1. Introduction
Surgical navigation can be considered as the evolution of stereotaxic surgery. One of the primary limitations of the earlier procedure was the lack of consideration for individual patient variability and the presence of pathological tissues [
1]. Only with the advent of CT and MRI technologies since the 1980s has it become possible to provide more precise and patient-specific data [
2].
The introduction and implementation of modern techniques, such as software systems for planning, rapid prototyping, intraoperative visualization and surgical navigation have revolutionized the preoperative approach to surgical procedures in maxillofacial surgery [
3,
4,
5].
Surgical navigation was first introduced in oral and maxillofacial surgery in the early 1990s, gradually assuming a more prominent role in medical practice within maxillofacial surgery units. This procedure’s advantages are particularly significant in areas associated with challenging and limited exposure. The three-dimensional complexity of facial anatomy needs accurate three-dimensional reconstruction to ensure functional recovery, favorable aesthetic outcomes, and a reduction in the need for repeated procedures [
6].
Navigation technology now plays an increasingly crucial role in oral and maxillofacial surgery. Presently, surgical navigation in maxillofacial surgery is predominantly employed in traumatology but also in procedures such as tumor resection and reconstruction, craniomaxillofacial malformation correction, implantology, orthognathic surgery, TMJ arthroplasty, and the removal of supernumerary teeth or foreign bodies [
3,
7,
8,
9].
Surgery navigation is particularly valuable in post-traumatic orbital wall reconstruction, representing the primary field of application in maxillofacial surgery. Novelli et al. (2014) emphasized the benefits of their protocol in the pre-surgical phase, enabling more precise diagnosis and better understanding of how defects can be reconstructed. Moreover, during surgery, it is helpful to verify whether the reconstruction matches with the pre-surgical planning, reducing operating time and standardizing the approach to orbital fracture treatment, thereby ensuring reproducibility [
4].
Surgical navigation is also a valuable technique for the accurate reconstruction of the facial mid-third, which is characterized by non-motile bones. However, its application in the jaw is limited by bone movements that reduce its accuracy [
10,
11,
12,
13]. Hirch et al. (2009) and Zhang et al. (2016) observed that real-time guidance offered by surgical navigation in mandibular reconstruction with vascularized fibula flap reduces the margin of error compared to free-hand techniques, decreases surgery duration, and demonstrates high accuracy and applicability [
14,
15,
16]. Sozzi et al. (2022) describe in their protocol how the positions of the screw holes for the reconstruction plate are pre-recorded on a 3D model of the patient’s jaw. Subsequently, before performing the resection, the screw holes are realized using a recorded drill, whose bit position can be tracked in real-time on CT images to ensure precise placement of the screws as per the planned location. Nevertheless, they noted that this method requires a substantial amount of pre-surgical setup time and a steep learning curve for the surgeon [
17].
The craniomaxillofacial area is characterized by numerous delicate vascular and nervous structures that must be preserved during surgery. The widespread use of piezoelectric tools has significantly advanced oral and maxillofacial surgery. In 2000, Vercellotti introduced piezoelectric surgery and developed an angulated, thin, and tapered cutting saw tip that is now widely used [
18]. Piezoelectric surgery offers several advantages, including the prevention or reduction of soft tissue injuries during osteotomies, minimal blood loss, less invasive approaches, and reduced postoperative pain [
19]. However, the use of a piezosurgical instrument requires a wide-open field for precise visualization of the instrument tip’s position. Bianchi et al. (2015) sought to combine the safety of piezosurgical instruments with the precise three-dimensional tip localization offered by surgical navigation, enabling not only sequential or indirect navigation but also direct and continuous navigation with a piezoelectric device [
20].
In our department we have 15 years of experience in surgical navigation and these findings strongly suggests us that the use of tracked tools in maxillofacial surgery has the potential for broader application.
According to the existing literature, there are limited publications exploring the possibilities of tracking new tools in oral and craniomaxillofacial surgery.
This study aims to showcase the clinical applications and indications of intraoperative tracking of various tools, such as drills, cutters, saws, chisel and piezoelectric instruments and to evaluate the accuracy of tool tracking in millimeters of error. Additionally, we will assess the advantages, disadvantages, potential errors, and complications associated with this surgical protocol.
2. Materials and Methods
2.1. Sample
The inclusion criteria were as follows: patients who underwent surgery at our center in the last 15 years, surgery assisted by the surgical navigation system, and the use of tracked tools during surgery. A total of 42 patients were included in the study, presenting the following pathologies: 1 case of facial dysmorphosis, 24 cases of oncologic surgery, 12 cases of traumatology and 5 cases of craniofacial malformations. The tracked instruments were the periosteal elevator, the mini-saw, the chisel, the drill and the piezoelectric device. The surgical treatment and the disease features are illustrated in
Table 1.
2.2. Surgical Navigation Protocol
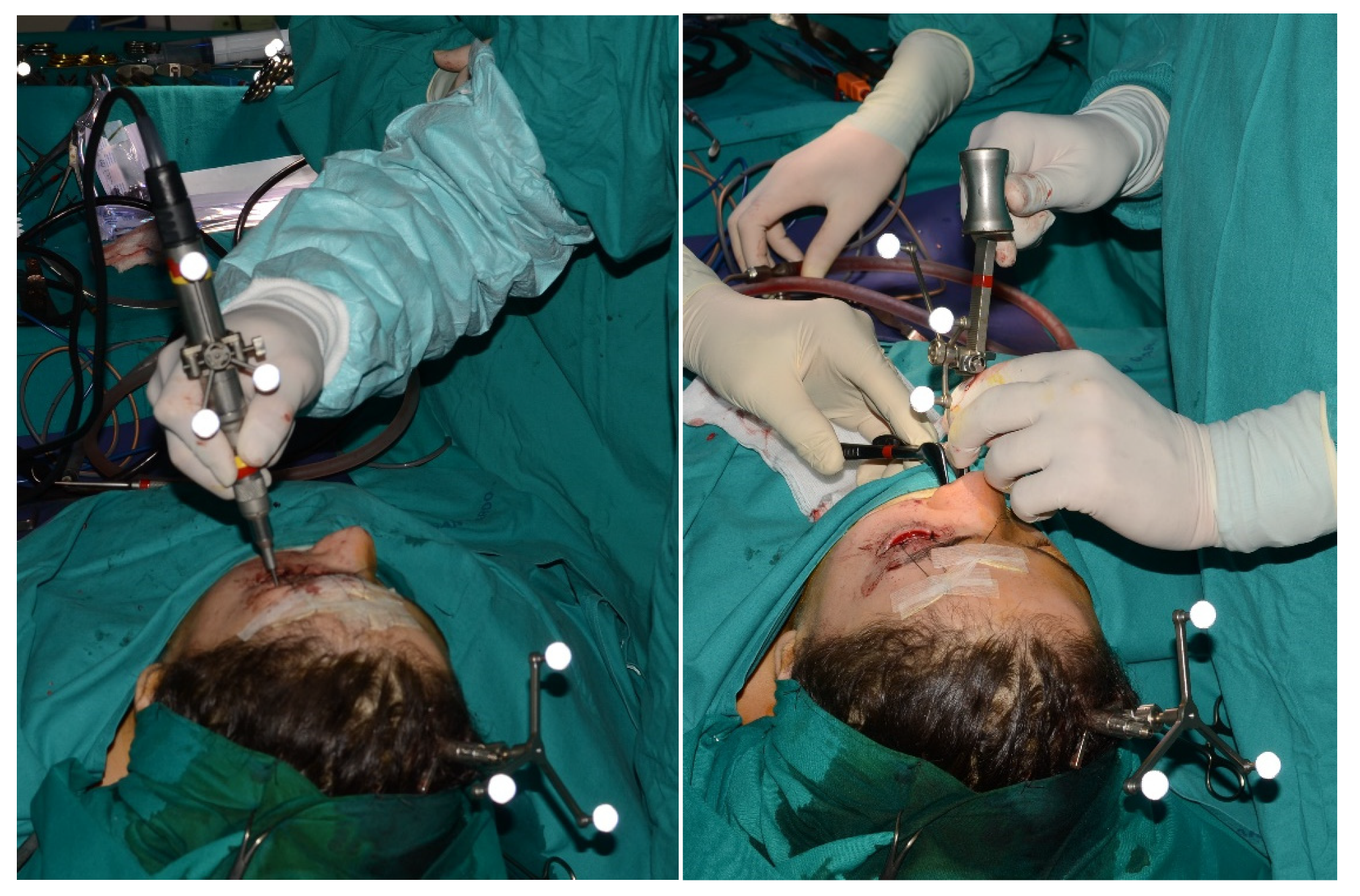
To perform the surgical navigation, an upper dental resin bite is realized. It is crucial that the bite fits one position only. At least 5 screws with different space vectors are then positioned on it, serving as fiducial markerpoints. In some cases, to enhance precision, an osseous screw is placed in the fronto-zygomatic region and it is removed after surgery. When mandibular navigation is required, since it is a mobile bone, a surgical bite that immobilizes the lower jaw through rigid intermaxillary fixation is then realized. This enables us to perform the procedure in the theatre in the same position as acquired during the preoperative CT scan. A high-definition CT scan, with a thickness of 0.6 mm, is carried out with the maxillary bite. Virtual planning is then performed using iPlan 3.0 software by Brainlab®. First, the fiducial markerpoints for navigator registration are identified. Subsequently, the resection of the lesion is designed, along with the tracing of any necessary osteotomies or the positioning points for anchoring osteosynthesis plates. Once these procedures are completed, the surgery can be performed.
In the operating room, the following steps are taken during the surgery. Firstly, the dynamic reference frame (DRF) is positioned for navigation in the contralateral parietal region. Next, the maxillary bite is positioned, and the previously marked points are registered. The accuracy was assessed in millimeters, and an error of less than 1.5 mm was deemed as acceptable. In order to track the tools, it was necessary to place the navigator’s reflective spheres on them.
In case of performing Virtual Surgical Simulation (VSS), a 3D model based on CT scan can be realized and used for pre-surgical navigation. A virtual planning is carried out using iPlan 3.0 software by Brainlab®, where well-defined anatomical markers such as the anterior nasal spine and the infraorbital foramen, among others, are positioned. Subsequently, navigated surgery is performed on the 3D model, after the placement of the DRF and the registration of the fiducial markepoints. Once the surgery is completed, the reference points for screw/plate placement or osteotomy tracing are stored in the system for later use in the operating room.
Surgical navigation workflow:
Maxillary bite realization
Definition of virtual fiducial markerpoints
-
Virtual Planning:
- (a)
Markerpoints identification
- (b)
Osteotomies tracing/ Resection margins definition/ Mapping of the position of osteosynthesis tools
Virtual Surgical Simulation (VSS)
-
Operating Room:
- (a)
Dynamic reference frame (DRF) placement
- (b)
Registration of Reference Points (error < 1.5 mm)
- (c)
Tracked tools Registration
We present 4 representative cases of our workflow and the possibilities of surgical navigation with tracked surgical tools.
3. Case 1: Right Coronoid Hyperplasia with mouth opening deficit
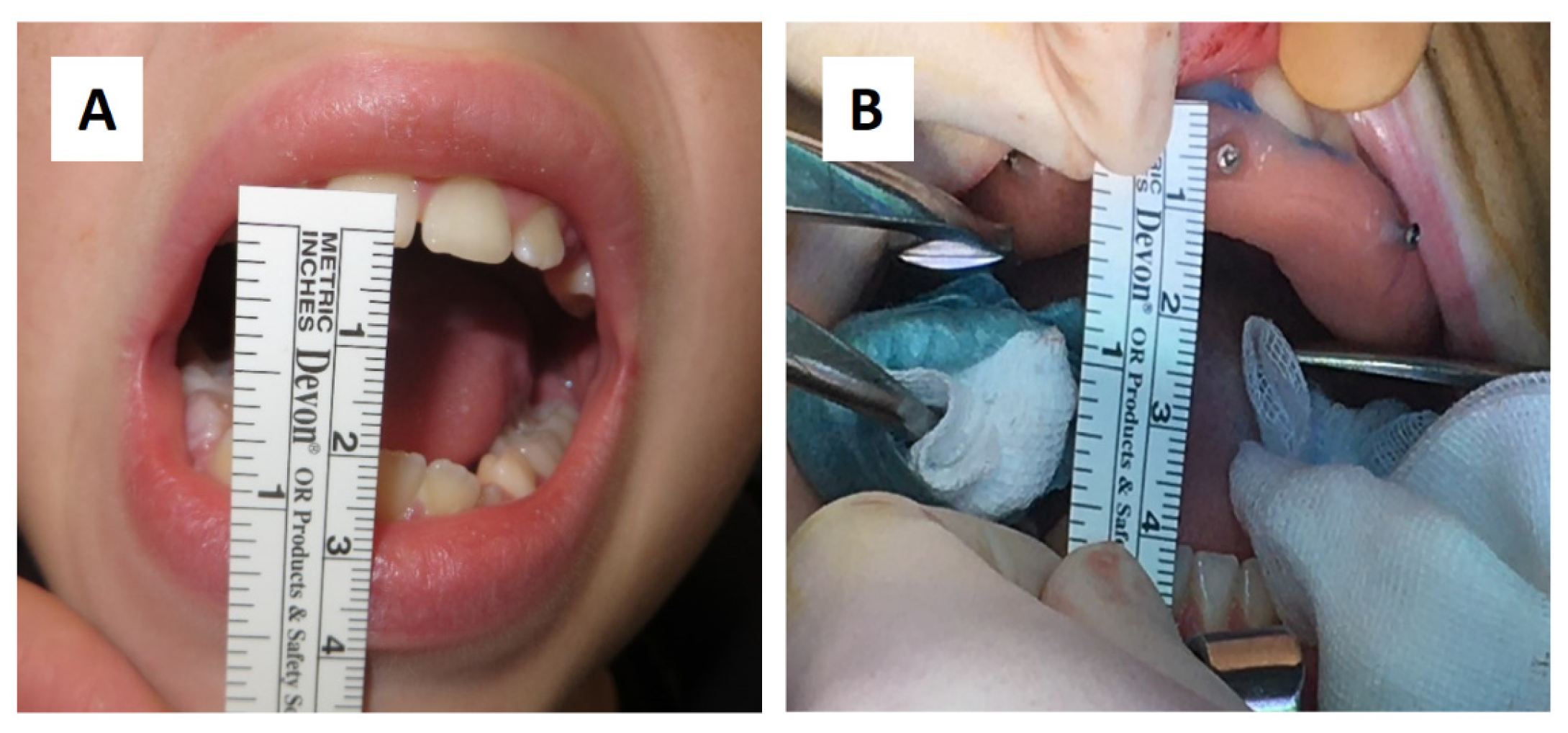
A sixteen-year-old male patient showed dento-facial asymmetry due to hypoplasia in the right mandibular region and hyperplasia of the right coronoid. The patient had previously undergone a coronoidectomy at another facility seven years before, which subsequently led to restricted mouth opening (17 mm) (
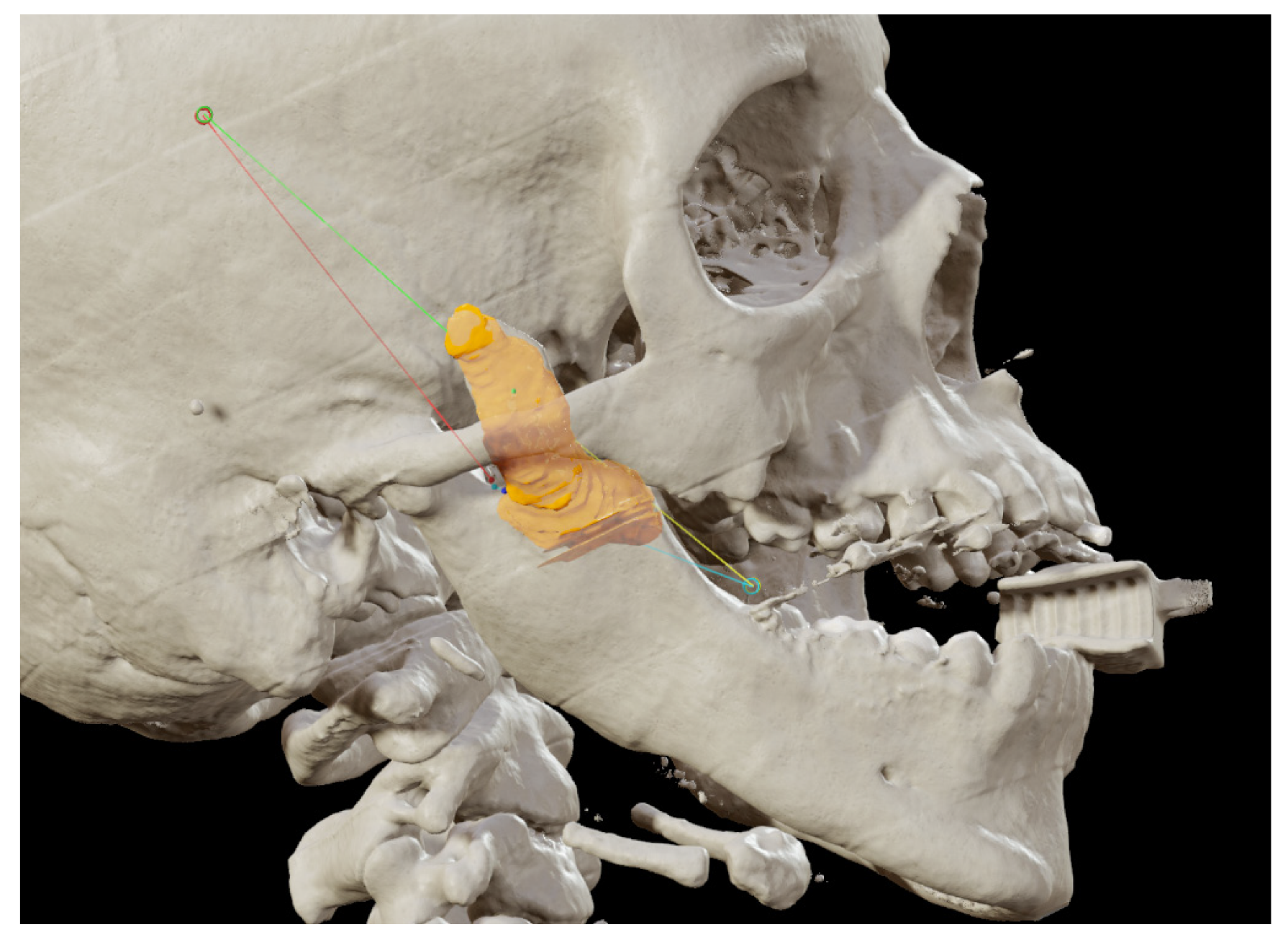
Figure 1A). A thick occlusal bite at maximal mouth opening has been realized, providing enough space for surgical procedure and maintaining the jaw in the same position during the preoperative CT acquisition and the surgical procedure. The surgery was meticulously planned by virtually tracing the coronoid ostectomy on a CT scan. The operation was performed under general anesthesia, utilizing the navigation system. The DRF system was positioned in the left parietal region, ensuring a navigation accuracy of < 0.5 mm. An incision was made from the right inferior vestibular fornix to the right superior vestibular fornix, allowing for visualization of the base of the coronoid process. Using surgical navigation, the sigmoid notch was precisely located, and the planned osteotomy (
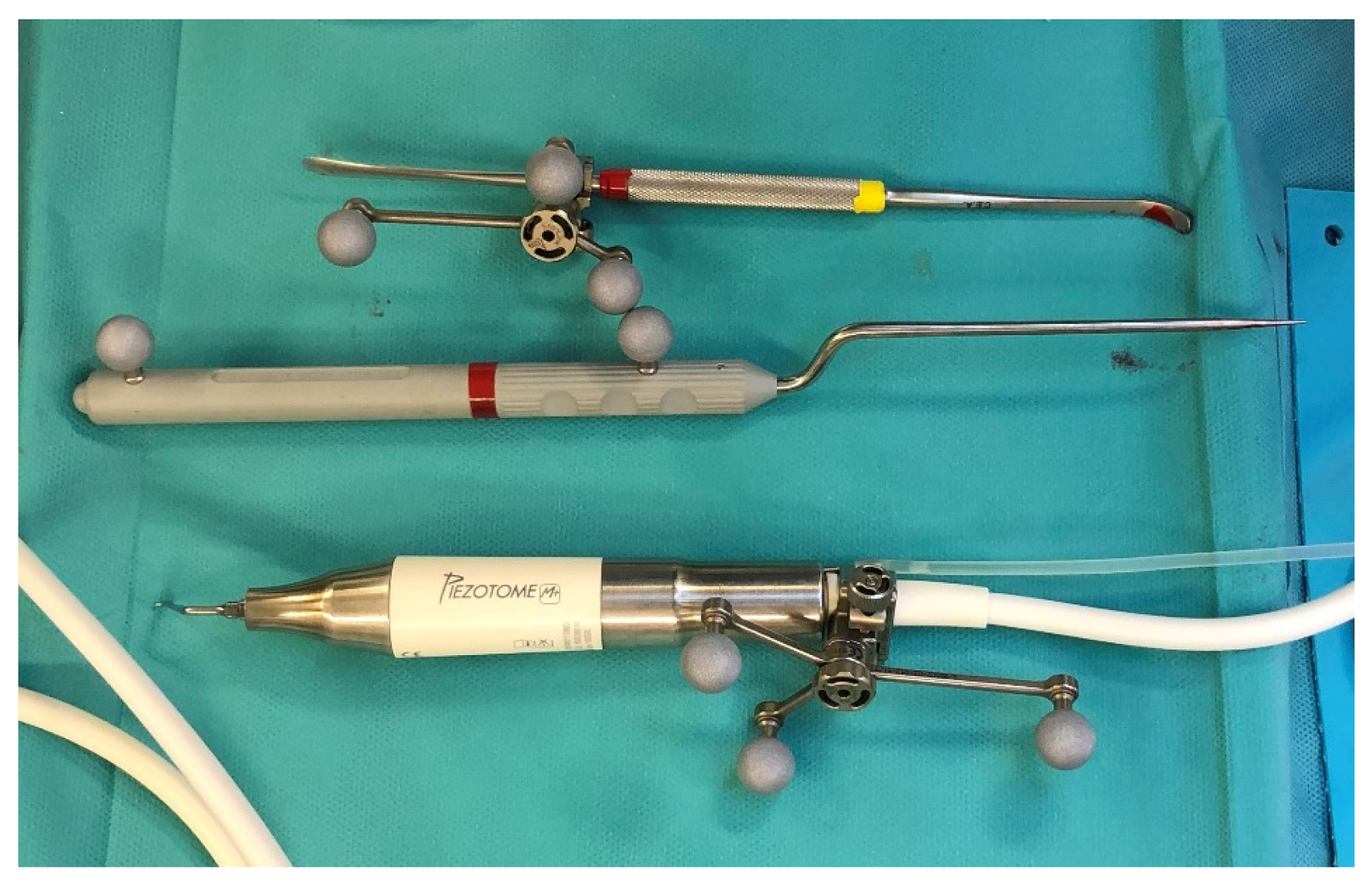
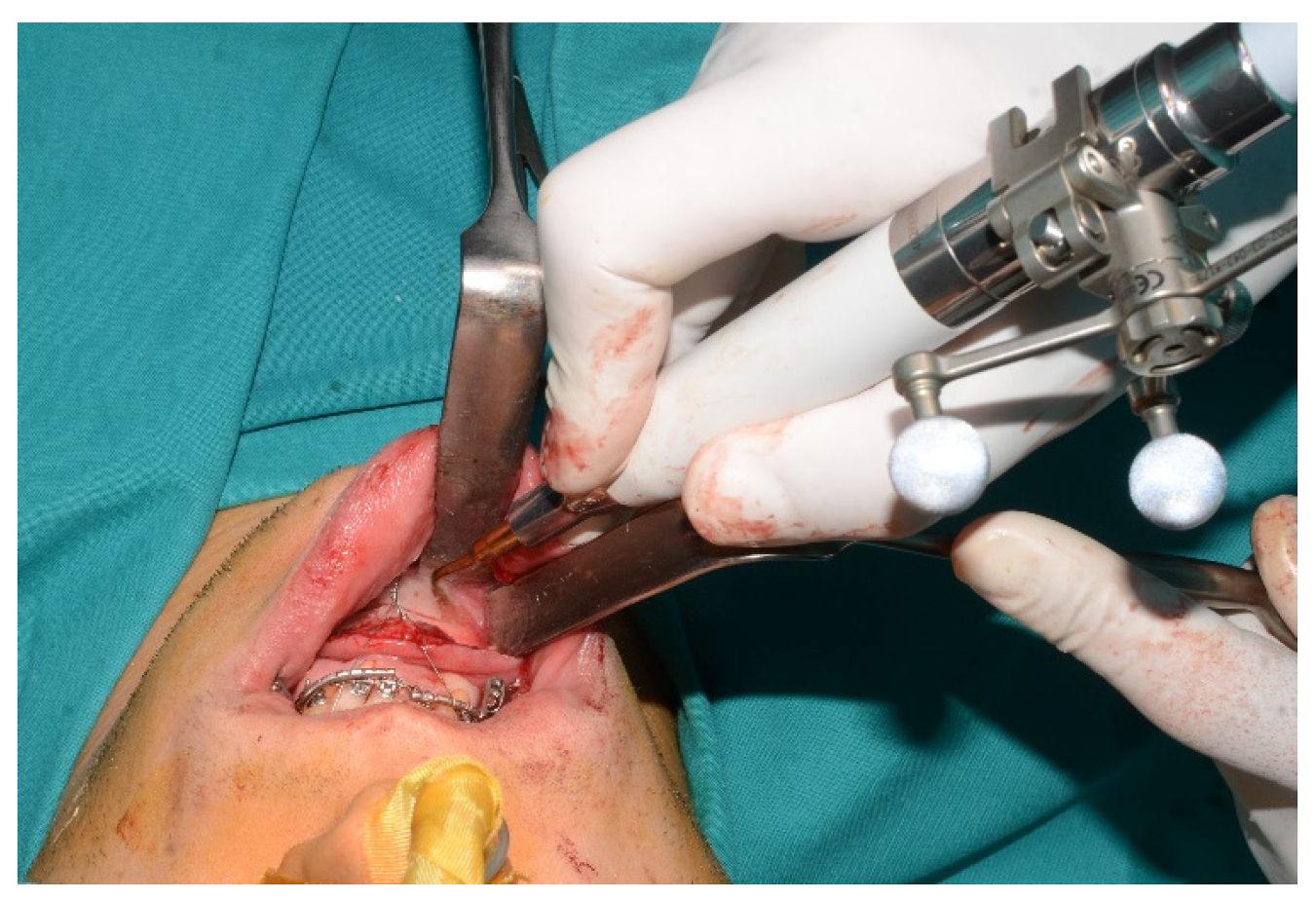
Figure 2) was performed real time tracking the position and the direction of periosteal elevator and piezoelectric tool (
Figure 3). The piezoelectric and the periosteal elevator were calibrated by attaching the tracking tool with three reflecting spheres to them handpiece, and the tip of the piezoelectric and the edge of the periosteal elevator were marked and utilized as a navigation reference point. Following the procedure, the oral cavity demonstrated a notable increase in opening (36 mm) (
Figure 1B).
4. Case 2: Nose-Orbital-Ethmoidal-Frontal ossifying fibroma Resection
The patient came to our attention at the age of 20 due to the persistence of a neoplasm in the left naso-ethmoid-frontal-orbital region. At the age of 7, she reported the development of a left nasal neoplasm, with a histological biopsy confirming a diagnosis of fibrous dysplasia. She had previously undergone multiple surgical procedures to remove the neoplasm in different facilities. A histological reevaluation of the histological slides was conducted, which yielded a diagnosis consistent with ossifying fibroma.
A preoperative CT scan was acquired, and with the assistance of iPlan 3.0 software by Brainlab, the resection margins of the neoplasm were prototyped (
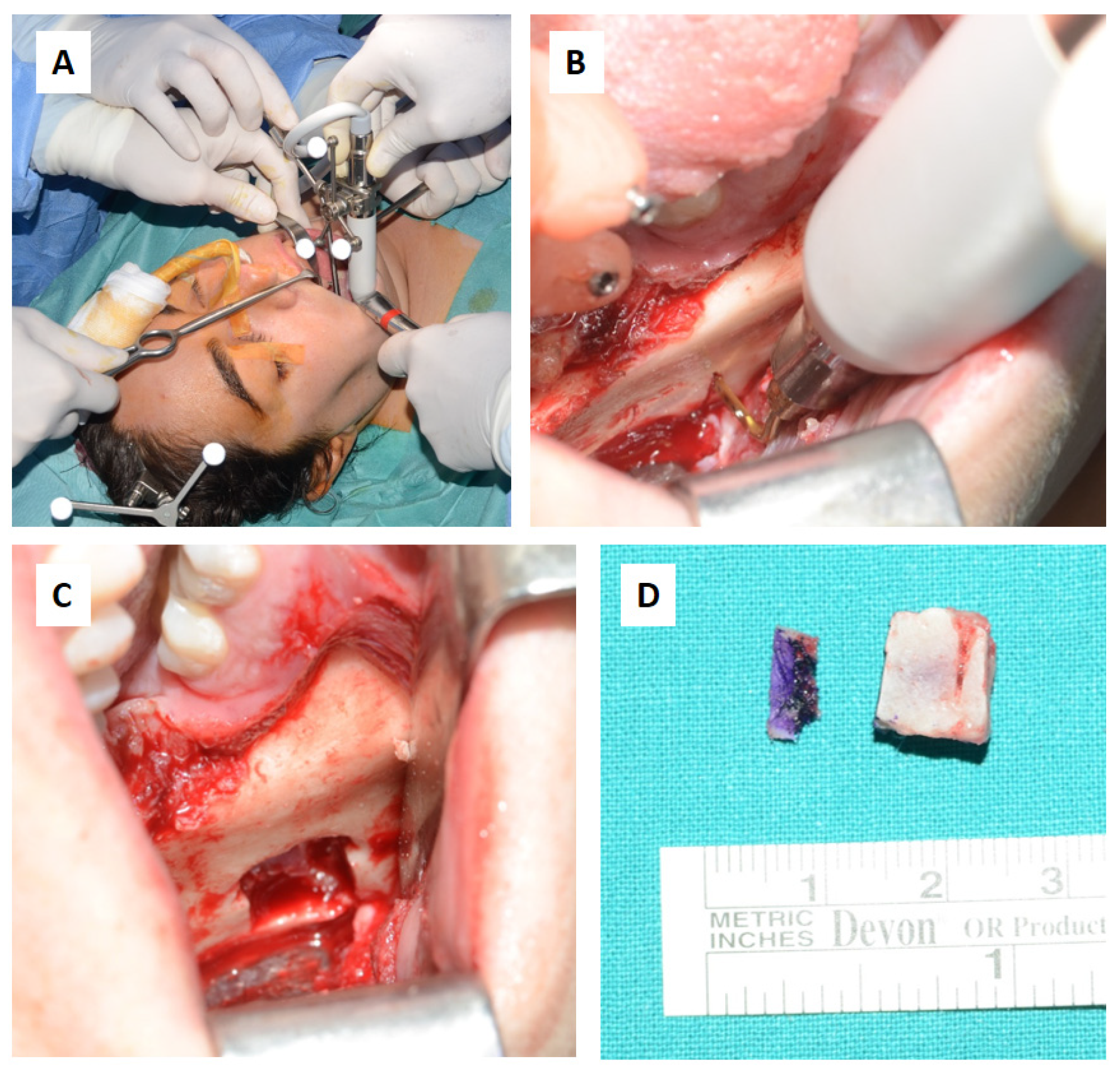
Figure 4). The operation was performed under general anesthesia, utilizing the navigation system. The DRF system was positioned in the right parietal region, ensuring a navigation accuracy of <0.5 mm. Surgical intervention was performed through a left transconjunctival retrocaruncular approach. The resection was performed using a “piecemeal” technique with tracked chisel and a mini-saw and the resection margins were monitored under the guidance of surgical navigation (
Figure 5). The instruments were registered by anchoring the three reflecting spheres tracking tool to the handpiece of tool and the edge of the chisel and the tip of the mini-sae was marked and used as a reference for navigation. The reconstruction of the medial wall and floor of the left orbit was made with a pre-molded titanium mesh.
A nine-year long-term follow-up has demonstrated excellent aesthetic results, preservation of extrinsic eye movements, and no neoplasm recurrence.
5. Case 3: Subapical (Köle) Osteotomy in Dentofacial Deformity
A 23-year-old patient presented with a class III dentoskeletal malocclusion, as well as transverse and sagittal hypoplasia of the upper maxilla. There was also an anterior mandibular incisal inclination that could not be orthodontically treated. Consequently, the following treatment plan was devised:
First step: SARPE (Surgical Assisted Rapid Palatal Expansion) and subapical (Köle) mandibular osteotomy followed by subsequent distraction osteogenesis.
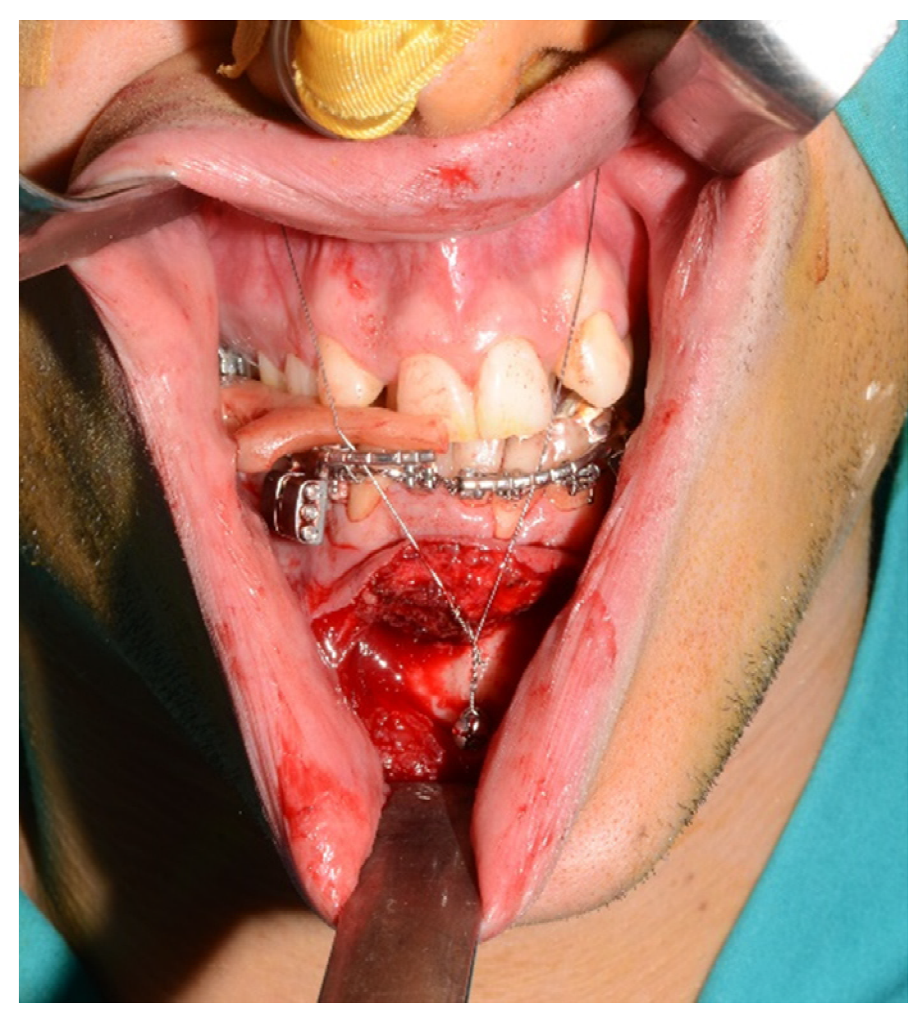
Second step: LeFort I maxillary osteotomy segmented into two pieces.
For the subapical osteotomy, it was decided to use the tracked tool technique with a piezoelectric scalpel to perform the procedure and avoid the risk of dental root injury, thereby enhancing surgical precision. A maxillary bite has been realized, and 5 screws with different space vectors were then placed on it, serving as fiducial marker points. The bite was used to maintain the mandible in the same position during the preoperative acquisition of CT scan and during the entire surgical procedure. Virtual planning was realized, with careful consideration of dental root preservation in the osteotomy tracing. During the surgical procedure, the Dynamic Reference Frame (DRF) was positioned in the left parietal bone, and the screws position on the bite were acquired as marker points. The same bite used during the CT image acquisition was utilized. Two screws were fixed on the maxillary bone, and one in the mandibular symphyseal region, and an intermaxillary block was set up to reproduce the position of the mandible as faithfully as possible during the CT image acquisition (
Figure 5).
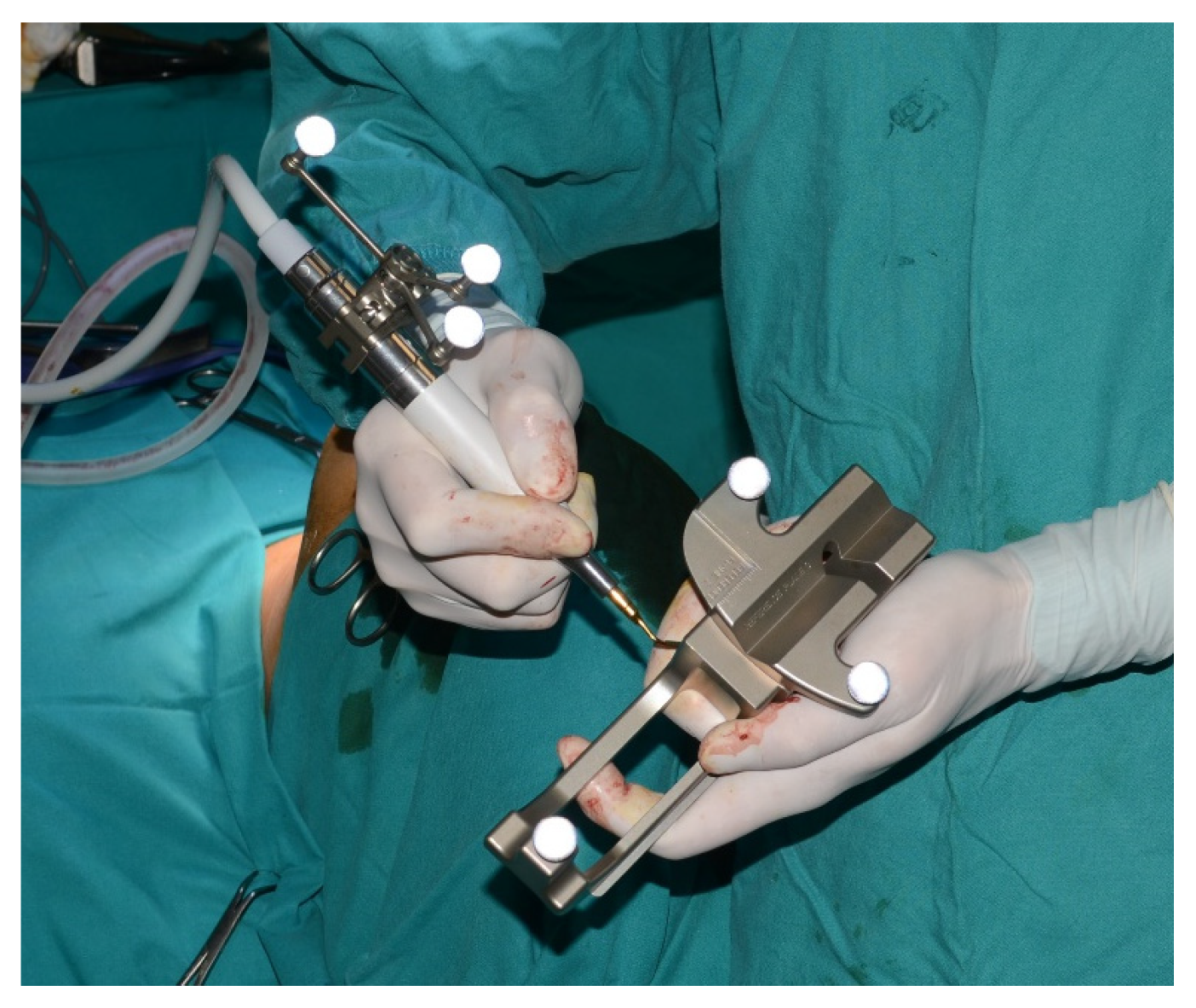
The piezoelectric was registered by anchoring the three reflecting spheres tracking tool to the handpiece of tool and the tip of the piezoelectric was marked and used as a reference for navigation (
Figure 6).
The navigation accuracy was less than 1 mm. The subapical osteotomy was performed as previously planned (
Figure 7 and
Figure 8).
One week after the surgery, an OPT (Orthopantomography) X-ray was performed, which showed the adequacy of the osteotomy and the preservation of dental roots (
Figure 9).
Three years later, the second surgical step was carried out.
6. Case 4: Removal of Recurrent Ameloblastoma at the Mandibular Angle
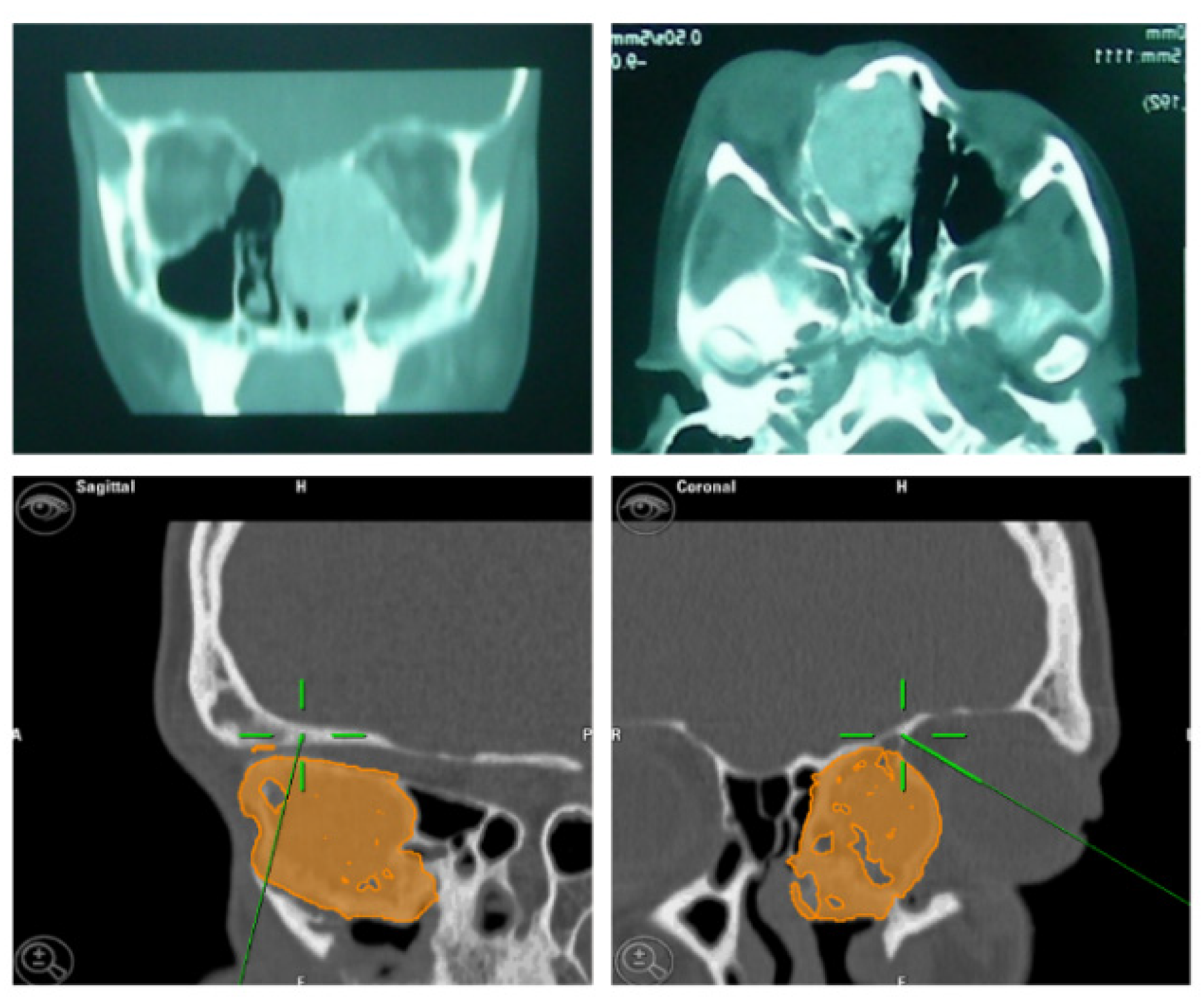
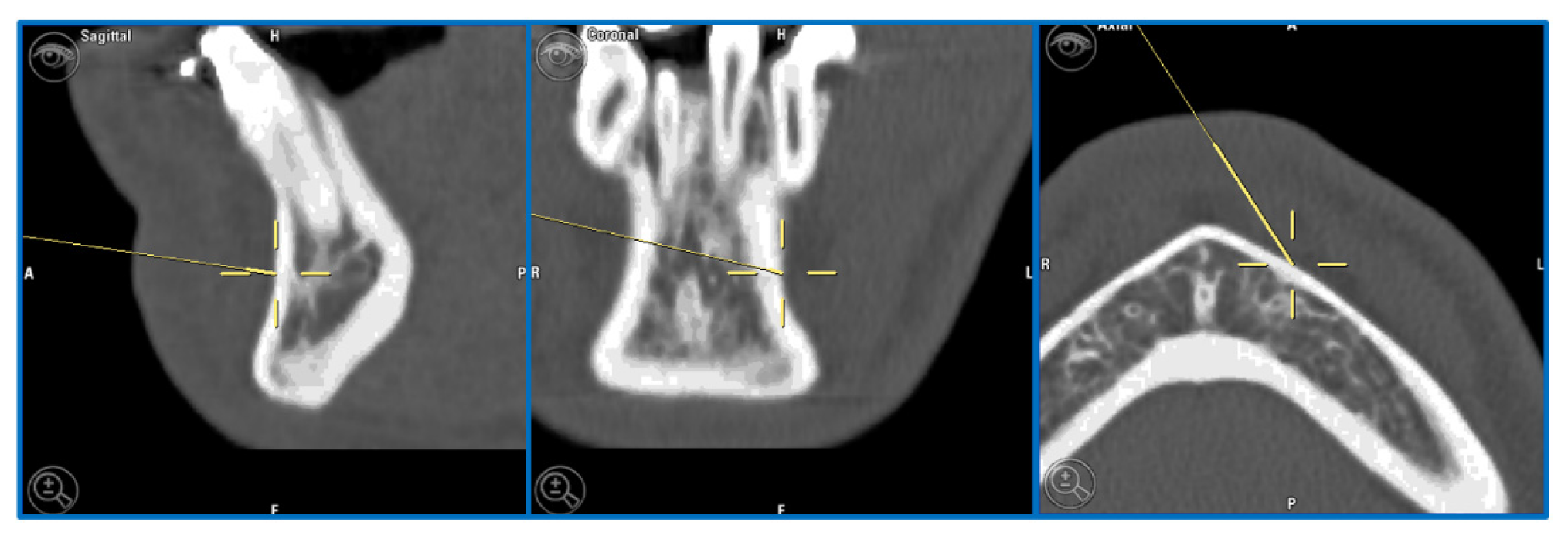
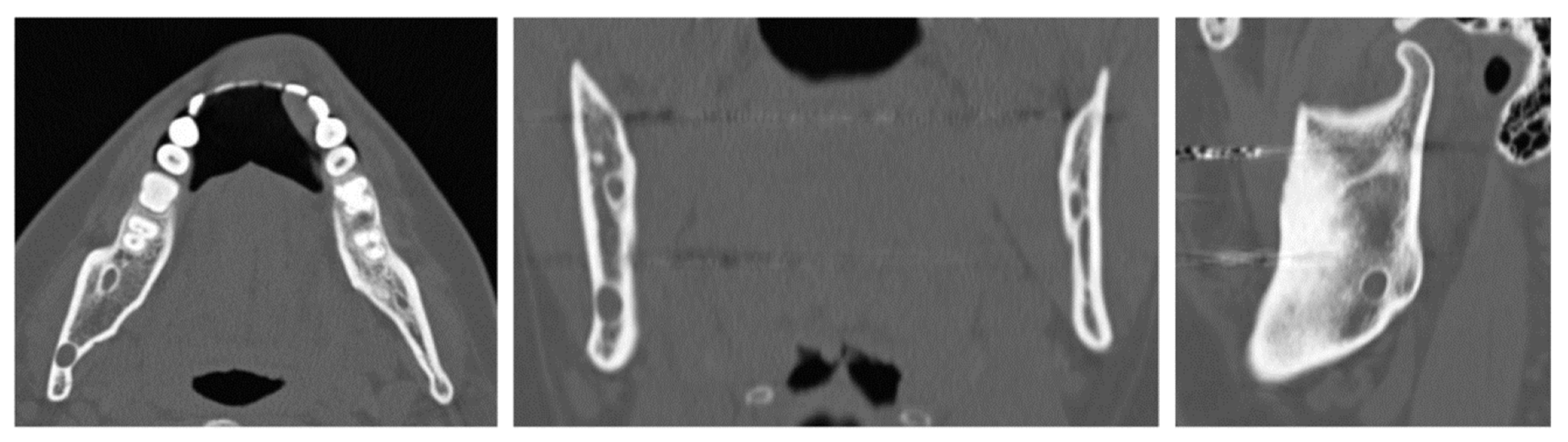
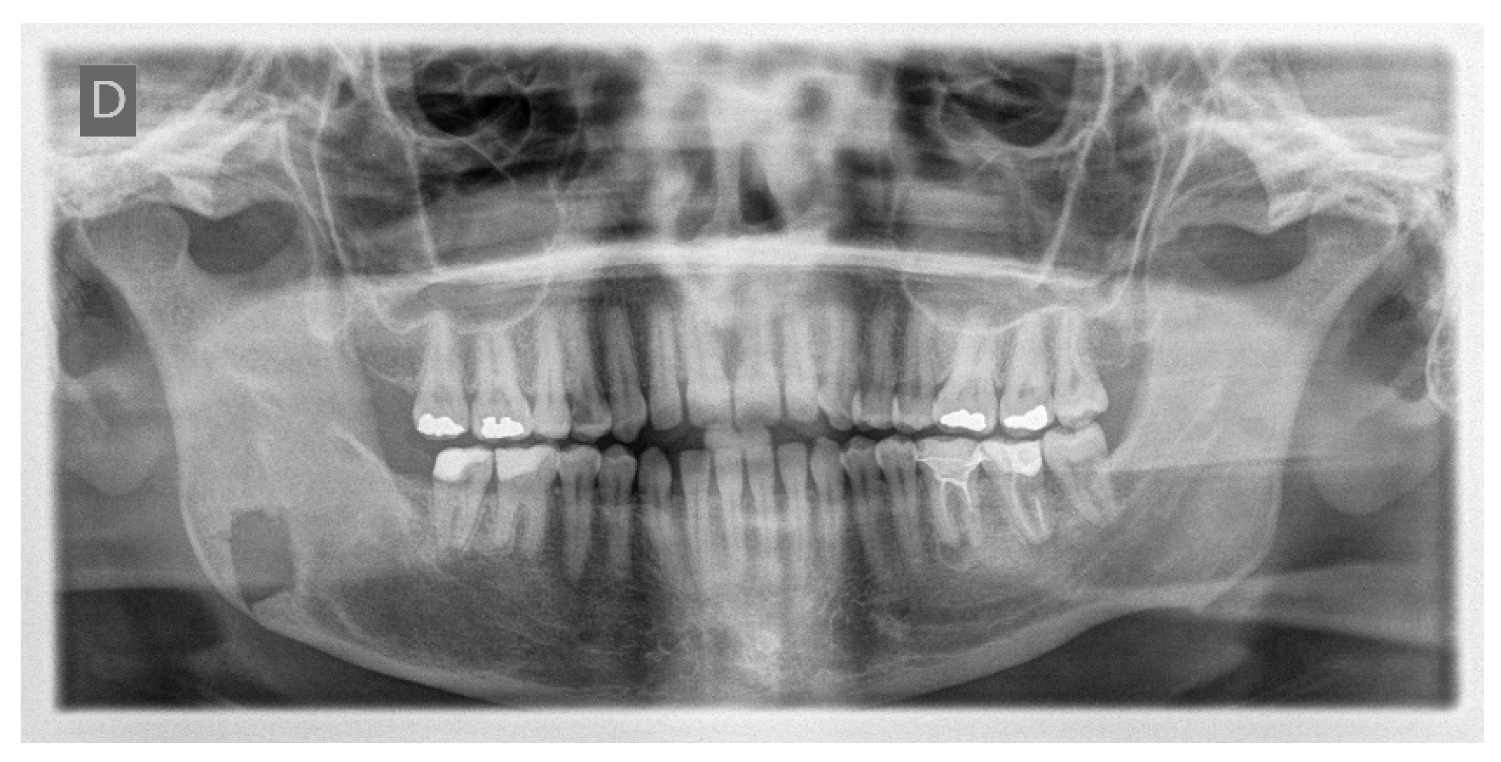
A 32-year-old patient came to our attention during an oncological follow-up, which included the execution of an OPT (Orthopantomography) X-ray, revealing a new osteolytic lesion at the right mandibular angle. In 1996, the patient underwent surgical removal of a unicystic ameloblastoma at another facility. We reviewed the patient’s most recent radiological documentation, which was an OPT X-ray taken 12 years earlier (8 years after the initial surgery) and showed no mandibular lesions. A mandibular CT scan was requested to better define the lesion (
Figure 10).
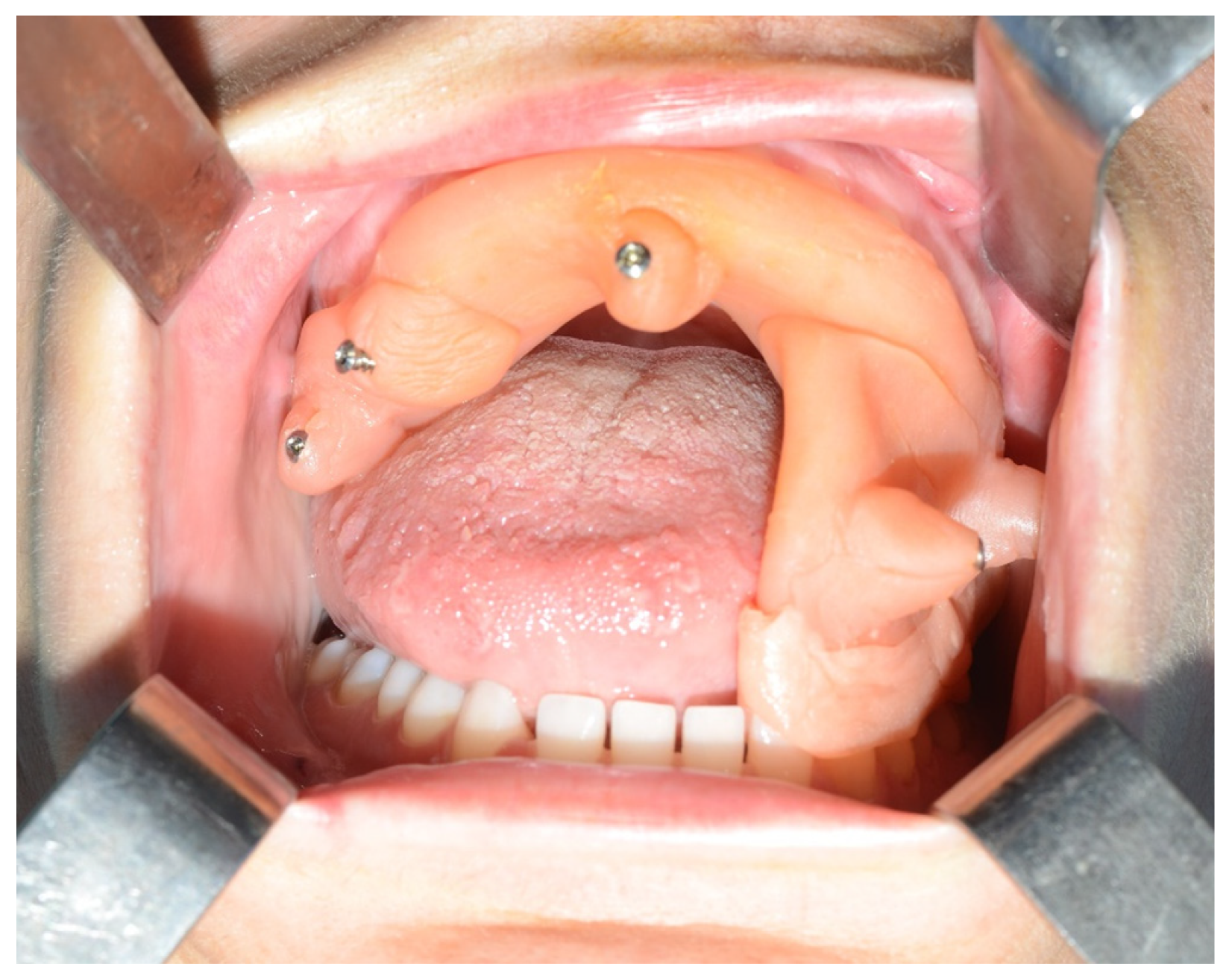
Given the suspicion of an ameloblastoma recurrence, surgical removal was recommended, and the decision was made to employ the technique of tracked instruments with surgical navigation. A maxillary bite was created, and 5 screws with varying spatial orientations were subsequently affixed to it, functioning as fiducial markers. This bite was employed to maintain the mandible in a consistent position both during the preoperative CT scan acquisition and throughout the entire surgical procedure (
Figure 11).
During the surgical procedure, the Dynamic Reference Frame (DRF) was positioned in the right parietal bone, and the positions of the screws on the bite were recorded as marker points. The same bite that was used during the CT image acquisition was utilized for this purpose. The navigation accuracy was less than 0.5 mm. The piezoelectric was registered by attaching a tracking tool with three reflecting spheres to the handpiece, and the tip of the piezoelectric was marked and utilized as a reference point for navigation. The navigation accuracy was found to be less than 1 mm.
Under the guidance of the tracked piezoelectric device, the resection of the lesion was carried out as per the preoperative plan, with macroscopically healthy bone margins preserved, including the lower and posterior mandibular border (
Figure 12).
The day following the surgery, a follow-up OPT X-ray was requested, which displayed the resection area and the integrity of the lower and upper borders of the mandible, in accordance with the preoperative virtual planning (
Figure 13).
Histological examination of the surgical resection confirmed the diagnosis of a recurrent unicystic ameloblastoma.
As of today, the patient undergoes annual visits in our Departement with OPT X-rays, with a follow-up duration of 9 years and no new recurrence detected.
7. Results
All the procedures were successfully performed, with no reported complications among the patients. Navigation accuracy measured was: <0.5 mm in 40.47% (17) of cases, <1 mm in 45.23% (19) of cases and <1.5 mm in 14.28% (6) of cases. The recorded mean accuracy was < 0.87 mm, with a standard deviation of 0.5 mm. Among all procedures performed with tracked instruments, the most frequently registered instrument was the drill (31 times), followed by the piezoelectric device (7 times), the periosteal elevator (6 times), the mini-saw (2 times), and then the chisel (1 time). In oncology cases, the recorded accuracy was < 0.5 mm in 9 instances, < 1 mm in 11 instances, and < 1.5 mm in 4 instances, with a mean accuracy of > 0.89 mm and a standard deviation of 0.36 mm. In traumatology cases, the recorded accuracy was < 0.5 mm in 6 instances, < 1 mm in 5 instances, and < 1.5 mm in 1 instance, with a mean accuracy of < 0.79 mm and a standard deviation of 0.33 mm. The only procedure performed in the surgical treatment of dentofacial dysmorphism with tracked instruments had a recorded accuracy of < 1 mm. In craniofacial malformation cases, the recorded accuracy was < 0.5 mm in 2 instances, < 1 mm in 2 instances, and < 1.5 mm in 1 instance, with a mean accuracy of < 0.9 mm and a standard deviation of 0.42 mm.
The osteotomy tracing was performed while confirming anatomical localization, concurrently adhering to the pre-planned virtual trajectory. In oncological cases, resections were performed with a macroscopically clear margin thank to concurrent navigation. Reconstructions using the free fibula flap closely matched the preoperatively programmed plan. Tracked instruments during navigation implied a reduction in surgical time, the use of a less invasive surgical approach, and a decrease in the risk of complications. In the treatment of endo-orbital pathology, where surgical approaches are minimal and there is a high density of anatomical noble structures, the tracking of the periosteal elevator allowed to perform a precise subperiosteal dissection. This enabled us to verify or shape the titanium mesh in real-time, generating outcomes closely aligned with the pre-planned ones.
Table 2.
Accuracy of surgical navigation registration and tracked instrument for patient and surgical disease area.
Table 2.
Accuracy of surgical navigation registration and tracked instrument for patient and surgical disease area.
| Disease |
Patient N° |
Tracked Tool |
Accuracy |
Disease |
Patient N° |
Tracked Tool |
Accuracy |
| Oncology |
1 |
Periosteal elevator |
< 0.5 mm |
|
22 |
Drill |
< 1 mm |
| 2 |
Mini-saw |
< 1 mm |
23 |
Drill |
< 0.5 mm |
| 3 |
Chisel and Mini-saw |
< 0.5 mm |
24 |
Drill |
< 1 mm |
| 4 |
Piezoelectric |
< 0.5 mm |
Traumatology |
25 |
Drill |
< 0.5 mm |
| 5 |
Drill |
< 1 mm |
26 |
Drill |
< 1 mm |
| 6 |
Drill |
< 1 mm |
27 |
Drill |
< 0.5 mm |
| 7 |
Drill |
< 1 mm |
28 |
Drill |
< 1 mm |
| 8 |
Drill |
< 1.5 mm |
29 |
Drill |
< 1 mm |
| 9 |
Drill |
< 0.5 mm |
30 |
Drill |
< 0.5 mm |
| 10 |
Drill |
< 1 mm |
31 |
Drill |
< 0.5 mm |
| 11 |
Drill |
< 1 mm |
32 |
Drill |
< 1 mm |
| 12 |
Drill |
< 1.5 mm |
33 |
Drill |
< 1.5 mm |
| 13 |
Drill |
< 1 mm |
34 |
Drill |
< 0.5 mm |
| 14 |
Drill |
< 0.5 mm |
35 |
Drill |
< 0.5 mm |
| 15 |
Drill |
< 1.5 mm |
36 |
Drill |
< 1 mm |
| 16 |
Drill |
< 1.5 mm |
Dento-facial Dysmorphosis |
37 |
Piezoelectric |
< 1 mm |
| 17 |
Drill |
< 0.5 mm |
Craniofacial Malformation |
38 |
Piezoelectric and periosteal elevator |
< 1.5 mm |
| 18 |
Drill |
< 1 mm |
39 |
Piezoelectric and periosteal elevator |
< 0.5 mm |
| 19 |
Drill |
< 0.5 mm |
40 |
Piezoelectric and periosteal elevator |
< 1 mm |
| 20 |
Drill |
< 0.5 mm |
41 |
Piezoelectric and periosteal elevator |
< 1 mm |
| 21 |
Drill |
< 1 mm |
42 |
Piezoelectric and periosteal elevator |
< 0.5 mm |
| Average accuracy |
< 0.87 mm |
| Standard deviation |
0.35 |
Table 3.
Accuracy average and standard deviation per surgical disease area.
Table 3.
Accuracy average and standard deviation per surgical disease area.
| Surgical disease area |
< 0.5 mm |
< 1mm |
< 1.5 mm |
Average accuracy |
Standard deviation |
| Oncology |
9 |
11 |
4 |
< 0.89 mm |
0.36 mm |
| Traumatology |
6 |
5 |
1 |
< 0.79 mm |
0.33 mm |
| Dento-facial Dysmorphosis |
/ |
1 |
/ |
1 mm |
/ |
| Craniofacial Malformation |
2 |
2 |
1 |
< 0.9 mm |
0.42 mm |
8. Discussion
The use of Surgery Navigation is extensively documented in the maxillofacial literature. There are few articles discussing the use of surgical navigation with tracked instrument. Our study presents our experience in the last 15 years using surgical navigation and surgical tracked tools, achieving satisfactory results with an error <1.5 mm, which we consider acceptable.
The use of surgical navigation with different tracked instruments enabled us to perform surgery under the constant real time monitoring of anatomical positions in reduced surgical fields. This allowed us to avoid damaging critical structures such as nerves, while also adhering to the osteotomy tracings, surgical resection boundaries, and accurately placing reconstructive materials in alignment with the pre-planned virtual program. All of this enables us to perform less invasive surgical approaches, thereby reducing postoperative complications. In cases in which the resection is carried out using a “piecemeal” technique and lesion margins are hard to be identified during surgery, as in the case 2, navigation allows to locate margins in real-time, ensuring confidence in the surgical approach, as already highlighted by Novelli et al. (2016) in their protocol [
21].
One of the advantages of virtual planning is the reproducibility of results, potentially allowing young and inexperienced surgeons to achieve the same outcomes as an expert surgeon, in case of accurate preoperative planning.
Nowadays CAD-CAM cutting guides enable the translation of virtual surgical planning into the operating room, albeit with several challenges, primarily related to their precise placement. Notably, positioning these surgical templates requires to perform wider surgical accesses, leading to increased disruption of surrounding healthy tissues and resulting in a more challenging postoperative period with an increased risk of complications. The adoption of intraoperative navigation has emerged as a valuable alternative, offering an effective cost-benefit balance for translating virtual surgical plans into practical procedures, and yielding favorable outcomes in terms of precision and safety [
22].
The tracking of surgical instruments was previously described by Bianchi et al. (2015), who performed craniofacial osteotomies for oncological reasons or in cases of orthognathic surgery in 18 patients using tracked drills, confirming the lower invasiveness of the surgery and greater precision. In some cases, the surgical time was longer than usual for a similar procedure. Robiony et al. (2019) performed nasal bone osteotomies using piezoelectric drill tracking [
22]. Recently, Alice Dean et al. (2022) reported 32 patients who underwent surgery using surgical navigation with tracked piezoelectric. The treatments spanned various disciplines including oncology, traumatology, and orthognathic surgery. The results affirm a reduction in surgical time, improved procedural safety, allowing for three-dimensional control over tumor resection or osteotomy depth [
23].
The limitations of using surgical navigation include the economic cost of the navigation device and software, the time required for pre-surgical planning, a steep learning curve for the surgeon and the necessity of an extra preoperative CT scan acquisition.
Currently, there are no studies in the available literature that have tracked surgical instruments other than the piezoelectric. In our experience, the use of different instruments allows the surgeon to better adapt to each anatomical region, selecting the most suitable instrument for the type of surgery required. All of this comes with the previously described advantages that the synergy of surgical navigation and tracking tools provides.
9. Conclusion
Surgical navigation using tracked instruments allows the synergistic implementation of the benefits of both techniques. This approach enables the surgeon to approach with greater precision, using less invasive surgical access and thereby reducing morbidity and complications. Furthermore, pre-planned virtual surgery facilitates the realization of potential osteotomic pathways, tumor resections, or placement of reconstructive materials, maintaining real time control over anatomy throughout. Additionally, this ensures reproducibility in surgeries, yielding comparable results regardless of the operating surgeon.
Surgery Navigation is revolutionizing the approach to complex craniomaxillofacial surgery, but nothing can substitute the experience of the operator. It is the synergy between this experience and the judicious application of Navigation Surgery that ultimately results in successful procedures.
References
- Collyer, J. Stereotactic navigation in oral and maxillofacial surgery. Br. J. Oral Maxillofac. Surg. 2010, 48, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Leksell, L.; Jernberg, B. Stereotaxis and tomography a technical note. Acta Neurochir. 1980, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Moretti, M.; Meazzini, M.C.; Cassé, C.M.A.; Mazzoleni, F.; Sozzi, D. Introduction to Surgical Navigation in Oral Surgery: A Case-Series. Oral 2023, 3, 146–154. [Google Scholar] [CrossRef]
- Novelli, G.; Tonellini, G.; Mazzoleni, F.; Bozzetti, A.; Sozzi, D. Virtual surgery simulation in orbital wall reconstruction: Integration of surgical navigation and stereolithographic models. J. Cranio-Maxillofacial Surg. 2014, 42, 2025–2034. [Google Scholar] [CrossRef]
- Lübbers, H.-T.; Jacobsen, C.; Könü, D.; Matthews, F.; Grätz, K.W.; Obwegeser, J.A. Surgical navigation in cranio-maxillofacial surgery: an evaluation on a child with a cranio-facio-orbital tumour. Br. J. Oral Maxillofac. Surg. 2011, 49, 532–537. [Google Scholar] [CrossRef]
- Bell, RB. Computer planning and intraoperative navigation in cranio-maxillofcial surgery. Oral Maxillofac Surg Clin North Am 2010, 22, 135–156. [Google Scholar] [CrossRef]
- Wu, J.; Sun, J.; Shen, S.G.; Xu, B.; Li, J.; Zhang, S. Computer-assisted navigation: its role in intraoperatively accurate mandibular reconstruction. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 134–142. [Google Scholar] [CrossRef]
- Gui, H. Wu J.Shen S.G.Bautista J.S.Voss P.J.Zhang S.Navigation-guided lateral gap arthroplasty as the treatment of temporomandibular joint ankylosis. J Oral Maxillofac Surg. 2014, 72, 128–138. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, T.; Zhang, Y.; An, J.; He, L. Application of a computer-assisted surgical navigation system in temporomandibular joint ankylosis surgery: a retrospective study. Int. J. Oral Maxillofac. Surg. 2016, 46, 189–197. [Google Scholar] [CrossRef]
- Wu, J.; Sun, J.; Shen, S.G.; Xu, B.; Li, J.; Zhang, S. Computer-assisted navigation: its role in intraoperatively accurate mandibular reconstruction. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 134–142. [Google Scholar] [CrossRef]
- Abbate, V.; Orabona, G.D.A.; Solari, D.; Bonavolontà, P.; Iaconetta, G.; Califano, L. Mandibular Surgical Navigation: An Innovative Guiding Method. J. Craniofacial Surg. 2017, 28, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Hohlweg-Majert, B.; Schön, R.; Schmelzeisen, R.; Gellrich, N.; Schramm, A. Navigational Maxillofacial Surgery Using Virtual Models. World J. Surg. 2005, 29, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shen, S.G.; Wang, X.; Zhang, L.; Zhang, S. The indication and application of computer-assisted navigation in oral and maxillofacial surgery—Shanghai's experience based on 104 cases. J. Cranio-Maxillofacial Surg. 2013, 41, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of Computer-Aided Design and Computer-Aided Manufacturing to Produce Orthognathically Ideal Surgical Outcomes: A Paradigm Shift in Head and Neck Reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 2115–2122. [Google Scholar] [CrossRef]
- Lei Zhang, Zhixu Liu, Biao Li, Hongbo Yu, Steve Guofang Shen, Xudong Wang, Evaluation of computer-assisted mandibular reconstruction with vascularized fibular flap compared to conventional surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016, 121, 139–148.
- Wu, J.; Sun, J.; Shen, S.G.; Xu, B.; Li, J.; Zhang, S. Computer-assisted navigation: its role in intraoperatively accurate mandibular reconstruction. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, D.; Filippi, A.; Canzi, G.; De Ponti, E.; Bozzetti, A.; Novelli, G. Surgical Navigation in Mandibular Reconstruction: Accuracy Evaluation of an Innovative Protocol. J. Clin. Med. 2022, 11, 2060. [Google Scholar] [CrossRef]
- Vercellotti, T. Piezoelectric surgery in implantology: a case report--a new piezoelectric ridge expansion technique. Int. J. Periodontics Restor. Dent. 2000, 20, 358–65. [Google Scholar]
- Spinelli, G.; Lazzeri, D.; Conti, M.; Agostini, T.; Mannelli, G. Comparison of piezosurgery and traditional saw in bimaxillary orthognathic surgery. J. Cranio-Maxillofacial Surg. 2014, 42, 1211–1220. [Google Scholar] [CrossRef]
- Bianchi, G. Badiali, L. Piersanti, C. Marchetti, Computer-assisted piezoelectric surgery: a navigated approach toward performance of craniomaxillofacial osteotomies. J Craniofac Surg. 2015, 26, 867–872. [Google Scholar] [CrossRef]
- Novelli, G.; Gramegna, M.; Tonellini, G.; Valente, G.; Boni, P.; Bozzetti, A.; Sozzi, D. Orbital Osteoblastoma: Technical Innovations in Resection and Reconstruction Using Virtual Surgery Simulation. Craniomaxillofacial Trauma Reconstr. 2016, 9, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ceccariglia, F.; Cercenelli, L.; Badiali, G.; Marcelli, E.; Tarsitano, A. Application of Augmented Reality to Maxillary Resections: A Three-Dimensional Approach to Maxillofacial Oncologic Surgery. J. Pers. Med. 2022, 12, 2047. [Google Scholar] [CrossRef] [PubMed]
- Massimo Robiony, Corrado Toro, Fabio Costa, Salvatore Sembronio, Francesco Polini, Massimo Politi, Piezosurgery: a new method for osteotomies in rhinoplasty. J Craniofac Surg. 2007, 18, 1098–1100.
- Dean, A.; Heredero-Jung, S.; Solivera, J.; Sanjuan, A.; Alamillos-Granados, F.J. Computer-assisted and navigated piezoelectric surgery: A new technology to improve precision and surgical safety in craniomaxillofacial surgery. Laryngoscope Investig. Otolaryngol. 2022, 7, 684–691. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
(A) Preoperative (17 mm) and (B) introperative (36 mm) mouth opening.
Figure 1.
(A) Preoperative (17 mm) and (B) introperative (36 mm) mouth opening.
Figure 2.
Coronoidectomy (yellow area) and preoperative planned osteotomy lines.
Figure 2.
Coronoidectomy (yellow area) and preoperative planned osteotomy lines.
Figure 3.
Tracked tools used during surgery.
Figure 3.
Tracked tools used during surgery.
Figure 4.
(A) Preoperative CT scan showing the tumor. (B) Virtual planning of resection margins.
Figure 4.
(A) Preoperative CT scan showing the tumor. (B) Virtual planning of resection margins.
Figure 4.
Tracked chisel and mini-saw.
Figure 4.
Tracked chisel and mini-saw.
Figure 5.
Intraoperative placement of the bite and setup of the intermaxillary block.
Figure 5.
Intraoperative placement of the bite and setup of the intermaxillary block.
Figure 6.
Registration of the piezoelectric tip and connecting it to the navigator with a calibration matrix.
Figure 6.
Registration of the piezoelectric tip and connecting it to the navigator with a calibration matrix.
Figure 7.
Surgical navigation with tracked piezoelectric.
Figure 7.
Surgical navigation with tracked piezoelectric.
Figure 8.
Subapical osteotomy (Köle) with tracked piezoelectric.
Figure 8.
Subapical osteotomy (Köle) with tracked piezoelectric.
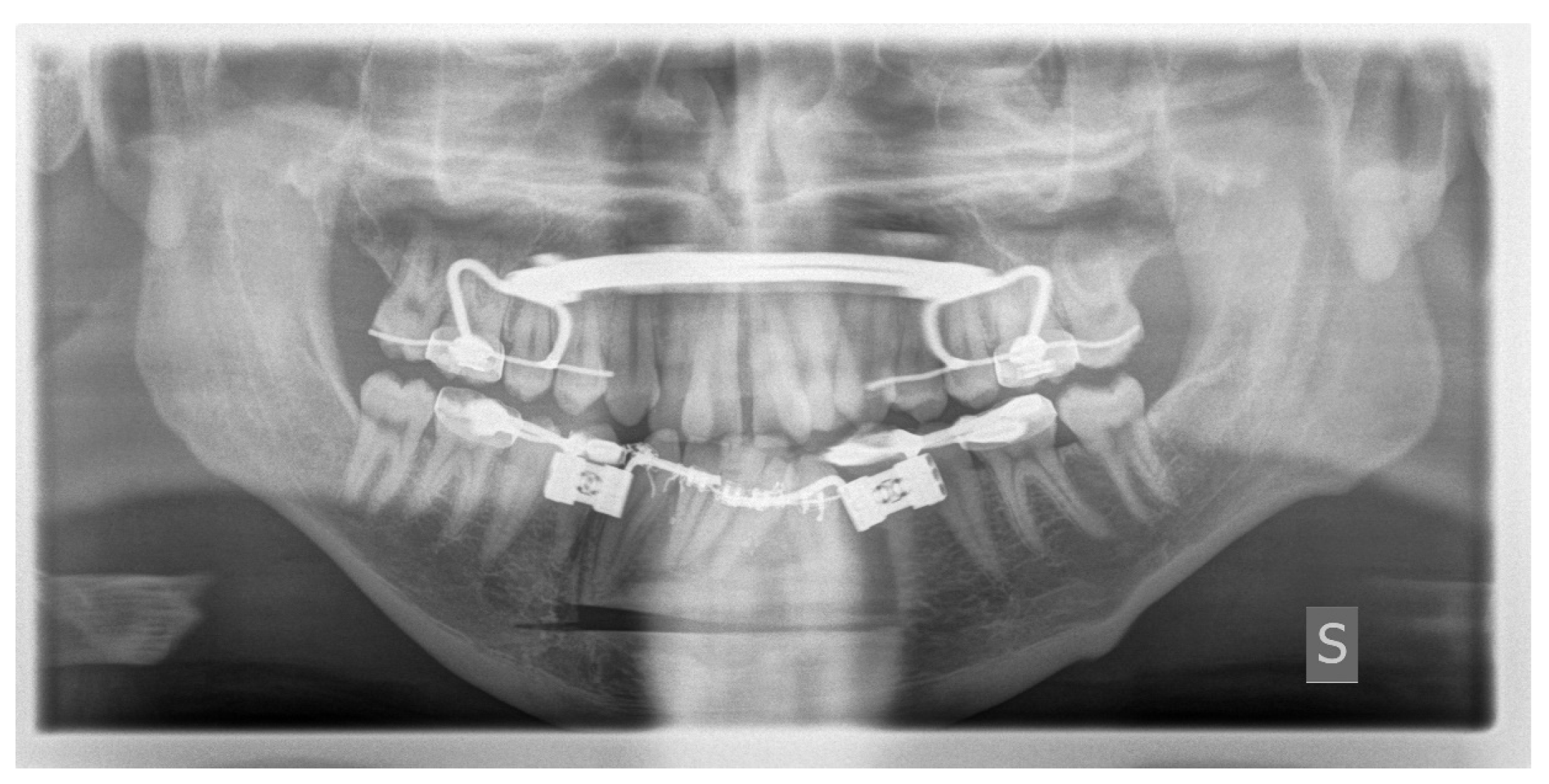
Figure 9.
Postoperative Orthopantomography X-ray.
Figure 9.
Postoperative Orthopantomography X-ray.
Figure 10.
Evidence on the mandibular CT scan of the appearance of a new osteolytic lesion, appreciable on axial, coronal, and sagittal views.
Figure 10.
Evidence on the mandibular CT scan of the appearance of a new osteolytic lesion, appreciable on axial, coronal, and sagittal views.
Figure 11.
Intraoperative placement of the same bite used for acquiring mandibular CT scan images. The bite enables the maintenance of the mandible in the same position as during the virtual surgical planning.
Figure 11.
Intraoperative placement of the same bite used for acquiring mandibular CT scan images. The bite enables the maintenance of the mandible in the same position as during the virtual surgical planning.
Figure 12.
Surgical steps: (A,B) Resection of the intraosseous osteolytic lesion under the guidance of the tracked piezoelectric device. (C) Complete removal of the mandibular segment containing the osteolytic lesion with macroscopically healthy bone margins preserved, including the lower and posterior mandibular border. (D) The excised surgical resection measured 10x10x7 mm.
Figure 12.
Surgical steps: (A,B) Resection of the intraosseous osteolytic lesion under the guidance of the tracked piezoelectric device. (C) Complete removal of the mandibular segment containing the osteolytic lesion with macroscopically healthy bone margins preserved, including the lower and posterior mandibular border. (D) The excised surgical resection measured 10x10x7 mm.
Figure 13.
Postoperative OPT X-ray showing the resection area and the integrity of the lower and upper borders of the mandible.
Figure 13.
Postoperative OPT X-ray showing the resection area and the integrity of the lower and upper borders of the mandible.
Table 1.
Characteristics of the cases operated using navigation surgery and tracking instruments technique.
Table 1.
Characteristics of the cases operated using navigation surgery and tracking instruments technique.
| Disease |
Area of treatment and pathology |
Nº |
Surgery |
Tracked Tool |
| Oncology |
Orbit |
Osteoblastoma |
1 |
Resection |
Periosteal elevator |
| Osteochondroma |
1 |
Resection |
Mini-saw |
| Nose-Orbital-Ethmoidal-Frontal ossifying fibroma |
1 |
Resection |
Chisel and Mini-saw |
| Mandible |
Squamous Cellular Carcinoma |
11 |
Resection + Fibular free flap |
Drill |
| Synovial Sarcoma |
1 |
Resection + Fibular free flap |
Drill |
| Adenoid Cystic Carcinoma |
1 |
Resection + Fibular free flap |
Drill |
| Ameloblastoma |
6 |
Resection + Fibular free flap |
Drill/Piezoelectirc |
| Odontogenic Keratocyst |
1 |
Resection + Fibular free flap |
Drill |
| Giant Cell Granuloma |
1 |
Resection + Fibular free flap |
Drill |
| Traumatology |
Orbital fractures |
12 |
Secondary orbital reconstruction (VSS) |
Drill |
| Dento-facial Dysmorphosis |
Skeletal class II |
1 |
Orthognatic Surgery: Subapical Osteotomy |
Piezoelectric drill |
| Craniofacial Malformation |
Pfeiffer Sd |
4 |
Supranumerari dental extractions |
Cutters |
| Coronoid Hyperplasia |
1 |
Coronoidectomy |
Piezoelectric and periosteal elevator |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).