4.1. Bottom-up procedure of fullerene organosols preparation
The variations of concentration and using different preparation methods, the fullerene spectra in high-solubility solvents stay almost unaffected. Contrary to it, a fundamental change is observed on going to polar solvents, and the DLS method firmly indicates appearance of colloidal species (
Figure 1).
Let us consider the bottom-up procedure. Dilution of molecular solutions with acetonitrile, ethanol, methanol, and other poor solvents leads to formation of colloidal solutions. In this case, the formation of colloidal species in solutions can be observed using UV-visible spectra. First it was demonstrated in the pioneering work by Sun and Bunker for fullerene C
70 in the toluene–acetonitrile binary solvent system [
45]. Since then, a number of such studies with C
60 and C
70 fullerenes was published; benzene, toluene, carbon disulfide and some other “good” solvents were used for preparation of the initial fullerene solutions [
21,
39,
40,
41,
42,
43,
44,
71,
72,
124,
125,
126].
Dilution of a fullerene solution in a polar solvent with another polar solvent is a special case [
27] that will be considered below. Some studies were performed in this laboratory [
70,
73,
98,
99,
101,
123].
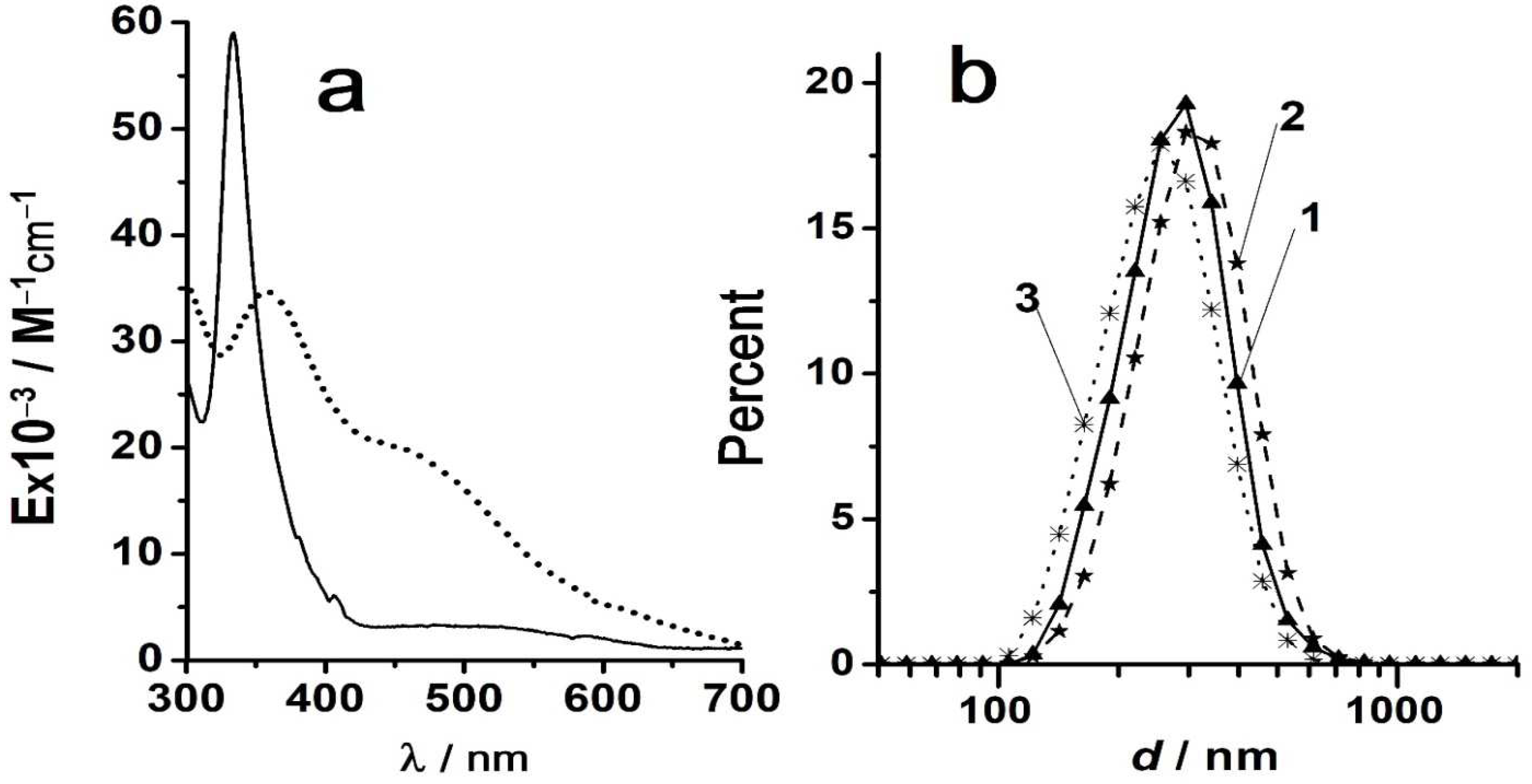
Figure 2 demonstrates the changes in the UV-visible absorption spectra of C
70 in toluene – acetonitrile along with the decrease in the CH
3CN concentration [
70,
99].
It is well documented that in the toluene–acetonitrile system, the solvent mixing regime is of key importance [
44,
46]. For example, dropwise or prompt adding of CH
3CN to a toluene solution of C
70 results in principally different absorption spectra [
46]. Molecule – aggregate transitions of fullerenes are accompanied by changes in fluorescence [
39,
43,
46].
It seems natural to reveal the critical composition of the binary solvent where the colloidal particles appear [
45]. Nath et al. [
42,
43] subjected this problem to detailed study. Basing on a number of binary solvent systems and using the
value as a characteristic of the solvent, they stated that C
60 forms molecular associates already at
about 12 – 13, while C
70 only at
27–31. Such difference is explained by a stronger interaction of C
70 with “good” solvents [
43]. These critical parameters were determined by both electronic absorption spectra and DLS, and the results agree [
42,
43]. It should be noted that in these works the
values of mixed solvents were calcullated using the additive scheme, which can lead to some (slight) errors [
73]. Also, it is well known that many properties of solutes can substantially differ in isodielectric solvents [
128].
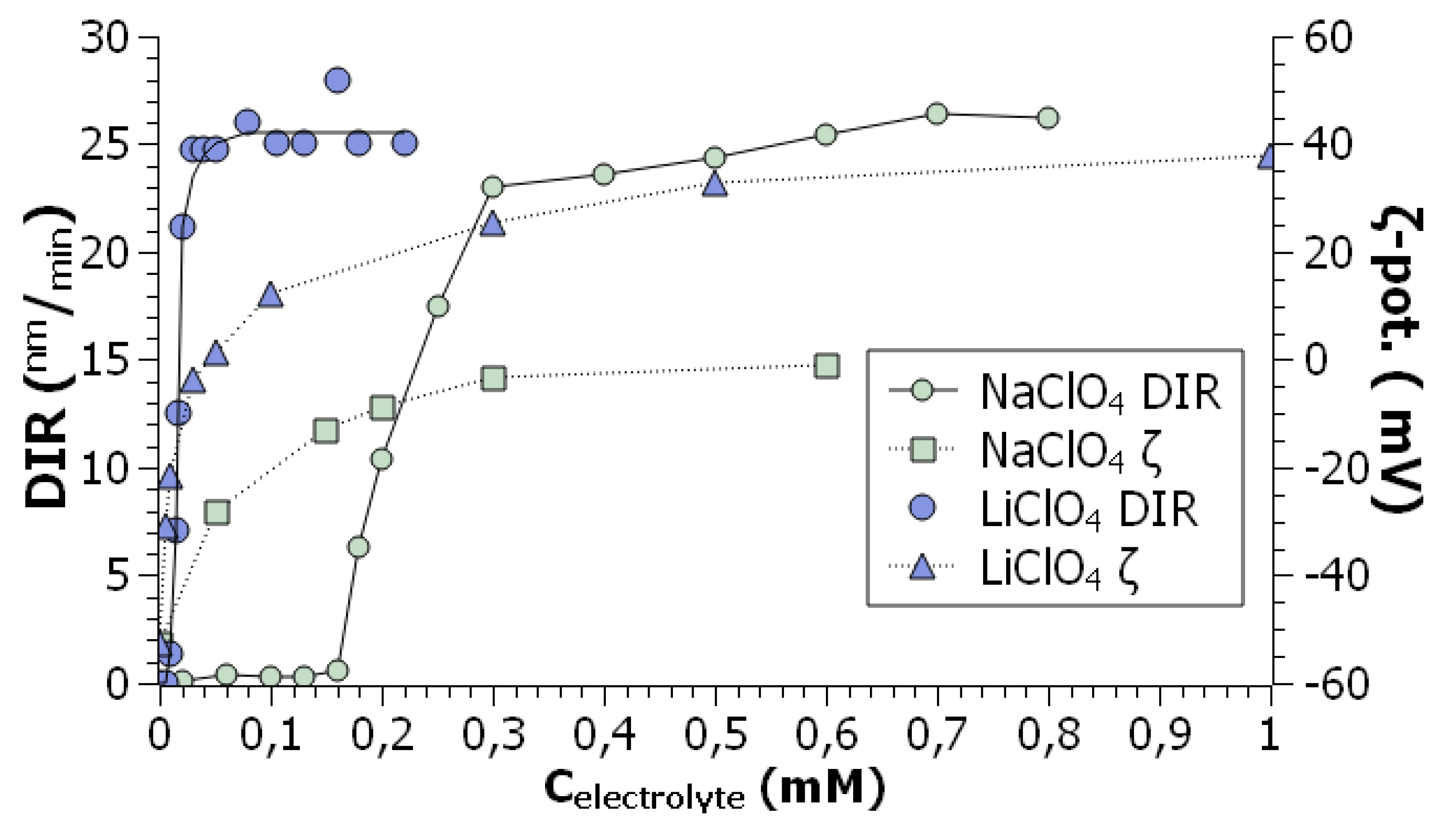
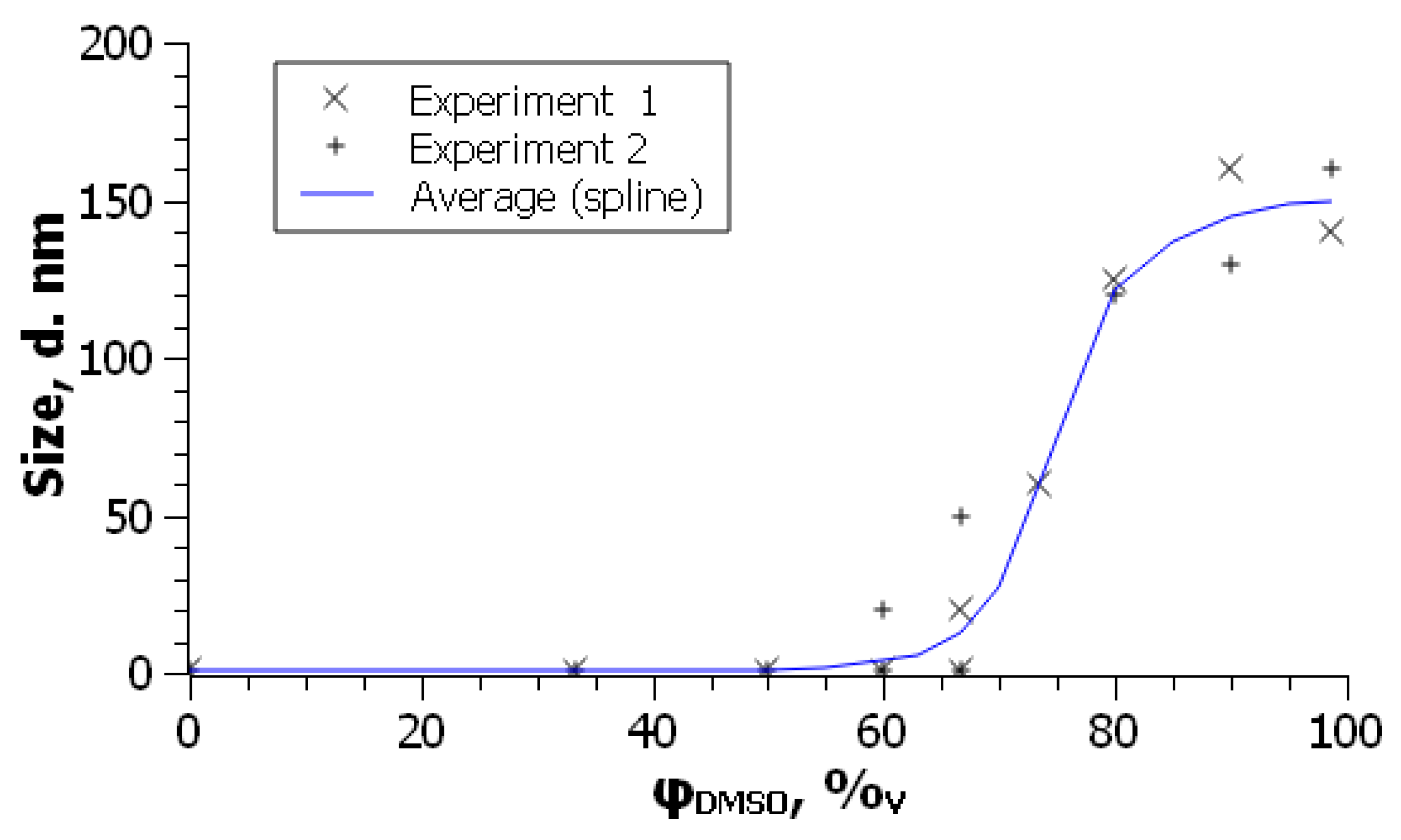
Two remarks should be made to this. First, even when the DLS method demonstrates the formation of colloidal particles and absence of molecular species, the UV-visible spectra retain the features of molecular absorption [
70,
73,
99,
101]. In other words, the onset concentration of the polar co-solvent is somewhat lower as determined by light scattering. For example, for the system presented in
Figure 3, the critical content of acetonitrile is 62 vol. % and 64 – 70 vol. % as obtained by DLS and UV-visible spectroscopy, respectively [
99]. Such findings can be explained either by hindering of observation of fullerene molecules in the presence of much stronger light scattering colloidal aggregates or by retaining aromatic solvation shells of fullerenes involved into the aggregates. Note that in a toluene –
n-hexane solvent system, the absorption spectrum of C
70 changes gradually along with rise of the aliphatic component, whereas no sign of colloidal particles is observed. This phenomenon is obviously caused by replacing the toluene molecules in the primary solvation shells of C
70 by
n-hexane [
99] and of C
60 by dichloromethane [
129].
Second, the fullerene concentration is an important factor. Sun and Bunker [
45] mentioned that in a toluene – 70 % acetonitrile mixture, the absorption spectrum of C
70 at concentration
8×10
–8 M is close to that in neat toluene. We confirmed this early statement using the DLS method: at C
70 concentration of 1.2×10
–7 M under the same conditions, only species with a size of ca. 1 nm were found [
70,
99].
More detailed consideration of the formation of fullerene colloidal species in the toluene–methanol system [
73] reveals, that the size of the aggregates decreases along with the increase in the polar solvent. This is in accord with the classical regularity formulated by Volmer [
130]: the lower the solubility is, the smaller the colloidal particles are formed. However, if the initial toluene solution of C
60 is prepared by a non-equilibrium method and oversaturated, the situation observed for a toluene–acetonitrile solvent system can be reverse [
129].
Interestingly, Pille et al. [
131] considered the alterations of the C
60 UV-visible absorption spectra on adding acetic acid, acetonitrile, methanol, DMSO, and DMF to a 1.3×10
–3 M toluene solution in terms of preferential solvation, not aggregation. The stock solution was prepared using stirring and sonication.
Recently, Kyzyma et al. [
129] conducted a detailed study of the C
60 – toluene – acetonitrile system using the UV-visible absorption spectra, TEM, DLS, SLS, SAXS, SANS, and LDI mass-spectrometry. Two series of experiments were performed, starting with C
60 solutions in toluene prepared by equilibrium and non-equilibrium (sonication) methods. The C
60 working concentrtions were (4.0–6.3)×10
–6 and (0.23–1.9)×10
–3 M, respectively. In all cases, adding of acetonitrile to the toluene solutions of C
60 favors aggregation. It is firmly proved that oxidation and illumination display pronounced influence on the aggregation processes. In the cited article [
129], the obtained data are compared with the results published by others.
In entire benzonitrile and benzyl alcohol, a threshold concentration of fullerene C
60 aggregation was reported [
63]. These polar solvents belong to the aromatic ones. For example, in benzonitrile, a solvent with
= 26, colloidal ≈ 250 nm-sized particles appear at 1×10
–4 M. Note, that though ultrasonication was used for preparation; larger particles may be removed by centrifugation and decantation [
63]. This critical concentration was estimated by both DLS and visible spectra at λ = 450 – 700 nm; the
nC
60 ⇌ (C
60)
n equilibrium is reversible [
63]. This is typical rather to lyophilic systems, like diphilic surfactants in water, which are characterized by a critical micelle concentration, CMC. However, the fundamental difference consists in the limiting solubility (here, it is 5.7×10
–4 M [
28,
35]), whereas for common surfactants in water, after reaching the CMC, the micellar solubility rises up to gelation. On the other hand, as it was mentioned in
Section 3.1, small fullerene aggregates can appear near the solubility limit even in “good” solvents [
21]. Obviously, the same takes place for the C
60 solutions in benzonitrile.
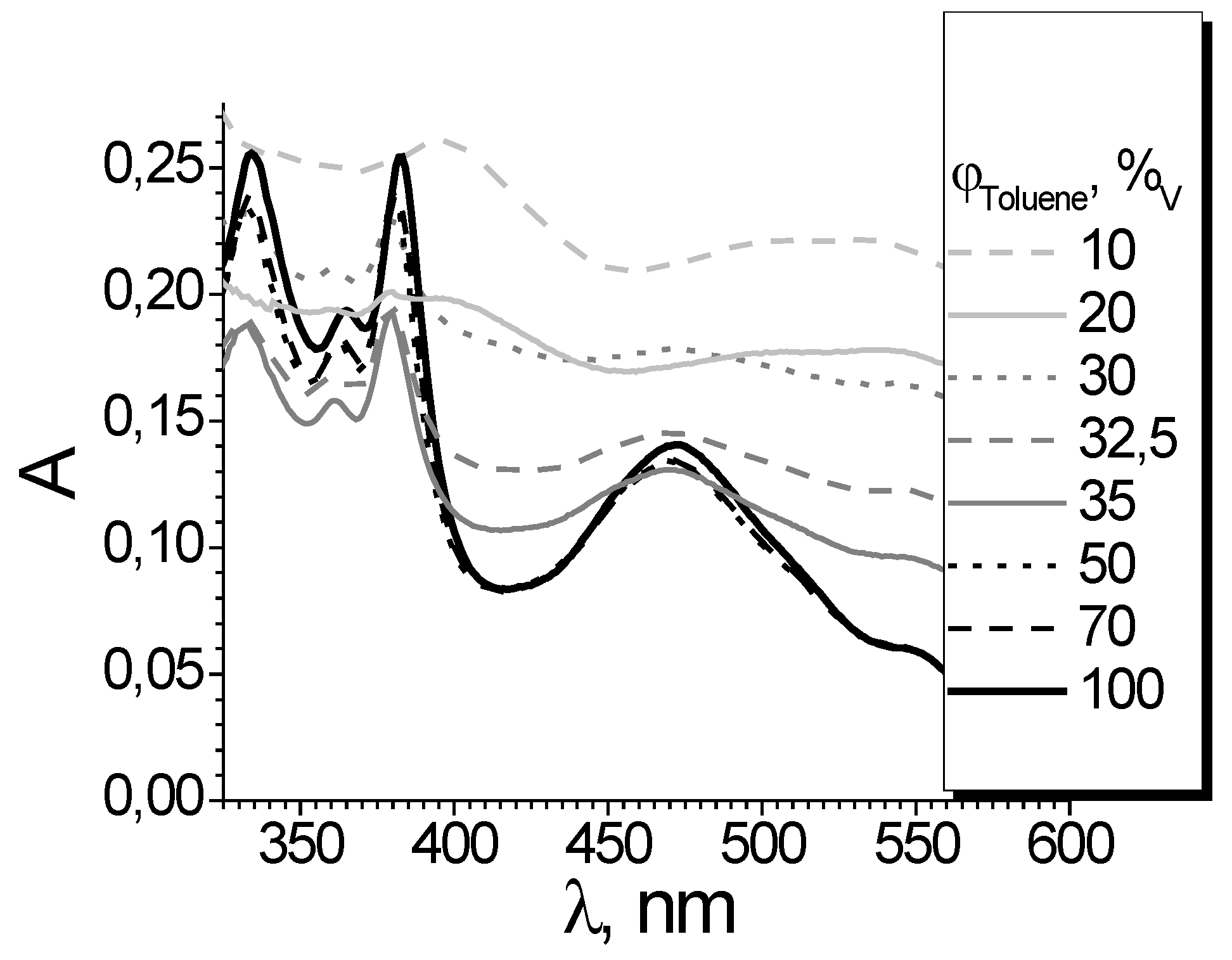
For C
60 in the toluene – acetone binary solvent system, the effects are similar to those observed for toluene or benzene mixtures with acetonitrile [
73]. If a benzene solution of C
70 is diluted by DMSO, the spectral changes resemble those presented in
Figure 2. At DMSO content of 33 vol. %, no sign of colloidal particles is observed (
Figure 4). The turning point of molecule – aggregate transition is about 60 vol. % DMSO [
74].
It should be noted that, contrary to the mixtures of a “good” solvents with methanol and acetonitrile, the size colloidal particles does not decrease near 100 % DMSO. Obviously, DMSO plays another role as compared with “poor” solvents, due to good solvation of C70 molecules. The properties of the DMSO-based systems will be considered in the next sections.
4.2. Top-down preparation of organosols and suspensions
Another way is the top-down preparation. This means breaking, grinding, mixing, and stirring of a solid sample in a polar solvent. Sonication and laser beam treatment can be also used. A more practical procedure was developed by Deguchi and Mukai [
132]. The top-down preparation of C
60 colloids in methanol, ethanol, 1-propanol, 2-propanol, 1-octanol, acetonitrile, and acetone was performed using 1–2 min hand-grinding of the solid sample in an agate mortor and sonication in a “poor” solvent [
132]. Furthermore, the same research group used this procedure to obtain stable graphite dispersion in aqueous acetone [
133].
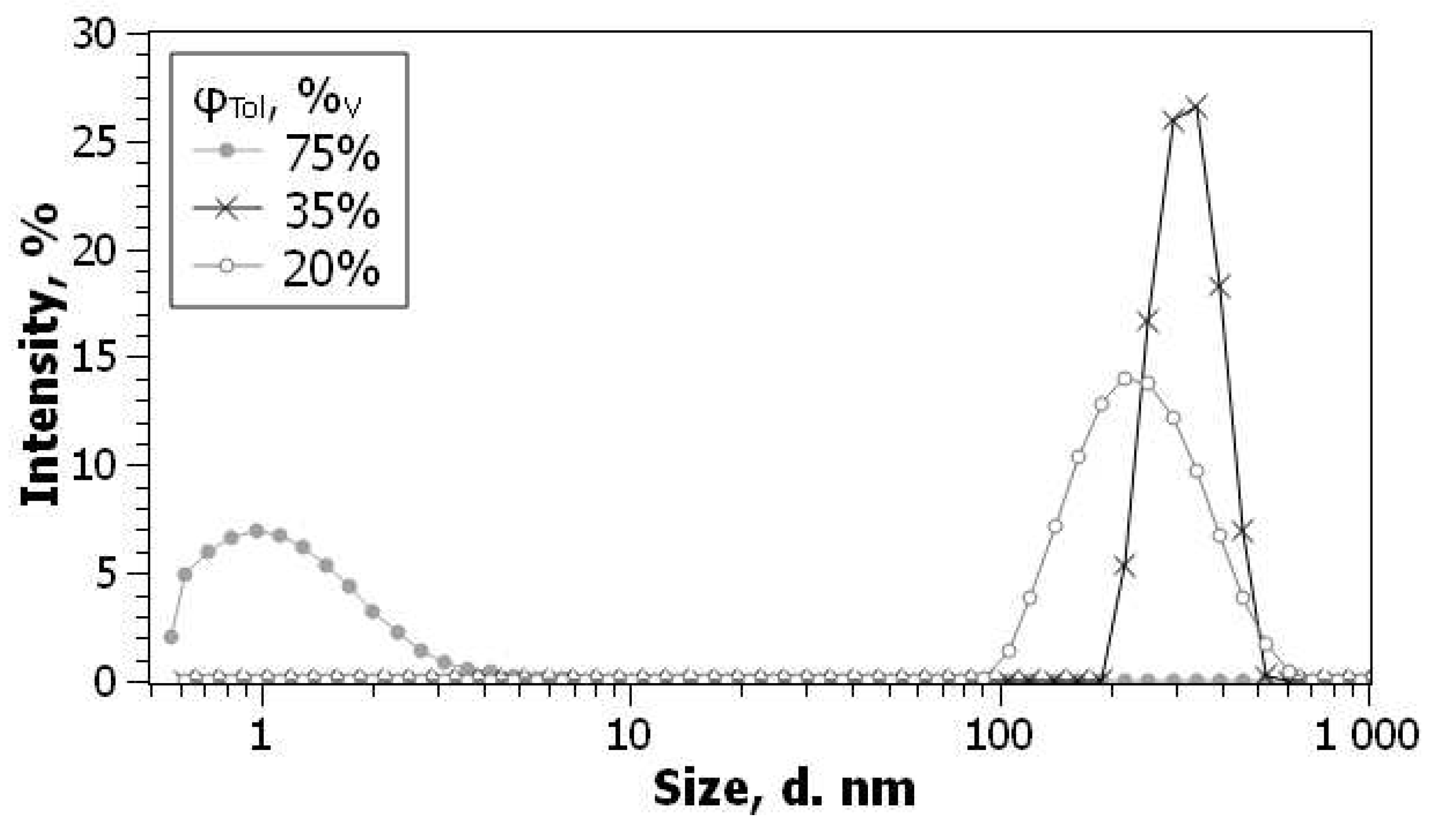
We have repeated this protocol for C
60 in acetonitrile with some modifications [
100,
129]. The size of particles as determined by DLS is 200–300 nm, in agreement with Deguchi and Murai [
131] and with our TEM data. The size distribution by number, scattering volume, and intensity is similar; the repeatability is medium, the polydispersity index, PDI, is 0.3 on average. Dilution with benzene (
Figure 5) allowed determination of the initial fullerene concentration in acetonitrile of ca. 3×10
–5 M.
Fullerene C
60 and C
70 solutions in
N-methylpyrrolidin-2-one, NMP (
= 32), are a special case [
17,
21,
125,
126,
127,
134,
135,
136,
137,
138,
139,
140,
141,
142,
143,
144,
145,
146,
147,
148]. While dilution of fullerene solutions in toluene, benzene, or CS
2 by acetonitrile, acetone, or alcohols is quite understandable from the point of view of colloid chemistry as an example of bottom-up preparation, the top-down preparation of fullerene colloids in the last “poor” solvents needs sonication. However, sonication is unnecessary in NMP. In earlier studies [
125,
126], C
60 and C
70 solutions in toluene were diluted with NMP in order to prepare colloidal solutions. Also, sonication was used for preparation of fullerene solutions in toluene, toluene – NMP mixtures, and in entire NMP. In other cited papers, the fullerene solutions were prepared directly by stirring the solid sample in NMP. The stirring time varied from 10–15 min [
138,
140,
143,
146,
147] to 1 h [
136,
139,
144], 6 h [
141], 24 h [
127] or four days [
135]. In some studies, initial solutions for investigation of the NMP–toluene systems were prepared either in toluene or in both polar and “good” nonpolar solvents [
140,
143,
144,
145].
Detailed studies of C
60 [
134,
135,
136,
138,
139,
140,
141,
144,
147] and C
70 [
143,
145,
146] in NMP-based systems made it possible to shed light upon the unusual properties of this solvent in respect to fullerenes. The main specific feature is the strong interaction between the fullerene and solvent molecules. Obviously, it is a kind of donor-acceptor interaction, which results in formation of charge transfer complexes [
139,
141,
144,
147] proved by the
1H NMR spectroscopy, quantum-chemical calculations [
139,
144], and mass spectra [
135,
144]. Another property is ageing of fullerene solutions over time, slow increasing in size up to ca. 500 nm as determined by DLS, SANS, and SAXS methods. The UV-visible spectra exhibit some bands characteristic for molecular absorption, but after preparation of solutions, smoothing of the absorption curve begins immediately, and the spectrum strongly resembles that shown in left hand side of
Figure 5. Importantly, using some assumptions it was demonstrated, that the Mie light scattering makes a negligible contribution it this case [
135].
Sun et al. [
149] studied the behavior of C
60 and C
70 in entire triethylamine. The UV-visible spectra of the solutions prepared either by sonication or without it exhibit a smoth curve. UV-visible spectra of freshly prepared solutions exhibit some features of molecular absorption. However, within 2 h, the spectra curve became completely structureless, like in the above mentioned case of NMP. However, the authors assume that the origin of the absorption changes is a chemical reaction, not a complex formation. This point of view is based on the NMR spectra and fluorescence data. This allows classifying triethylamine as a “reactive” solvent.
Returning to the NMP, it should be concluded that, in any case, the reason for the ease of dissolution is the strong interaction of this electron-donor solvent with fullerenes. In toluene–NMP binary solvents, formation of 55–60 nm particles occurs within ca. 1 h [
125,
126]. A set of works were devoted to absorption spectra of fullerenes in the toluene – NMP system at different sequence of components adding [
125,
126,
140,
141,
143,
144,
145,
146]. These data demonstrate peculiarities of competition between these two solvents in the solvation shells of fullerene molecules. Because of some information on the irreversible changes of the fullerene solutions [
140], the NMP may be (partly) attributed to the reactive ones.
Some studies were devoted to kinetics of fullerene aggregation [
21,
150]. The nucleation process was studied in detail be Tropin, Avdeev, Aksenov and their colleagues in NMP [
21,
137,
142,
151,
152]. These authors managed to desctibe the experimental data using the model of complex formation between fullerene and solvent.
Obviously, the electron-donor properties of solvents are of the crucial role in solvation of fullerenes, which are Lewis acids [
74]. While the value of the relative permittivity,
, is used to characterise the solvent polarity, the Gutmann’s donor number, DN, describes the cationophilic properties [
128,
153]. In
Table 2, both parameters for some selected solvents are given (mainly at 25
oC).
Among the non-hydrogen bond solvents, first three in
Table 2 are typical protophobic (cationophobic) ones, while the last five are protophilic (cationophilic). Pyridine dissolves the C
60 fullerene, and aggregates were observed via DLS [
154]. The preparation of solutions of C
60 in DMF was described at least by two research groups [
155,
156]. However, recently the colloidal solutions in this solvent were obtained even easier [
74]. Note that some organic solvents may become unstable over time. For example, DMF can decompose to formic acid and dimethylamine [
157]. In our study, we used freshly purified and distilled DMF.
Although solutions of fullerenes in DMSO and DMSO – water solvents were already obtained in 1993 and were used for spreading at the water/air interface [
64], these systems were not further considered in more detail. Wang et al. [
64] reported no details of the preparation of solutions. For DMSO, the sonication is undesirable (After even several minutes of sonication, substantial amounts of acidic admixtures appear in DMSO; colloidal particles of fullerenes became positively charged and unstable [
74]) but fortunately not necessary [
74], analogous to the case of NMP. We prepared C
60 and C
70 colloidal solutions in DMSO and DMF by hand grinding in an agate mortar and 3 h mixing with a magnetic stirrer [
74]. The solutions are rather stable over time and contain ≈ 200 – 250 nm-sized particles with substantially negative electrokinetic potential. The repeatability of the preparation procedure was good.
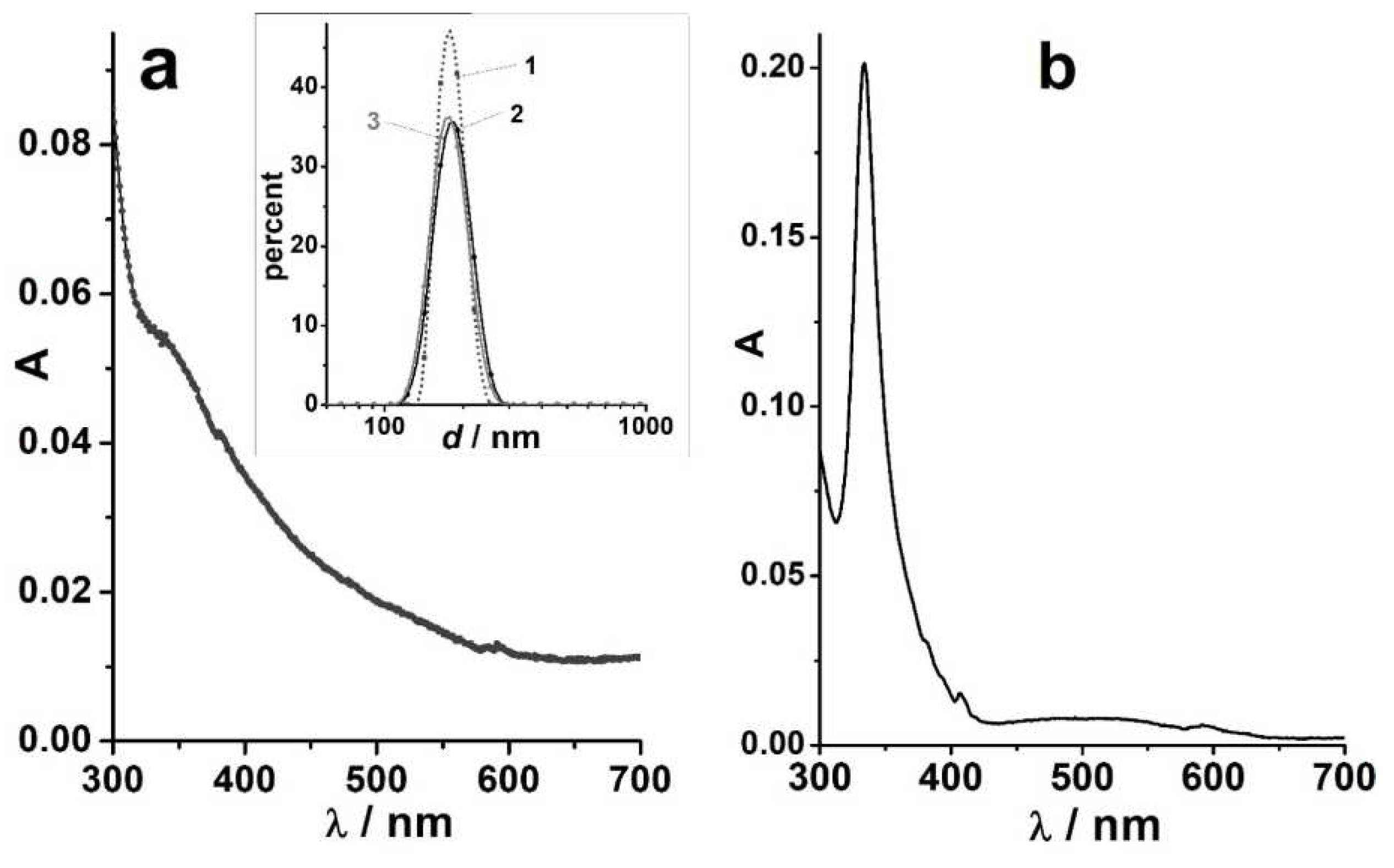
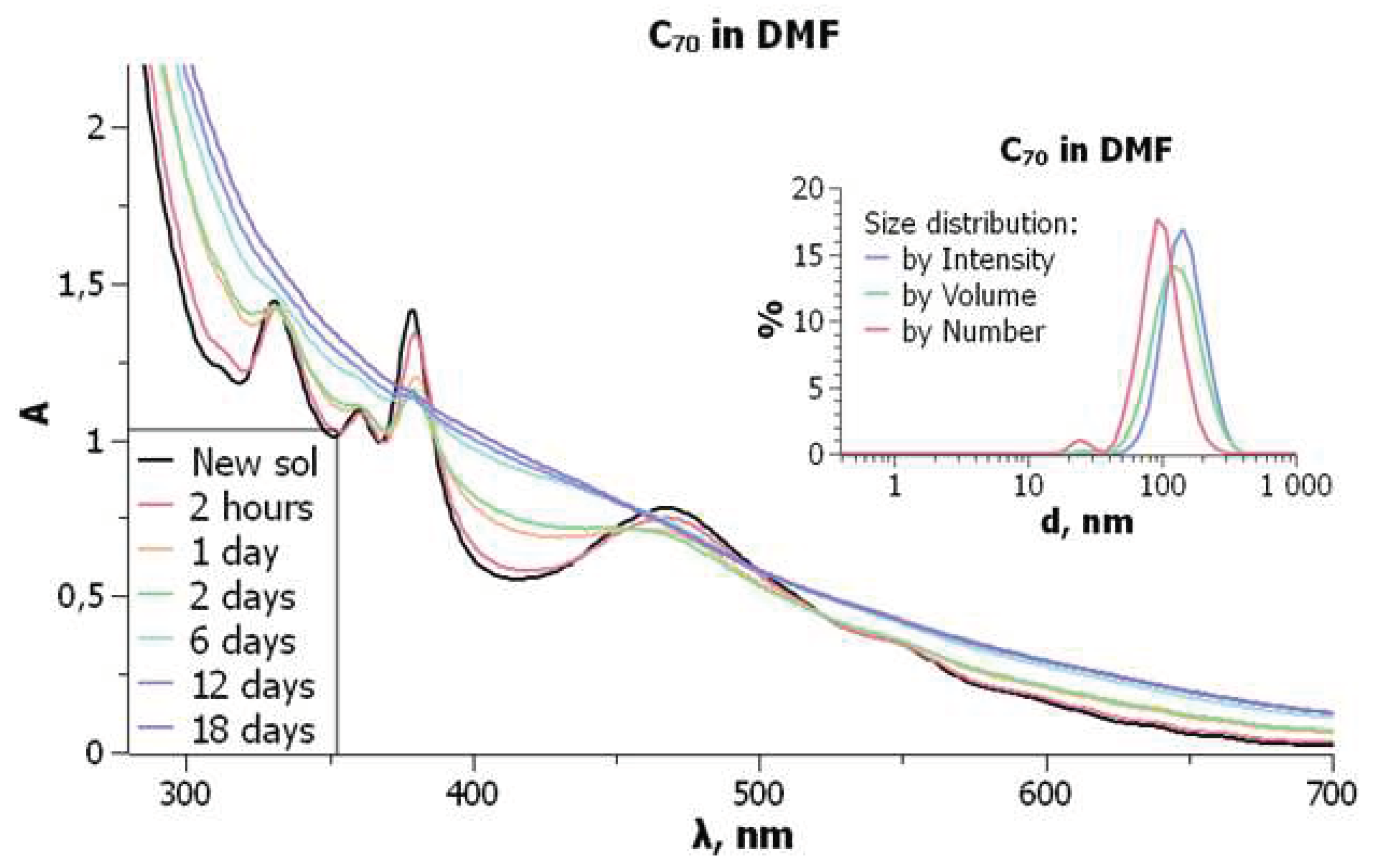
UV-visible absorption spectra are typified in
Figure 6. Particle sizes and size distribution do not undergo major changes over time. In the freshly prepared solutions of C
70 in DMF, the molecular bands are observed with the
, nm (
×10
–3 M
–1cm
–1) values of 331 (55.7); 360 (43.2), 380 (56.0); and 467 (31.4). However, smoothing of the spectral curve begins shortly after the preparation of solutions. For the C
60 solution in DMF, typical molecular band of 332–333 nm was observed immediately after preparation.
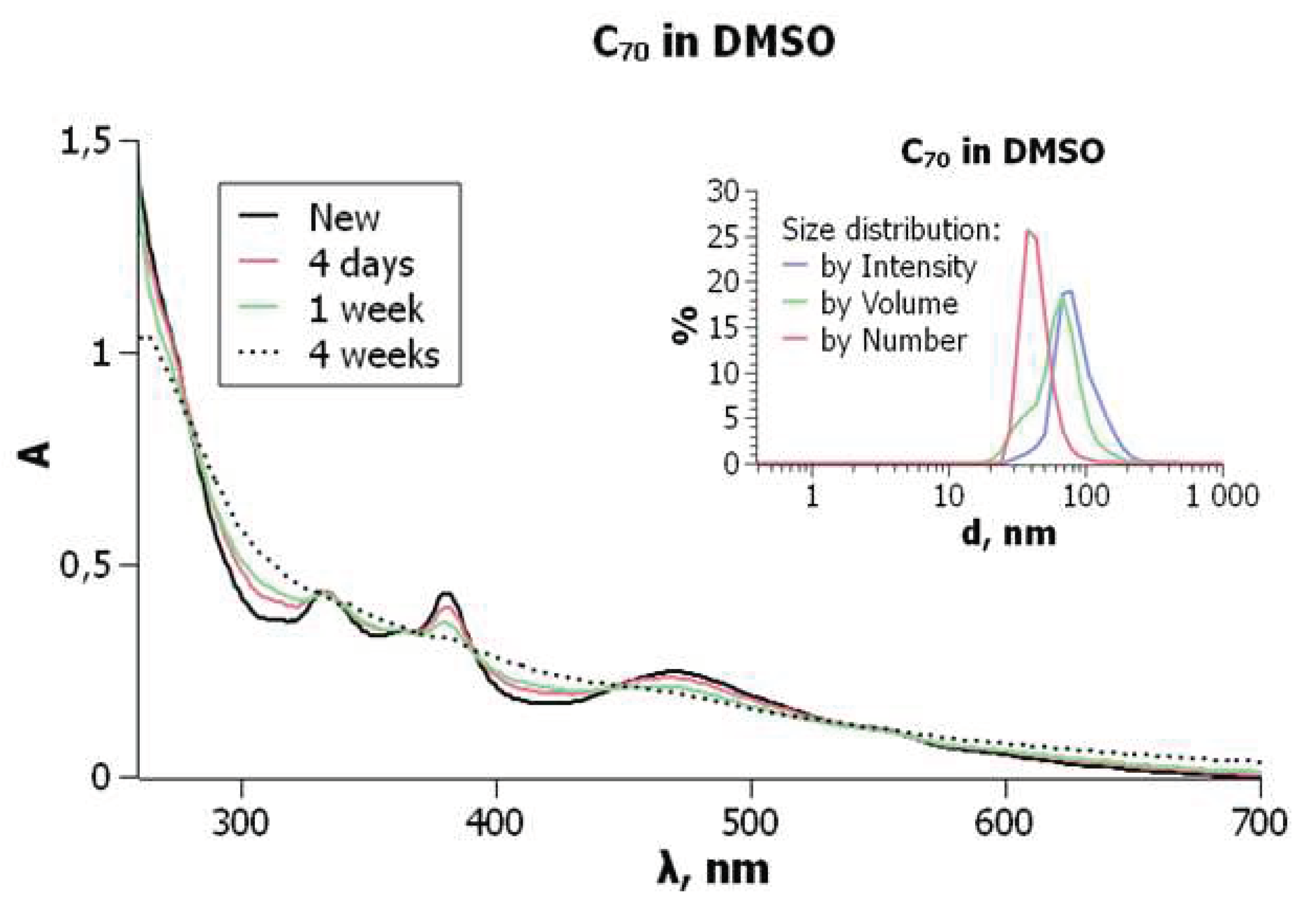
Similar results were obtained in DMSO: C70 exhibits molecular bands of 333, 363, 380, and 470 nm, and smoothing of the curve takes place (Figure 7).
Despite a significant variety of solvent compositions of organosols (suspensions) of fullerenes, they have one important property in common. Namely, colloidal particles are always negatively charged [
44]. Now we have a set of electrokinetic potential values in different solvents, calculated using the Henry equation (Ohshima approximation) [
158,
159]. They are compiled in
Table 3. (The overcharging phenomenon in the presence of electrolytes will be considered in
Section 5.)
These results are in agreement with the earlier published work by Alargova et al. [
44], where the
= –(32.5 – 38.5) mV in acetonitrile for colloids if C
60, C
70, and their mixtures was reported. Their calculations were processed using the Smolukhowski equation; for recalculation to Henry equation there are –(48.8 – 57.8) mV.
The origin of the charge of fullerene colloidal species in polar organic solvents is of special interest. The transfer of electrons from the solvent molecules to fullerenes is obviously the most probable path [
44]. Also, disproportionation of the C
60 molecules into oppositely charged radicals can also take place [
41]. In the latter case, it favors aggregation even in a “good” solvent; this process can be suppressed by introducing a radical scavenger [
41]. Indeed, we have demonstrated that if a benzene solution of C
60 is mixed with an equal volume of acetonitrile in the presence of 2,6-di-
tert-butyl-4-methylphenol, a substantial decrease in the
value accompanied by a jump of the particle size takes place [
101]. Within a period of 1 h, the average size reaches 1700 nm, and then the precipitation of fullerene takes place, while
approaches zero [
101]. If, however, the radical scavenger is added at 1.5 h after formation of the colloid, no changes are observed. In a set of C
60 colloidal solutions in acetonitrile, prepared by hand-grinding and sonication [
100], the
value was within the range of –(42 – 63) mV, average value
= – 48 mV. If acetonitrile contains a radical scavenger 2,6-di-
tert-butyl-4-methylphenol, the interfacial charge is greatly reduces;
is about –10 mV. This further confirms that free radicals are a source of charge formation.
Similar, but not expressed changes are observed in acetonitrile with 1 vol. % toluene [
101]. For C
70 colloids, the above effects were almost imperceptible.
Polar basic solvents can readily be mixed with water. This was demonstrated by Mrzel et al. [
154] for pyridine. These authors underline the difference between the C
60 aggregates in pyridine and nanocapsulates of fullerene in pyridine – water mixtures [
154]. DMSO-based organo-hydrosols of C
60 and C
70 were prepared by Wang et al. [
64] and in our study [
74]. Chaban et al. [
161] studied the C
60 – water – DMSO system using the molecular dynamics simulations and predicted good solvation of fullerene molecules by DMSO and fullerene aggregation. The same was demonstrated for the DMF – water systems [
74]; Yang et al. [
156] added water to the C
60 deposit after evaporation of DMF. Dilution of fullerene solution in NMP with water also results in organo-hydrosols [
135,
136,
138,
146,
148]. In all these hybride sols, the fullerene particles are also negatively charged.
Concluding, fullerenes in polar solvents exist as colloidal systems. However, all these solvents exhibit individual feachures. While methanol, acetonitrile, and acetone are typical “poor” solvents with C
60 solubilities of 3.3×10
–8, 5.6×10
–7, and 1.4×10
–6 M, respectively [
35], for cationophilic DMF, pyridine, and NMP this key parameter is higher: 3.75×10
–5, 8.3×10
–4, and 1.2×10
–3 M, respectively [
35]. On the other hand, the solubility of C
60 in benzonitrile, a polar solvent with the lowest DN value among collected in
Table 2, is with 5.7×10
–4 M substantial. Obviously, the reason is the aromatic nature of this solvent, which favors solvation of fullerenes. Moreover, just in this solvent single fullerene molecules predominate within a pronounced concentration range, before the aggregation is observed [
63]. The same was reported by Nath et al. for the benzyl alcohol [
42]; these authors underline the “intermediate” character of polar aromatic solvents.
It can be argued that even for the few organic liquids discussed above, it is possible to divide them into three sub-groups of “poor” solvents.
Though NMP should be considered rather as a “poor” solvent, with some features of “reactive” ones, Stuart et al. [
127] prepared a C
60 solution in acetonitrile by ten-fold dilution of the initial NMP solution with another “poor” solvent CH
3CN. Alargova et al. [
44] consider NMR as a polar solvent, which “exhibits good solubility of C
60 (comparable to that in toluene), being an exception among the good solvents for fullerenes.“ Their experiments showed that dilution of C
60 solution in NMP with acetonitrile resulted in formation of colloidal particles similar to those obtained using aromatic solvents instead of NMP [
44]. However, it was firmly proved that, despite relatively easy dissolution of fullerenes in NMP, all solutes within a short interval of time transfer into colloidal state [
134,
135,
136,
138,
139,
140,
141,
143,
144,
145,
146,
147]. The same is true, e.g., for DMSO [
74].
So, if such solvents can be classified as “high-solubility” to some extent, they dissolve fullerenes only in the form of colloidal aggregates. On the contrary, aromatic solvents dissolve fullerenes in molecular form under equilibrium conditions.
4.3. Fullerenes in room temperature ionic liquids, RTIL
Room temperature ionic liquids, RTIL, like fullerenes, have long ceased to be exotic compounds, but their combination has been relatively little studied to date. Theoretical studies on C
60 with were performed by Fileti, Chaban, and Maciel [
162,
163,
164,
165] and Garcia et al. [
166,
167], whereas Pádua group performed both experimental [
168,
169,
170] and theoretic studies [
168].
In the first article [
168], interaction of C
60 with four ionic liquids having the same anion, bis(trifluoromethanesulfonyl)amide (Ntf
2−), and differing in the lengths of the alkyl chains of the 1-alkyl-3-methylimidazolium cations, from ethyl to
n-decyl, was described. Soid fullerene was dissolved in CH
2Cl
2 using 5 min sonication and prolonged stirring. After mixing with the RTIL 1-decyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide and evaporating the molecular solvent, UV-visible spectra at C
60 concentrations up to 6.25×10
–4 M demonstrated pronounced difference from that in CH
2Cl
2. Some difference in the behavior of the fluorinated fullerene, C
60F
48, was observed [
168]. In the next study, it was shown that the enthalpy of mixing of decylmethylimidazolium bis(trifluoromethanesulfonyl)amide with 1,2-dichlorobenzene is more negative in case if the organic solvent contains C
60 [
169]. More detailed comparison of C
60 and C
60F
48 demonstrated, that their solubilities in [bmim]
+[Ntf
2−] are 7×10
–5 and 6×10
–4 M (here, bmim
+ means 1-butyl-3-methylimidazolium cation). Whereas colloidal partilcles of C
60 are large, up to 5×10
4 nm and sedimentates even at low concentrations, while C
60F
48 exists as solvated isolated molecules or small aggregates and sedimentates only at concentration of 1.5 g/L. However, the smoothing of absorption curve also takes place for C
60F
48.
The solubility of C
70 in imidazolium-, ammonium-, and phosphonium-based RTIL was found even earlier in the course of fluorescence studies; Cl
– and Ntf
2− were used as counter ions [
171]. The procedure was as described above. The solubility of C
70 varied from 0 in [bmim
+][BF
4–] to 9.5×10
–5 M in [methyltrioctylammonium
+][Ntf
2−]. Although the authors exclude the formation of suspensions, this conclusion is probably based on visual observation. In any case, the absorption curve is smooth. Hence, some kinds of aggregates seem to be probable.
Maciel and Fileti [
162] used molecular dynamics simulations for estimating the solvation energy of C
60 in ethylammonium nitrate and 1-butyl-3-methylimidazolium tetrafluoroborate. The solvation of the fullerene by nitrate is substantially better than by the BF
4–. The energy of C
60 transfer from the first to the second RTIL was estimated as 235 kJ mole
–1 (±1 %) [
162]. The modeling predicts separation of two C
60 by a bmim
+ cation [
163,
164]. At the same time, fullerenes are Lewis acids. In the last work, Fileti and Chaban theoretically predicted a jump of the C
60 solubility in [bmim
+][BF
4–] at high temperatures, up to 380 K [
164]. This interesting conclusion should be verified experimentally.
Garcia et al. [
166,
167] presented important results on analyzing the interactions of 24 different RTIL with C
60 using molecular dynamics and DFT methods. Analysis of structural, dynamic, and energetic factors were analysed to clarify their role on the behavior of the above systems. In particular, the role of the
interactions is important, which is in line with other theoretical considerations [
162,
163,
164]. Useful guidelines are provided for selecting an RTIL suitable for fullerene solvation; rationalization means an adequate cation-anion choice [
167].
Recently, Cardoso and Colherinhas [
172] published a detailed molecular dynamics investigation of C
60 with [bmim
+][PF
6–] and water using polarization effects. In order to observe the impact of fullerene – solvent electronic interactions, the NMR and electronic absorption spectra were calculated using the GIAO-DFT and TD-DFT methodology [
171]. These authors also compared the solvation of C
60 in water, DMSO, and DMF and demonstrated a better solvation in the last organic solvents [
171].
It can be concluded, that in RTIL, also known as “green” solvents due to their negligible vapor pressure, the C
60 and C
70 fullerenes exist in form of aggregates. As for the permittivity of RTIL, even estimating this value is not an easy task. For [bmim
+][PF
6–], and estimate
10 can be accepted [
173,
174]. Hence, the RTIL solvents are on the border between polar and non-polar ones; they should be ascribed to “poor” solvents. Also, the large variability of RTIL should be taken into account; in this case, the above mentioned theoretical modellin can be useful for correct choice of solvents.
Campisciano et al. [
175] covalently attached imidazolium groups to the C
60 molecule and thus obtained a valuable supramolecular system for providing Suzuki and Mizoroki–Heck reactions in aqueous media. The smoothing of the spectral curve in the UV-visible range is pronounced. In any case, such a modification makes it possible to transfer the fullerene to water. Basing on molecular dynamics method, Fileti and Chaban [
165] recommended the imidazolium ionic liquid [bmim
+][BF
4–] for dispersion of fullerene in water. In this sense, the RTIL is one of the ionic compounds capable of stabilizing colloidal fullerene particles in hydrosols.
4.4. Hydrosols of fullerenes: Preparation.
In water, fullerenes exist in colloid state. Because the electrophilic properties of C
60 and C
70 are pronounced, a number of studies were directed to their biological activity and possible application in medicine [
6,
9,
11,
12,
14,
15,
176,
177]. On the other hand, a plenty of information on cytotoxicity, photocytotoxicity, genotoxicity (DNA damage), etc. is available [
24,
25]. Many studies are devoted to the behavior of fullerenes in soils, freshwater, etc. [
9,
10,
16,
177,
178,
179,
180].
As result, an impressive number of different procedures and protocols of preparation of fullerene hydrosols and aqueous suspensions is accumulated; see, e.g., recent review papers [
15,
16,
24,
25]. This set of methods that have been tried for the preparation of hydrosols and aqueous suspensions of fullerenes can serve as an excellent teaching example for a university course in colloid chemistry.
Some of the colloidal solutions are rather suspensions, than sols, first of all those prepared by top-down method, whereas hydrosols are first of all prepared by the bottom-up way. However, following many authors, we use these terms here mainly as synonyms. For orientation in this data set, special designations for the most popular preparation methods gradually appeared, such as, e.g., son/nC60. Here “son” and “nC60” denote sonication and aggregate formation, respectively.
First approach was the stepwise solvent exchange going from C
60 in benzene to tetrahydrofurane, then to acetone, and finally to water; the final fullerene concentration was 2×10
–6 M [
181]. If the initial fullerene solution is prepared in toluene and successively diluted with tetrahydrofurane, acetone and water, the target system is designated as TTA/
nC
60. In this case, even a C
60 concentration of 0.002 M can be reached [
182]; the procedure was subsequently reconsidered [
183]. Dilution of tetrahydrofurane solution of fullerene with water leads to hydrosols of THF/
nC
60 type [
184,
185]. First, a fullerene solution in THF is prepared in inert atmosphere, and after mixing with water the organic solvent is evaporated [
184]. Dilution of C
60 and C
70 solutions in polar solvents
N-methylpyrrolidone and dimethylformamide with water results in NMP/
nC
60, NMP/
nC
70 [
186], and DMF/
nC
60 [
155] colloidal systems. New versions of such approach were developed by the Ausman group [
183,
187]. These authors proposed a new procedure using C
60 solution in hexane, diluted with
iso-propanol and water [
187]; under such conditions, hexane is evaporated first.
An approach basing on introduction of a surfactant sodium dodecylsulfate (SDS) has been proposed. A fullerene solution in a good solvent is added to aqueous acetone containing SDS, and organic solvents are removed by distillation [
188,
189,
190]. Here, SDS stabilizes the final C
60 colloidal particles; however, utilization of surfactants, polymers, etc., is beyond the shape of this minireview.
Another bottom-up method was already reported as early as 1997. It consists in introduction of THF solution of the C
60 anion radical into water; oxidation with atmospheric oxygen results in formation of a hydrosol [
191]. This procedure was revisited after two decades [
192]. Reaction with KOH and oxidation results in formation of fullerenol; the NIR spectra of fullerene anion radical and the properties of hydrosol of fullerenol is recently reported [
193].
Preparation of colloidal solutions can be performed using sonication. Solutions of C
60 were prepared in DMF and THF by stirring and then, instead of mixing with water, the latter was added and accompanied with sonication only after the organic solvents evaporation [
156]. The authors of the original article designate thus obtained as DMF/
nC
60 and THF/
nC
60 [
156]. A versatile analysis of thus obtained systems disclosed substantial chemical changes of the fullerene. Not only sonication but the nature of the organic solvent plays a role in these alterations [
156].
More popular is the sonication method consisting in ultrasonic extraction from toluene to water [
194]. The hydrosols prepared in this bottom-up way are designated as son/
nC
60, or tol/
nC
60, or SON/
nC
60. Contrary to the above mentioned procedures this allows to receive much higher fullerene concentrations, up to 0.001 M [
195] and particle size down to 20 nm [
16].
This method works well not only for C
60, but also for C
70 [
196,
197,
198], and C
76, C
84 [
196] and is permanently modified [
196,
199,
200,
201,
202,
203]. So, if the pH of the water phase is elevated to 10, the negative charge of the colloidal particles increases thus making them more stable [
199]. Also, SDS was added in order to stabilize the particles that appear during the ultrasound extraction; the surfactant was removed from the final colloid
via dialysis [
203]. Some authors used addition of small amounts of ethanol to the aqueous phase [
196,
200,
204]. Slight heating to 40
0C and passing nitrogen to remove toluene traces is proposed [
200]. For the same purpose, 15 min boiling of the final hydrosol can be used [
201]. Heating the system during the sonication process up to 60
0C allows substantially decrease the size of colloidal particles [
202].
The disadvantages of the method include the occurrence of the (possible) fullerene oxidation and other chemical reactions under conditions of sonication. So, reactive oxygen species are readily formed such as superoxide ion, singlet oxygen, etc. [
16]. Conversion of toluene into the benzoic acid and benzoate is also reported [
205,
206].
In 2022, two new approaches to the C
60 hydrosols preparation were published [
14,
15]. Merland et al. modified the extraction/sonication method and developed an emulsification-evaporation process in the presence of an amphiphilic polymer. Instead of toluene, trichloromethane or carbon disulfide was used. Kop et al. [
14] proposed fullerene – curcumin antioxidant system. These authors prepared the C
60 hydrosols by 9-fold dilution of a solution in NMP with water, followed by stirring, dialysis, and filtration though a 450 nm-pored filter. The sols were stabilized by Tween-80, polyvinyl pyrrolidone, cyclodextrin, and curcumin with different combinations.
As an example of a hydrosol of C
70, the results of our recent study are presented in Figure 8 and Figure 9 [
198]. This colloid system of son/
nC
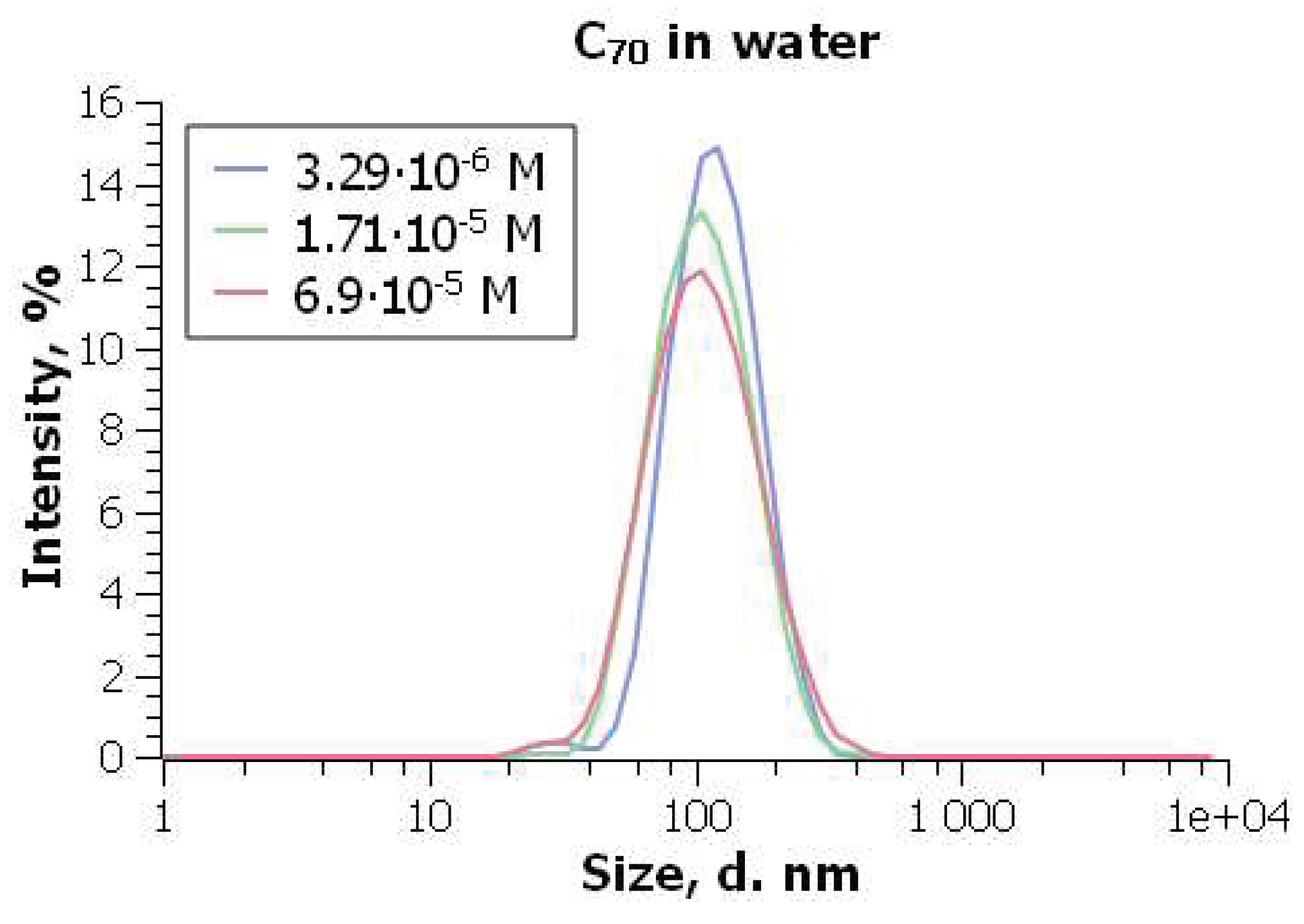
70 type was prepared by Dr. Vladimir Klochkov using highly pure benzene instead of toluene. At the C
70 concentration of 3.3×10
–6 M and 25
0C, the size of particles is 97±3 nm (Figure 8) and
= –40±4 mV [
198].
Figure 8.
Particle size distribution in the son/
nC
70. From ref. [
198] with permission.
Figure 8.
Particle size distribution in the son/
nC
70. From ref. [
198] with permission.
Increase in concentration displays insignificant alterations of the particle size; PDI is always around 0.2 [
198]. The above characterization of this C
70 hydrosol is in agreement with the results by Aich et al. [
196].
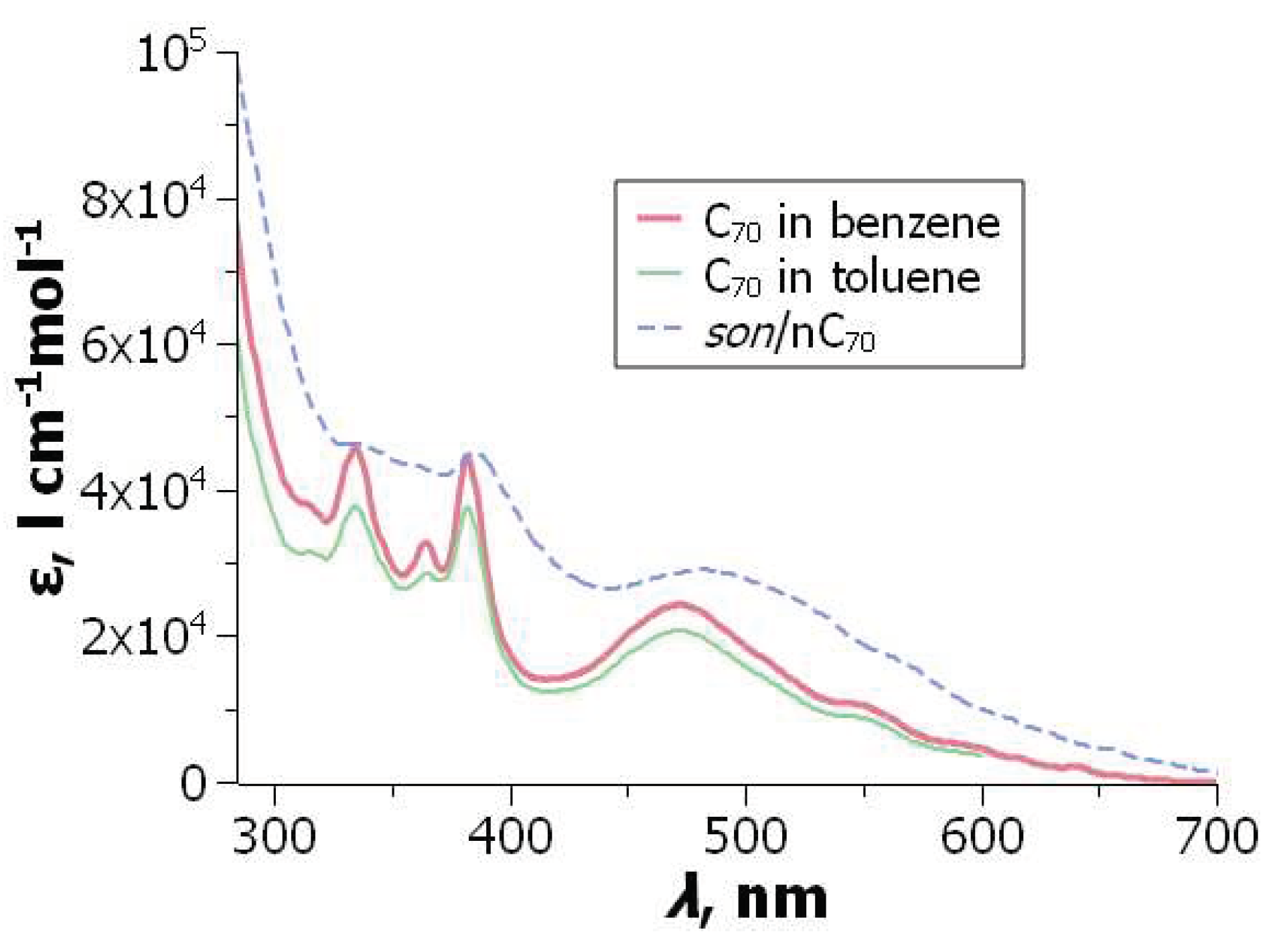
Figure 9.
UV-visible absorption spectra of C
70 in good solvents and in the son/
nC
70 hydrosol. From ref. [
198] with permission. .
Figure 9.
UV-visible absorption spectra of C
70 in good solvents and in the son/
nC
70 hydrosol. From ref. [
198] with permission. .
The size/
values for C
70 hydrosols, available in the literature, as as follows: 92±14 nm/–39±4 mV [
196]; 175±5 nm/ –34.4±0.7 mV [
197]; ≈ 100 nm/–21.7 mV [
177] (in the last paper, the fullerene concentration was 8.9×10
–5 M). As a rule, data are published without specifying the type of equation used for
calculation. As mentioned above, we used the Henry–Ohshima equation, which corresponds to the Hückel equation in the absence of foreign electrolytes.
It is important to note that relatively small variations in this preparation method often lead to formation of hydrosols with significantly different properties. This is a typical sign of hydrophobicity of the colloidal system of interest. Given the widespread use of this method, it is important to try to standardize the synthetic protocol. Such attempts were made by Mikheev et al., who prepared standard samples of fullerene hydrosols [
206,
207]. Note, that in these works as well in the study by Kyzyma et al. [
186], sonication was used not only for the toluene – water system, but also at preparation of the initial toluene solution of fullerenes. Contrary to it, we use to prepare the stock solutions in toluene and benzene without sonication, keeping the solid sample in the good solvent within ca. 2 weeks [
198].
Finally, the top-down methods have been used, first of all, prolonged stirring of the solid samples of fullerene in water. Thus obtained colloid systems were designated as aq/
nC
60, aqu/
nC
60, stir/
nC
60, or STI/
nC
60. Sometimes stirring continued even 1075 days [
208]. Murdianti et al. [
209] presented experimental evidence that suspension formation does not occur in an inert atmosphere. They attach a key importance to the formation of fullerene oxide C
60O for the appearance of colloidal particles [
209]. Basing on detailed studies, it was concluded that it is impossible to obtain identical results by mixing in water [
210].
Mixing and stirring can be performed in the presence of salts of organic acids. For example, if citrate is used, the colloid is named as cit/
nC
60 [
211]. Some authors used sonication in water and named the colloid as aq/SON/
nC
60 [
210], while others crushed a solid fullerene sample in water by a laser beam [
212]. Hand grinding of the solid sample in a mortar followed by transfer to water and sonication was named mechano-assisted reduction of size, or MARS/
nC
60 [
213,
214], is preferably carried out on with the addition of SDS [
25].
In any case, filtration of the resulting colloid solutions using 220 or 450 nm pore filter is highly recommended.
4.5. Hydrosols of fullerenes: Key properties.
Briefly summarizing the numerous data accumulated to date, we can give the following characteristics of hydrosols or suspensions of fullerenes. These are typical hydrophobic colloidal nanodispersed systems, with size of negatively charged particles mainly from ≈ 40 to ≈ 200 nm, and with fullerene concentration as a rule below 0.01 mass %. Although it is obvious that the authors of various works sought to obtain the maximum concentration of fullerene, the final concentrations depend to a large extent on the method of preparation used. Other properties of the colloids also strongly depend on the preparation protocol. Such scatter of the properties of the colloidal solutions prepared by different methods or even by the same methods but by different authors gives additional support to the idea of pronounced hydrophobic character of the systems of interest.
Since the initial weight of fullerene usually cannot be completely transferred into water, it is necessary to have a method for determining the fullerene concentration in solution. A common method consists in deposition of fullerene colloidal species by Mg(ClO
4)
2, NaCl, NaNO
3 or/and acetic acid followed by extraction with toluene and absorbance measurements [
24,
25,
183,
187]. New method basing on the light scattering has also been developed [
210].
As for determination of particle size and polydispersity of fullerene aqueous dispersions, the dynamic light scattering method is used first of all. However, indirect method basing on UV-visible absorption spectra [
213,
215,
216] may also be used. For example, Deguchi et al. [
213] proposed an equation that describes the dependence of the absorption maximum in the region of 340–350 nm on the hydrodynamic diameter of the colloidal particle determined using the DLS method, Equation (1).
The discussion of applicability of this and other equations can be found in a previous review [
25]. A more detailed characterization provides for knowledge about impurities, first of all oxidation products [
156,
183,
187,
206,
209].
The origin of the negative charge of the colloidal particles is of special interest because it allows to shed additional light on the nature of the fullerene/water interface. Several popular explanations of the charge origin have been considered and discussed in previous reviews [
24,
25]. The most probable reasons are as follows. (i) Adsorption of the HO
– ions, which is typical for many hydrophobic surfaces such as oil droplets [
217] and gas bubbles [
218]. (ii) Localized hydrolysis caused by electrophilic properties of fullerenes, which are in fact Lewis acids [
24,
25,
219]; it also favors the additional formation of the HO
– ions. The role of HO
– ions in formation of the surface charge is supported both by additional stabilization at pH above 7 and coagulation at pH 1–2 [
25]. (iii) Quantum-chemical calculations made by Choi et al. [
220] revealed that the interactions between fullerene and water molecules lead to charge transfer and polarization, thus making a contribution to the negative charging of the fullerene aggregates in water. This explanation is in line with our concept (ii).
In any case, the negative charge is a main factor of the aggregative stability of colloids including fullerene sols and suspensions. Noneman et al. [
221] performed a detailed molecular dynamics modeling of mixtures of C
60 with C
60O in water. The main idea of this and previous works of this group [
183,
187,
209] consists in stabilization of C
60 colloids in water by admixtures of C
60O. The latter to some extent plays the role of a diphilic compound, a kind of stabilizing surfactant [
221]. This is quite plausible, but the fullerene oxide itself cannot cause the surface charge, and it is the charge screening by electrolytes that leads to coagulation.
According to molecular simulations made by Hinkle and Phelan [
222], the Gibbs solvation energy,
, of C
60 in water (–50.9 kJ mol
–1) is more negative than that reported by Stukalin et al. [
93]. However, the energy of transfer from water to methanol and ethanol is –68.1 and –86.5 kJ mol
–1, respectively. The
value of C
60 hydration is negative. These parameters, however, refer to the C
60 molecule and not to the colloidal particle. Voronin et al. [
223] estimated the negative entropic
value by studying a son/nC
60 hydrosol using DLS method at different temperatures, atomic force microscopy, and isothermal titration calorimetry. Based on the combined equation of the first and second laws of thermodynamics, they stated that the C
60 fullerene aggregation in aqueous solution is entropically driven, occurring with nearly zero enthalpy change. However, the use of a thermodynamic approach for a hydrophobic colloidal system is clearly inappropriate.
Recently, Godínez-Pastor and González-Melchor [
224] published a detailed molecular dynamics study of the behavior of the fullerene/water system under liquid and liquid–vapor conditions. A computational study of fullerene/water systems under liquid phase and liquid-vapor conditions was performed uing atomistic model. At 300 K and 1 bar, fullerene aggregates were observed. The aggregation was less defined at 373 K and 10 to 24 kbar [
224].
In addition to the hydrosols and aqueous suspensions of fullerenes, the C
60 layers on the water/air interface should be mentioned. Kolker and Borovkov [
225] used cyclohexane as spreading solvent and prepared a diluted highly homogeneous 2D system C
60 – H
2O. They also showed that under compressing, instead of true Langmuir monolayers, polylayers up to hexalayers can arise. These systems can be considered as an interfacial colloid system; the cited article also provides a review of the relevant literature data [
225].
Another interesting issue is the partition of fullerenes between water and nonpolar solvents. Mikheev et al. [
226] presented a detailed quantitative study of partition of C
60 and C
70 between mutually saturated water and toluene. The specificity of their approach consists in using sonication; the ratio of concentration of fullerenes in aqueous and organic phases was relatively similar when the quasi-equilibrium state was reached from both sides. The distribution constants for the two fullerenes were found to be 6 and 2, respectively [
226]. Note, that Jafvert and Kulkarni [
227] determined the partition constants of C
60 between toluene or 1-octanol and water, 2.8×10
8 and 4.7×10
6, respectively. These values for mutually saturated solvents were obtained using three different procedures, and describe the equilibrium of single C
60 molecules in different solvents. The solubility of fullerene in water was estimated as 1×10
11 M [
227]. Such difference of partition constants is understandable because in the first paper [
226] fullerene is in colloid state in water and, probably, in toluene under conditions of sonication.
This problem is to some extent connected with the partition of nanoparticles between water and organic solvents, including 1-octanol [
228]. The last concept was criticized by Praetorius et al. [
229], because for lyophobic systems the thermodynamic approach is not correct. Their paper is entitled “The road to nowhere: equilibrium partition coefficients for nanoparticles” [
229]. Basing on approach developed by Hill [
230], Shchukin et al. [
231] mentioned that a disperse system with very small particles can be conditionally regarded as a one-phase colloidal solution containing large "molecules". However, for partition of charged particles of a lyophobic system between two liquid phases it can be really misleading.
The colloidal particles, including those of fullerenes in water are charged, which hinders their transfer into the organic phase. However, Mikheev et al. [
226] explained their results by transferring of single molecules. They estimated a maximum concentration of C
60 in water produced from a toluene solution by the solvent-exchange procedure, i.e., in the son/nC
60 sol, as 5.6×10
–4 M. Though their results are self-consistent, it would be worthwhile to process the experiments using other parameters of sonication. For example, Scharff et al. managed to prepare a son/nC
60 colloid with concentration of 1.9×10
–3 M [
232].