Introduction
The placental growth includes extensive vasculogenesis and angiogenesis controlled by multifunctional angiogenic factors expressed by trophoblasts, immune cells, endothelial cells, and also secreted into the maternal circulation [
1,
2]. Human Leukocyte Antigen-G (HLA-G) is a member of class Ib histocompatibility family expressed in the placenta [
3]. Soluble (s)HLA-G isoforms include HLA-G1 and HLA-G5-7. sHLA-G is detectable in both extravillous and villous cytotrophoblasts as well as in the chorion membrane and in syncytiotrophoblasts [
3,
4]. sHLA-G isoforms play critical roles in the trophoblast invasion, remodeling of spiral arteries, fetal development, and materno-fetal immune tolerance [
3,
5]. Increasing findings support the role of HLA-G in complications associated with pregnancy. The level of total soluble and membrane-bound HLA-G was decreased significantly in case of preeclamptic placenta and maternal serum compared with placenta and maternal serum of pregnant women with no relevant complications [
6,
7]. An increased level of HLA-G5 in the placenta [
8], and in maternal serum [
9] was observed in preeclampsia (PE), whereas a diminished sHLA-G1 level was described in PE [
9] in the third trimester. Decreased sHLA-G level and reduced membrane-bound HLA-G protein expression was linked to recurrent miscarriage [
10].
Placental Protein-13 (PP13) is a member of the galectin family and is synthesized in syncytiotrophoblasts [
11]. PP13 promotes the migration and differentiation of trophoblasts, and is implicated in the vascular remodeling of spiral arteries in early pregnancy. Moreover, it contributes to the immunoregulation by inducing apoptosis of maternal T-cells [
12,
13]. PP13 can predict preeclampsia in the first trimester [
14,
15]. Maternal serum PP13 level is lower in pregnancies complicated with gestational diabetes mellitus (GDM) [
16], however diminished levels of PP13 were found in non-complicated pregestational diabetes mellitus [
17].
Both sHLA-G and PP13 can be detected in the maternal blood [
14,
15,
16,
18,
19,
20,
21,
22] and amniotic fluid [
19,
23,
24,
25]. The interrelation of the fetoplacental growth during gestation and the levels of these angiogenic factors in maternal serum and amniotic fluid have not been investigated yet. Therefore, this study aims to determine sHLA-G and PP13 protein levels related to sonographic parameters of the fetus and placenta during mid-trimester as well as to perinatal outcome.
Study design
A prospective, cross-sectional cohort study was conducted in pregnant women undergoing amniocentesis at the Department of Obstetrics and Gynecology, University of Szeged, Hungary between January 2019 and December 2020. During the study period, all singleton pregnancies with increased risk of chromosomal abnormality, where amniocentesis (AC) was performed between 16+0 and 22+6 weeks of gestation, were recruited into our study. The indications for AC were increased nuchal translucency (NT) at first trimester scan (≥2MoM for gestational age) (n = 20), chromosome aberration or gene disorder concerning the previous pregnancy (n = 11), and advanced maternal age (n = 40).
Exclusion criteria of the study were identified as follows: multiple pregnancies, fetal or neonatal structural or genetic anomaly, improper localization of the placenta for sonography (placenta praevia, posterior placenta), pathological placentation (placenta accreta spectrum), self-reported drugs, alcohol, caffeine or nicotine abuse, or exposure to circulatory medication (oxerutins, calcium dobesilate), systemic disease (e. g., any type of pregestational diabetes mellitus, autoimmune disease, vasculitis, hemophylia, thrombophylia, HIV infection).
Women with complications during late pregnancy (GDM treated with diet (n = 12), hypertensive related diseases (n = 11), small for gestational age (SGA) at delivery (n = 4), large for gestational age at delivery (n = 10)) were not excluded from the study.
The study protocol was approved by the Clinical Research Ethics Committee of the University of Szeged (Ref. no.:09/2017). The study was carried out according to the Principles of the Declaration of Helsinki. We obtained written informed consent from all participants.
Conventional 2-dimensional (2-D) sonographic examinations
All patients were categorized by the measurement of the crown rump length (CRL). NT and anatomic assessment between 11+0 and 13+6 weeks were performed by conventional methods. Ultrasound examination took place before measuring AC to determine the number of fetuses, fetal biometry, fetal anomalies, placental location and the amount of amniotic fluid. Fetal weight was estimated according to the method of Hadlock et al. [
26] after measuring the necessary sonographic parameters (biparietal diameter, head circumference, abdominal circumference and femur length). Estimated fetal weight percentile was calculated according to the local standards [
27]. The ultrasound investigations were conducted by J.S., A.S. and A.M.
Volume acquisition
Acquisition of the images used for the determination of placental volume and 3-dimensional Power Doppler (3-DPD) indices were obtained at the time of the visit. All 3-D scans were performed by A.S. Voluson 730 Expert ultrasound machine (GE Medical Systems, Kretztechnik GmbH & Co OHG, Austria) equipped with a multifrequency probe (2–5MHz) that was used to acquire all images. Each sample was examined using the 3-D rendering mode, in which the color and grey value information were processed and combined to give a 3-D image (mode cent; smooth: 4/5; FRQ: low; quality: 16; density: 6; enhance: 16; balance: 150; filter: 2; actual power: 2dB; pulse repetition frequency: 0.9). We used fast low-resolution acquisition to avoid any kind of artefacts. The 3-D static volume box was placed over the highest villous vascular density zone at the umbilical cord insertion [
28]. Each image was recovered from the disc in succession for processing. During gestation we recorded one sample from each patient.
Determination of Power Doppler Indices
The stored volumes were further analyzed using the Virtual Organ Computer-aided Analysis (VOCAL) program pertaining to the computer software 4DView (GE Medical Systems, Austria, version 10.4) by the same expert in 3-D analysis (A.S.). Image used for recovering from the hard disc was captured and processed using the multiplanar system. The spherical sample volume was constantly 28 ml. The VOCAL program calculated automatically the grey and color scale values in a histogram from the acquired spherical sample volume in all cases. The combined use of power Doppler with three-dimensional ultrasound provides the possibility of quantifying blood in motion within a volume of interest. Three indices were calculated, namely vascularization index (VI), flow index (FI) and vascularization flow index (VFI) as estimates of the percentage of the volume filled with detectably moving blood. VI (expressed as a percentage) is the proportion of color voxels in the studied volume, representing the proportion of blood vessels within the tissue. FI (expressed as a scale of 0–100) is the average value of all color voxels, representing the average power Doppler amplitude within blood vessels. VFI (expressed as a scale of 0–100) is the average color value of all grey and color voxels, a product of the number of color voxels as a percentage and the relative amplitude of these voxels [
29,
30].
The intraobserver errors were evaluated by repeated measurements of the 3-DPD indices at the initiation of the study. The intra-class correlation coefficients for all Doppler indices were excellent (0.99) for all indices.
Amniocentesis procedure
The patients were informed about the procedure and possible complications, before a consent form was signed prior to the procedure. All procedures were performed by the same expert operator (J.S.) at the outpatient unit, who followed the standard protocol. A local antiseptic was applied to the skin. A 22-gauge spinal needle was inserted under continuous ultrasound guidance and needle insertion through placenta was avoided. Amniotic fluid (8–10 mL) was aspirated and the first 2 mL of each sample was discarded to prevent the contamination with maternal cell. Blood-contaminated amniotic fluid was not utilized. Fetal heart rate was evaluated after the procedure and no stillbirth or premature rupture was observed. Following amniocentesis, anti-D immunoglobulin was administered, when it was necessary.
Samples
Amniotic fluid and maternal venous blood were collected from each patient at the time of amniocentesis. Blood samples were centrifuged at 3400RPM for 15 minutes. Serum and amniotic fluid samples were stored at -80 °C until assayed.
Enzyme-linked immunosorbent assay (ELISA)
Human PP13 and human sHLA-G in maternal serum and amniotic fluid were determined by ELISA. The laboratory staff who performed the assays were blinded to the pregnancy outcome, and the clinician recruiting the women did not participate at analyzing the samples.
Concentration of sHLA-G were measured by kits from Elabscience Biotechnology Corporation (Houston, TX, USA). The sensitivity of the assay was 0.38 ng/ml. The intra- and inter-assay coefficients of variation were <10% according to the manufacturer.
Human PP13 levels were determined by Cusabio kits (Wuhan Huamei Biotec Co. LTD. Wuhan, China). The sensitivity of the assay was <3.9 pg/ml. The intra- (<8%) and inter- (<10%) assay coefficient of variation according to the manufacturer.
Data and statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as numbers and percentages. The relationship between the level of angiogenic factors (HLA-G5 and PP13) and other continuous variables was assessed using Pearson’s correlation coefficients and regression analyses. Multiple linear regression was adjusted for maternal age, BMI at the time of genetical consultation, number of previous pregnancies and gestational age at the time of amniocentesis, as these factors determine the actual placental volume and fetal weight. Correlation coefficients (B) were calculated for both univariate and multiple linear regression, whereas standardized coefficients (ß) were given for univariate analyses and semi-partial correlations (r) for multivariable regression. Independent samples t-tests were used to determine, whether the angiogenic factor levels in body fluid were different in complicated pregnancies vs. healthy controls. Paired samples t-test was applied to analyze the differences between the serum and amniotic levels of the analytes. The two-tailed statistical significance level was set at 5%, and p-values were adjusted using a Holm–Bonferroni correction for multiple comparisons.
Results
Demographic characteristics of the group is shown in
Table 1. As expected, amniocentesis has been proposed mostly for pregnant women with advanced age (mean 34.52 years). Women who participated in this study had a mean body mass index (BMI) of 26.98 kg/m
2 and approximately one third (32.39%) of the participants were primiparous. The mean gestational age during amniocentesis was 18+2 weeks. Sonographic parameters are presented in
Table 2. The mean percentiles of the anthropometric measurements were near the median. We found relatively large variation of intrauterine placental volume and perfusion indices.
Table 3 gives an overview of the levels of angiogenic factors in the amniotic fluid samples. The mean concentrations of angiogenic factors were as follows: 8.68 pg/ml for PP-13 in amniotic fluid and 204.23 pg/ml for PP-13 in serum, 55.89 ng/ml for sHLA-G in amniotic fluid and 55.84 ng/ml for sHLA-G in serum.
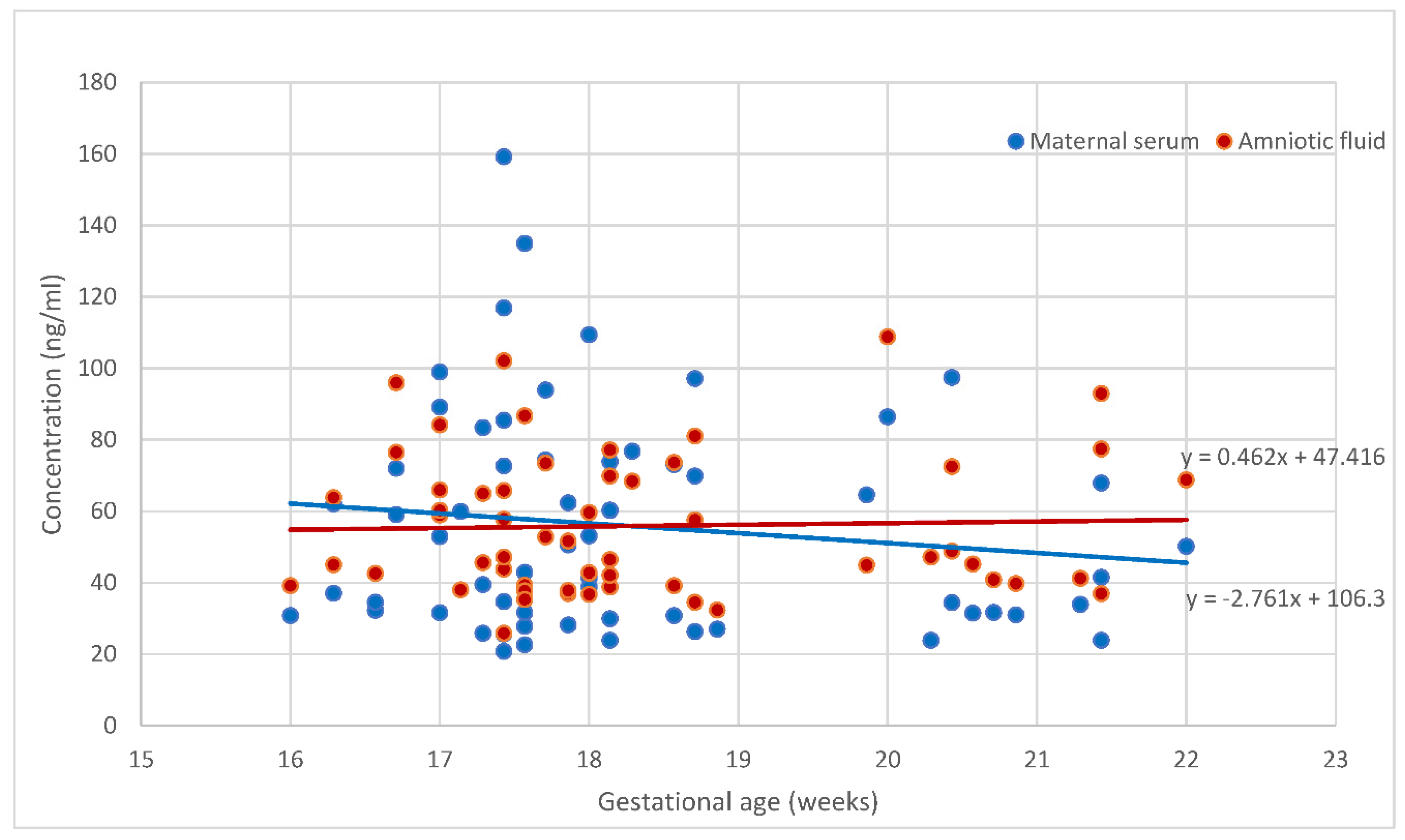
Figure 1 and
Figure 2 demonstrate the placental angiogenic factor levels in the body fluids according to the gestational age. The sHLA-G levels were steady during this gestational period. PP13 concentration in amnion was unchanged between 16+0 and 22+6 weeks of gestation, while the concentrations of PP13 in the serum slightly decreased, but the reduction was below statistical significance. The concentrations in serum and amniotic fluid were significantly different for PP13 (p<0.001), but not for the sHLA-G (p>0.05).
When regrouping was performed and uncomplicated pregnancies were compared to those complicated with hypertensive disorders during pregnancies, diabetes in pregnancy, small or large for gestational age at pregnancy, then no placental biomarkers exhibited significant difference with regard to any of the measured factors in the serum and in the amniotic fluid (data will publish later).
Table 4 displays the correlations to the levels of angiogenic factors. An inverse correlation emerged between sHLA-G levels and PP13 levels in the serum in univariate and multivariate analyses (r = -0.186, B = -0.07, ß
2 = 0.14 and B = -0.07, r
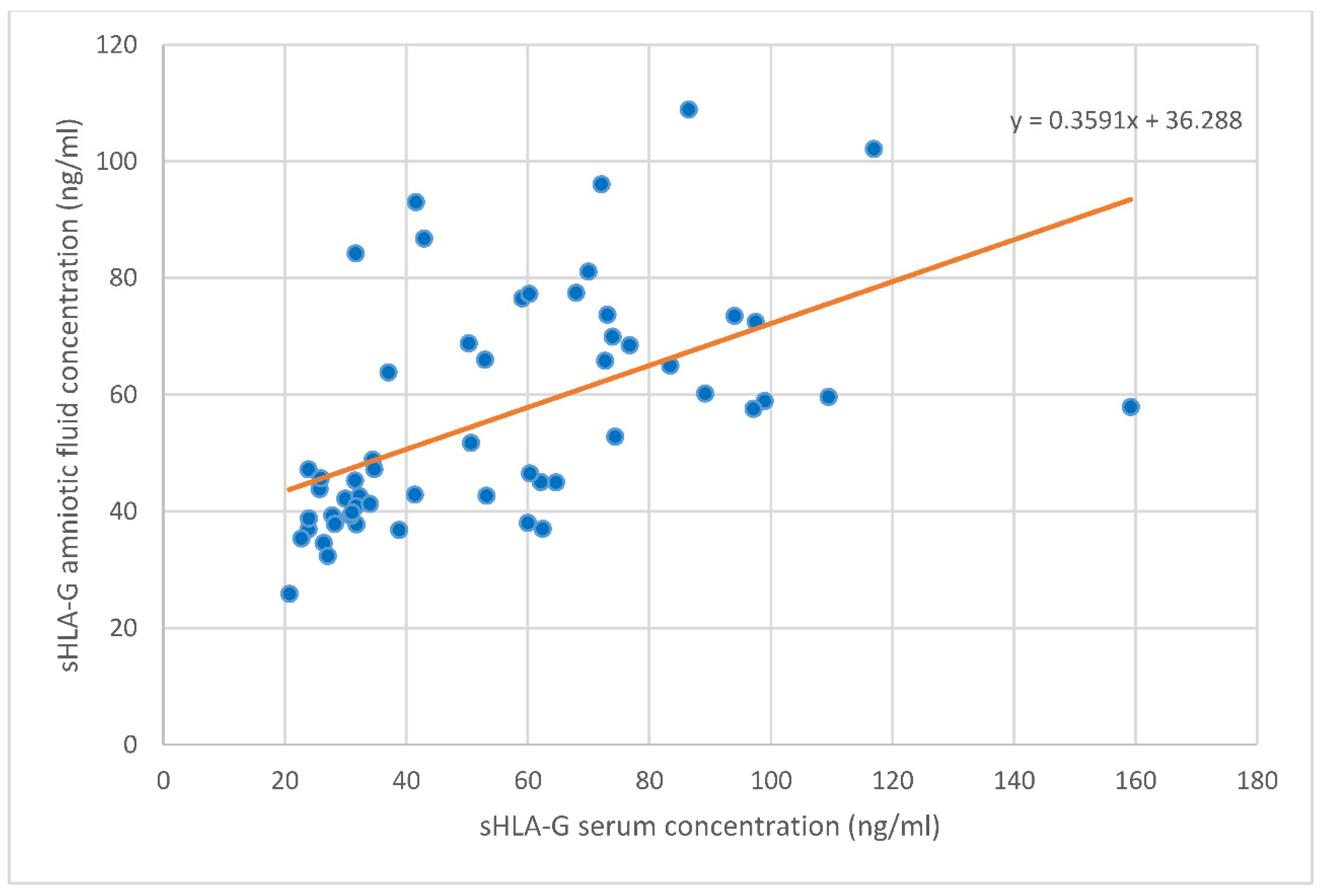
2 = 0.16, respectively). Whereas sHLA-G levels in the serum and amniotic fluid appeared to be strongly correlated positively with each other (univariate analysis: r = 0.662, B = 0.36, ß
2 = 0.29 and multivariate analysis: B = 0.37, r
2 = 0.30, respectively,
Figure 3). No other correlations were found between the angiogenic factor levels in serum and amniotic fluid.
Table 5 demonstrates the sonographic correlates of sHLA-G levels. The sHLA-G serum concentrations tended to be higher with larger placental volumes (univariate analysis: r = 0.142, B = 0.09, ß
2 = 0.09 and multivariate analysis: B = 0.09, r
2 = 0.06, respectively). There was a strong indirect correlation between VFI and sHLA-G levels in amniotic fluid (univariate analysis: r = -0.450, B = -1.90, ß
2 = 0.14 and multivariate analysis: B = -2.00, r
2 = 0.13, respectively).
The correlations between PP13 levels and sonographic features are displayed in
Table 6. The PP13 concentration in the amniotic fluid proved a negative correlation with fetal weight (univariate analysis: r = -0.102, B = -0.01, ß
2 = 0.07 and multivariate analysis: B = -0.01, r
2 = 0.06, respectively) and gestational age at delivery (univariate analysis: r = -0.155, B = -2.64, ß
2 = 0.15 and multivariate analysis: B = -3.02, r
2 = 0.18, respectively). An inverse correlation was observed between the level of PP13 in the amniotic fluid and fetal abdominal circumference at amniocentesis (univariate analysis: r = -0.098, B = 0.31, ß
2 = 0.15 and multivariate analysis: B = 0.49, r
2 = 0.09, respectively).
Discussion
Placental growth encompasses extensive vascularization in the chorionic villi which is regulated by angiogenic factors [
5,
10,
25,
31]. Circulating angiogenic factors have been shown to be associated with fetoplacental growth [
9,
10,
14,
25,
32] and pregnancy disorders [
14,
32]. In this study, we observed that sHLA-G concentration in the maternal serum and amniotic fluid is stable in the mid-trimester, despite the expansive increase of the trophoblasts, fetal membrane epithelial and immune cells secreting sHLA-G proteins. The unchanged longitudinal concentration of the sHLA-G in the body fluids is in correlation with the results reported by others [
9,
23,
32,
33,
34].
Moreover, there is a paucity in the literature on the soluble HLA-G levels in the amniotic fluid [
7,
24,
35]. A new and interesting finding is that the amniotic sHLA-G level correlates highly with that of the maternal peripheral blood level at the time of amniocentesis. The amniotic sHLA-G is mainly synthetized by villous trophoblasts and therefore it is of fetal origin. The strong positive correlation of the amniotic sHLA-G level with the maternal serum level remains to be elusive [
19]. One of the possible explanations may be that extravillous trophoblasts (EVT) produce sHLA-G both to maternal serum and amniotic fluid.
Furthermore, one of the principal results of the present study is that sHLA-G level in maternal serum is a valuable marker of placental growth, but not for fetal growth. This can be explained by the fact that increased amount of EVT cells in a larger placenta can promote an elevated serum sHLA-G level in pregnant women. On the other hand, the elevated secretion of sHLA-G by maternal monocytes can be stimulated by the increased expression of antigens presented by larger EVT volume [
5,
35].
Conversely, the sHLA-G level in the amnion was negatively correlated with the vascular perfusion of the placenta. We assume, that the sHLA-G secretion may be reactive to reduced blood flow and vascular network in the expanding placenta as gestation advances, since sHLA-G facilitates the process of vasculogenesis [
9]. The expansion of placental volume may outweigh the increasing trends of capillary branching. To evaluate the placental perfusion in-vivo, we used “Mercé-type sonobiopsy” which is a reproducible and validated method [
36,
37,
38], and by obtaining a representative sample of the placental tree it is applicable throughout the whole pregnancy in contrast to other methods [
29], when the entire placenta needs to be visualized [
39,
40]. No significant correlation was found between the fetal growth parameters measured by ultrasound examination and the corresponding level of sHLA-G in different body fluids. One study [
9] has found statistically significant correlation between sHLA-G1 levels in the maternal serum and neonatal birth weight and placental weight at delivery. This is not in contradiction of our results since we detected all the sHLA-G molecules in the serum and in the amnion.
The importance of sHLA-G was supported by its lower serum level in PE according to several reports [
6,
24,
34], whereas a higher sHLA-G level in the amniotic fluid as well as in maternal blood indicates the immune response to maternal infection, inducing preterm birth [
7,
32,
35].
We found a small, non-significant decreasing tendency in PP13 concentration in the maternal serum and a constant PP13 level in the amniotic fluid between the 16th and 23rd weeks of gestation. Other studies reporting on a minimal elevation of serum PP13 concentration throughout all the trimesters [
41,
42,
43,
44]. Moreover, most of the studies on PP13 serum concentrations focus on its prognostic performance in the field of pregnancy outcome in the first trimester and at term [
42]. Our further interesting finding is that there is a decimal range difference between the levels in the serum and in amniotic fluid, which was also reported by Sammar et al. [
43] in both complicated (PE) and uncomplicated pregnancies in the third trimester. Like others [
41,
42,
43,
44], we noticed a wide range of distribution of PP13 levels both in the serum and amniotic fluid. Only two earlier studies have evaluated the secretion of PP13 into the amniotic fluid [
43,
45]. Sammar et al. [
43] concluded that PP13 level measured in the amniotic fluid that was derived from Cesarean section is steady between 30 and 42 weeks of gestation, while a declining trend for PP13 level can be described in PE between 26 and 36 weeks of gestation. Inconsistently, the amount of PP13 detected in amniotic fluid was twice as much as in serum samples in case of a small-scale study [
45].
In our dataset, there was no difference in the serum PP13 levels between the women with pregnancy related hypertensive disorders or SGA and controls. This was supported by Burger et al. [
45] indicating that the serum PP13 levels are lower in the first trimester in these types of complications, whereas the levels are higher in the third trimester, and the transition between the low to the high levels occurs between 16th and 20th gestational week [
45], which includes the gestational range of our study. Several studies highlight the predictive value of serum PP13 concentration during the first trimester in developing preeclampsia [
20,
42,
46], whereas no evidence has been raised on corresponding tendency in the second trimester [
47].
Our study revealed, that PP13 levels in amniotic fluid in the second trimester can predict the birth weight, which has not been proved previously. There is reverse correlation between PP13 and birth weight. An additional finding is that the PP13 levels in body fluids are not related to the size of the placenta. However, the opposite was confirmed by Sharawand et al. [
48], describing that maternal serum PP13 level is associated with placental volume in the first trimester. On the other hand, it is unclear, why the amniotic PP13 levels prognosticate lower birth weight at delivery, since it is secreted from placental trophoblasts and is involved in normal implantation and placentation [
42].
The strength of our study is that a robust, large-scale amniocentesis study was performed in one center in respect of the prognostic efficacy of angiogenic biomarkers for pregnancy outcome. The limitation of the study is that the samples were harvested from a high-risk population and hence generalizability of the study results is not possible for the whole population.
Our conclusion of particular value is to provide that maternal sHLA-G and PP13 concentrations in the serum correlate negatively with each other and sHLA-G levels in different body fluids are strongly positively correlated with each other. PP13 decreases slightly in the maternal serum, but not in the amniotic fluid, whereas HLA-G is unchanged in both compartments in the mid-pregnancy, in line with other published studies. Amniotic PP13 might be used as predictor of pregnancy outcome, while sHLA-G is a potent biomarker in placental development. Also, this study indicates that late complications during pregnancy are not associated with altered level of sHLA-G and PP13 taken at amniocentesis
Author Contributions
Conceptualization, M.V., Z.K., J. S.; methodology, M.V., Z.K., J. S.; formal analysis, A.M. writing—original draft preparation, M.V., Z.K.; writing—review and editing, M.V., Z.K., A.S., J.S., J.S.Jr.; supervision, G.N., Sz.V.; data collection, M.V., J.S.Jr., and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
Hetényi Grant of Albert Szent-Györgyi Medical School of the University of Szeged.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available by the corresponding authors on request.
Acknowledgments
The authors would like to thank to Fredrik Johansson for the help in statistical calculations.
Conflicts of Interest
the authors declare no conflict of interest.
References
- Cuffe JSM, Holland O, Salomon C, Rice GE, Perkins A V. Review: Placental derived biomarkers of pregnancy disorders. Placenta. 2017;54. [CrossRef]
- Chen DB, Zheng J. Regulation of Placental Angiogenesis. Microcirculation. 2014;21(1). [CrossRef]
- Le Bouteiller P. Human villous trophoblast and the lack of intron 4-retaining soluble HLA-G secretion: Beware of possible methodological biases. Mol Hum Reprod. 2005;11(10). [CrossRef]
- Morales PJ, Pace JL, Platt JS, Langat DK, Hunt JS. Synthesis of β2-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology. 2007;122(2). [CrossRef]
- Xu X, Zhou Y, Wei H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front Immunol. 2020;11. [CrossRef]
- Yie SM, Li LH, Li YM, Librach C. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am J Obstet Gynecol. 2004;191(2). [CrossRef]
- Tantengco OAG, Richardson L, Lee A, Kammala A, Silva M de C, Shahin H; et al. Histocompatibility antigen, class i, g (Hla-g)’s role during pregnancy and parturition: A systematic review of the literature. Life. 2021;11(10). [CrossRef]
- Emmer PM, Joosten I, Schut MH, Zusterzeel PLM, Hendriks JCM, Steegers EAP. Shift in expression of HLA-G mRNA spliceforms in pregnancies complicated by preeclampsia. J Soc Gynecol Investig. 2004;11(4). [CrossRef]
- Rizzo R, Andersen AS, Lassen MR, Sørensen HC, Bergholt T, Larsen MH; et al. Soluble Human Leukocyte Antigen-G isoforms in maternal plasma in early and late pregnancy. American Journal of Reproductive Immunology. 2009;62(5). [CrossRef]
- Barbaro G, Inversetti A, Cristodoro M, Ticconi C, Scambia G, Di Simone N. HLA-G and Recurrent Pregnancy Loss. Int J Mol Sci. 2023;24(3). [CrossRef]
- Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot-Leyden Crystal protein. Placenta. 1999;20(8). [CrossRef]
- Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z; et al. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271(6). [CrossRef]
- Gadde R, Dayanand CD, Sheela SR. Placental protein 13: An important biological protein in preeclampsia. J Circ Biomark. 2018;7. [CrossRef]
- Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H; et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound in Obstetrics and Gynecology. 2006;27(1). [CrossRef]
- Khalil A, Cowans NJ, Spencer K, Goichman S, Meiri H, Harrington K. First trimester maternal serum placental protein 13 for the prediction of pre-eclampsia in women with a priori high risk. Prenat Diagn. 2009;29(8). [CrossRef]
- Unverdorben L, Hüttenbrenner R, Knabl J, Jeschke U, Hutter S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta. 2015;36(2). [CrossRef]
- Kuc S, Wortelboer EJ, Van Rijn BB, Franx A, Visser GHA, Schielen PCJI. Evaluation of 7 serum biomarkers and uterine artery doppler ultrasound for first-trimester prediction of preeclampsia: A systematic review. Obstet Gynecol Surv. 2011;66(4). [CrossRef]
- Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000;183(3). [CrossRef]
- Rebmann V, Pfeiffer K, Päßler M, Ferrone S, Maier S, Weiss E; et al. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53(1). [CrossRef]
- Chafetz I, Kuhnreich I, Sammar M, Tal Y, Gibor Y, Meiri H; et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197(1). [CrossRef]
- Alegre E, Díaz-Lagares A, LeMaoult J, López-Moratalla N, Carosella ED, González A. Maternal antigen presenting cells are a source of plasmatic HLA-G during pregnancy: Longitudinal study during pregnancy. Hum Immunol. 2007;68(8). [CrossRef]
- Shaikly VR, Morrison IEG, Taranissi M, Noble C V., Withey AD, Cherry RJ; et al. Analysis of HLA-G in Maternal Plasma, Follicular Fluid, and Preimplantation Embryos Reveal an Asymmetric Pattern of Expression. The Journal of Immunology. 2008;180(6). [CrossRef]
- Hackmon R, Hallak M, Krup M, Weitzman D, Sheiner E, Kaplan B; et al. HLA-G antigen and parturition: Maternal serum, fetal serum and amniotic fluid levels during pregnancy. Fetal Diagn Ther. 2004;19(5). [CrossRef]
- Hackmon R, Koifman A, Hyobo H, Glickman H, Sheiner E, Geraghty DE. Reduced third-trimester levels of soluble human leukocyte antigen G protein in severe preeclampsia. Am J Obstet Gynecol. 2007;197(3). [CrossRef]
- Sammar M, Drobnjak T, Mandala M, Gizurarson S, Huppertz B, Meiri H. Galectin 13 (PP13) facilitates remodeling and structural stabilization of maternal vessels during pregnancy. Int J Mol Sci. 2019;20(13). [CrossRef]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985 Feb;151(3):333–7. [CrossRef]
- K. Joubert. Magyar születéskori testtömeg- és testhossz-standardok az 1990-96. évi országos élveszületési adatok alapján. Magy Noorv Lapja. 2000;63(12):155–63.
- Suranyi A, Kozinszky Z, Molnar A, Nyari T, Bito T, Pal A. Placental three-dimensional power Doppler indices in mid-pregnancy and late pregnancy complicated by gestational diabetes mellitus. Prenat Diagn. 2013 Oct;33(10):952–8. [CrossRef]
- Molnar A, Suranyi A, Nyari T, Nemeth G, Pal A. Examination of placental three-dimensional power Doppler indices and perinatal outcome in pregnancies complicated by intrauterine growth restriction. Int J Gynaecol Obstet. 2015 Apr;129(1):5–8. [CrossRef]
- Lai PK, Wang YA, Welsh AW. Reproducibility of regional placental vascularity/perfusion measurement using 3D power Doppler. Ultrasound Obstet Gynecol. 2010 Aug;36(2):202–9. [CrossRef]
- Sikovanyecz J, Vincze M, Földesi I, Németh G, Kozinszky Z. Angiogenic factors measured in aspirated placental tissue between the 10 + 6 and 18 + 3 weeks of gestation. Reprod Biol. 2021;21(4). [CrossRef]
- Beneventi F, Locatelli E, De Amici M, Martinetti M, Spinillo A. Soluble HLA-G concentrations in obese women during pregnancy and in cord blood. J Reprod Immunol. 2017;119. [CrossRef]
- Krop J, Van Der Keur C, Kapsenberg JM, Den Hollander F, Van Der Hoorn MLP, Heidt S; et al. Soluble HLA-G blood levels are not increased during ongoing pregnancy in women with a history of recurrent pregnancy loss. J Reprod Immunol. 2022;153. [CrossRef]
- Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H. Placental abruption is associated with decreased maternal plasma levels of soluble HLA-G. J Clin Immunol. 2003;23(4). [CrossRef]
- Kusanovic JP, Romero R, Jodicke C, Mazaki-Tovi S, Vaisbuch E, Erez O; et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22(12). [CrossRef]
- Merce LT, Barco MJ, Bau S. Reproducibility of the study of placental vascularization by three-dimensional power Doppler. J Perinat Med. 2004;32(3):228–33. [CrossRef]
- Merce LT, Barco MJ, Bau S, Kupesic S, Kurjak A. Assessment of placental vascularization by three-dimensional power Doppler “vascular biopsy” in normal pregnancies. Croat Med J. 2005 Oct;46(5):765–71.
- Tuuli MG, Houser M, Odibo L, Huster K, Macones GA, Odibo AO. Validation of placental vascular sonobiopsy for obtaining representative placental vascular indices by three-dimensional power Doppler ultrasonography. Placenta. 2010 Mar;31(3):192–6. [CrossRef]
- Rizzo G, Capponi A, Pietrolucci ME, Aiello E, Arduini D. First trimester placental volume and three dimensional power doppler ultrasonography in type I diabetic pregnancies. Prenat Diagn. 2012 May;32(5):480–4. [CrossRef]
- de Paula CFS, Ruano R, Campos JADB, Zugaib M. Quantitative analysis of placental vasculature by three-dimensional power Doppler ultrasonography in normal pregnancies from 12 to 40 weeks of gestation. Placenta. 2009 Feb;30(2):142–8. [CrossRef]
- Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B; et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Archiv. 2008;453(4). [CrossRef]
- Huppertz B, Sammar M, Chefetz I, Neumaier-Wagner P, Bartz C, Meiri H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn Ther. 2008;24(3). [CrossRef]
- Sammar M, Nisemblat S, Fleischfarb Z, Golan A, Sadan O, Meiri H; et al. Placenta-bound and body fluid PP13 and its mRNA in normal pregnancy compared to preeclampsia, HELLP and preterm delivery. Placenta. 2011;32(SUPPL. 1). [CrossRef]
- Moslemi Zadeh N, Naghshvar F, Peyvandi S, Gheshlaghi P, Ehetshami S. PP13 and PAPP-A in the First and Second Trimesters: Predictive Factors for Preeclampsia? ISRN Obstet Gynecol. 2012;2012. [CrossRef]
- Burger O, Pick E, Zwickel J, Klayman M, Meiri H, Slotky R; et al. Placental protein 13 (PP-13): Effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta. 2004;25(7). [CrossRef]
- Wu P, Van Den Berg C, Alfirevic Z, O’brien S, Röthlisberger M, Baker PN; et al. Early pregnancy biomarkers in pre-eclampsia: A systematic review and meta-analysis. Int J Mol Sci. 2015;16(9). [CrossRef]
- Spencer K, Cowans NJ, Chefetz I, Tal J, Kuhnreich I, Meiri H. Second-trimester uterine artery Doppler pulsatility index and maternal serum PP13 as markers of pre-eclampsia. Prenat Diagn. 2007;27(3). [CrossRef]
- Sahraravand M, Järvelä IY, Laitinen P, Tekay AH, Ryynänen M. The secretion of PAPP-A, ADAM12, and PP13 correlates with the size of the placenta for the first month of pregnancy. Placenta. 2011;32(12). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).