Submitted:
10 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Clinical samples
2.2. Immunohistochemistry (IHC)
2.3. Immunofluorescence staining
2.4. Quantification and statistical analysis
3. Results
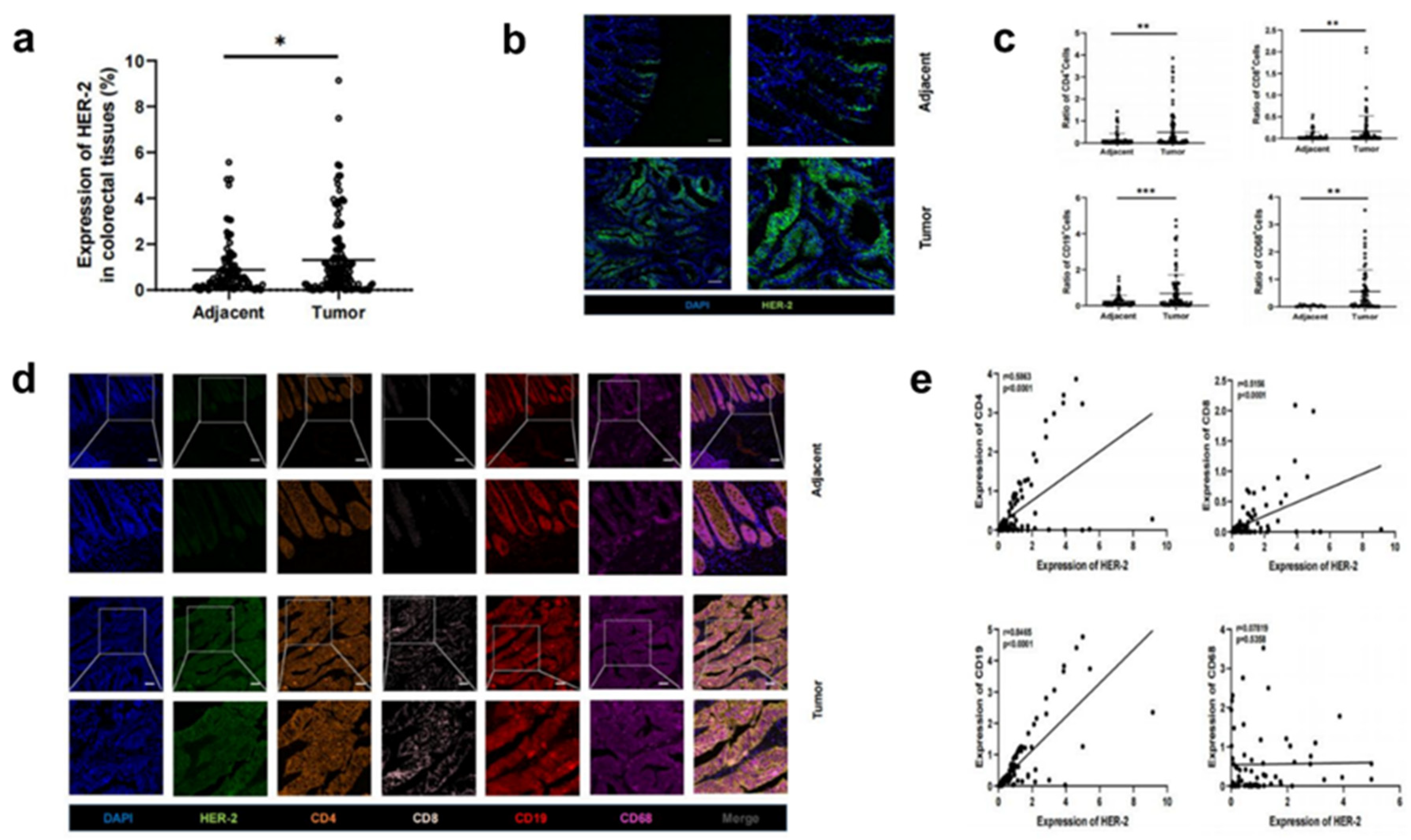
3.1. Increased HER-2 expression and increased number of immune cells in CRC tissues compared to paracancerous tissues
3.2. Increased HER-2 expression in CRC tissues is accompanied by increased infiltration of immune cells
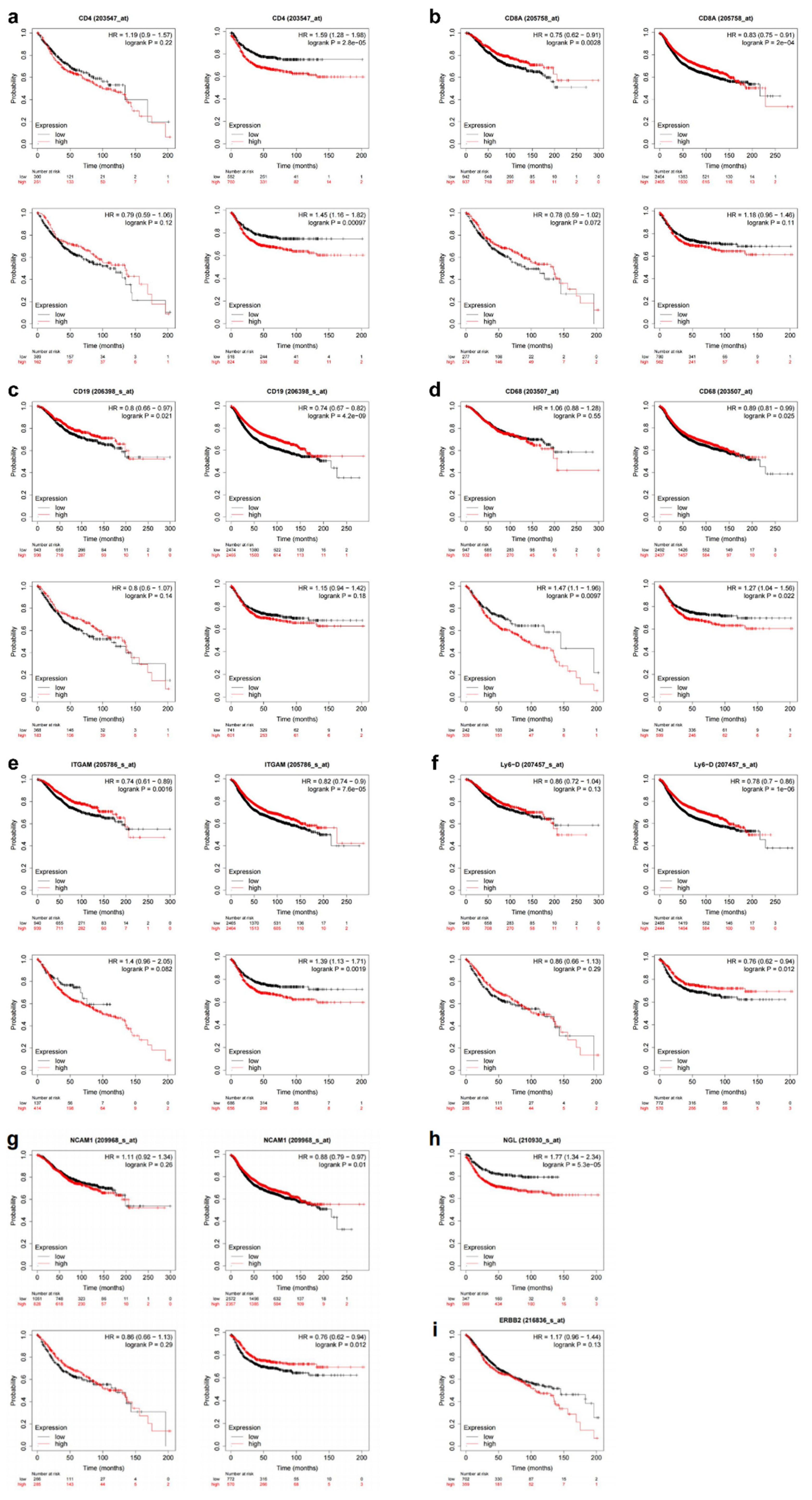
3.3. Upregulation of HER-2 expression is a risk factor for death in CRC patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H. , et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal For Clinicians, 2021. 71(3): p. 209-249. [CrossRef]
- Siegel, R.L. , et al., Cancer statistics, 2022. CA Cancer J Clin, 2022. 72(1): p. 7-33.
- Richman, S.D. , et al., HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. The Journal of Pathology, 2016. 238(4): p. 562-570. [CrossRef]
- Siena, S. , et al., Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Annals of Oncology: Official Journal of the European Society For Medical Oncology, 2018. 29(5): p. 1108-1119.
- Eiermann, W. , Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: pivotal trial data. Annals of Oncology: Official Journal of the European Society For Medical Oncology, 2001. 12 Suppl 1: p. S57-S62. [CrossRef]
- Bang, Y.-J. , et al., Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England), 2010. 376(9742): p. 687-697. [CrossRef]
- Keam, S.J. , Trastuzumab Deruxtecan: First Approval. Drugs, 2020. 80(5): p. 501-508. [CrossRef]
- Sartore-Bianchi, A. , et al., Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. The Lancet. Oncology, 2016. 17(6): p. 738-746. [CrossRef]
- Siena, S. , et al., Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. The Lancet. Oncology, 2021. 22(6): p. 779-789. [CrossRef]
- Strickler, J.H. , et al., Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA Oncol, 2022. 8(5): p. 760-769.
- Clifton, G.T. , Peoples, and E. George, Immunotherapy as a partner for HER2-directed therapies. Expert Review of Anticancer Therapy, 2021. 21(7): p. 739-746.
- Pagès, F. , et al., International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet (London, England), 2018. 391(10135): p. 2128-2139. [CrossRef]
- Mlecnik, B. , et al., Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. Journal of the National Cancer Institute, 2018. 110(1). [CrossRef]
- Denkert, C. , et al., Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. The Lancet. Oncology, 2018. 19(1): p. 40-50. [CrossRef]
- Nuciforo, P. , et al., A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Annals of Oncology: Official Journal of the European Society For Medical Oncology, 2018. 29(1): p. 170-177. [CrossRef]
- Griguolo, G. , et al., Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. Journal For Immunotherapy of Cancer, 2019. 7(1): p. 90. [CrossRef]
- Slamon, D.J. , et al., Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, N.Y.), 1987. 235(4785): p. 177-182.
- [Chinese protocol of diagnosis and treatment of colorectal cancer of the National Health Commission (2023 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi = Chinese Journal of Gastrointestinal Surgery, 2023. 26(6): p. 505-528.
- Zhao, Y. , et al., XBP1 regulates the protumoral function of tumor-associated macrophages in human colorectal cancer. Signal Transduction and Targeted Therapy, 2021. 6(1): p. 357. [CrossRef]
- Valtorta, E. , et al., Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Modern Pathology: an Official Journal of the United States and Canadian Academy of Pathology, Inc, 2015. 28(11): p. 1481-1491.
- Lánczky, A. and B. Győrffy, Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. Journal of Medical Internet Research, 2021. 23(7): p. e27633. [CrossRef]
- Győrffy, B. , Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Computational and Structural Biotechnology Journal, 2021. 19: p. 4101-4109. [CrossRef]
- Siegel, R.L. , et al., Cancer statistics, 2022. CA: a Cancer Journal For Clinicians, 2022. 72(1).
- Benson, A.B. , et al., NCCN Guidelines Insights: Colon Cancer, Version 2.2018. Journal of the National Comprehensive Cancer Network: JNCCN, 2018. 16(4): p. 359-369.
- Benson, A.B. , et al., Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN, 2021. 19(3): p. 329-359.
- De Cuyper, A., M. Van Den Eynde, and J.-P. Machiels, HER2 as a Predictive Biomarker and Treatment Target in Colorectal Cancer. Clinical Colorectal Cancer, 2020. 19(2): p. 65-72. [CrossRef]
- Wu, S.-W., C. -C. Ma, and Y. Yang, The prognostic value of HER-2/neu overexpression in colorectal cancer: evidence from 16 studies. Tumour Biology: the Journal of the International Society For Oncodevelopmental Biology and Medicine, 2014. 35(11): p. 10799-10804.
- Wu, Q.B. and G.P. Sun, Expression of COX-2 and HER-2 in colorectal cancer and their correlation. World J Gastroenterol, 2015. 21(20): p. 6206-14.
- Li, C. , et al., HER-2 overexpression and survival in colorectal cancer: a meta-analysis. Journal of Zhejiang University. Science. B, 2014. 15(6): p. 582-589. [CrossRef]
- Dieci, M.V., F. Miglietta, and V. Guarneri, Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells, 2021. 10(2). [CrossRef]
- Pernas, S. and S.M. Tolaney, Clinical trial data and emerging strategies: HER2-positive breast cancer. Breast Cancer Research and Treatment, 2022. 193(2): p. 281-291. [CrossRef]
- Harris, S.J. , et al., Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biology & Medicine, 2016. 13(2): p. 171-193. [CrossRef]
- Park, S. , et al., The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell, 2010. 18(2): p. 160-170.
- Di Modica, M. , et al., Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition. Oncotarget, 2016. 7(1): p. 255-265. [CrossRef]
- Lasry, A., A. Zinger, and Y. Ben-Neriah, Inflammatory networks underlying colorectal cancer. Nature Immunology, 2016. 17(3): p. 230-240. [CrossRef]
- Gall, V.A. , et al., Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Research, 2017. 77(19): p. 5374-5383. [CrossRef]
- Garaud, S. , et al., Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight, 2019. 5(18). [CrossRef]
- Li, G.-M. , Mechanisms and functions of DNA mismatch repair. Cell Research, 2008. 18(1): p. 85-98. [CrossRef]
- Fan, A. , et al., Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci, 2021. 17(14): p. 3837-3849. [CrossRef]
- Zhang, Q.-w. , et al., Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS One, 2012. 7(12): p. e50946. [CrossRef]
- Dost Gunay, F.S. , et al., Tumor-associated Macrophages and Neuroendocrine Differentiation Decrease the Efficacy of Bevacizumab Plus Chemotherapy in Patients With Advanced Colorectal Cancer. Clinical Colorectal Cancer, 2019. 18(2): p. e244-e250. [CrossRef]
- Miao, H. , et al., Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nature Communications, 2016. 7: p. 11716. [CrossRef]
- Nielsen, S.R. and M.C. Schmid, Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators of Inflammation, 2017. 2017: p. 9624760. [CrossRef]
- Kim, S. , et al., WNT5A augments cell invasiveness by inducing CXCL8 in HER2-positive breast cancer cells. Cytokine, 2020. 135: p. 155213.
- Yuan, Q. , et al., Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Frontiers In Immunology, 2022. 13: p. 951137. [CrossRef]
- Mohamed, M.M. , et al., Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. The International Journal of Biochemistry & Cell Biology, 2014. 46: p. 138-147. [CrossRef]
- Ahmed, S. , et al., IL-8 secreted by tumor associated macrophages contribute to lapatinib resistance in HER2-positive locally advanced breast cancer via activation of Src/STAT3/ERK1/2-mediated EGFR signaling. Biochimica Et Biophysica Acta. Molecular Cell Research, 2021. 1868(6): p. 118995.
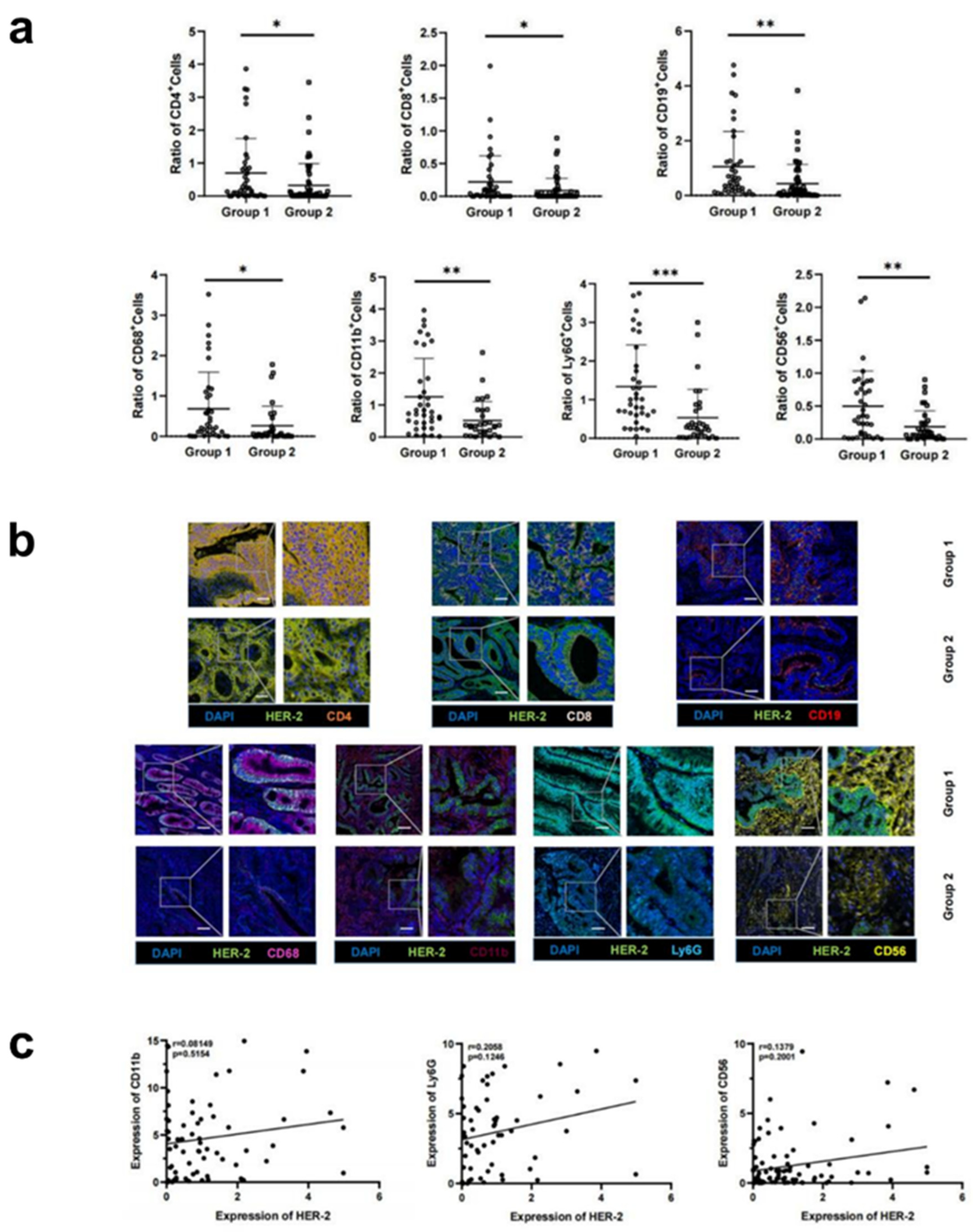
| cell | Mean ± SD | t-test | ||
|---|---|---|---|---|
| HER-2 up-regulated |
HER-2 non-up-regulated |
t | p | |
| CD4+ | 0.70 ± 0.37 | 0.32 ± 0.17 | 2.15 | 0.03 |
| CD8+ | 0.22 ± 0.13 | 0.09 ± 0.06 | 2.18 | 0.03 |
| CD68+ | 0.31 ± 0.19 | 0.12 ± 0.08 | 2.41 | 0.02 |
| CD19+ | 1.05 ± 0.62 | 0.43 ± 0.21 | 3.02 | <0.01 |
| LY6G+ | 1.34 ± 0.81 | 0.53 ± 0.23 | 3.58 | <0.01 |
| CD56+ | 0.50 ± 0.31 | 0.19 ± 0.1 | 3.20 | <0.01 |
| CD11b+ | 1.26 ± 0.74 | 0.52 ± 0.24 | 3.11 | <0.01 |
| Factor | Variables | Proportion |
|---|---|---|
| Age | ≥ 60 | 50 (51.5%) |
| < 60 | 47 (48.5%) | |
| Gender | Male | 57 (58.9%) |
| Female | 40 (41.2%) | |
| Tumor position | Ascending colon | 12(12.4%) |
| Transverse colon | 1(1.0%) | |
| Descending colon | 11(11.3%) | |
| Sigmoid colon | 25(25.8%) | |
| Rectum | 48(49.5%) | |
| T Stage | 1 | 3 (3.1%) |
| 2 | 21 (21.6%) | |
| 3 | 42 (43.3%) | |
| 4 | 31 (32.0%) | |
| N Stage | 0 | 49(50.5%) |
| 1 | 30(30.9%) | |
| 2 | 18(18.6%) | |
| M Stage | 0 | 94(96.9%) |
| 1 | 3(3.1%) | |
| Lymphovascular invasion | Yes | 32(33.0%) |
| No | 65(67.0%) | |
| Large vessel invasion | Yes | 15(15.5%) |
| No | 82(84.5%) | |
| Tumor budding | Yes | 24(24.7%) |
| No | 73(75.3%) | |
| Peripheral nerve invasion | Yes | 32(33.0%) |
| No | 65(67.0%) | |
| CEA | <5 | 59(60.8%) |
| ≥ 5 | 38(39.2%) | |
| HER-2 | - | 48(49.5%) |
| + | 39(40.2%) | |
| ++ | 7(7.2%) | |
| +++ | 3(3.1%) | |
| Differentiation | 1 | 9(9.3%) |
| 2 | 20(20.6%) | |
| 3 | 64(66.0%) | |
| 4 | 2(2.1%) | |
| 5 | 2(2.1%) | |
| Chemotherapy | Yes | 83(85.6%) |
| No | 14(14.4%) | |
| Recurrence-free survival (Recurrence event=1) |
0 | 73(75.3%) |
| 1 | 24(24.7%) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Age | 0.485 | 0.987(0.953-1.023) | ||
| Gender | 0.651 | 1.208(0.533-2.736) | ||
| Tumor position | 0.583 | 1.093(0.794-1.506) | ||
| T stage | 0.037 | 1.804(1.024-3.179) | 0.924 | 1.031(0.554-1.919) |
| N stage | 0.001 | 2.454(1.434-4.199) | 0.001 | 3.238(1.648-6.363) |
| M stage | 0.001 | 13.182(2.777-62.578) | 0.001 | 97.428(5.881-1613.942) |
| Lymphovascular invasion | 0.918 | 1.048(0.433-2.534) | ||
| Large vessel invasion | 0.102 | 2.269(0.827-6.224) | ||
| Tumor budding | 0.100 | 2.185(0.861-5.547) | ||
| Peripheral nerve invasion | 0.078 | 2.104(0.921-4.806) | ||
| CEA | 0.004 | 1.007(1.002-1.011) | 0.838 | 0.999(0.992-1.007) |
| HER-2 | 0.047 | 2.317(1.011-5.309) | 0.009 | 3.421(1.359-8.613) |
| Differentiation | 0.426 | 0.807(0.476-1.368) | ||
| Chemotherapy | 0.513 | 0.698(0.238-2.048) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





