1. Introduction
The Food and Agriculture Organization of the United Nations estimates that upwards of 1.3 billion metric tons of food is wasted after harvest every year, which is enough to feed more than 1.9 billion people or roughly ¼ of the world population(Food and Agriculture Organization of the United Nations 2022). This does not include the food loss that occurs prior to harvest. Fruit, especially those of climacteric disposition, are both valuable and perishable. Maintenance of fruit constitution during-and post-harvest is a complex task involving coordination between growers, storage houses, distributors, cold-chain transporters, and the demands of the market. The complexity of this multi-faceted system results in wastage. Research investigating specifics of ripening and potential post-harvest control mechanisms is seen as a viable approach to reducing the amount of food spoilage.
The 2-carbon gaseous molecule 1-methylcyclopropene (1-MCP) was identified and patented as an inhibitor of ethylene action in 1996. 1-MCP competitively binds to ethylene receptors, thereby delaying ripening, preserving fruit flesh firmness, and extending storage life in climacteric fruits(Edward C Sisler and Blankenship 1996; E. C. Sisler and Blankenship 1993). This compound has been used to great effect in apple (Malus domestica) post-harvest systems producing reliable and repeatable results. The use of 1-MCP in research settings has bolstered some understanding of ethylene action. For example, there seems to be a compounding ‘time X concentration’ relationship for 1-MCP in most fruit and vegetable crops, where higher concentrations or extended treatment times show greater inhibition ripening (E C Sisler et al. 1997), a relationship in agreement with ethylene research data.
In some instances, rather than shed light on the complex patterns of ripening processes, research into the effects of 1-MCP treatments in various crops has revealed counter-intuitive and highly variable results. In Pineapple, it was observed that ethylene levels remain elevated longer and decline slower in 1-MCP treated fruits than in non-treated(Selvarajah, Bauchot, and John 2001). Ethylene biosynthesis has even been shown to increase upon application of 1-MCP in figs (Sozzi et al. 2005), jujube (Zozio et al. 2014), and mango (Wang et al. 2009). Stone fruits such as apricots, peaches and plums have been reported to develop internal browning, flesh breakdown and flesh reddening when stored at low temperatures after 1-MCP treatment(Watkins 2007). These studies highlight just how much of a knowledge gap still exists in the collective understanding of 1-MCP and its interactions with plants.
Observations of extreme variation, and in some cases, lack of reproducibility in 1-MCP treatment experiments point towards the role of physiological and genetic variability across different species. Recent research demonstrates a shift away from commercial application (i.e., physiology) research and move towards molecular and biochemical studies of fruit interactions with 1-MCP (Zhang et al. 2020). Additionally, the inability to standardize consistent applications of 1-MCP have been problematic given it is a highly volatile molecule. Several studies have shown 1-MCP-fruit interactions are most likely due to inconsistent 1-MCP applications, as concentrations even in the parts per billion range are effective at eliciting ripening inhibition – for example in European pear (MacLean et al. 2007; Ekman et al. 2004). Experimentation at such an intricate biological level must minimize independent variable disparity to obtain results with a high level of confidence. Furthermore, interest to explore 1-MCP response at an increasingly granular level must be paired with increased focus on how to apply 1-MCP in a quantifiable, replicable, and consistent manner.
To address the technical gap for 1-MCP application, we have developed a standardized and reproducible 1-MCP application and quantitation method to reduce variability in 1-MCP related post-harvest experiments. Because such low concentrations of 1-MCP are effective at inhibiting ethylene related response in fruits, it is important that application methods be accurate and reproducible. The use of non-reactive acrylic chambers that are very effective at maintaining constant headspace concentrations can aid in the standardization process(Vallejo and Beaudry 2006). Adoption of this methodology is expected to allow for greater control of a crucial independent variable in post-harvest horticultural studies (i.e., 1-MCP application rate), thereby limiting observed variation in the biological realm and enabling additional research in the area of 1-MCP with its interactions in various plant systems to develop efficient methods for post-harvest waste reduction.
2. Materials and Methods
Materials and reagent acquisition
1-MCP-cyclodextrin inclusion complex 3.3% active ingredient was sourced from Chesen Biochem Co. Ltd (Hefei, Anhui, China). 110L acrylic desiccator chambers (catalog number 1400-1-AB) were sourced from Cleatech Cleanroom and Laboratory Solutions (Orange, CA, USA). Cis-2 butene and 1-Butene standards were sourced from Sigma Aldrich (St. Louis, MO, USA). Sodium hydroxide was sourced from Fisher Scientific (Hampton, NH, USA).
1. -MCP Theoretical yield calculations
Yield calculations were made assuming complete liberation of 1-MCP active ingredient from the cyclodextrin inclusion complex. Utilizing the ideal gas law (PV=nRT), corrections were made for elevation (Pullman WA, 717m) and temperature (20oC) to give the maximum theoretical yield of 1-MCP for the chamber headspace.
1. -MCP application and sampling
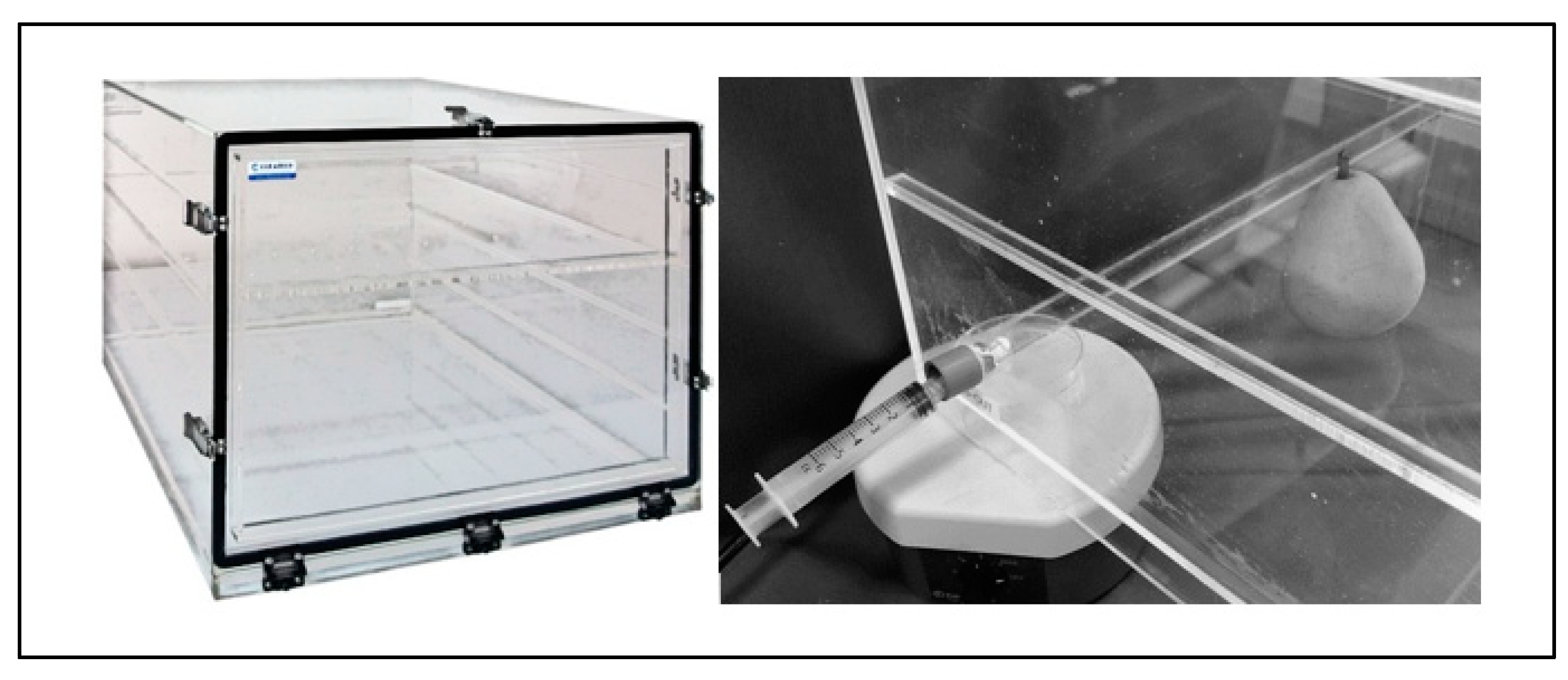
1-MCP compound was weighed into glass dishes containing a magnetic stir bar and placed in the desiccation cabinets near one of the septum-lined injection ports and the chamber was sealed by closing the door and fastening the clasps. The chamber was elevated such that a magnetic stir plate could be placed beneath, facilitating agitation of the 1-MCP compound Figure 1. With agitation, a 7.5% (w/v) sodium hydroxide solution was administered to the 1-MCP-containing plate from the outside of the chamber by needle and syringe through the septa. Only enough sodium hydroxide solution required to saturate the 1-MCP powder was administered. Agitation with stir bar ensured that all 1-MCP/cyclodextrin compound entered solution and increases the probability of active ingredient liberation near or at theoretical yield for a given temperature and pressure. After roughly 10 minutes, when the 1-MCP cyclodextrin complex was completely dissolved into solution, a 500 µL headspace sample was taken from a septa-lined injection port opposite the sample petri dish. Another sample was taken in the same manner 24 hours after sample hydration to ensure chambers remained airtight.
Surrogate standard application and sampling
Cis-2-butene(Nations 2006) and 1-butene(Lee et al. 2006) surrogate standards were chosen for their similarity in chemical composition and structure to 1-MCP as well as their prior use in 1-MCP related research. Dilutions of pure standards were prepared in syringe and injected into the chambers. Surrogate standard application did not require the use of the sodium hydroxide hydration method as the gasses were supplied in pure format. After 10 mins of equilibration time, headspace samples were removed and analyzed in the same manner as 1-MCP. No 24-hour samples were taken of surrogate standards.
Figure 1.
Photo showing Cleatech desiccation chamber (left) and compound hydration arrangement (right). Approximately 2mL 7.5% sodium hydroxide is injected into the 1-MCP cyclodextrin containing petri dish during constant agitation from the magnetic stir plate underneath. Constant agitation increases the probability of active ingredient liberation at or near the theoretical yield by ensuring all 1-MCP/cyclodextrin compound enters solution.
Figure 1.
Photo showing Cleatech desiccation chamber (left) and compound hydration arrangement (right). Approximately 2mL 7.5% sodium hydroxide is injected into the 1-MCP cyclodextrin containing petri dish during constant agitation from the magnetic stir plate underneath. Constant agitation increases the probability of active ingredient liberation at or near the theoretical yield by ensuring all 1-MCP/cyclodextrin compound enters solution.
The 1-MCP detection and quantification
Samples were analyzed via gas chromatography on an HP 5890 GC fitted with an Agilent HP Plot-Q-15m X 0.5mm column with 18µm phase. Oven, injector, and detector temperatures were 120, 140, and 200oC, respectively. Nitrogen was used as a carrier gas at a flow rate of 5 mL min-1. Sample injection volume was 500µL and all samples were analyzed in triplicate. 1-MCP peaks were distinct, with retention times always preceding Cis-2-butene and succeeding 1-Butene (Fig. S3). Because of this unique peak profile, the 1-MCP peak could be clearly identified for every sample.
3. Results
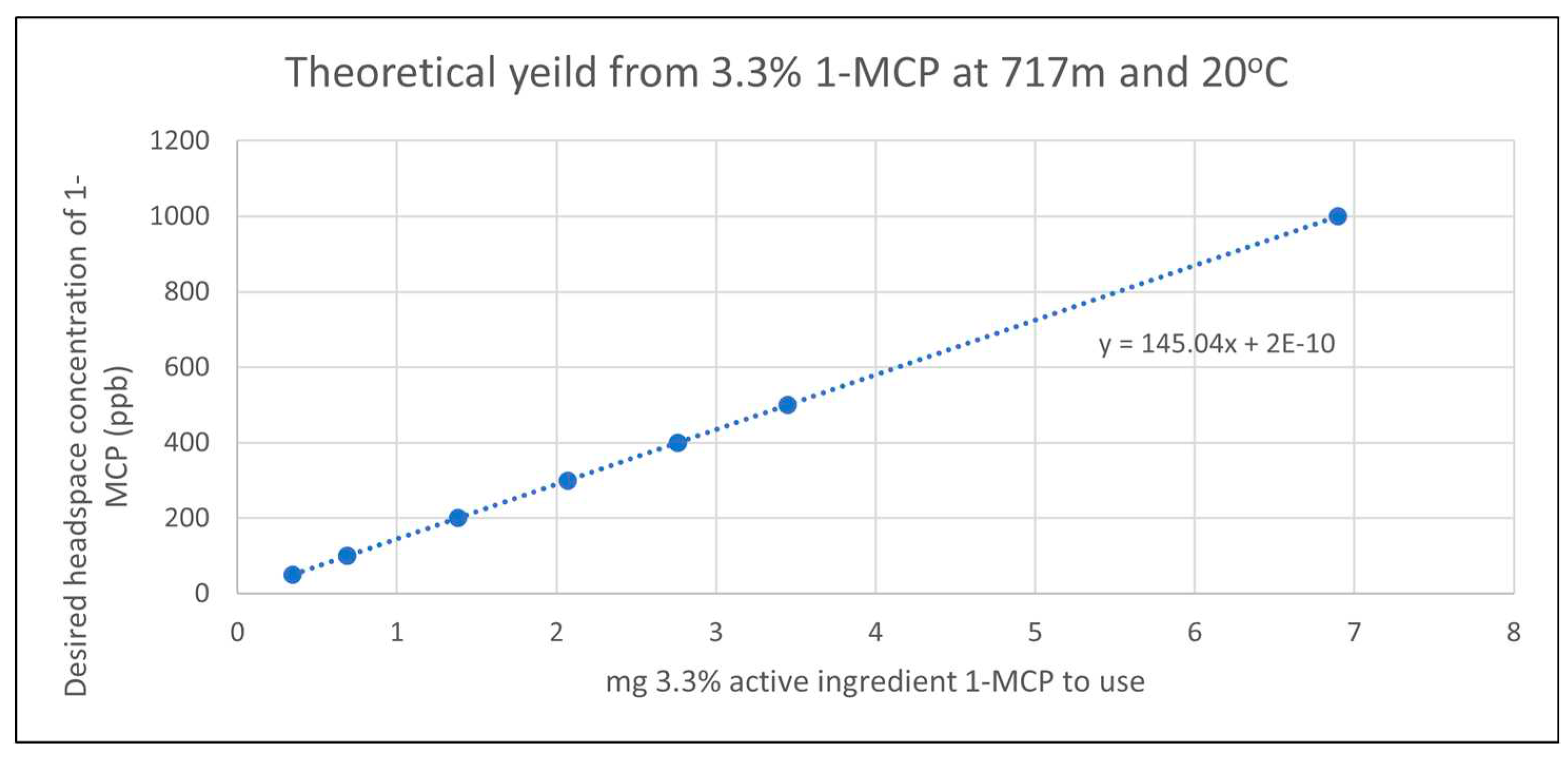
A formulation of 3.3% active ingredient 1-MCP surrounded by a cyclic oligosaccharide was necessary to weigh and apply the compound as 1-MCP at physiologically relevant temperatures is a gas. Concentrations ranging from 50 to 500 parts per billion 1-MCP were tested. The theoretical yield assuming complete liberation of active ingredient from cyclodextrin inclusion complex was determined using the ideal gas law coefficients where corrections were made for air pressure at elevation 717m above sea level, 20oC, and the formula weight of 1-MCP at 54.9g mol -1. The linear regression relating mass of 1-MCP inclusion product to theoretical headspace concentration in the 110L application chamber can be seen in Figure 2.
Figure 2.
A linear regression accounting for % active ingredient, pressure and temperature was developed to determine to appropriate mass of 1-MCP/cyclodextrin compound to obtain a given desired headspace concentration in the test chambers. Theoretical yield of 1-MCP from cyclodextrin inclusion complex assuming complete liberation of active ingredient upon hydration with 7.5% sodium hydroxide. Mass of 1-MCP product is shown on the x-axis and theoretical headspace concentration of 1-MCP is on the y-axis. The relationship is assumed to be linear.
Figure 2.
A linear regression accounting for % active ingredient, pressure and temperature was developed to determine to appropriate mass of 1-MCP/cyclodextrin compound to obtain a given desired headspace concentration in the test chambers. Theoretical yield of 1-MCP from cyclodextrin inclusion complex assuming complete liberation of active ingredient upon hydration with 7.5% sodium hydroxide. Mass of 1-MCP product is shown on the x-axis and theoretical headspace concentration of 1-MCP is on the y-axis. The relationship is assumed to be linear.
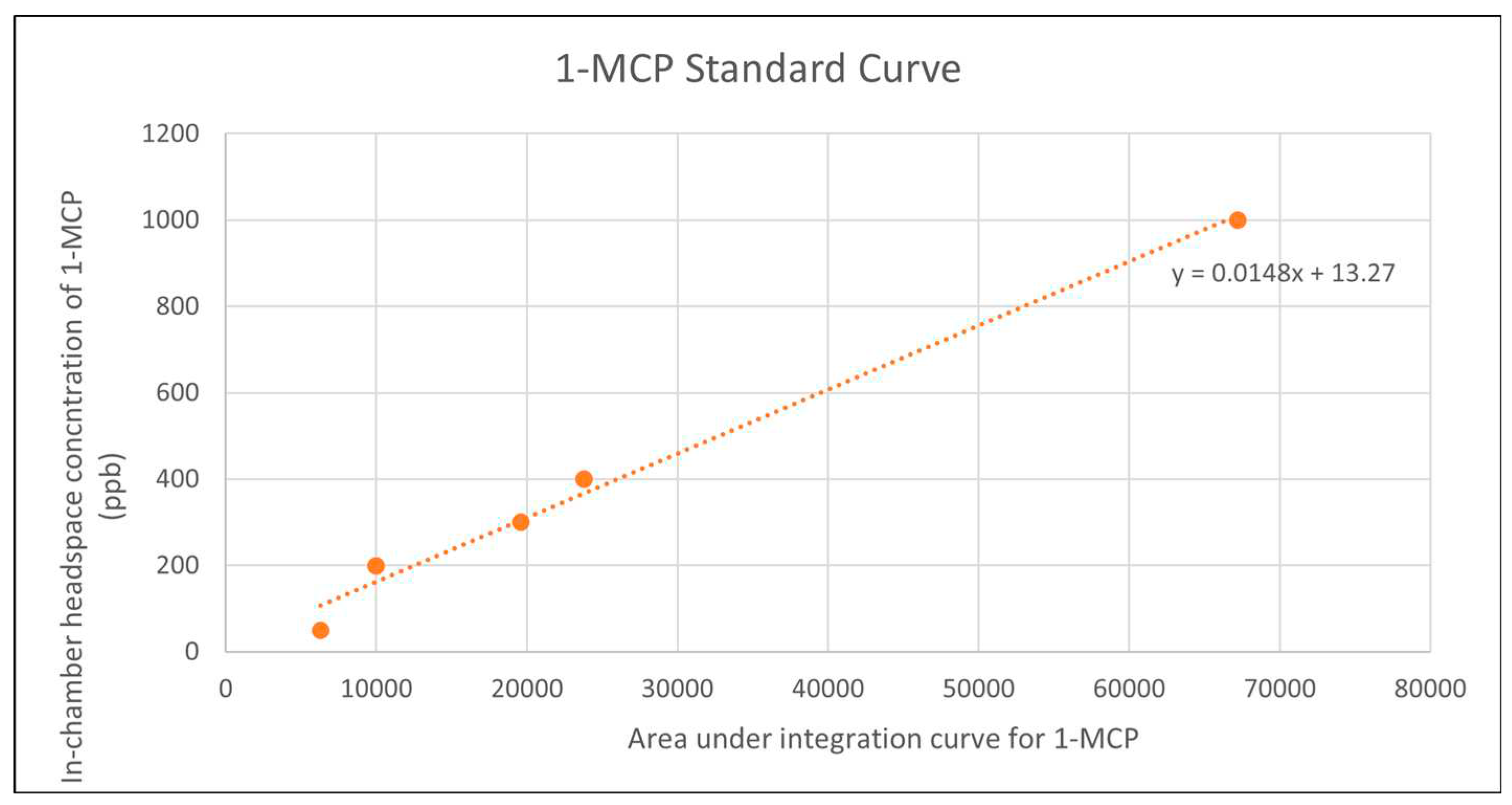
Dilutions of surrogate standards were used to generate standard curves and determine appropriate integrator time scales Supplementary File 1. Only then were 1-MCP dilutions run in parallel with blanks. The similarity in chemical makeup and structure between the surrogate standards and 1-MCP and the absence of peaks in blank samples could be leveraged to ensure accurate detection of 1-MCP without the use of a mass spectrometer. The use of surrogate standards was necessary as 1-MCP is typically supplied and used by industry as an impure compound (i.e. contained within an inclusion complex) making detection by chromatography difficult. By first running dilutions of pure surrogate standards, the retention time of the1-MCP peak could be estimated and observed carefully in later 1-MCP headspace measurements. Appropriate masses determined from the theoretical yield regression equation were used for 1-MCP standard curve development. Care was taken to maintain constant agitation by stirring of the inclusion complex during hydration to avoid aggregation of particles as previously reported (Ariyanto and Yoshii 2019). Remarkably, concentrations as low as 50ppb were easily observed as distinct peaks. The relationship between headspace 1-MCP concentration based on theoretical yield and area under the curve remained linear throughout all concentrations tested (50-500 ppb) Figure 3.
Figure 3.
1-MCP Standard curve generated by gas chromatography in 110L test chambers. Mass-concentration relationships derived by the ideal gas law. The relationship remains linear throughout all concentrations tested.
Figure 3.
1-MCP Standard curve generated by gas chromatography in 110L test chambers. Mass-concentration relationships derived by the ideal gas law. The relationship remains linear throughout all concentrations tested.
4. Conclusions
Molecular and biochemical studies exploring 1-MCP-fruit interactions need to be replicable. Variability in response to 1-MCP is often high, resulting in inconclusive outcomes(Zhang et al. 2020). If the observed variation is exclusively biological, then the role of 1-MCP in a given experiment can be elucidated. The method and approach reported here provides a framework for accurate 1-MCP application and quantitation for use in post-harvest research and aims to minimize experimental variation by standardizing 1-MCP application. By sourcing high-quality 1-MCP-cyclodextrin product and carefully accounting for pressure and temperature, theoretical yield estimations can be generated for the application volume. 1-MCP application in non-reactive gas tight chambers minimizes leakage and ensures theoretical yield closely matches quantitative measurement. Furthermore, constant agitation of the 1-MCP/cyclodextrin during hydration by magnetic stir-plate increases the probability of complete liberation of the active ingredient from inclusion complex by ensuring compound solubilization. Utilization of inexpensive, reliable surrogate standard gases such as 1-butene and Cis-2 butene helps in identifying 1-MCP chromatogram peaks and helps validate the concentrations reliably. It is expected that adoption of these considerations and methodology will facilitate further understanding of 1-MCP-fruit interactions and help maximize experimental reproducibility and confidence in results.
Supplementary Materials
Additional file 1: Figure S1. Cis-2-butene standard curve (50-1000 ppb) generated in 110L Cleatech chambers. Additional file 2: Figure S2. 1-butene standard curve (50-1000 ppb) generated in 110L Cleatech chambers. Additional file 3: Figure S3. Figure S3 Representative chromatograph depicting 1-butene (3.948), 1-methylcyclopropene (4.148), and cis-2-butene (4.731) peaks. Additional file 4: Table S1. Alkenes standard curves.
Author Contributions
ES and AD conceived and designed the study. ES and DSM collected data and interpreted data. JB and AD provided revisions and critique. All authors read and approved the final manuscript.
Funding
This research was supported by a Washington State Department of Agriculture Specialty Crop Block Grant, Fresh and Processed Pear Research Subcommittee to AD. Work in the Dhingra lab was supported by a Washington State University Agriculture Center Research Hatch Grant WNP00011 and startup funds from Texas A&M AgriLife Research, Texas A&M University.
Data Availability Statement
All data used during this study are included in this published article and its Additional Files.
Acknowledgments
The authors are grateful to Ray Schmitten, Blue Bird Growers (Peshastin, WA) and Blue Star Growers (Cashmere, WA) for providing pears for research.
Conflicts of Interest
The authors declare no conflict of interest.
References
-
The State of Food Security and Nutrition in the World 2022; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022; ISBN 9789251364994.
- Sisler, E. C. & Blankenship, S. M. Method of counteracting an ethylene response in plants. ( 1996.
- Sisler, E.C.; Blankenship, S.M. Diazocyclopentadiene (DACP), a light sensitive reagent for the ethylene receptor in plants. Plant Growth Regul. 1993, 12, 125–132. [Google Scholar] [CrossRef]
- Sisler, E. C. , Serek, M., Serek, S. E. C. & Si, £ C. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant. 100, 577–582 (1997).
- Selvarajah, S.; Bauchot, A.; John, P. Internal browning in cold-stored pineapples is suppressed by a postharvest application of 1-methylcyclopropene. Postharvest Biol. Technol. 2001, 23, 167–170. [Google Scholar] [CrossRef]
- Sozzi, G.; A Abraján-Villaseñor, M.; Trinchero, G.D.; A Fraschina, A. Postharvest response of ‘Brown Turkey’ figs (Ficus carica L.) to the inhibition of ethylene perception. J. Sci. Food Agric. 2005, 85, 2503–2508. [Google Scholar] [CrossRef]
- Zozio, S.; Servent, A.; Hubert, O.; Hiol, A.; Pallet, D.; Mbéguié-A-Mbéguié, D. Physicochemical and biochemical characterization of ripening in jujube (Ziziphus mauritiana Lamk) fruits from two accessions grown in Guadeloupe. Sci. Hortic. 2014, 175, 290–297. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Feng, X.; Lin, L.; Zhao, Y.; Jiang, W. Effects of 1-MCP and exogenous ethylene on fruit ripening and antioxidants in stored mango. Plant Growth Regul. 2009, 57, 185–192. [Google Scholar] [CrossRef]
- Watkins, C.B. The effect of 1-MCP on the development of physiological storage disorders in horticultural crops. Stewart Postharvest Rev. 2007, 3, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.-M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- MacLean, D. D. , Murr, D. P., DeEll, J. R., Mackay, A. B. & Kupferman, E. M. Inhibition of PAL, CHS, and ERS1 in ‘Red d’Anjou’ Pear (Pyrus communis L.) by 1-MCP. Postharvest Biol. Technol. 45, 46–55 (2007).
- Ekman, J. H. , Clayton, M., Biasi, W. V. & Mitcham, E. J. Interactions between 1-MCP concentration, treatment interval and storage time for ‘Bartlett’ pears. Postharvest Biol. Technol. 31, 127–136 (2004).
- Vallejo, F. & Beaudry, R. Depletion of 1-MCP by ‘non-target’ materials from fruit storage facilities. Postharvest Biol. Technol. 40, 177–182 (2006).
- Ariyanto, H.D.; Yoshii, H. Effect of stepwise humidity change on the release rate constant of 1-methylcyclopropene (1-MCP) in a cyclodextrin inclusion complex powder. Food Packag. Shelf Life 2019, 21. [Google Scholar] [CrossRef]
- Nations, F. and A. O. of the U. FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES 1-Methylcyclopropene. (2006).
- Lee, Y. S. , Beaudry, R., Kim, J. N. & Harte, B. R. Development of a 1-Methylcyclopropene (1-MCP) Sachet Release System. J. Food Sci. 71, (2006).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).