Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Participants
2.2. Study Design
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snow, J.D. ON THE INHALATION OF THE VAPOUR OF ETHER IN SURGICAL OPERATIONS. Br. J. Anaesth. 1953, 25, 253–267. [Google Scholar] [CrossRef]
- Mendelson, C.L. The Aspiration of Stomach Contents into the Lungs During Obstetric Anesthesia. Am. J. Obstet. Gynecol. 1946, 52, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, X.; Huo, Y.; Mi, M.; Peng, G.; Zhang, C.; Jiang, Y.; Zhou, Y.; Zhao, X.; Li, T.; et al. Abbreviated Perioperative Fasting Management for Elective Fresh Fracture Surgery: Guideline Adherence Analysis. BMC Musculoskelet. Disord. 2022, 23, 688. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Xu, H.-Z.; Han, M.-L. Enhanced Recovery after Surgery Strategy to Shorten Perioperative Fasting in Children Undergoing Non-Gastrointestinal Surgery: A Prospective Study. World J. Clin. Cases 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Liang, X.-Q.; Li, Y.-T.; Wang, D.-X. Preoperative Carbohydrate Loading with Individualized Supplemental Insulin in Diabetic Patients Undergoing Gastrointestinal Surgery: A Randomized Trial. Int. J. Surg. 2022, 98, 106215. [Google Scholar] [CrossRef] [PubMed]
- Frykholm, P.; Disma, N.; Andersson, H.; Beck, C.; Bouvet, L.; Cercueil, E.; Elliott, E.; Hofmann, J.; Isserman, R.; Klaucane, A.; et al. Pre-Operative Fasting in Children: A Guideline from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2022, 39, 4–25. [Google Scholar] [CrossRef]
- Beck, C.E.; Rudolph, D.; Mahn, C.; Etspüler, A.; Korf, M.; Lüthke, M.; Schindler, E.; Päukert, S.; Trapp, A.; Megens, J.H.A.M.; et al. Impact of Clear Fluid Fasting on Pulmonary Aspiration in Children Undergoing General Anesthesia: Results of the German Prospective Multicenter Observational (NiKs) Study. Pediatr. Anesth. 2020, 30, 892–899. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.C.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced Recovery after Surgery: A Consensus Review of Clinical Care for Patients Undergoing Colonic Resection. Clin. Nutr. 2005, 24, 466–477. [Google Scholar] [CrossRef]
- Rizvanović, N.; Nesek Adam, V.; Čaušević, S.; Dervišević, S.; Delibegović, S. A Randomised Controlled Study of Preoperative Oral Carbohydrate Loading versus Fasting in Patients Undergoing Colorectal Surgery. Int. J. Colorectal Dis. 2019, 34, 1551–1561. [Google Scholar] [CrossRef]
- Carvalho, C.A.L. de B.; Carvalho, A.A. de; Preza, A.D.G.; Nogueira, P.L.B.; Mendes, K.B.V.; Dock-Nascimento, D.B.; Aguilar-Nascimento, J.E. Benefícios Metabólicos e Inflamatórios Da Abreviação Do Jejum Pré-Operatório Em Cirurgia Pediátrica. Rev. Colégio Bras. Cir. 2020, 47, e20202353. [Google Scholar] [CrossRef]
- Akbuğa, G.A.; Başer, M. Effect of Preoperative Oral Liquid Carbohydrate Intake on Blood Glucose, Fasting-Thirst, and Fatigue Levels: A Randomized Controlled Study. Braz. J. Anesthesiol. Engl. Ed. 2021, S0104001421001329. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, B.; Dong, Q.; Zhao, Z.; Yu, Y. Effect of Preoperative Oral Carbohydrate Administration on Patients Undergoing Cesarean Section with Epidural Anesthesia: A Pilot Study. J. Perianesth. Nurs. 2021, 36, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, J. Preoperative Carbohydrate Loading in Gynecological Patients Undergoing Combined Spinal and Epidural Anesthesia. J. Invest. Surg. 2020, 33, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Abdelmalak, B.B.; Weigel, W.A.; Harbell, M.W.; Kuo, C.I.; Soriano, S.G.; Stricker, P.A.; Tipton, T.; Grant, M.D.; Marbella, A.M.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Preoperative Fasting: Carbohydrate-Containing Clear Liquids with or without Protein, Chewing Gum, and Pediatric Fasting Duration—A Modular Update of the 2017 American Society of Anesthesiologists Practice Guidelines for Preoperative Fasting. Anesthesiology 2023, 138, 132–151. [Google Scholar] [CrossRef] [PubMed]
- de-Aguilar-Nascimento, J.E.; Salomão, A.B.; Waitzberg, D.L.; Dock-Nascimento, D.B.; Correa, M.I.T.D.; Campos, A.C.L.; Corsi, P.R.; Portari Filho, P.E.; Caporossi, C. ACERTO Guidelines of Perioperative Nutritional Interventions in Elective General Surgery. Rev. Colégio Bras. Cir. 2017, 44, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Nascimento, J.; Dias, A.; Dock-Nascimento, D.; Correia, M.; Campos, A.; Portari-Filho, P.; Oliveira, S. Actual Preoperative Fasting Time in Brazilian Hospitals: The BIGFAST Multicenter Study. Ther. Clin. Risk Manag. 2014, 107. [Google Scholar] [CrossRef]
- Merchant, R.N.; Chima, N.; Ljungqvist, O.; Kok, J.N.J. Preoperative Fasting Practices Across Three Anesthesia Societies: Survey of Practitioners. JMIR Perioper. Med. 2020, 3, e15905. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.M.; Daliya, P.; Lewis-Lloyd, C.; Adiamah, A.; Malcolm, F.L.; Boyd-Carson, H.; Couch, D.; Herrod, P.J.J.; Hossain, T.; Couch, J.; et al. Fasting and Surgery Timing (FaST) Audit. Clin. Nutr. 2021, 40, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, A.; Young, A.; Mudge, A.; Banks, M.; Bauer, J. EXploring Practice Gaps to Improve PERIoperativE Nutrition CarE (EXPERIENCE Study): A Qualitative Analysis of Barriers to Implementation of Evidence-Based Practice Guidelines. Eur. J. Clin. Nutr. 2019, 73, 94–101. [Google Scholar] [CrossRef]
- Cho, E.-A.; Huh, J.; Lee, S.H.; Ryu, K.-H.; Shim, J.-G.; Cha, Y.-B.; Kim, M.S.; Song, T. Gastric Ultrasound Assessing Gastric Emptying of Preoperative Carbohydrate Drinks: A Randomized Controlled Noninferiority Study. Anesth. Analg. 2021, 133, 690–697. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Ahn, J.H.; Shim, J.-G.; Lee, S.H.; Ryu, K.-H.; Lee, S.-H.; Cho, E.-A. Gastric Emptying of Preoperative Carbohydrate in Elderly Assessed Using Gastric Ultrasonography: A Randomized Controlled Study. Medicine (Baltimore) 2021, 100, e27242. [Google Scholar] [CrossRef] [PubMed]
- Bisinotto, F.M.B.; Silveira, L.A.M. da; Rossi, T.C.; Martins, L.B.; Zago, G.P.; Mendonça, M.A.L. Estudo comparativo do esvaziamento gástrico entre uma solução isotônica e um suplemento nutricional por meio da ultrassonografia. Braz. J. Anesthesiol. 2019, 69, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Roughead, T.; Sewell, D.; Ryerson, C.J.; Fisher, J.H.; Flexman, A.M. Internet-Based Resources Frequently Provide Inaccurate and Out-of-Date Recommendations on Preoperative Fasting: A Systematic Review. Anesth. Analg. 2016, 123, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Zia, F.; Cosic, L.; Wong, A.; Levin, A.; Lu, P.; Mitchell, C.; Shaw, M.; Rosewarne, F.; Weinberg, L. Effects of a Short Message Service (SMS) by Cellular Phone to Improve Compliance with Fasting Guidelines in Patients Undergoing Elective Surgery: A Retrospective Observational Study. BMC Health Serv. Res. 2021, 21, 27. [Google Scholar] [CrossRef]
- van Noort, H.H.J.; Eskes, A.M.; Vermeulen, H.; Besselink, M.G.; Moeling, M.; Ubbink, D.T.; Huisman–de Waal, G.; Witteman, B.J.M. Fasting Habits over a 10-Year Period: An Observational Study on Adherence to Preoperative Fasting and Postoperative Restoration of Oral Intake in 2 Dutch Hospitals. Surgery 2021, 170, 532–540. [Google Scholar] [CrossRef]
| . | n (%) | Mean (SD) | Median (IQR) |
|---|---|---|---|
| SC | |||
| 1 | 249 (85) | ||
| 2 | 44 (15) | ||
| ASA | |||
| 1 | 135 (46.1) | ||
| 2 | 140 (47.8) | ||
| 3 | 7 (2.4) | ||
| 4 | 2 (0.7) | ||
| Gender | |||
| Female | 188 (64.2) | ||
| Male | 104 (35.5) | ||
| Age (in years) | 290 (99) | 37.36 (21.95) | 39 (16.8-56) |
| 0 to 9 years | 44 (15.18) | ||
| 10 to 14 years | 25 (8.6) | ||
| 15 to 24 years | 23 (7.9) | ||
| 25 to 59 years | 145 (50.0) | ||
| from 60 | 53 (18.3) | ||
| BMI (kg/m²) | 190 (64.8) | 26.32 (7.85) | 25.6 (22.1-29.4) |
| Height (m) | 185 (63.1) | 1.62 (1) | 1.6 (1.5-1.7) |
| Weight (kg) | 269 (91.8) | 60.5 (26.1) | 63 (48-75) |
| Shift | |||
| Morning | 129 (44) | ||
| Afternoon | 164 (56) | ||
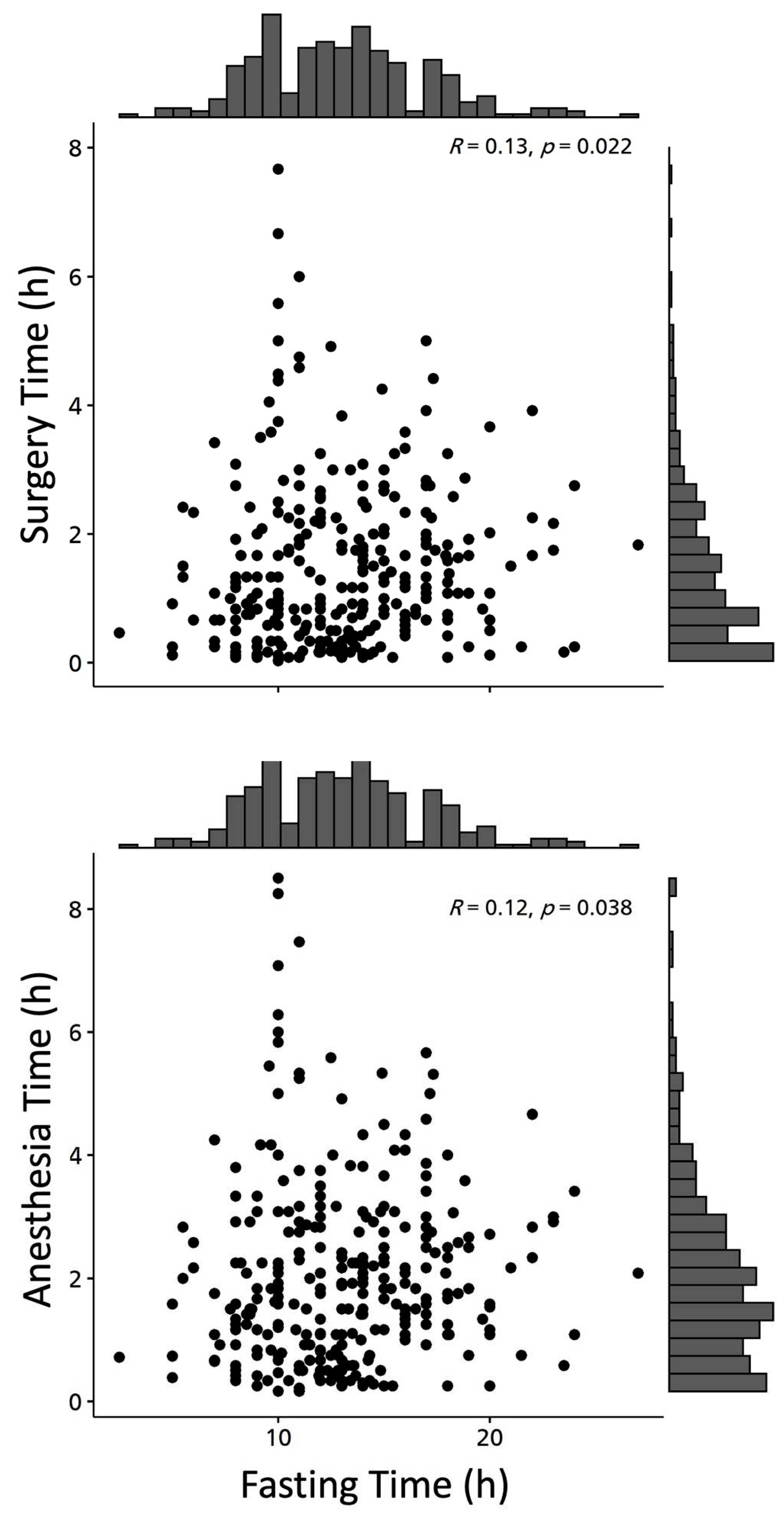
| Anesthesia Time (h) | 293 (100) | 2.06 (1.47) | 1.8 (1-2.8) |
| Surgery Time (h) | 293 (100) | 1.45 (1.26) | 1.1 (0.5-2.1) |
| Type of Surgery | |||
| Open Abdominal | 53 (18.1) | ||
| Abdominal by video | 47 (16) | ||
| Endoscopy | 25 (8.5) | ||
| Pediatric | 55 (18.8) | ||
| Plastic | 12 (4.1) | ||
| Thoracic | 6 (2) | ||
| Others | 95 (32.4) | ||
| Type of Anesthesia | |||
| General Anesthesia | 169 (57.7) | ||
| Neuraxial blockade | 68 (23.2) | ||
| Combined | 21 (7.2) | ||
| Sedation | 35 (11.9) | ||
| Hypotension | |||
| Yes | 19 (6.5) | ||
| No | 274 (93.5) | ||
| Nausea/vomiting | |||
| Yes | 3 (1) | ||
| No | 290 (99) | ||
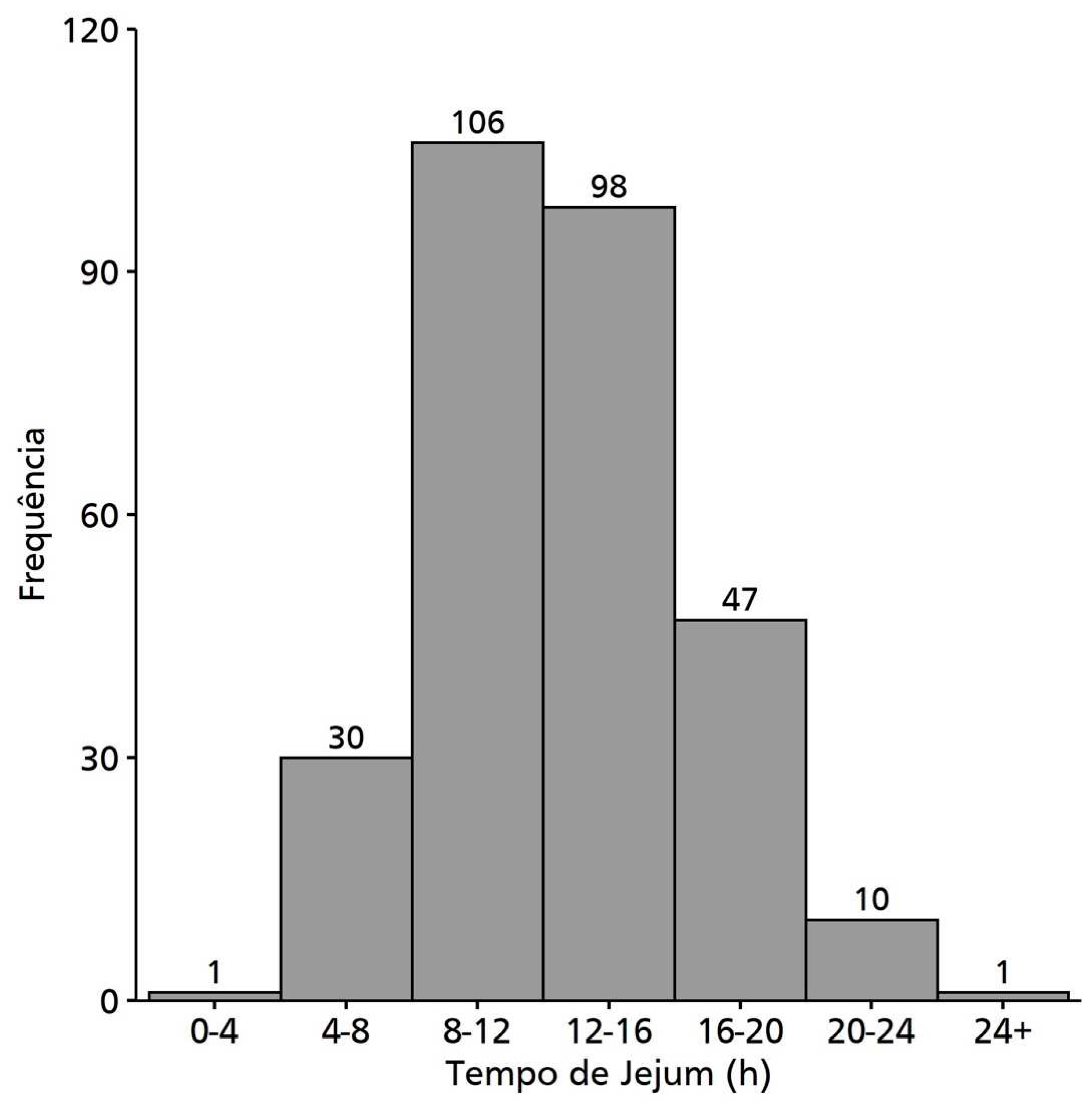
| Fasting (h) | 293 (100) | 13 (3.98) | 12.8 (10-15.5) |
| 0-8 | 31 (10.6) | ||
| 8-12 | 106 (36.2) | ||
| 12-16 | 98 (33.4) | ||
| >16 | 58 (19.8) |
| Fasting (h) | ||||||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | p-value | ||||
| SC | ||||||
| 1 | 13.16 (4.08) | 13 (10-16) | 0.092 | |||
| 2 | 12.04 (3.19) | 12.4 (9.8-14) | ||||
| ASA | ||||||
| 1 | 13.05 (4.41) | 13 (10-15.5) | 0.814 | |||
| 2 | 13.1 (3.71) | 13 (10-16) | ||||
| 3 | 12.19 (1.3) | 12.5 (10.5-13) | ||||
| 4 | 11 (1.41) | 11 (10-0) | ||||
| Gender | ||||||
| Female | 13.09 (4.12) | 12.6 (10-15.9) | 0.824 | |||
| Male | 12.8 (3.73) | 13 (10-15) | ||||
| Age (in years) | ||||||
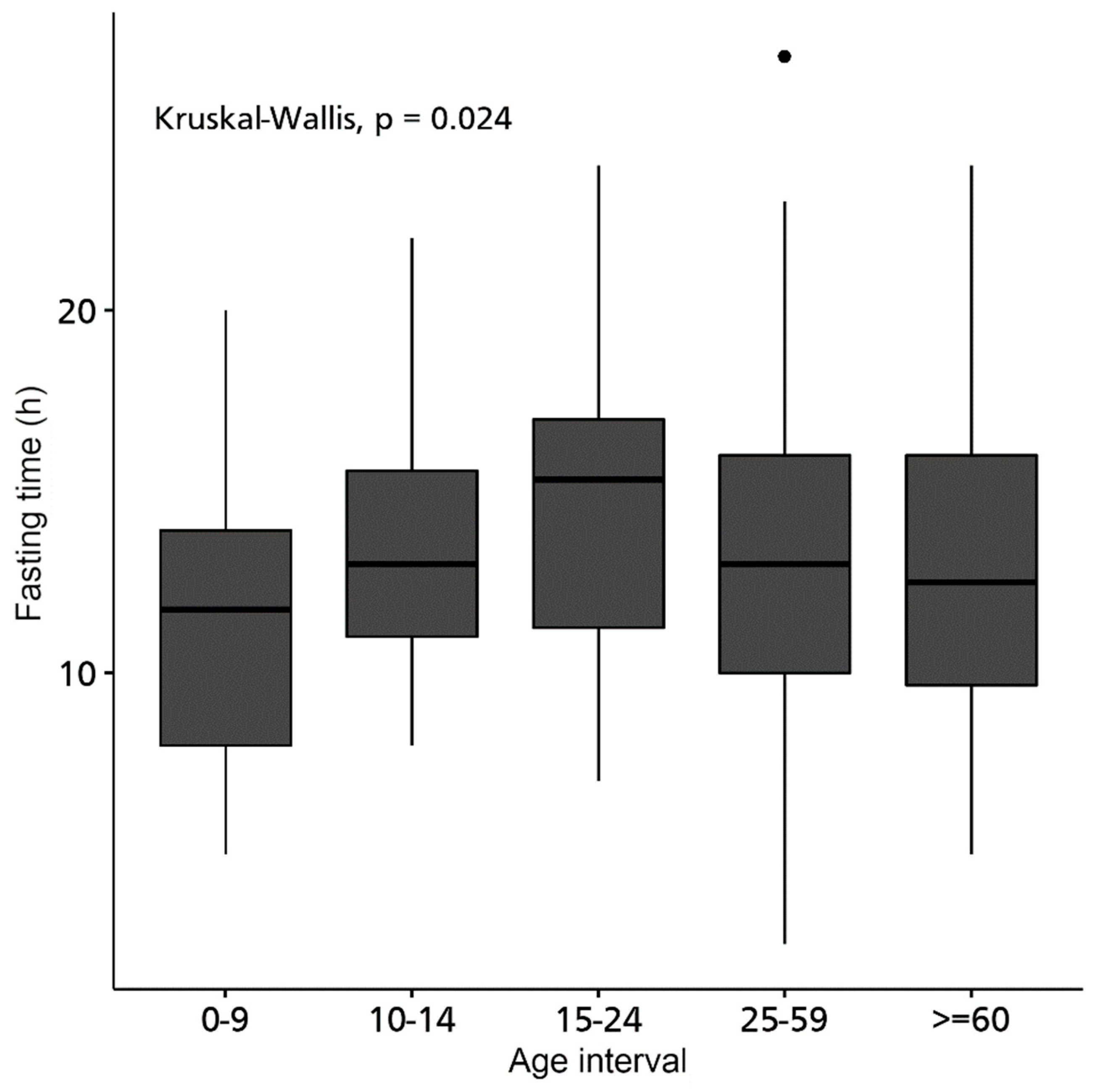
| 0 to 9 years to | 11.41 (3.37) | 11.75 (8-13.98) | 0.021 | |||
| 10 to 14 years a,b | 13.41 (3.37) | 13 (11-16.29) | ||||
| 15 to 24 years b | 14.55 (4.56) | 15.33 (10.5-17) | ||||
| 25 to 59 years a,b | 13.25 (4) | 13 (10-16) | ||||
| From 60 a,b | 12.8 (4.17) | 12.5 (9.62-16) | ||||
| Shift | ||||||
| Morning | 11.92 (2.48) | 12 (10-13.5) | <0.001 | |||
| Afternoon | 13.84 (4.67) | 14.9 (9.7-17) | ||||
| Type of Surgery | ||||||
| Open Abdominal | 13.7 (4.22) | 14 (10-17) | 0.072 | |||
| Abdominal by video | 12.78 (4.21) | 11.3 (9.7-16) | ||||
| Endoscopy | 11.98 (3.34) | 12 (9.7-13.8) | ||||
| Pediatric | 11.93 (3.59) | 12 (9-14) | ||||
| Plastic | 11.87 (2.11) | 12 (10-13.8) | ||||
| Thoracic | 14 (2.37) | 14 (11.8-16.3) | ||||
| Others | 13.67 (4.2) | 13.7 (10.5-16) | ||||
| Type of Anesthesia | ||||||
| General Anesthesia | 13.12 (4.07) | 12.8 (10-16) | 0.402 | |||
| Neuroaxial blockade | 13.21 (4.17) | 13 (9.9-16) | ||||
| Combined | 13.28 (3.39) | 14 (10-15.3) | ||||
| Sedation | 11.79 (3.36) | 12.5 (9.5-13.7) | ||||
| Hypotension | ||||||
| Yes | 14.01 (4.37) | 14 (11-17.2) | 0.257 | |||
| No | 12.93 (3.95) | 12.8 (10-15.4) | ||||
| Fasting (h) | |||||
|---|---|---|---|---|---|
| <9h (n=39) |
9-13 (n=109) |
13-17 (n=89) |
>17 (n=56) |
p-value | |
| SC. n (%) | |||||
| 1 | 31 (12.4) | 90 (36.1) | 76 (30.5) | 52 (20.9) | 0.250 Q |
| 2 | 8 (18.2) | 19 (43.2) | 13 (29.5) | 4 (9.1) | |
| ASA. n (%) | |||||
| 1 | 21 (15.6) | 46 (34.1) | 41 (30.4) | 27 (20) | 0.427 Q |
| 2 | 17 (12.1) | 51 (36.4) | 43 (30.7) | 29 (20.7) | |
| 3 | 0 (0) | 5 (71.4) | 2 (28.6) | 0 (0) | |
| 4 | 0 (0) | 2 (100) | 0 (0) | 0 (0) | |
| Gender. n (%) | |||||
| Female | 25 (13.3) | 72 (38.3) | 55 (29.3) | 36 (19.1) | 0.964 Q |
| Male | 14 (13.5) | 37 (35.6) | 33 (31.7) | 20 (19.2) | |
| Age. Median (IQR) | 37 (6-58.5) | 39 (16.5-56.5) | 39 (17-53.5) | 41 (22.5-56.5) | 0.591 K |
| Age group. n (%) | |||||
| 1 to 9 years | 12 (27.3) | 16 (36.4) | 14 (31.8) | 2 (4.5) | 0.051 Q |
| 10 to 14 years | 1 (4) | 11 (44) | 7 (28) | 6 (24) | |
| 15 to 24 years | 3 (13) | 4 (17.4) | 8 (34.8) | 8 (34.8) | |
| 25 to 59 years | 15 (10.3) | 55 (37.9) | 46 (31.7) | 29 (20) | |
| from 60 | 8 (15.1) | 21 (39.6) | 13 (24.5) | 11 (20.8) | |
| BMI (kg/m²). Median (IQR) | 25.6 (20.4-28.2) | 26.4 (23-30.5) | 25.2 (21.8-29.8) | 25.4 (23.1-28.3) | 0.825 K |
| Height (m). Median (IQR) | 160 (150-163) | 159 (153-165) | 158 (149-165) | 159 (155.5-166) | 0.565 K |
| Weight (kg). Median (IQR) | 56.5 (22-68) | 63.5 (47-74) | 64 (48-76) | 64.5 (55.5-80) | 0.100 K |
| Round. n (%) | |||||
| Morning | 11 (8.5) a | 70 (54.3) b | 45 (34.9) a,b | 3 (2.3) c | <0.001 Q |
| Afternoon | 28 (17.1) a | 39 (23.8) b | 44 (26.8) a,b | 53 (32.3) c | |
| Anesthesia Time (h). Median (IQR) | 1.5 (0.9-2.1) | 1.8 (0.8-3.1) | 1.8 (1.1-2.3) | 2.3 (1.3-3) | 0.070 K |
| Surgery Time (h). Median (IQR) | 0.8 (0.5-1.3) a | 1 (0.3-2.3) b,c | 1 (0.5-1.8) a,b | 1.7 (0.9-2.3) c | 0.038 K |
| Type of Surgery. n (%) | |||||
| Open Abdominal | 7 (13.2) | 15 (28.3) | 17 (32.1) | 14 (26.4) | 0.161 Q |
| Abdominal by video | 6 (12.8) | 22 (46.8) | 8 (17) | 11 (23.4) | |
| Endoscopy | 3 (12) | 12 (48) | 8 (32) | 2 (8) | |
| Pediatric | 12 (21.8) | 22 (40) | 15 (27.3) | 6 (10.9) | |
| Plastic | 1 (8.3) | 7 (58.3) | 4 (33.3) | 0 (0) | |
| Thoracic | 0 (0) | 2 (33.3) | 3 (50) | 1 (16.7) | |
| Others | 10 (10.5) | 29 (30.5) | 34 (35.8) | 22 (23.2) | |
| Type of Anesthesia. n (%) | |||||
| General Anesthesia | 22 (13) | 64 (37.9) | 47 (27.8) | 36 (21.3) | 0.294 Q |
| Locks | 12 (17.6) | 20 (29.4) | 21 (30.9) | 15 (22.1) | |
| Combined | 2 (9.5) | 7 (33.3) | 9 (42.9) | 3 (14.3) | |
| Sedation | 3 (8.6) | 18 (51.4) | 12 (34.3) | 2 (5.7) | |
| Hypotension. n (%) | |||||
| Yes | 1 (5.3) | 8 (42.1) | 4 (21.1) | 6 (31.6) | 0.349 Q |
| No | 38 (13.9) | 101 (36.9) | 85 (31) | 50 (18.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





