Submitted:
11 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Demographic Characteristics
2.3. Vitamin B12 Measurement
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Vitamin B12 Correlations with Sleep Quality, Daytime Sleepiness, and Insomnia Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef]
- Cho, Y.W.; Shin, W.C.; Yun, C.H.; Hong, S.B.; Kim, J.; Earley, CJ. Epidemiology of insomnia in Korean adults: prevalence and associated factors. J Clin Neurol 2009, 5, 20–23. [Google Scholar] [CrossRef]
- Hossain, J.L.; Shapiro, C.M. The prevalence, cost implications, and management of sleep disorders: an overview. Sleep Breath 2002, 6, 85–102. [Google Scholar] [CrossRef]
- Minowa, M.; Okawa, M. Uchiyama, M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol 2000, 10, 79–86. [Google Scholar]
- Sutton, D.A.; Moldofsky, H. Badley, E.M. Insomnia and health problems in Canadians. Sleep 2001, 24, 665–670. [Google Scholar] [CrossRef]
- Wong, W.S.; Fielding, R. Prevalence of insomnia among Chinese adults in Hong Kong: a population-based study. J Sleep Res 2011, 20, 117–126. [Google Scholar] [CrossRef]
- Riemann, D.; Berger, M.; Voderholzer, U. Sleep and depression – results from psychobiological studies: an overview. Biol Psychol 2001, 57, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Liao, D.; Pejovic, S.; Calhoun, S.; Karataraki, M.; & Bixler, E.O.; & Bixler, E. O. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care 2009, 32, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Cooper, D.; D'Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Y.; Zhang, X.; Wei, X.; He, J. Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer 2014, 134, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Kalesan, B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res 2009, 18, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Afaghi, A.; O’Connor, H.; Chow, C.M. Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci 2008, 11, 146–154. [Google Scholar] [CrossRef]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res 2014, 23, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Bertisch, S.M.; Sillau, S.; de Boer, I.H.; Szklo, M.; Redline, S. 25-Hydroxyvitamin D concentration and sleep duration and continuity: Multi-Ethnic Study of Atherosclerosis. Sleep 2015, 38, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr Res 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Ursin, R. Serotonin and sleep. Sleep Med Rev 2002, 6, 55–67. [Google Scholar] [CrossRef]
- Sowa-Kućma, M.; Legutko, B.; Szewczyk, B.; Novak, K.; Znojek, P.; Poleszak, E.; Papp, M.; Pilc, A.; Nowak, G. A ntidepressant-like activity of zinc: further behavioral and molecular evidence. J Neural Transm 2008, 115, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Honma, K.; Kohsaka, M.; Fukuda, N.; Morita, N.; Honma, S. Effects of vitamin B12 on plasma melatonin rhythm in humans: increased light sensitivity phase-advances the circadian clock? Experientia 1992, 48, 716–720. [Google Scholar] [CrossRef]
- Ji, X.; Grandner, M.A.; Liu, J. The relationship between micronutrient status and sleep patterns: a systematic review. Public Health Nutr 2017, 20, 687–701. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B. Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gamaldo, A.A.; Canas, J.A.; Beydoun, H.A.; Shah, M.T.; McNeely, J.M.; Zonderman, A.B. Serum nutritional biomarkers and their associations with sleep among US adults in recent national surveys. PLoS One 2014, 9, e103490. [Google Scholar] [CrossRef] [PubMed]
- Al-Musharaf, S.; Alabdulaaly, A.; Bin Mujalli, H.; Alshehri, H.; Alajaji, H.; Bogis, R.; Alnafisah, R.; Alfehaid, S.; Alhodaib, H.; Murphy, A.M.; Hussain, S.D.; Sabico, S.; McTernan, P.G.; Al-Daghri, N. Sleep quality is associated with vitamin B12 status in female Arab students. Int. J. Environ. Res. Publ. Health 2021, 18, 4548. [Google Scholar]
- Okawa, M.; Takahashi, K.; Egashira, K.; Furuta, H.; Higashitani, Y.; Higuchi, T.; Ichikawa, H.; Ichimaru, Y.; Inoue, Y.; Ishizuka, Y.; Ito, N.; Kamei, K.; Kaneko, M.; Kim, Y.; Kohsaka, M.; Komori, T.; Kotorii, T.; Matsumoto, M.; Mishima, K.; Mizuki, Y.; … Takahashi, S. Vitamin B12 treatment for delayed phase syndrome: a multicenter double-blind study. Psychiatry Clin Neurosci 1997, 51, 275–279. [Google Scholar] [CrossRef]
- Takahashi, K.; Okawa, M.; Matsumoto, M.; Mishima, K.; Yamadera, H.; Sasaki, M.; Ishizuka, Y.; Yamada, K.; Higuchi, T.; Okamoto, N.; Furuta, H.; Nakagawa, H.; Ohta, T.; Kuroda, K.; Sugita, Y.; Inoue, Y.; Uchimura, N.; Nagayama, H.; Miike, T.; Kamei, K. Double-blind test on the efficacy of methylcobalamin on sleep–wake rhythm disorders. Psychiatry Clin Neurosci 1999, 53, 211–213. [Google Scholar] [CrossRef]
- Mayer, G.; Kroger, M. Meier-Ewert, K. Effects of vitamin B12 on performance and circadian rhythm in normal subjects. Neuropsychopharmacology 1996, 15, 456–464. [Google Scholar] [CrossRef]
- Sato-Mito, N.; Shibata, S.; Sasaki, S.; Sato, K. Dietary intake is associated with human chronotype as assessed by both morningness–eveningness score and preferred midpoint of sleep in young Japanese women. Int J Food Sci 2011, 62, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med 2015, 53, 1149–1159. [Google Scholar] [CrossRef]
- Soysal, P.; Smith, L.; Dokuzlar, O.; Isik, A.T. Relationship Between Nutritional Status and Insomnia Severity in Older Adults. J Am Med Dir Assoc 2019, 20, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.S.; Begum, K. Comparison of nutrient intake by sleep status in selected adults in Mysore, India. Nutr Res Pract 2011, 5, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Okamoto, N.; Nishimoto, M.; Hoshino, R.; Ohara, K.; Ohashi, Y.; Kawaguchi, K. A Multicenter Study of the Effects of Vitamin B12on Sleep-Waking Rhythm Disorders: In Shizuoka Prefecture. Psychiatry Clin. Neurosci 1992, 46, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, Z.; Yao, N.; Zhang, X.; Ge, Q. The independent association between vitamin B12 and insomnia in Chinese patients with type 2 diabetes mellitus: a cross-sectional study. Nutr Diabetes 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- O'Logbon, J.; Crook, M.; Steed, D.; Harrington, D.J.; Sobczyńska-Malefora, A. Ethnicity influences total serum vitamin B12 concentration: a study of Black, Asian and White patients in a primary care setting. J Clin Pathol 2022, 75, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef]
- Duffy, J.F.; Zitting, K.M.; Chinoy, E.D. Aging and circadian rhythms. Sleep Med Clin 2015, 10, 423–434. [Google Scholar] [CrossRef]
- Channer-Wallen, T.; Dawson, P.; Thomas-Brown, P.G.; Gossell-Williams, M. Lack of association between serum vitamin B12 and nocturnal sleep parameters following cyanocobalamin supplementation in healthy adults. Heliyon 2022, 8, e08831. [Google Scholar] [CrossRef]
- Condo, D.; Lastella, M.; Aisbett,B. ; Stevens, A.; Roberts, S. Sleep duration and quality are associated with nutrient intake in elite female athletes. J Sci Med Sport 2022, 25, 345–350. [Google Scholar] [CrossRef]
- Jahrami, H.; Alekri, E.; BaHammam, A.S.; Alsalman, A.; Bragazzi, N.L.; Alhaj, O.; Saif, Z. The association between micronutrient status and sleep quality in patients with depression: a case-control study. Sleep Breath 2021, 25, 1571–1579. [Google Scholar] [CrossRef]
- Heybeli, C.; Soysal, P.; Oktan, M.A.; Smith, L.; Çelik, A.; Kazancioglu, R. Associations between nutritional factors and excessive daytime sleepiness in older patients with chronic kidney disease. Aging Clin Exp Res 2022, 34, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Koc Okudur, S.; Soysal, P. Excessive Daytime Sleepiness is Associated With Malnutrition, Dysphagia, and Vitamin D Deficiency in Older Adults. J Am Med Dir Assoc 2021, 22, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, I.; Yingling, K.; Bukamur, H.; Abusnina, W. Vitamin B12 Deficiency: A Rare Cause of Excessive Daytime Sleepiness. J Clin Sleep Med 2019, 15, 1365–1367. [Google Scholar] [CrossRef] [PubMed]

| Total population according to Vitamin B12 status |
||||
|---|---|---|---|---|
| Total population |
Vitamin B12 ≥342 pg/mL | Vitamin B12 <342 pg/mL | p-value | |
| N=512 | N=256 | N=256 | ||
| Demographics | ||||
| Gender, males (%) | 180 (35%) | 84 (33%) | 96 (38%) | 0.343 |
| Menopause (%) | 249 (75%) | 129 (75%) | 120 (75%) | 0.997 |
| Age, years | 64 ± 16 | 63 ± 16 | 65 ± 15 | 0.144 |
| Age ≥ 60 years | 323 (63%) | 151 (59%) | 172 (67%) | 0.160 |
| BMI (kg/m2) | 30 ± 16 | 30 ± 19 | 30 ± 11 | 0.903 |
| BMI≥30, n (%) | 168 (33%) | 84 (33%) | 84 (33%) | 0.966 |
| Smoking status | ||||
| Never, n (%) | 272 (53%) | 148 (58%) | 124 (49%) | |
| Current/, n (%) | 107 (21%) | 56 (22%) | 51 (20%) | |
| Former, n (%) | 133 (26%) | 51 (20%) | 82 (32%) | 0.066 |
| Co-morbidities | ||||
| Hypertension | 260 (51%) | 130 (51%) | 130 (51%) | 0.999 |
| Diabetes Type 2 | 97(19%) | 38 (15%) | 59 (23%) | 0.128 |
| Hyperlipidemia | 279 (55%) | 133 (52%) | 146 (57%) | 0.463 |
| COPD | 38 (7%) | 18 (7%) | 20 (8%) | 0.163 |
| Asthma | 20 (4%) | 15 (6%) | 5 (2%) | 0.136 |
| Coronary Artery Disease | 35 (7%) | 20 (8%) | 15 (6%) | 0.529 |
| Atrial fibrillation | 23 (5%) | 13 (5%) | 10 (4%) | 0.837 |
| Cerebrovascular Disease | 11 (2%) | 7 (3%) | 4 (1%) | 0.429 |
| Cardiovascular Disease | 74 (15%) | 43 (17%) | 31 (12%) | 0.216 |
| Depression (on medications) |
59 (11%) | 33 (13%) | 26 (10%) | 0.533 |
| Total population according to Vitamin B12 status |
||||
|---|---|---|---|---|
| Total population |
Vitamin B12 ≥342 pg/mL | Vitamin B12 <342 pg/mL | p-value | |
| N=512 | N=256 | N=256 | ||
| Daytime sleepiness | ||||
| ESS | 6 (4, 10) | 6 (4, 10) | 6 (4, 10) | 0.908 |
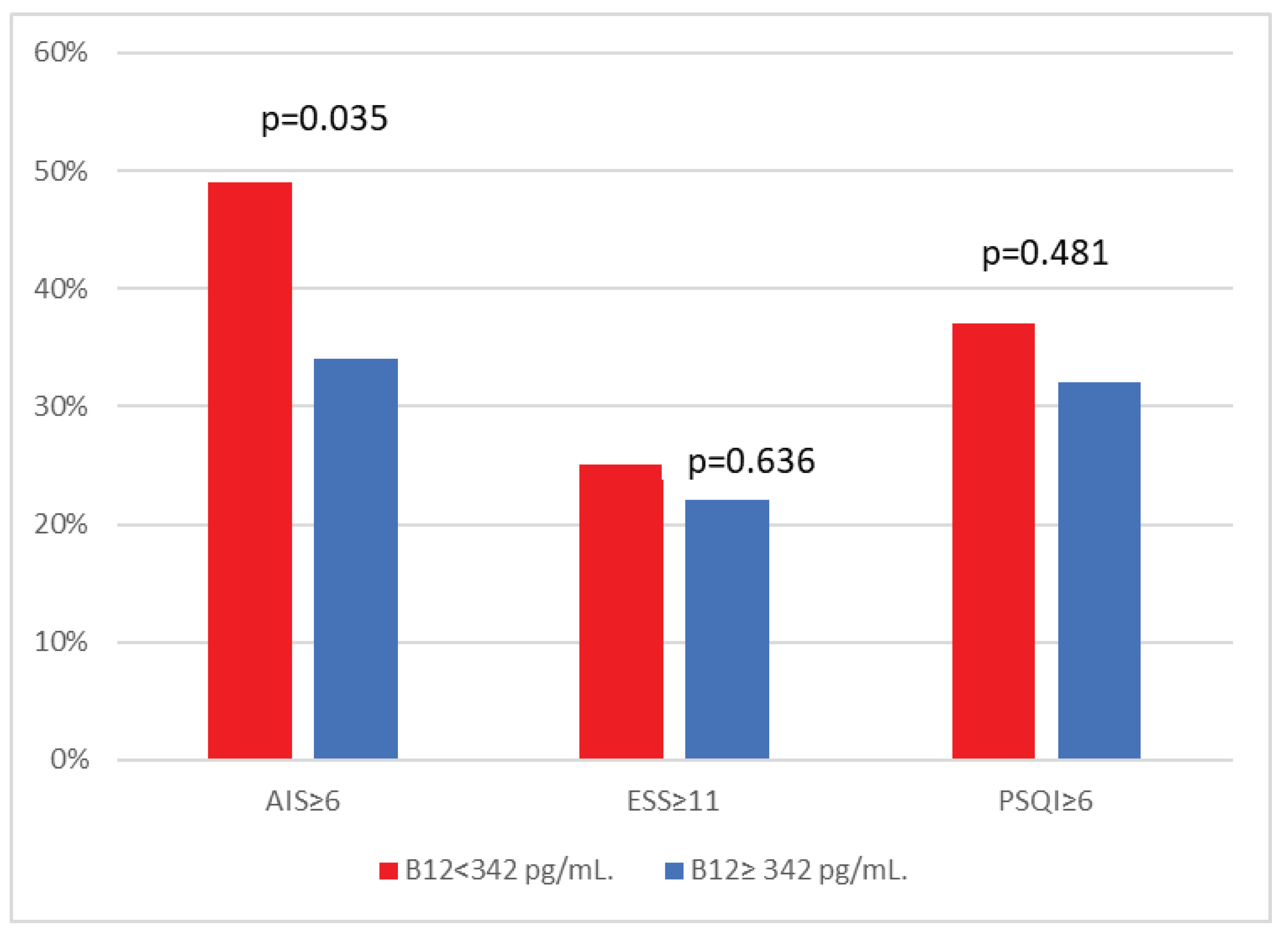
| ESS≥11 (%) | 120 (23%) | 56 (22%) | 64 (25%) | 0.636 |
| Insomnia symptoms | ||||
| Athens Insomnia Scale Score | 5 (3,6) | 4 (3, 6) | 5 (3, 6) | 0.419 |
| Athens Insomnia Scale Score≥6 (%) | 212 (41%) | 87 (34%) | 125 (49%) | 0.035 |
| Sleep Quality | ||||
| PSQI | 5 (3, 6) | 4 (3, 6) | 5 (3, 6) | 0.286 |
| PSQI≥6 | 177 (35%) | 82 (32%) | 95 (37%) | 0.481 |
| B | S.E. | p-value | OR (95%CI) | |
| Males versus Females | 1.098 | 0.385 | 0.004 | 2.998 (1.410-6.374) |
| Age >60 years | -0.169 | 0.366 | 0.669 | 0.844 (0.388-1.835) |
| Body mass index≥30 | 0.759 | 0.342 | 0.027 | 2.135 (1.091-4.178) |
| Current/Former smoking | 0.362 | 0.397 | 0.362 | 1.436 (0.660-3.126) |
| Hypertension | 0.139 | 0.393 | 0.723 | 1.159 (0.532-2.484) |
| Diabetes type 2 | 0.443 | 0.429 | 0.302 | 1.557 (0.671-3.614) |
| Cardiovascular disease | -0.554 | 0.521 | 0.288 | 0.575 (0.207-1.597) |
| COPD | 0.189 | 0.546 | 0.729 | 1.208 (0.414-3.521) |
| Depression | -0.050 | 0.573 | 0.931 | 0.952 (0.310-2.924) |
| Vitamin B12 <342 | 0.101 | 0.517 | 0.762 | 1.106 (0.576-2.125) |
| B | S.E. | p-value | OR (95%CI) | |
| Females versus Males | 1.266 | 0.397 | 0.001 | 3.547 (1.630-7.718) |
| Age >60 years | -0.129 | 0.375 | 0.730 | 0.879 (0.422-1.831) |
| Body mass index≥30 | 0.073 | 0.318 | 0.818 | 1.076 (0.577-2.009) |
| Current/Former smoking | 0.227 | 0.356 | 0.523 | 1.255 (0.625-2.520) |
| Hypertension | 1.229 | 0.368 | 0.001 | 3.419 (1.661-7.035) |
| Diabetes type 2 | -0.467 | 0.424 | 0.270 | 0.627 (0.273-1.438) |
| Cardiovascular disease | 0.526 | 0.453 | 0.246 | 1.692 (0.696-4.112) |
| COPD | 0.133 | 0.603 | 0.826 | 1.142 (0.350-3.724) |
| Depression | -0.467 | 0.472 | 0.322 | 0.627 (0.249-1.579) |
| Vitamin B12 <342 | 0.890 | 0.308 | 0.004 | 2.434 (1.331-4.452) |
| B | S.E. | p-value | OR (95%CI) | |
| Females versus Males | 0.318 | 0.460 | 0.489 | 1.374 (0.558-3.384) |
| Age >60 years | 0.385 | 0.469 | 0.410 | 1.470 (0.588-3.679) |
| Body mass index≥30 | 0.305 | 0.424 | 0.471 | 1.357 (0.591-3.116) |
| Current/Former smoking | -0.624 | 0.424 | 0.141 | 0.536 (0.233-1.229) |
| Hypertension | 0.028 | 0.438 | 0.948 | 1.029 (0.436-2.430) |
| Diabetes type 2 | 0.275 | 0.522 | 0.599 | 1.316 (0.473-3.662) |
| Cardiovascular disease | 0.262 | 0.592 | 0.658 | 1.300 (0.408-4.144) |
| COPD | 1.307 | 0.767 | 0.089 | 3.693 (0.821-16.614) |
| Depression | 0.540 | 0.631 | 0.392 | 1.716 (0.498-5.918) |
| Vitamin B12 <342 | 0348 | 0.376 | 0.354 | 1.416 (0.678-2.958) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





