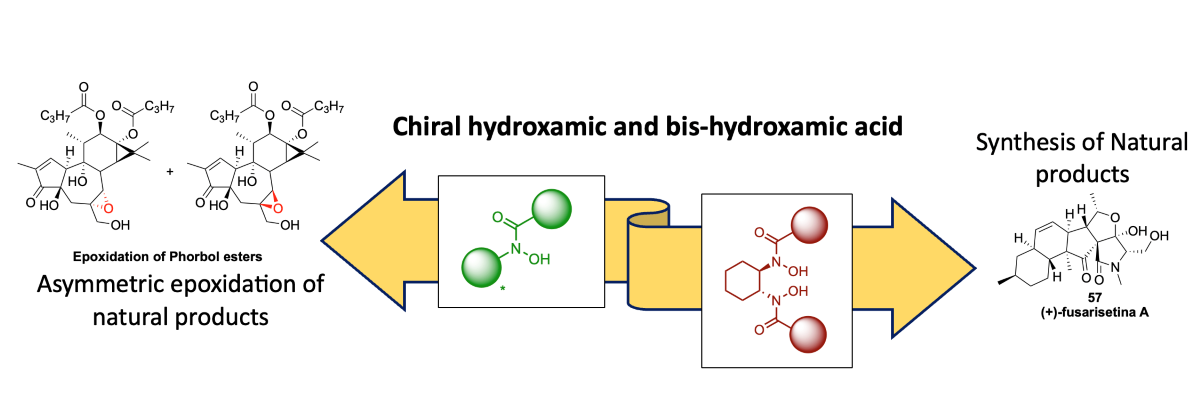
1. Introduction
The synthesis of natural products has long been a fascinating pursuit in organic chemistry, driven by the remarkable structural complexity and diverse array of biological activities exhibited by these compounds. Natural products serve as a wellspring of inspiration for the development of new drugs, agrochemicals, and materials[
1]. Asymmetric synthesis, which aims to access enantiomerically pure compounds, plays a pivotal role in the construction of complex natural product scaffolds with desired stereochemistry. Over the years, the development of efficient chiral ligands has revolutionized asymmetric synthesis, enabling chemists to achieve high selectivity and efficiency in their synthetic endeavors[
2].
Among the various classes of chiral ligands, hydroxamic acids have emerged as versatile tools for asymmetric synthesis. Hydroxamic acids possess a distinctive molecular structure characterized by a hydroxylamine (-NOH) functional group attached to a carbonyl group. This arrangement imparts them with a unique set of properties that make them highly effective ligands in coordination chemistry. The hydroxamic acid moiety can form coordination complexes with transition metal catalysts, leading to the formation of chiral catalysts capable of inducing asymmetric transformations[
3].
Chiral hydroxamic acid (HA) and Bis-hydroxamic acid (BHA) ligands have gained significant attention and found widespread application in asymmetric synthesis due to their ability to provide exceptional stereochemical control. The design and development of these ligands have been driven by the need to achieve high enantioselectivity, regioselectivity, and stereoselectivity in a variety of synthetic transformations. The inherent flexibility and modifiability of hydroxamic acid ligands allow for fine-tuning of their properties, enabling chemists to tailor them for specific reactions and target molecules[
4,
5].
In the realm of natural product synthesis, chiral hydroxamic acid ligands have made remarkable contributions, offering elegant solutions to challenging synthetic problems. Their effectiveness in accessing enantiopure compounds has enabled chemists to construct complex natural product architectures, reproduce their biological activities, and explore structure-activity relationships. By harnessing the power of these ligands, synthetic chemists have achieved impressive advancements in various aspects of asymmetric synthesis pertaining to natural products[
6,
7].
One area where chiral hydroxamic acid ligands have demonstrated exceptional utility is in enantioselective carbon-carbon (C-C) bond formation. The formation of C-C bonds is a key step in constructing the carbon framework of natural products[
6]. Chiral hydroxamic acid ligands have been successfully employed in asymmetric allylation reactions, aldol reactions, Mannich reactions, and other important C-C bond-forming transformations. Through careful ligand design and optimization, chemists have achieved high levels of enantioselectivity, enabling the synthesis of complex natural product motifs with precise stereochemistry[
7].
In addition to C-C bond formation, the functionalization of carbon-hydrogen (C-H) bonds represents an important strategy in natural product synthesis. Direct C-H functionalization allows chemists to access molecular complexity efficiently by utilizing unactivated carbon-hydrogen bonds as functional handles. Chiral hydroxamic acid ligands have been instrumental in developing transition metal-catalyzed C-H activation reactions with high enantioselectivity. These reactions have enabled the selective formation of chiral centers in the target molecules, thus providing access to diverse natural products that were previously challenging to synthesize[
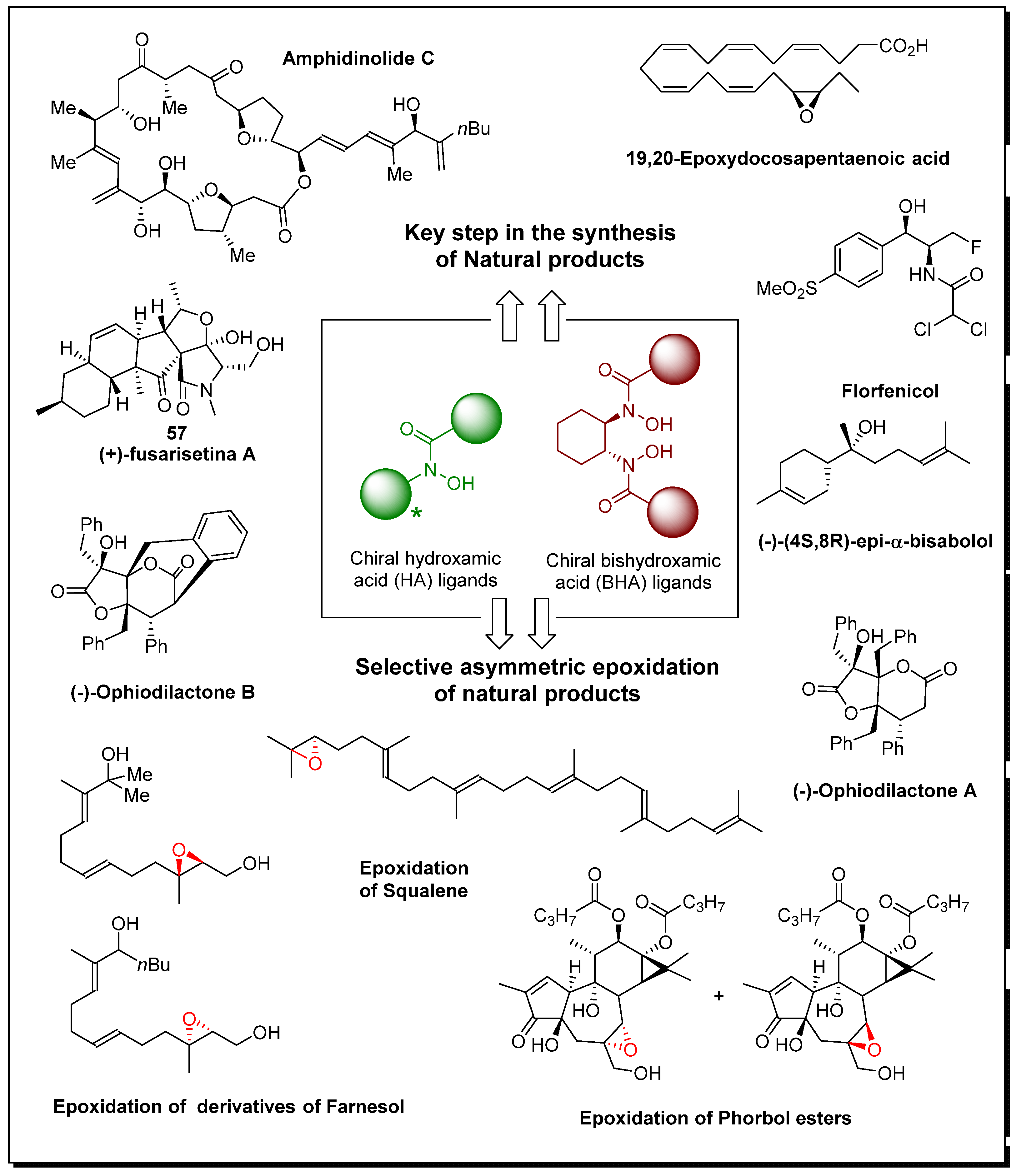
3]. Furthermore, chiral hydroxamic acid ligands have demonstrated their efficacy in asymmetric transformations involving heteroatoms. Natural products frequently contain heterocyclic motifs that contribute to their biological activities. Chiral hydroxamic acid ligands have been successfully employed in asymmetric amination reactions, amidation reactions, and epoxidations, enabling the controlled formation of chiral heterocycles with high enantioselectivity. The ability to selectively introduce heteroatoms into complex natural product scaffolds expands the synthetic possibilities and provides access to a broader range of natural product structures (
Figure 1).
Moreover, the impact of chiral HA and BHA ligands extends beyond their application in specific transformations. These ligands have played a pivotal role in the total synthesis of numerous natural products, showcasing their versatility and efficiency. By strategically incorporating chiral hydroxamic acid ligands in key steps, synthetic chemists have successfully achieved the total synthesis of complex alkaloids, polyketides, terpenoids, and other structurally intricate natural products. These achievements not only facilitate the acquisition of biologically active compounds but also deepen our understanding of complex relationships[
8,
9].
In this review, we aim to provide a comprehensive overview of the remarkable contributions of chiral hydroxamic acid and bis-hydroxamic acid ligands in the asymmetric synthesis of natural products. Natural products have long captivated the attention of organic chemists due to their intricate structural complexity and diverse biological activities. Asymmetric synthesis, which enables access to enantiomerically pure compounds, has played a pivotal role in the construction of complex natural product scaffolds with precise stereochemistry. Within this context, chiral hydroxamic acid and bis-hydroxamic acid ligands have emerged as versatile tools, offering unique properties that facilitate high selectivity and efficiency in synthetic endeavors. By examining key examples from the literature, we will explore the pivotal role of these ligands in the synthesis of natural products, highlighting their impact on enantioselective carbon-carbon bond formation, functionalization of carbon-hydrogen bonds, heteroatom-based transformations, and their contribution to total synthesis efforts. Through this review, we aim to shed light on the versatility and potential of chiral HA and BHA ligands as valuable tools in the asymmetric synthesis of natural products.
2. Total Synthesis of Natural Compounds
2.1. Total synthesis of α-bisabolol
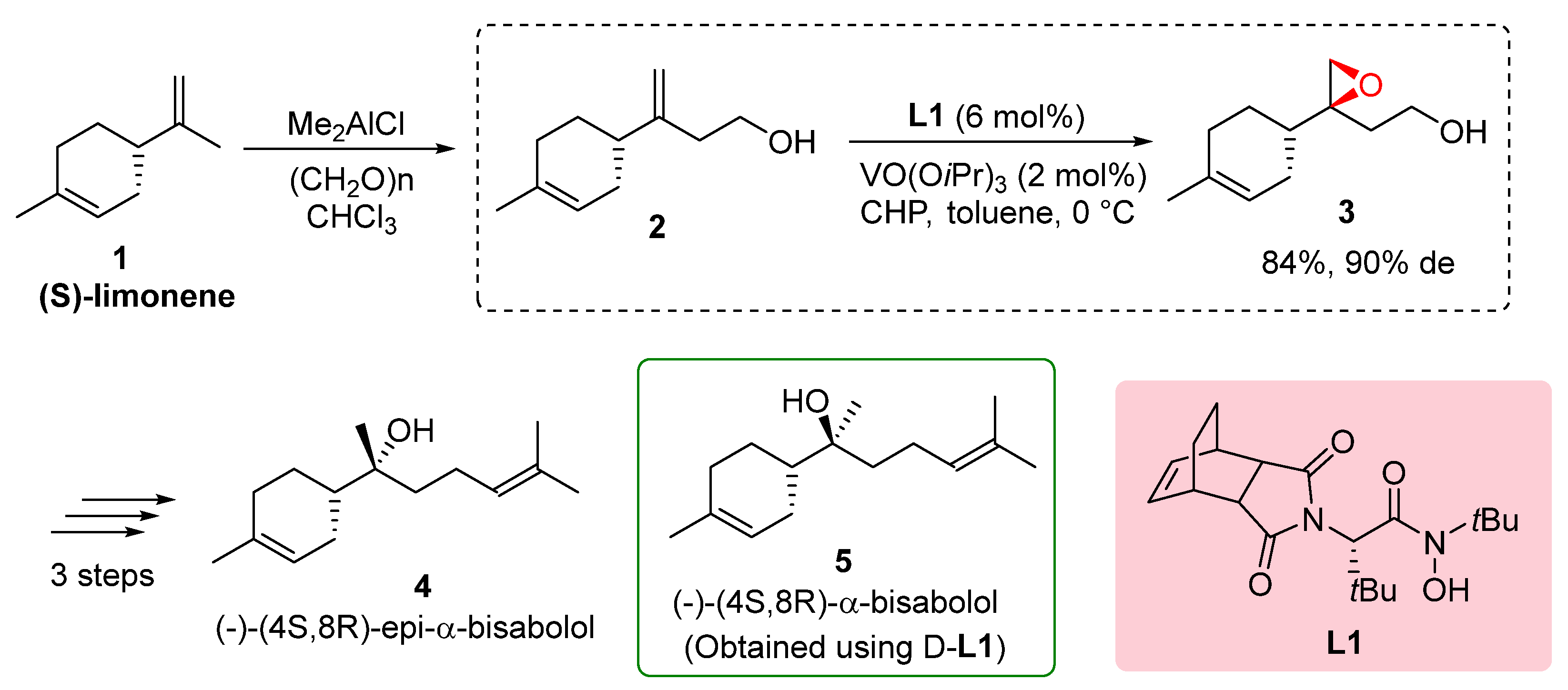
The total synthesis of (-)-α-bisabolol, a fragrance compound derived from (
S)-limonene
1, was successfully achieved through the utilization of hydroxamic acid ligands
L1, as demonstrated by Yamamoto in 2003. The synthetic route involved several key steps starting with the hydroxymethylation of (
S)-limonene
1, resulting in the formation of (
S)-alcohol
2. Diastereoselective epoxidation utilizing hydroxamic acid ligand
L1 proved highly effective, yielding epoxy alcohol
3 with a good overall yield of 84% and a high diastereomeric excess of 90% (de). Subsequent reduction of epoxy alcohol
6 produced diol, which was further subjected to tosylation and coupling with isopropenyl magnesium bromide, ultimately leading to the synthesis of (-)-(
4S,
8S)-α-bisabolol
4. The overall yield from (
S)-limonene was reported to be 21%. Additionally, the study explored the use of hydroxamic acid ligand
L1, resulting in the synthesis of (-)-(
4S,
8R)-epi-α-bisabolol
4 (
Scheme 1). These findings underscore the remarkable efficiency of hydroxamic acid ligands in controlling diastereoselectivity and achieving high yields in the synthesis of complex molecules, thereby providing a practical and promising approach for accessing natural products such as (-)-α-bisabolol. These results highlight the significance of natural product synthesis and the role of hydroxamic acid ligands in the development of efficient synthetic methodologies.[
10]. α-Bisabolol is a significant monocyclic sesquiterpene derived from essential oils of various edible and ornamental plants. It possesses diverse biological activities, including anticancer, antimicrobial, and anti-inflammatory properties. Its therapeutic potential and natural occurrence in essential oils make α-bisabolol an intriguing target for total synthesis and the development of synthetic strategies using hydroxamic acid ligands. The utilization of hydroxamic acid ligands offers a practical approach for accessing natural products like α-bisabolol, enabling further exploration of its therapeutic applications and expanding our knowledge of its biological activities.[
11]
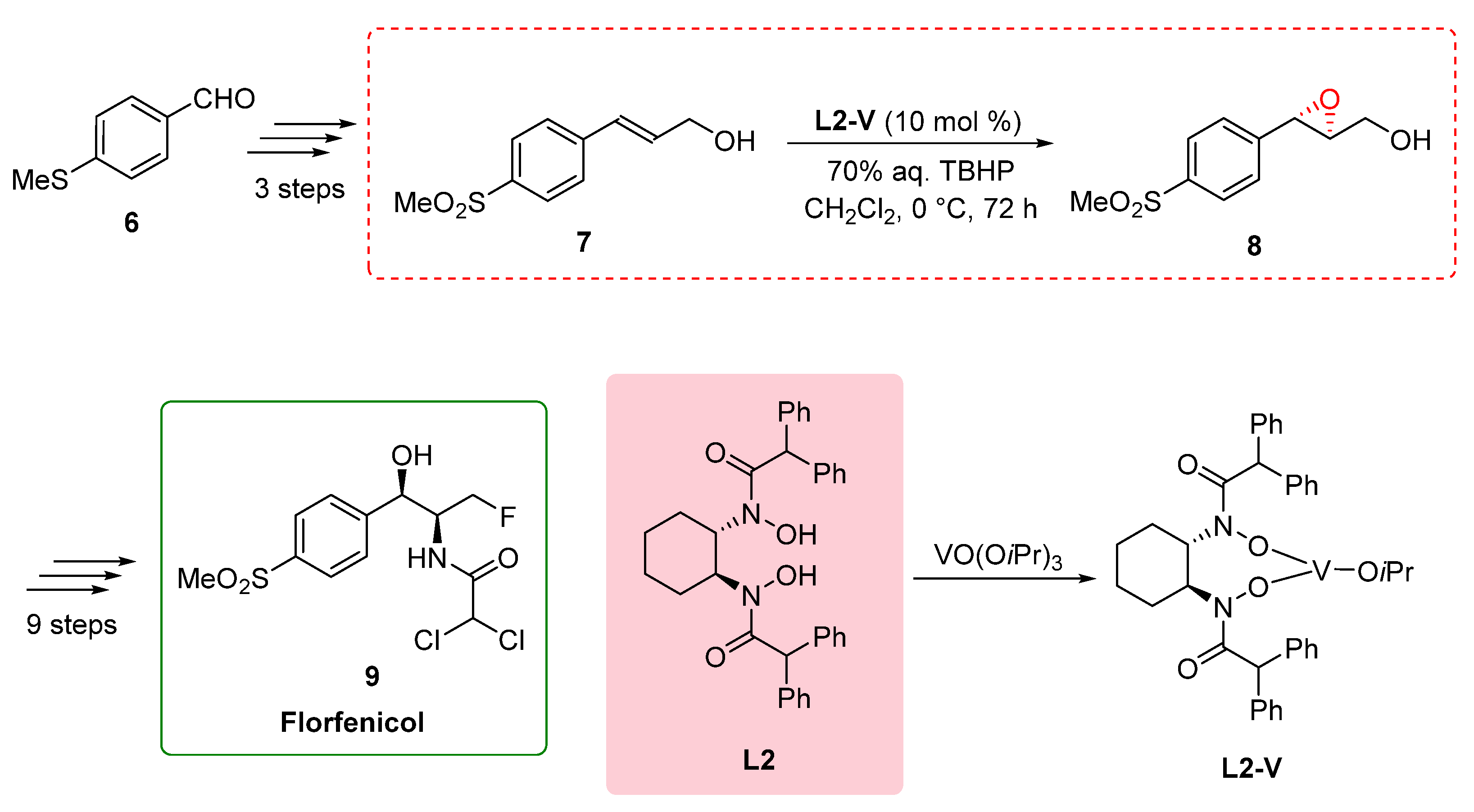
2.2. Total synthesis of Florfenicol
The total synthesis of Florfenicol 9, as reported by Chen in 2011, involved a concise and efficient route with a significant emphasis on the utilization of a chiral bishydroxamic acid (BHA) ligand L2. Florfenicol, a fluorinated analog of thiamphenicol, is a broad-spectrum antibiotic widely used in aquaculture species as well as various livestock species, including bovine, porcine, and chicken, for the treatment of infections.
The synthetic pathway commenced with the transformation of 4-methylthiobenzaldehyde 6, leading to the formation of allylic alcohol
7 in three sequential steps. The subsequent critical focus was directed towards the synthesis of the pivotal intermediate (
2S,
3S)-epoxide
8. While the initial consideration was the Sharpless epoxidation protocol employing anhydrous tert-butyl hydroperoxide (TBHP), its limitations for scale-up necessitated an alternative strategy. Consequently, the authors employed a modified procedure involving 1.5 equivalents of 70% aqueous TBHP and Yamamoto's vanadium catalyst
L2-V at 0°C for 72 hours [
8]. This innovative approach successfully furnished (
2S,
3S)-epoxide
8 with a yield of 75% and an enantiomeric excess of 90%. Further recrystallization enhanced the enantiomeric excess to 95%. Subsequently, an additional nine steps were executed to achieve the desired product, Florfenicol
9, with an overall yield of 37% [
12]. The successful total synthesis of Florfenicol demonstrates the significant contribution of chiral BHA ligands in the synthesis of important pharmaceutical compounds. The broad-spectrum antibiotic properties and widespread applications of Florfenicol highlight the importance of efficient synthetic methodologies to access such valuable compounds. By utilizing the chiral BHA ligand-enabled key step in the synthesis, chemists can efficiently access Florfenicol, enabling its availability for the treatment of infections in aquaculture species and livestock. This synthesis showcases the versatility and efficacy of chiral BHA ligands in the construction of complex pharmaceutical molecules, further underscoring their significance in the field of organic synthesis.[
13]
Scheme 2.
Chiral BHA ligand-enabled key step in the total synthesis of Florfenicol.
Scheme 2.
Chiral BHA ligand-enabled key step in the total synthesis of Florfenicol.
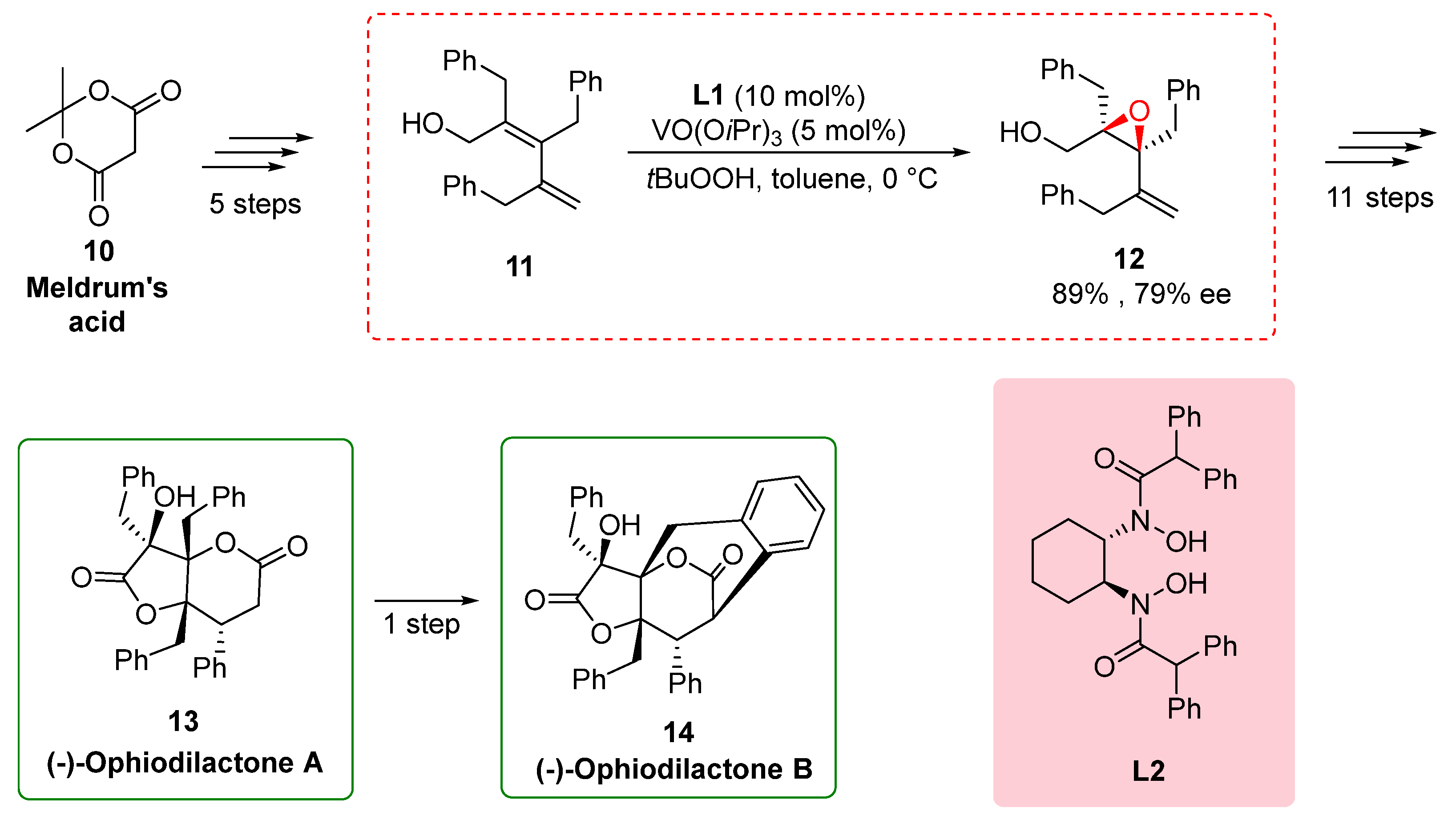
2.3. Total synthesis of (-)-ophiodilactones A and B
In a study conducted by Hatakeyama in 2014 [
14], the total synthesis of (-)-ophiodilactones A and B was achieved using a chiral bishydroxamic acid (BHA) ligand. The synthesis involved several key steps, including Stille coupling and asymmetric epoxidation. Previous reports had shown low enantioselectivity in the epoxidation step, prompting the researchers to employ a method developed by Yamamoto and co-workers [
8]. By utilizing tert-butyl hydroperoxide and a vanadium catalyst in the presence of a BHA ligand, they successfully obtained epoxy alcohol
12 with improved enantioselectivity of 79%. Subsequently, a sequence of 11 steps led to the synthesis of ophiodilactone A
13. The synthesis involved various transformations, including Swern oxidation, Grignard reaction, and hydrogenation, culminating in the desired compound 1. Furthermore, ophiodilactone B
14 was obtained by direct oxidative coupling reactions of C-H and Ar–H bonds of ophiodilactone A. The strategic use of the BHA ligand in the asymmetric epoxidation step played a pivotal role in improving the enantioselectivity and enabling the synthesis of ophiodilactones A and B. The use of the BHA ligand in the asymmetric epoxidation step played a crucial role in improving the enantioselectivity of the synthesis. By implementing the method developed by Yamamoto and co-workers, the researchers achieved higher enantioselectivity in the formation of epoxy alcohol
11. This success enabled the subsequent transformations, leading to the synthesis of γ-lactones
5 and
12 and ultimately facilitating the synthesis of ophiodilactone A
13. The strategic incorporation of the BHA ligand not only controlled the stereochemistry of the synthesized molecules but also provided valuable insights into the synthesis of ophiodilactones and their potential applications in medicinal chemistry [
15].
Scheme 3.
Chiral BHA in total synthesis of (-)-Ophiodilactone A and (-)-Ophiodilactone B.
Scheme 3.
Chiral BHA in total synthesis of (-)-Ophiodilactone A and (-)-Ophiodilactone B.
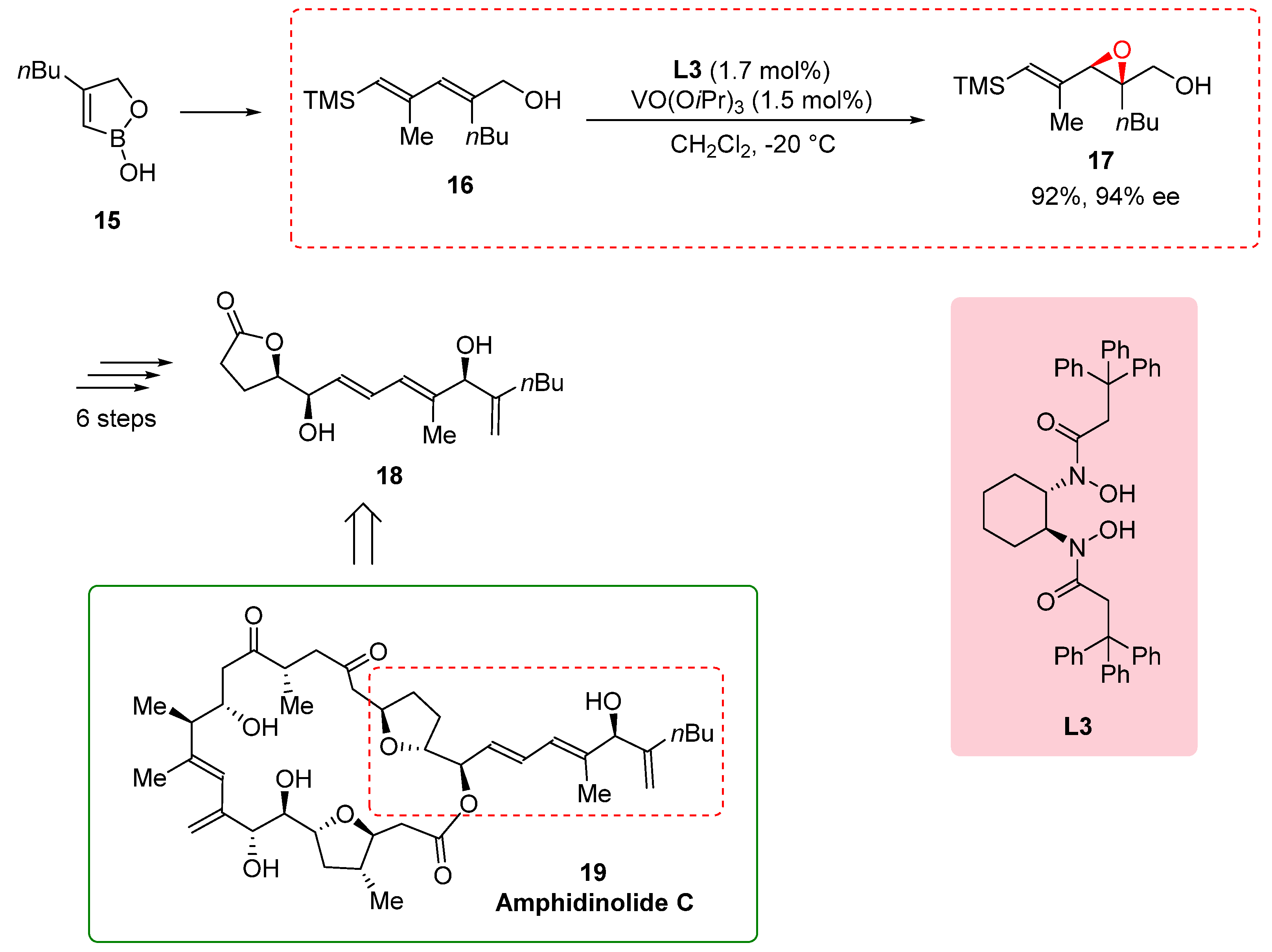
2.4. Synthesis of the side chain of amphidinolide C
In the synthesis of the side chain of amphidinolide C, a complex natural product with potent antineoplastic activity, the epoxidation of allylic alcohol
16 played a crucial role in achieving the desired structure and functionality. However, the commonly used Sharpless epoxidation method did not provide the required enantioselectivity for the formation of epoxy alcohol
17. To overcome this challenge, Fenneteau et al. (2015) turned to the use of a BHA ligand, specifically the
L3-Vanadium complex, known for its ability to control stereoselectivity in reactions. By incorporating the BHA ligand into the epoxidation reaction, the researchers aimed to enhance the enantioselectivity and obtain the desired epoxy alcohol
17 with the desired stereochemistry. The BHA ligand, in conjunction with the Vanadium catalyst, proved highly effective in improving the enantioselectivity of the epoxidation reaction, resulting in a remarkable enantiomeric excess of 94% and a yield of 92%. This successful application of the BHA ligand demonstrates its versatility and effectiveness in achieving challenging synthetic goals, particularly in the synthesis of complex natural products. The synthesis of the side chain of amphidinolide C continued with six additional steps, ultimately yielding the desired side chain
18 (
Scheme 4) [
16]. Amphidinolide C, the first twenty-five-membered macrocyclic lactone with potent antineoplastic activity. Its complex structure and biological activity make it an intriguing target for synthesis. The utilization of the BHA ligand in the synthesis of the side chain of amphidinolide C highlights its importance in controlling the stereochemistry and achieving the desired structural features. By employing the BHA ligand, researchers could enhance the selectivity and efficiency of transformations, enabling the synthesis of complex natural products with significant biological activities [
17].
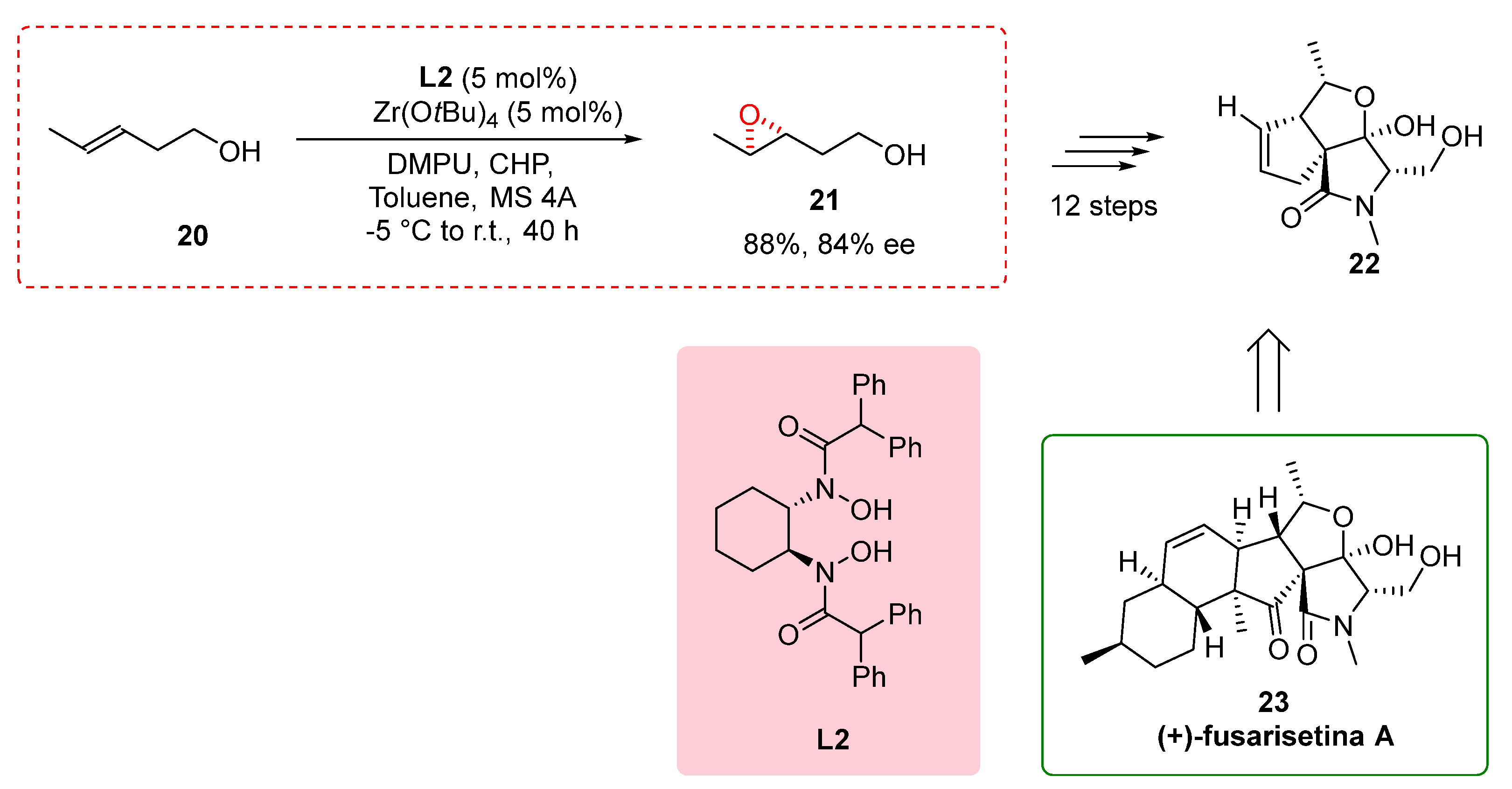
2.5. Partial Synthesis of (+)-Fusarisetin A
In their study, Kohyama et al. (2015) utilized a novel methodology in the partial synthesis of (+)-fusarisetin A, a pentacyclic fungal metabolite known for its intriguing biological properties, including its role as an acinar morphogenesis inhibitor [
18]. The researchers specifically focused on the transformation of the homoallylic alcohol 20, employing the conditions described by Yamamoto and coworkers [
8]. By utilizing chiral bishydroxamic acid ligand
L2, Zn(OtBu)
4 and DMPU, they successfully formed the corresponding epoxide
21 with an impressive enantiomeric excess of 84% and a yield of 88%. Further, through an additional twelve steps, the desired compound
22 was obtained, representing a significant milestone in the synthesis of (+)-fusarisetin A. These steps involved various transformations, including functional group manipulations, ring formations, and stereochemical control, enabling the construction of the intricate pentacyclic structure of (+)-fusarisetin A. Notably, the study revealed the critical role of the metal core in controlling the enantioselectivity of the reaction, with chiral BHA ligands
L2 and hafnium catalyst leading to improved enantiomeric excesses [
19]. The successful application of this methodology in the partial synthesis of (+)-fusarisetin A not only enhances our understanding of the compound's synthetic pathway but also highlights its potential for the efficient synthesis of complex natural products and other valuable compounds. The ability to selectively control the enantioselectivity of reactions by carefully selecting the appropriate metal catalyst opens up new avenues for accessing chiral compounds such as (+)-fusarisetin A.
Scheme 5.
Partial Synthesis of (+)-Fusarisetin A.
Scheme 5.
Partial Synthesis of (+)-Fusarisetin A.
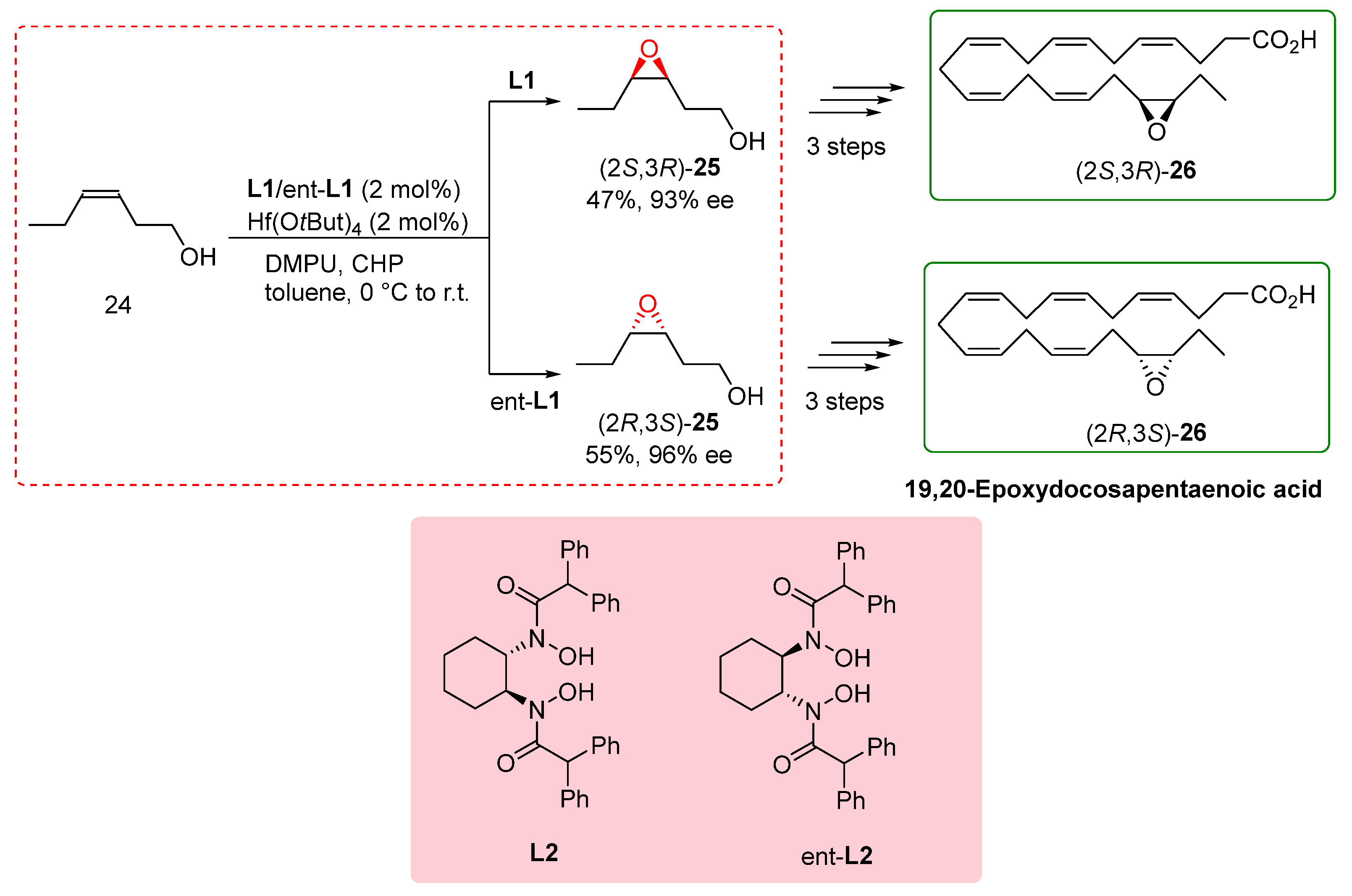
2.6. Total Synthesis of 19,20-Epoxydocosapentanoic Acid
The total synthesis of fatty acids is a challenging task, especially when large quantities of materials are required for biological assays. Within this context, the synthesis of specific unsaturated fatty acids containing an epoxide group is of particular importance as they play crucial roles as endogenous lipids.[
20] To address this challenge, Cinelli et al. reported the total synthesis of 19,20-epoxydocosapentanoic acid (19,20-EDP), a significant compound with biological relevance. Previous synthetic routes for fatty acids did not offer a direct method for the epoxidation of alcohols, resulting in poor outcomes. In their study, the researchers focused on the transformation of homoallylic alcohol
24 into the corresponding epoxy alcohols (
S,R) and (
R,S)-
25. They employed
L2 and ent-
L2 ligands in conjunction with Hf(OtBu)
4 as the catalyst. Although the yield and enantioselectivity achieved were modest and lower than reported values, this study represented the first report of utilizing BHA ligands in the total synthesis of these exciting compounds.
Scheme 6 illustrates the key steps involved in the total synthesis of 19,20-EDP. Despite the challenges faced in achieving high yields and enantioselectivity, this work showcased the potential of BHA ligands in the total synthesis of complex fatty acids, expanding the synthetic scope and paving the way for the efficient synthesis of important bioactive compounds. [
21] This study not only highlights the significance of direct epoxidation methods in fatty acid synthesis but also emphasizes the potential of BHA ligands in enabling the total synthesis of challenging compounds. By utilizing these ligands, researchers can advance the understanding of fatty acid chemistry and pursue the efficient synthesis of biologically relevant molecules.
3. Asymmetric Epoxidation of natural products
3.1. Epoxidation of Phorbol esters
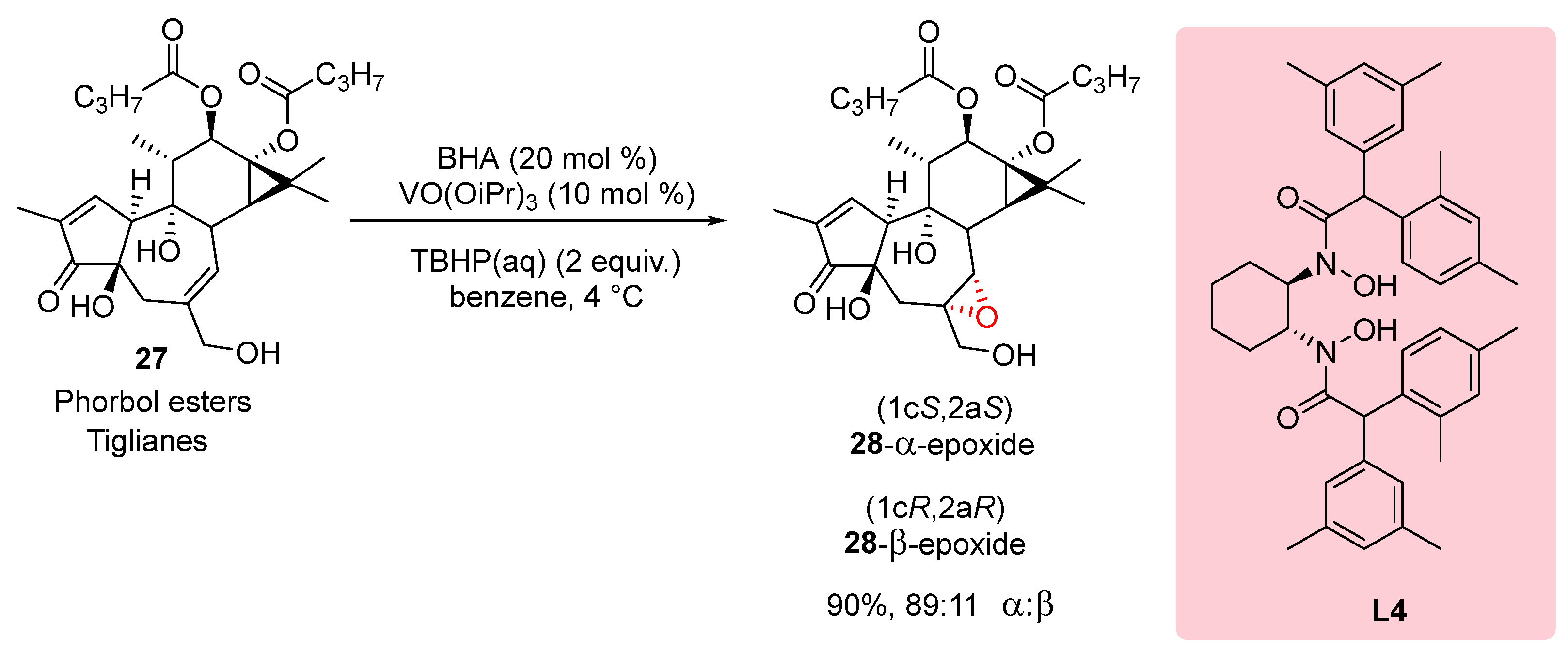
In 2015, Wender et al. conducted a study on the epoxidation of diterpenes, specifically daphnanes and tiglianes, at the C
6-C
7 position. They investigated the diastereoselectivity of the reaction by altering the steric size of the ligand. The researchers used phorbol ester
27 as a test substrate and observed that the choice of ligand influenced the diastereoselectivity. In
Scheme 7, it is shown that when using V(V)-ligand
L4, the reaction exhibited a diastereoselectivity ratio of 89:11 in favor of the
28-α-epoxide. This demonstrates the ability to modulate the diastereoselectivity of the reaction by varying the steric size of the ligand. This study highlights the importance of ligand design in controlling the stereochemistry of epoxidation reactions in the synthesis of diterpenes. By optimizing the ligand, researchers can achieve the desired diastereoselectivity, enabling the selective synthesis of specific stereoisomers.[
22]
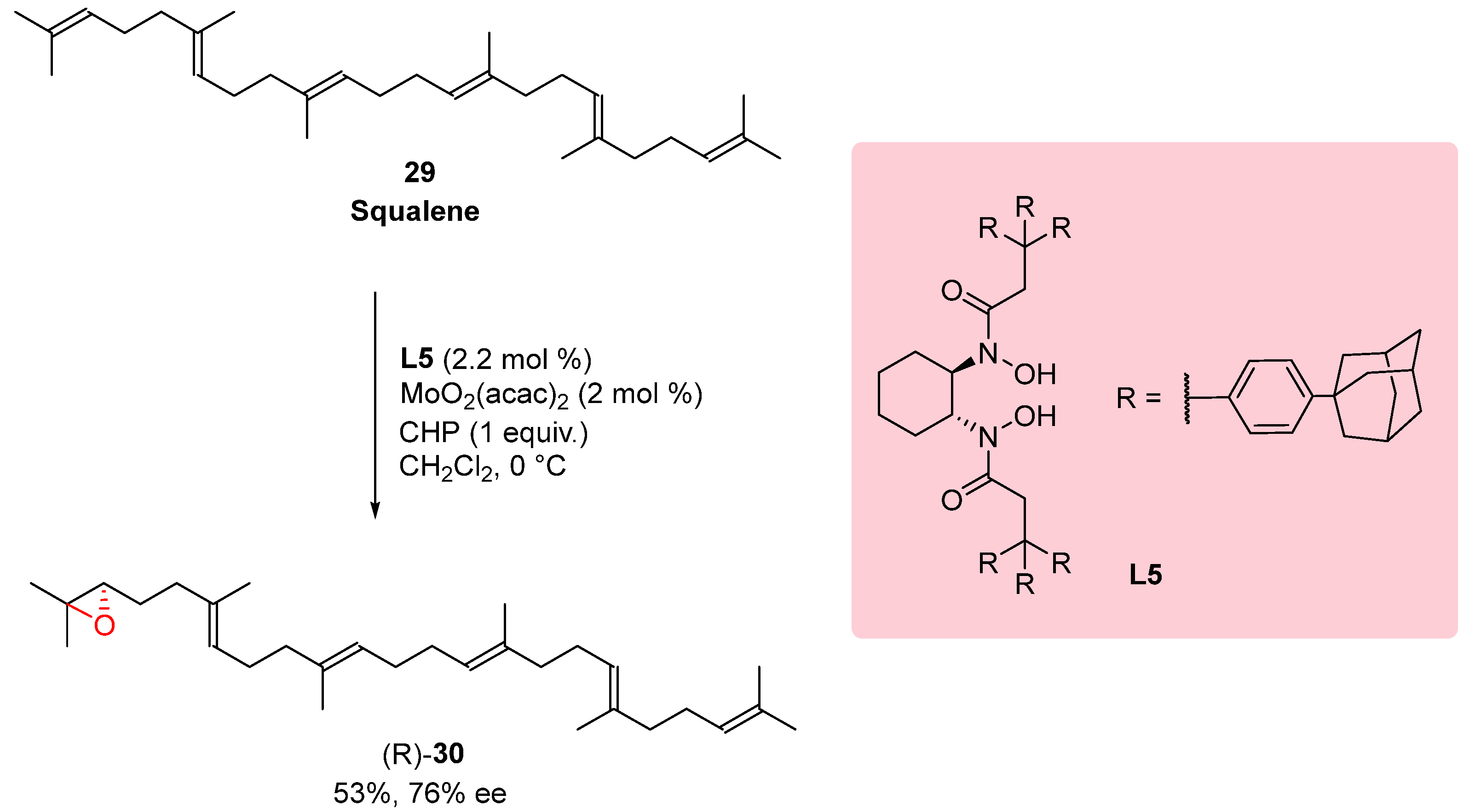
3.2. Epoxidation of Squalene
In the synthetic endeavor depicted in
Scheme 8, squalene
29, a fundamental biogenetic precursor for the synthesis of steroids and polycyclic terpenoids, underwent a similar reaction under specific conditions. This experiment resulted in the formation of 2,3-epoxysqualene
30 with a notable enantioselectivity of 76%. Squalene, a triterpene hydrocarbon, serves as a vital intermediate in the biosynthesis of various biologically active molecules. To introduce an epoxide group at the desired position of the squalene molecule, the researchers employed reaction conditions specifically tailored for this transformation. Through the utilization of these optimized reaction conditions, the desired 2,3-epoxysqualene
30 was obtained with a significant enantioselectivity of 76%. The enhanced enantioselectivity indicates the successful application of the chosen ligand or catalyst system in controlling the stereochemistry of the reaction. This achievement demonstrates the potential of utilizing tailored ligands or catalysts, such as the one employed in this study, to achieve desired stereochemical outcomes in the synthesis of complex natural products and biogenetic precursors. By fine-tuning reaction conditions and leveraging appropriate catalyst systems, researchers can achieve improved enantioselectivity, opening avenues for the efficient synthesis of diverse and biologically significant molecules.[
23]
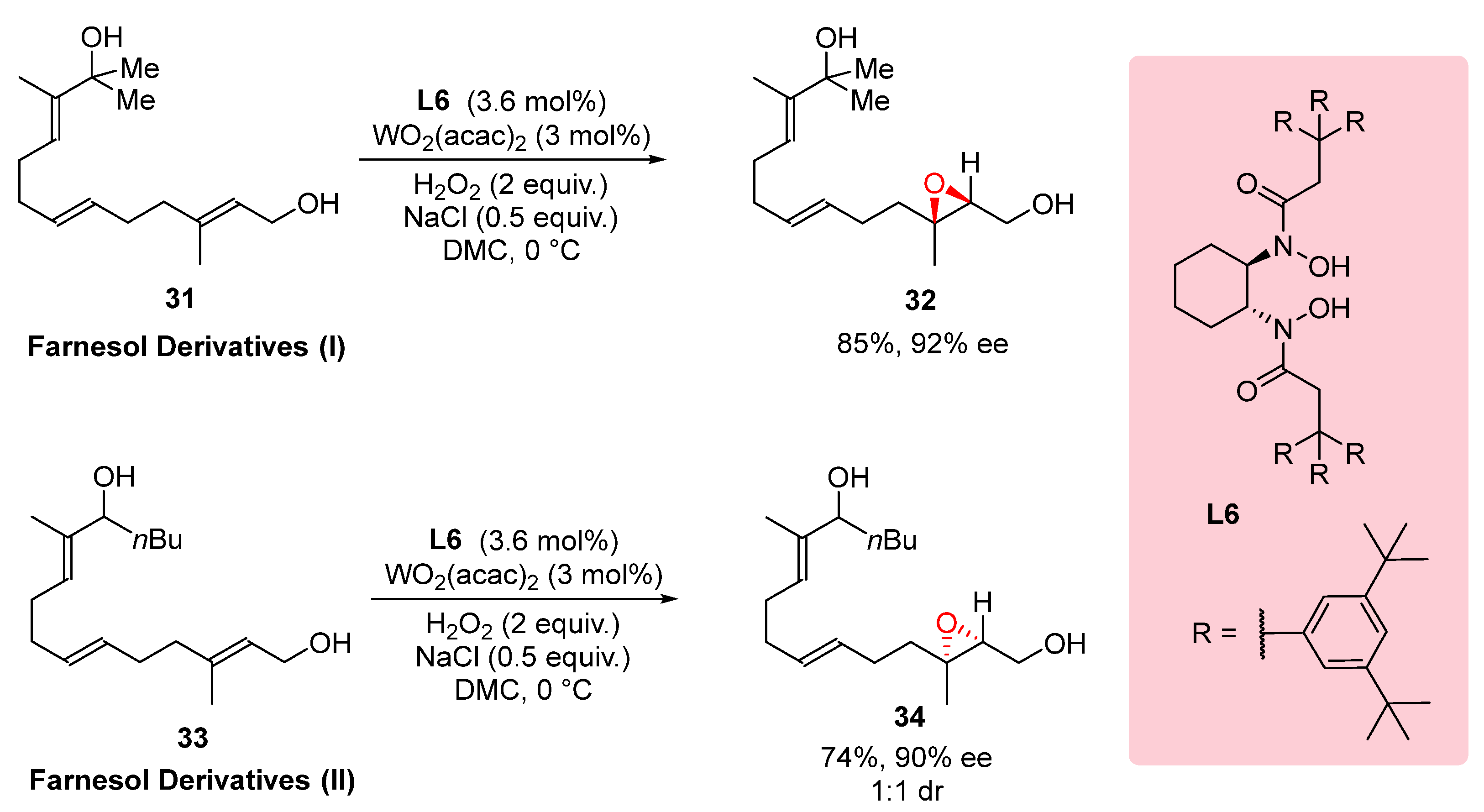
3.3. Epoxidation of Farnesol Derivatives
The regioselective synthesis of compounds bearing multiple olefin and alcohol moieties is a challenging task in organic chemistry. In the study conducted by Wang et al. (2014), the potential of two farnesol derivatives,
31 and
33, as precursors for such compounds was investigated using a catalyst system. By employing this catalyst system, the desired products were obtained with remarkable regioselectivity. The synthetic strategy involved the selective functionalization of specific positions on the farnesol derivatives, leading to the formation of compounds with multiple olefin and alcohol moieties. The high regioselectivity achieved in this transformation highlights the efficiency and selectivity of the catalyst system, offering new opportunities for the synthesis of complex molecules with diverse functionalities. The results presented in
Scheme 9 demonstrate the successful application of this methodology in the regioselective synthesis of compounds bearing three olefins and two alcohol moieties, providing valuable building blocks for further exploration in organic synthesis and the development of novel materials and bioactive compounds.[
24]
4. Discussion
The utilization of bishydroxamic acid (BHA) ligands in the total synthesis of natural products holds great promise for the future development of efficient synthetic methodologies. As researchers continue to explore and optimize the use of BHA ligands, several future perspectives and challenges can be identified.
Firstly, the design and development of new BHA ligands with enhanced catalytic properties and broader substrate scope are areas of ongoing research. The exploration of ligand modifications, such as structural variations and the introduction of different functional groups, can provide valuable insights into the factors that influence reactivity and selectivity. These advancements will contribute to the expansion of the synthetic toolbox and enable the synthesis of diverse natural product scaffolds.
Furthermore, the investigation of alternative metal catalysts in conjunction with BHA ligands represents an exciting direction for future research. While the current focus has been primarily on vanadium-based catalysts, the exploration of other transition metals could offer new opportunities for controlling stereochemistry and achieving unique reactivity profiles. The development of more sustainable and readily available metal catalysts will contribute to the practicality and scalability of BHA-mediated transformations.
In addition to ligand and catalyst development, addressing scalability and practicality challenges is crucial for the wider adoption of BHA ligands in industrial settings. Efforts should be directed toward developing scalable and cost-effective synthetic methodologies that are amenable to large-scale production. This requires optimization of reaction conditions, catalyst loading, and purification strategies to ensure high yields and product purity.
Lastly, the integration of BHA ligands in multistep synthesis and their application in complex natural product total syntheses represent important future directions. The development of streamlined and efficient synthetic routes will allow for the synthesis of intricate natural product structures with high stereochemical control. The combination of BHA ligands with other powerful synthetic tools, such as organocatalysis and transition metal catalysis, holds great potential for unlocking new synthetic strategies and expanding the boundaries of chemical synthesis.
The future of BHA ligands in the total synthesis of natural products is promising, with opportunities for ligand design, exploration of alternative metal catalysts, scalability optimization, and integration into multistep syntheses. Addressing these challenges will contribute to the advancement of synthetic methodologies and enable the efficient and sustainable synthesis of complex natural products with important biological activities.
5. Conclusions
In conclusion, the remarkable contributions of chiral hydroxamic acid ligands in the asymmetric synthesis of natural products have unveiled their transformative potential in organic chemistry. These ligands have revolutionized the field by providing a powerful tool for controlling stereochemistry and achieving high enantioselectivity. Through various case studies, it is evident that chiral hydroxamic acid ligands play a pivotal role in catalyzing key reactions such as epoxidation, oxidation, and other transformations crucial for natural product synthesis. Their unique properties, including metal chelation and stereocontrol abilities, have enabled improved yields, enhanced enantioselectivity, and streamlined synthetic routes. The versatility of these ligands has been observed in the synthesis of diverse natural product scaffolds, contributing to drug discovery and development. Ongoing research and development in this area promise even greater advancements and exciting discoveries facilitated by these exceptional ligands.
Acknowledgments
The authors acknowledge the financial support provided by CONAHCyT for the budget allocated to the Instituto de Ecología, A.C. The postdoctoral fellowship of TJP (grant number I1200/320/2022-MOD.ORD./09/2022) and the postgraduate scholarship of KIVH were also funded by CONAHCyT. We are grateful for their support in conducting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baran, S.P. Natural Product Total Synthesis: As Exciting as Ever and Here to Stay. J. Am. Chem. Soc. 2018, 140, 4751–4755. [Google Scholar] [CrossRef]
- Su, B.; Hartwig, J.F. Development of Chiral Ligands for the Transition-Metal-Catalyzed Enantioselective Silylation and Borylation of C−H Bonds. Angew. Chem. Int. Ed. 2021, 61, e202113343. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, R.C.; Palermo, R.E.; Sharpless, K.B. Chiral hydroxamic acids as ligands in the vanadium catalyzed asymmetric epoxidation of allylic alcohols by tert-butyl hydroperoxide. J. Am. Chem. Soc. 1977, 99, 1990–1992. [Google Scholar] [CrossRef]
- Li, Z.; Yamamoto, H. Hydroxamic Acids in Asymmetric Synthesis. Acc. Chem. Res. 2012, 46, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Pawar, T.J.; Bonilla-Landa, I.; Reyes-Luna, A.; Barrera-Méndez, F.; Enríquez-Medrano, F.J.; Díaz-de-León-Gómez, R.E.; Olivares-Romero, J.L. Chiral Hydroxamic Acid Ligands in Asymmetric Synthesis: The Evolution of Metal-Catalyzed Oxidation Reactions. ChemistrySelect 2023, 8, e202300555. [Google Scholar] [CrossRef]
- Sawano, T.; Yamamoto, H. Substrate-Directed Catalytic Selective Chemical Reactions. J. Org. Chem. 2018, 83, 4889–4904. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Yamamoto, H. Substrate Directed Asymmetric Reactions. Chem Rev. 2018, 118, 3391–3446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Basak, A.; Kosugi, Y.; Hoshino, Y.; Yamamoto, H. Enantioselective Epoxidation of Allylic Alcohols by a Chiral Complex of Vanadium: An Effective Controller System and a Rational Mechanistic Model. Angew. Chem. Int. Ed. 2005, 44, 4389–4391. [Google Scholar] [CrossRef]
- Whitesell, J.K. C2 symmetry and asymmetric induction. Chem. Rev. 1989, 8, 1581–1590. [Google Scholar] [CrossRef]
- Makita, N.; Hoshino, Y.; Yamamoto, H. Asymmetric Epoxidation of Homoallylic Alcohols and Application in a Concise Total Synthesis of (−)-α-Bisabolol and (−)-8-epi-α-Bisabolol. Angew. Chem. Int. Ed. 2003, 2003 42, 941–943. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Z.-H.; Zhao, L.; Xiong, F.-J.; He, Q.-Q.; Chen, F.-E. An efficient enantioselective synthesis of florfenicol via a vanadium-catalyzed asymmetric epoxidation. Tetrahedron: Asymmetry 2011, 22, 1337–1341. [Google Scholar] [CrossRef]
- Papich, M.G. Florfenicol. In Saunders Handbook of Veterinary Drugs (Fourth Edition); 2016; pp. 327–329. [Google Scholar] [CrossRef]
- Matsubara, T.; Takahashi, K.; Ishihara, J.; Hatakeyama, S. Total synthesis of (-)-ophiodilactone A and (-)-ophiodilactone B. Angew. Chem. Int. Ed. Engl. 2014, 53, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, R.; Fujita, T.; Matsunaga, S. Ophiodilactones A and B, Cytotoxic Tetrameric Phenylpropanoids, from the Ophiuroid Ophiocoma scolopendrina. J. Org. Chem. 2009, 74, 4396–4399. [Google Scholar] [CrossRef] [PubMed]
- Fenneteau, J.; Vallerotto, S.; Ferrié, L.; Figadère, B. Liebeskind–Srogl cross-coupling on γ-carboxyl-γ-butyrolactone derivatives: application to the side chain of amphidinolides C and F. Tetrahedron Lett. 2015, 56, 3758–3761. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ishibashi, M.; Walchli, M.R.; Nakamura, H.; Hirata, Y.; Sasaki, T.; Ohizumi, Y. Amphidinolide C: the first twenty-five membered macrocyclic lactone with potent antineoplastic activity from the cultured dinoflagellate Amphidinium sp. J. Am. Chem. Soc. 1988, 110, 490–494. [Google Scholar] [CrossRef]
- Xu, J.; Caro-Diaz, E.J.E.; Trzoss, L.; Theodorakis, E.A. Nature-Inspired Total Synthesis of (−)-Fusarisetin A. J Am Chem Soc 2012, 134, 5072–5075. [Google Scholar] [CrossRef]
- Kohyama, A.; Kanoh, N.; Kwon, E.; Iwabuchi, Y. An enantiocontrolled entry to the tricyclic polar segment of (+)-fusarisetin. Tetrahedron Letters 2016, 57, 517–519; [Google Scholar] [CrossRef]
- Morin, C.; Fortin, S.; Rousseau, E. 19,20-EpDPE, a bioactive CYP450 metabolite of DHA monoacyglyceride, decreases Ca2+ sensitivity in human pulmonary arteries. Am. J. Physiol. Heart. Circ. 2011, 301, H1311–H1318. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Lee, K.S.S. Asymmetric Total Synthesis of 19,20-Epoxydocosapentaenoic Acid, a Bioactive Metabolite of Docosahexaenoic Acid. J. Org. Chem. 2019, 84, 15362–15372. [Google Scholar] [CrossRef]
- Boudreault, P.L.; Mattler, J.K.; Wender, P.A. Studies on the regio- and diastereo-selective epoxidation of daphnanes and tiglianes. Tetrahedron Lett. 2015, 56, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Barlan, A.U.; Basak, A.; Yamamoto, H. Enantioselective Oxidation of Olefins Catalyzed by a Chiral Bishydroxamic Acid Complex of Molybdenum. Angew. Chem. Int. Ed. 2006, 45, 5849–5852. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamamoto, H. Tungsten-Catalyzed Asymmetric Epoxidation of Allylic and Homoallylic Alcohols with Hydrogen Peroxide. J. Am. Chem. Soc. 2014, 136, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).