza, T.C.; Bezerra, K.G.O.; Durval, I.J.B.; Converti, A.; Sarubbo, L.A. Oil spills: impacts and perspectives of treatment technologies with focus on the use of green surfactants. Environ. Monit. Assess. 2022, 194, 143. https://doi.org/10.1007/s10661-022-09813-z. [CrossRef]
- Sarubbo, L.A.; Silva, M. da G.C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. https://doi.org/10.1016/j.bej.2022.108377. [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: a review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. https://doi.org/10.1080/21655979.2022.2074621. [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactants: Production and Application Prospects in the Food Industry. Biotechnol. Prog. 2020. https://doi.org/10.1002/btpr.3030. [CrossRef]
- Paulino, B.N.; Pessôa, M.G.; Mano, M.C.R.; Molina, G.; Neri-Numa, I.A.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. https://doi.org/10.1007/s00253-016-7980-z. [CrossRef]
- Hayes, D.G.; Smith, G.A. Biobased Surfactants: Overview and Industrial State of the Art. Biobased Surfactants Synth. Prop. Appl. 2019, 3–38. https://doi.org/10.1016/B978-0-12-812705-6.00001-0. [CrossRef]
- Fernández Cirelli, A.; Ojeda, C.; Castro, M.J.L.; Salgot, M. Surfactants in sludge-amended agricultural soils: a review. Environ. Chem. Lett. 2008, 6, 135–148. https://doi.org/10.1007/s10311-008-0146-1. [CrossRef]
- Kandasamy, R.; Rajasekaran, M.; Venkatesan, S.K.; Uddin, M. New Trends in the Biomanufacturing of Green Surfactants: Biobased Surfactants and Biosurfactants. In ACS Symposium Series; American Chemical Society, 2019; Vol. 1329, pp. 243–260.
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018. https://doi.org/10.1002/btpr.2682. [CrossRef]
- Zahed, M.A.; Mohammad, ·; Matinvafa, A.; Azari, · Aryandokht; Mohajeri, L. Biosurfactant, a green and effective solution for bioremediation of petroleum hydrocarbons in the aquatic environment. Discov. Water 2022 21 2022, 2, 1–20. https://doi.org/10.1007/S43832-022-00013-X. [CrossRef]
- Silva, M. da G.C.; de Medeiros, A.O.; Sarubbo, L.A. Biosurfactant: A Biodegradable Antimicrobial Substance. In Biodegradable Materials and Their Applications; Inamuddin, T.A., Ed.; Wiley, 2022; pp. 471–486.
- Santos, D.; Rufino, R.; Luna, J.; Santos, V.; Sarubbo, L. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. https://doi.org/10.3390/ijms17030401. [CrossRef]
- Silva, M. da G.C.; Sarubbo, L.A. Synthetic and biological surfactants used to mitigate biofouling on industrial facilities surfaces. Biointerface Res. Appl. Chem. 2022, 12, 2560–2585. https://doi.org/10.33263/BRIAC122.25602585. [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.C.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: a way towards cleaner production. Microb. Cell Fact. 2021, 20, 120. https://doi.org/10.1186/s12934-021-01613-3. [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial Surfactants: The Next Generation Multifunctional Biomolecules for Applications in the Petroleum Industry and Its Associated Environmental Remediation. Microorg. 2019, Vol. 7, Page 581 2019, 7, 581. https://doi.org/10.3390/MICROORGANISMS7110581. [CrossRef]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: a clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Bio/Technology 2018, 17, 591–602. https://doi.org/10.1007/s11157-018-9477-y. [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. https://doi.org/10.1007/S00253-010-2498-2/TABLES/2. [CrossRef]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and characterisation of short chain rhamnolipid production in a previously uninvestigated, non-pathogenic marine pseudomonad. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. https://doi.org/10.1007/S00253-018-9202-3. [CrossRef]
- Rawat, G.; Dhasmana, A.; Kumar, V. Biosurfactants: the next generation biomolecules for diverse applications. Environ. Sustain. 2020, 3, 353–369. https://doi.org/10.1007/s42398-020-00128-8. [CrossRef]
- Wang, H.; Roelants, S.L.K.W.; To, M.H.; Patria, R.D.; Kaur, G.; Lau, N.S.; Lau, C.Y.; Van Bogaert, I.N.A.; Soetaert, W.; Lin, C.S.K. Starmerella bombicola: recent advances on sophorolipid production and prospects of waste stream utilization. J. Chem. Technol. Biotechnol. 2019, 94, 999–1007. https://doi.org/10.1002/JCTB.5847. [CrossRef]
- Fei, D.; Zhou, G.W.; Yu, Z.Q.; Gang, H.Z.; Liu, J.F.; Yang, S.Z.; Ye, R.Q.; Mu, B.Z. Low-Toxic and Nonirritant Biosurfactant Surfactin and its Performances in Detergent Formulations. J. Surfactants Deterg. 2020, 23, 109–118. https://doi.org/10.1002/JSDE.12356. [CrossRef]
- Rawat, G.; Dhasmana, A.; Kumar, V. Biosurfactants: the next generation biomolecules for diverse applications. Environ. Sustain. 2020, 3, 353–369. https://doi.org/10.1007/s42398-020-00128-8. [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. https://doi.org/10.1016/J.COCIS.2020.03.013. [CrossRef]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. https://doi.org/10.1016/j.procbio.2019.06.027. [CrossRef]
- Datta, P.; Tiwari, P.; Pandey, L.M. Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J. Pet. Sci. Eng. 2020, 195, 107612. https://doi.org/10.1016/J.PETROL.2020.107612. [CrossRef]
- Carolin C, F.; Kumar, P.S.; Ngueagni, P.T. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. https://doi.org/10.1016/J.JHAZMAT.2020.124827. [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. https://doi.org/10.1111/1751-7915.13704. [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Moldes, A.B.; Cruz, J.M. Biodegradability Study of the Biosurfactant Contained in a Crude Extract from Corn Steep Water. J. Surfactants Deterg. 2020, 23, 79–90. https://doi.org/10.1002/jsde.12338. [CrossRef]
- Silva, E.J.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B. Biointerfaces 2018, 172, 127–135. https://doi.org/10.1016/j.colsurfb.2018.08.034. [CrossRef]
- Chaprão, M.J.; da Silva, R. de C.F.S.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments. Ecotoxicology 2018, 27, 1310–1322. https://doi.org/10.1007/s10646-018-1982-9. [CrossRef]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharm. 2020, Vol. 12, Page 1099 2020, 12, 1099. https://doi.org/10.3390/PHARMACEUTICS12111099. [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arh. Hig. Rada Toksikol. 2010, 61, 95–110. https://doi.org/10.2478/10004-1254-61-2010-1943. [CrossRef]
- Silva, R. de C.F.S.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. https://doi.org/10.1007/s11356-021-16483-w. [CrossRef]
- Brasileiro, P.P.F.; De Almeida, D.G.; De Luna, J.M.; Rufino, R.D.; Dos Santos, V.A.; Sarubbo, L.A. Optimization of biosurfactant production from Candida guiliermondii using a Rotate Central Composed Design. Chem. Eng. Trans. 2015, 43, 1411–1416. https://doi.org/10.3303/CET1543236. [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environ. Technol. Innov. 2021, 24, 102090. https://doi.org/10.1016/J.ETI.2021.102090. [CrossRef]
- Martinez-Burgos, W.J.; Bittencourt Sydney, E.; Bianchi Pedroni Medeiros, A.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Junior Letti, L.A.; Thomaz Soccol, V.; de Melo Pereira, G.V.; et al. Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresour. Technol. 2021, 341, 125795. https://doi.org/10.1016/j.biortech.2021.125795. [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. https://doi.org/10.1016/j.ecoenv.2019.109607. [CrossRef]
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An overview of current research and developments in biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. https://doi.org/10.1016/j.jiec.2021.05.017. [CrossRef]
- Rostás, M.; Blassmann, K. Insects had it first: surfactants as a defence against predators. Proc. R. Soc. B Biol. Sci. 2009, 276, 633–638. https://doi.org/10.1098/rspb.2008.1281. [CrossRef]
- Fukuoka, T.; Yoshida, S.; Nakamura, J.; Koitabashi, M.; Sakai, H.; Abe, M.; Kitamoto, D.; Kitamoto, H. Application of Yeast Glycolipid Biosurfactant, Mannosylerythritol Lipid, as Agrospreaders. J. Oleo Sci. 2015, 64, 689–695. https://doi.org/10.5650/jos.ess15017. [CrossRef]
- Trinchera, A.; Baratella, V. Use of a Non-Ionic Water Surfactant in Lettuce Fertigation for Optimizing Water Use, Improving Nutrient Use Efficiency, and Increasing Crop Quality. Water 2018, 10, 613. https://doi.org/10.3390/w10050613. [CrossRef]
- Abagandura, G.O.; Park, D.; Bridges, W.C. Surfactant and irrigation impacts on soil water content and leachate of soils and greenhouse substrates. Agrosystems, Geosci. Environ. 2021, 4, 1–11. https://doi.org/10.1002/agg2.20153. [CrossRef]
- Hassan, M.N.; Namood-e-Sahar; Zia-Ul-Husnain Shah, S.; Afghan, S.; Hafeez, F.Y. Suppression of red rot disease by Bacillus sp. based biopesticide formulated in non-sterilized sugarcane filter cake. BioControl 2015, 60, 691–702. https://doi.org/10.1007/s10526-015-9673-4. [CrossRef]
- Ławniczak, Ł.; Marecik, R.; Chrzanowski, Ł. Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 2013, 97, 2327–2339. https://doi.org/10.1007/s00253-013-4740-1. [CrossRef]
- Luzuriaga-Loaiza, W.P.; Schellenberger, R.; De Gaetano, Y.; Obounou Akong, F.; Villaume, S.; Crouzet, J.; Haudrechy, A.; Baillieul, F.; Clément, C.; Lins, L.; et al. Synthetic Rhamnolipid Bolaforms trigger an innate immune response in Arabidopsis thaliana. Sci. Rep. 2018, 8, 8534. https://doi.org/10.1038/s41598-018-26838-y. [CrossRef]
- Nguyen, B.V.G.; Nagakubo, T.; Toyofuku, M.; Nomura, N.; Utada, A.S. Synergy between Sophorolipid Biosurfactant and SDS Increases the E ffi ciency of P. aeruginosa Bio fi lm Disruption. 2020. https://doi.org/10.1021/acs.langmuir.0c00643. [CrossRef]
- 111. Karamchandani, B.M.; Pawar, A.A.; Pawar, S.S.; Syed, S.; Mone, N.S.; Dalvi, S.G.; Rahman, P.K.S.M.; Banat, I.M.; Satpute, S.K. Biosurfactants’ multifarious functional potential for sustainable agricultural practices. Front. Bioeng. Biotechnol. 2022, 10. https://doi.org/10.3389/fbioe.2022.1047279. [CrossRef]
- Liu, Z.; Li, Z.; Zhong, H.; Zeng, G.; Liang, Y.; Chen, M.; Wu, Z.; Zhou, Y.; Yu, M.; Shao, B. Recent advances in the environmental applications of biosurfactant saponins: A review. J. Environ. Chem. Eng. 2017, 5, 6030–6038. https://doi.org/10.1016/J.JECE.2017.11.021. [CrossRef]
- Sales da Silva, I.G.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil Bioremediation: Overview of Technologies and Trends. Energies 2020, 13, 4664. https://doi.org/10.3390/en13184664. [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. https://doi.org/10.1016/j.jhazmat.2014.12.009. [CrossRef]
- Mata-Sandoval, J.C.; Karns, J.; Torrents, A. Influence of Rhamnolipids and Triton X-100 on the Biodegradation of Three Pesticides in Aqueous Phase and Soil Slurries. J. Agric. Food Chem. 2001, 49, 3296–3303. https://doi.org/10.1021/jf001432w. [CrossRef]
- Odukkathil, G.; Vasudevan, N. Enhanced biodegradation of endosulfan and its major metabolite endosulfate by a biosurfactant producing bacterium. J. Environ. Sci. Health. B. 2013, 48, 462–469. https://doi.org/10.1080/03601234.2013.761873. [CrossRef]
- Wattanaphon, H.T.; Kerdsin, A.; Thammacharoen, C.; Sangvanich, P.; Vangnai, A.S. A biosurfactant from Burkholderia cenocepacia BSP3 and its enhancement of pesticide solubilization. J. Appl. Microbiol. 2008, 105, 416–423. https://doi.org/10.1111/J.1365-2672.2008.03755.X. [CrossRef]
- Sarubbo, L.A.; Rocha, R.B.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015, 31, 707–723. https://doi.org/10.1080/02757540.2015.1095293. [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental Applications of Biosurfactants: Recent Advances. Int. J. Mol. Sci. 2011, 12, 633–654. https://doi.org/10.3390/ijms12010633. [CrossRef]
- Mulligan, C.N.; Wang, S. Remediation of a heavy metal-contaminated soil by a rhamnolipid foam. Eng. Geol. 2006, 85, 75–81. https://doi.org/10.1016/j.enggeo.2005.09.029. [CrossRef]
- Colores, G.M.; Macur, R.E.; Ward, D.M.; Inskeep, W.P. Molecular Analysis of Surfactant-Driven Microbial Population Shifts in Hydrocarbon-Contaminated Soil. Appl. Environ. Microbiol. 2000, 66, 2959. https://doi.org/10.1128/AEM.66.7.2959-2964.2000. [CrossRef]
- Reid, R.; Hayes, J. Mechanisms and control of nutrient uptake in plants. Int. Rev. Cytol. 2003, 229, 73–114. https://doi.org/10.1016/S0074-7696(03)29003-3. [CrossRef]
- Mitra, G.N. Regulation of Nutrient Uptake by Plants; Springer India: New Delhi, 2015; ISBN 978-81-322-2333-7.
- Adhikari, S.; Anuragi, H.; Chandra, K.; Tarte, S.H.; Dhaka, S.R.; Jatav, H.S.; Hingonia, K. Molecular basis of plant nutrient use efficiency - concepts and challenges for its improvement. Sustain. Plant Nutr. 2023, 107–151. https://doi.org/10.1016/B978-0-443-18675-2.00001-8. [CrossRef]
- Osman, K.T. Plant Nutrients and Soil Fertility Management. In Soils; Springer Netherlands: Dordrecht, 2013; pp. 129–159.
- Dietel, K.; Beator, B.; Budiharjo, A.; Fan, B.; Borriss, R. Bacterial Traits Involved in colonization of Arabidopsis thaliana roots by Bacillus amyloliquefaciens FZB42. Plant Pathol. J. 2013, 29, 59–66. https://doi.org/10.5423/PPJ.OA.10.2012.0155. [CrossRef]
- Le Mire, G.; Siah, A.; Brisset, M.-N.; Gaucher, M.; Deleu, M.; Jijakli, M. Surfactin Protects Wheat against Zymoseptoria tritici and Activates Both Salicylic Acid- and Jasmonic Acid-Dependent Defense Responses. Agriculture 2018, 8, 11. https://doi.org/10.3390/agriculture8010011. [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R. de C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. https://doi.org/10.1016/J.EJBT.2021.02.002. [CrossRef]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surfaces B Biointerfaces 2019, 181, 77–84. https://doi.org/10.1016/j.colsurfb.2019.05.032. [CrossRef]
- Sobrinho, H.B.S.; Luna, J.M.; Rufino, R.D.; Porto, A.L.F.; Sarubbo, L.A. Assessment of toxicity of a biosurfactant from Candida sphaerica UCP 0995 cultivated with industrial residues in a bioreactor. Electron. J. Biotechnol. 2013, 16. https://doi.org/10.2225/vol16-issue4-fulltext-4. [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; de Campos-Takaki, G.M. Evaluation Antimicrobial and Antiadhesive Properties of the Biosurfactant Lunasan Produced by Candida sphaerica UCP 0995. Curr. Microbiol. 2011, 62, 1527–1534. https://doi.org/10.1007/s00284-011-9889-1. [CrossRef]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surfaces B Biointerfaces 2011, 84, 1–5. https://doi.org/10.1016/j.colsurfb.2010.10.045. [CrossRef]
- Rufino, R.D.; de Luna, J.M.; de Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. https://doi.org/10.1016/j.ejbt.2013.12.006. [CrossRef]
- Kim, P.I.; Bai, H.; Bai, D.; Chae, H.; Chung, S.; Kim, Y.; Park, R.; Chi, Y.-T. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 2004, 97, 942–949. https://doi.org/10.1111/j.1365-2672.2004.02356.x. [CrossRef]
- Boyette, C.D.; Lynn Walker, H.; Abbas, H.K. Biological Control of Kudzu ( Pueraria lobata ) with an Isolate of Myrothecium verrucaria. https://doi.org/10.1080/09583150120093031 2010, 12, 75–82. https://doi.org/10.1080/09583150120093031. [CrossRef]
- Rodriguez, S.B.; Mahoney, N.E. Inhibition of Aflatoxin Production by Surfactants. Appl. Environ. Microbiol. 1994, 60, 106–110. https://doi.org/10.1128/aem.60.1.106-110.1994. [CrossRef]
- Krzyzanowska, D.M.; Potrykus, M..; Golanowska, M..; Polonis, K.A..; Gwizdek-Wisniewska, E..; Lojkowska, and Jafra, S. Rhizosphere bacteria as potential biocontrol agents against soft rot caused by various Pectobacterium and Dickeya spp. strains. J. Plant Pathol. 2012, 94, 367–78.
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid Biosurfactants as New Players in Animal and Plant Defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5095. https://doi.org/10.3390/IJMS11125095. [CrossRef]
- Kim, S.K.; Kim, Y.C.; Lee, S.; Kim, J.C.; Yun, M.Y.; Kim, I.S. Insecticidal Activity of Rhamnolipid Isolated from Pseudomonas sp. EP-3 against Green Peach Aphid (Myzus persicae). J. Agric. Food Chem. 2011, 59, 934–938. https://doi.org/10.1021/jf104027x. [CrossRef]
- Kruijt, M.; Tran, H.; Raaijmakers, J.M. Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267. J. Appl. Microbiol. 2009, 107, 546–556. https://doi.org/10.1111/j.1365-2672.2009.04244.x. [CrossRef]
- Velho, R. V.; Medina, L.F.C.; Segalin, J.; Brandelli, A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. (Praha). 2011, 56, 297–303. https://doi.org/10.1007/S12223-011-0056-7. [CrossRef]
- Haddad, N.I.A.; Wang, J.; Mu, B. Isolation and characterization of a biosurfactant producing strain, Brevibacilis brevis HOB1. J. Ind. Microbiol. Biotechnol. 2008, 35, 1597–1604. https://doi.org/10.1007/S10295-008-0403-0. [CrossRef]
- Nielsen, T.H.; Sørensen, J. Production of Cyclic Lipopeptides by Pseudomonas fluorescens Strains in Bulk Soil and in the Sugar Beet Rhizosphere. Appl. Environ. Microbiol. 2003, 69, 861–868. https://doi.org/10.1128/AEM.69.2.861-868.2003. [CrossRef]
- Kim, P. Il; Ryu, J.; Kim, Y.H.; Chi, Y.-T. Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010, 20, 138–45.
- Stahlman, P.W.; Currie, R.S.; El-Hamid, M.A. Nitrogen Carrier and Surfactant Increase Foliar Herbicide Injury in Winter Wheat ( Triticum aestivum ). Weed Technol. 1997, 11, 7–12. https://doi.org/10.1017/S0890037X00041257. [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B. Biointerfaces 2013, 102, 202–209. https://doi.org/10.1016/J.COLSURFB.2012.08.008. [CrossRef]
- Silva, S.N.R.L.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf. B. Biointerfaces 2010, 79, 174–83. https://doi.org/10.1016/j.colsurfb.2010.03.050. [CrossRef]
- Luiz, V.; Lovaglio, R.B.; Tozzi, H.H.; Contiero, J. Rhamnolipids : A New Application in Seeds Development. 2015, 1, 100–106.
- Santos, A.P.P.; Silva, M.D.S.; Costa, E.V.L.; Rufino, R.D.; Santos, V.A.; Ramos, C.S.; Sarubbo, L.A.; Porto, A.L.F. Production and characterization of a biosurfactant produced by Streptomyces sp. DPUA 1559 isolated from lichens of the Amazon region. Brazilian J. Med. Biol. Res. 2018, 51. https://doi.org/10.1590/1414-431x20176657. [CrossRef]
- Dusane, D.H.; Zinjarde, S.S.; Venugopalan, V.P.; McLean, R.J.C.; Weber, M.M.; Rahman, P.K.S.M. Quorum sensing: implications on rhamnolipid biosurfactant production. Biotechnol. Genet. Eng. Rev. 2010, 27, 159–184. https://doi.org/10.1080/02648725.2010.10648149. [CrossRef]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants. Minireview. Environ. Microbiol. 2001, 3, 229–236. https://doi.org/10.1046/j.1462-2920.2001.00190.x. [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. Prog. Biol. Control 2020, 21, 275–293. https://doi.org/10.1007/978-3-030-53238-3_16. [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. https://doi.org/10.1016/j.crmicr.2021.100094. [CrossRef]
- Nishio, E.; Ichiki, Y.; Tamura, H.; Morita, S.; Watanabe, K.; Yoshikawa, H. Isolation of Bacterial Strains that Produce the Endocrine Disruptor, Octylphenol Diethoxylates, in Paddy Fields. Biosci. Biotechnol. Biochem. 2002, 66, 1792–1798. https://doi.org/10.1271/bbb.66.1792. [CrossRef]
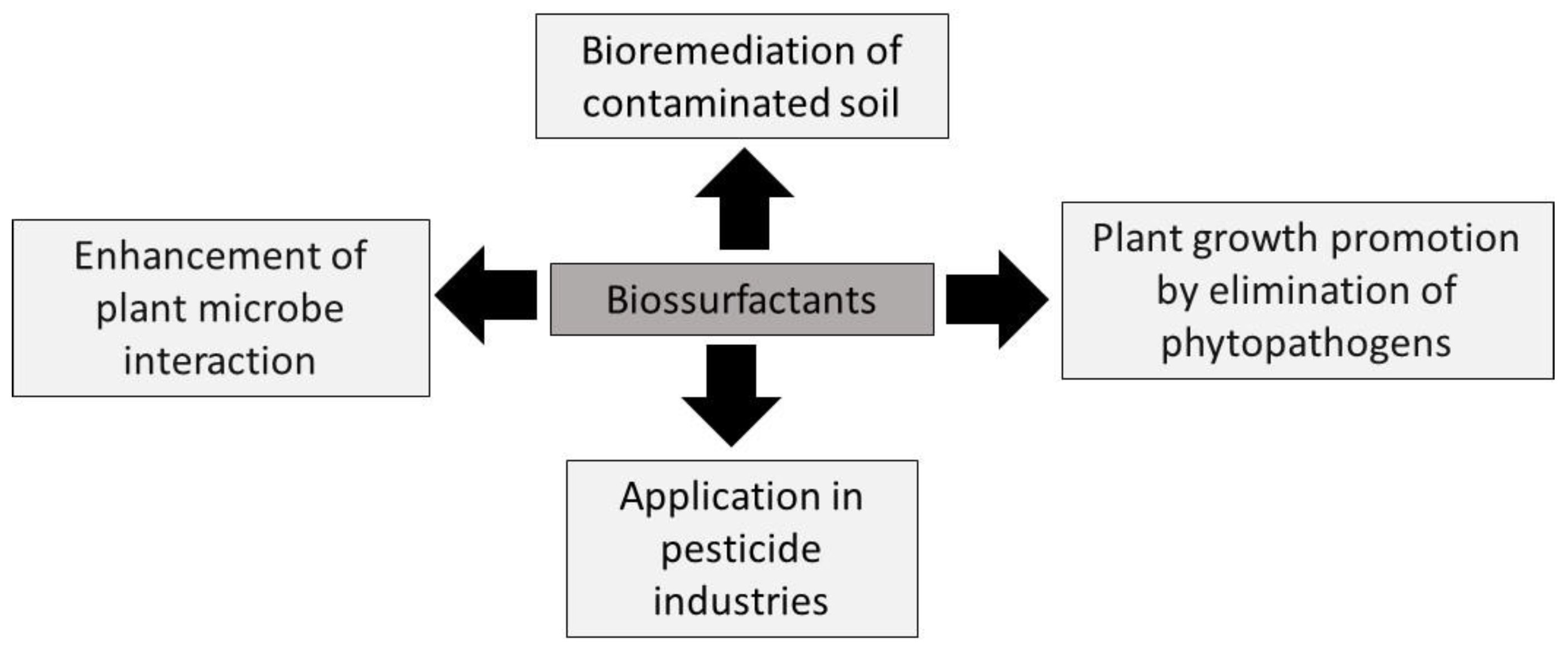
| Trends in biosurfactant application in agriculture | Other promising applications of biosurfactants in agriculture |
|---|---|
| Development of more effective and affordable biopesticides and biofertilizers [104]. | Utilization of biosurfactants in irrigation systems [105,106]. |
| Biocontrol of plant pathogens [107]. | Use of biosurfactants to enhance biofuel production [108]. |
| Stimulation of plant growth [109]. | Removal of biofilms in irrigation systems [110]. |
| Stabilization of pesticide and fertilizer emulsions [111]. | Application of biosurfactants for remediation of contaminated soils [112]. |
| Enhancement of herbicide and foliar nutrient absorption [104]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





