Introduction
The wound healing criteria are controlled by a variety of variables like the sort of wound, the fluid amount produced by the wound, and the patient’s overall health status [
1]. The use of appropriate wound dressing is essential for preventing complications such as infection and promoting optimal wound healing [
2]. Because of their excellent properties, biopolymers are extensively used as wound care materials [
3]. Biopolymers can aid in the recovery process by facilitating tissue regeneration, controlling inflammation, and acting as a scaffold for cellular growth and migration [
4,
5]. Carrageenan, α (1–4)-3,6-anhydro-d-galactose and β (1–3)-d-galactose, is a type of biopolymer derived from red seaweed that has been used in wound healing applications [
6,
7,
8,
9,
10]. It is a polysaccharide formed from galactose and 3, 6-anhydrogalactose repeating units, and the characteristics are determined by its molecular weight, degree of sulfation, and structure [
11]. Carrageenan has antimicrobial properties against a variety of bacteria such as
Staphylococcus aureus and
Pseudomonas aeruginosa, both of which can infect wounds and slow healing [
12]. However, more research is needed to fully understand its potential benefits and limitations in wound healing [
13].
Polyacrylamide, poly (2-propenamide), is a synthetic polymer that has been used in wound healing. It promotes wound healing by creating a moist environment. The moist environment assists in the prevention of wound desiccation, which can impede healing. Polyacrylamide protects the wound from external contaminants by maintaining the moist barrier while allowing oxygen and nutrients to reach the wound site [
14,
15,
16,
17]. It can also provide mechanical support to the wound site. The hydrogel can form a soft, conformable gel that can fill irregular wound shapes and provide a Cushing effect, which can reduce mechanical stress on the wound and minimize pain.
Cetrimide, alkyltrimethylammonium bromide, is a quaternary ammonium compound with antiseptic properties [
18]. It is commonly used as an ingredient in various pharmaceutical and personal care products [
19]. Cetrimide is primarily used for its antimicrobial effects [
20], helping to prevent or treat infections caused by bacteria and fungi [
21].
The reaction of the sulfonic acid group (-SO
3H) with the amide group (-CONH
2) can lead to the formation of sulfonamide (-SO
2NH-) group. This reaction can occur in both organic and inorganic chemistry contexts. The resulting sulfonamide product can have a range of applications in organic synthesis, pharmaceuticals, and materials science. Sulfonamide compounds are often used as drugs for their antibacterial, antifungal, and diuretic properties [
22]. It is well known that sulfa drugs (Sulfonamides), are a group of synthetic antimicrobial agents that have been widely used to treat bacterial infections. They work by inhibiting the growth and reproduction of bacteria, specifically by interfering with the biosynthesis of folic acid, which is essential for bacterial cell metabolism. Sulfonamides are bacteriostatic, meaning that they inhibit the growth of bacteria rather than directly killing them [
23]. They interfere with PABA (p-aminobenzoic acid) in the biosynthesis of folic acid leading to impaired DNA, RNA, and protein synthesis, and ultimately bacterial cell death.
κ-carrageenan/polyacrylamide double network, DN hydrogels covalently cross-linked were prepared using UV-initiator in the presence of KCl as an ionic charge carrier and N, N-methylenebisacrylamide as cross-linker to be used as self-healing materials [
24,
25]. Although DN hydrogels have excellent mechanical properties, however, most of them exhibit negligible fatigue resistance by the effect of irreversibility covalent bonds [
26].
Physical cross-linking methods such as ionic cross-linking is an efficient route to prepare three-dimensional network polymers without using toxic chemical agents. The chemicals used in chemical cross-linking are often toxic and the residual cross-linker must be removed before use in biomedical applications [
27].
In this study, the self-crosslinked κ-carrageenan/polyacrylamide hydrogel film was prepared successfully for the first time without using an initiator and cross-linkers by manual casting method. Cetrimide (CI) was added to the network structure to obtain a material that has a dual effect of healing and anti-inflammatory. The structures of κ-CAR/PAAm/CI films were studied by FT-IR. The thermal and mechanical properties were investigated. Antimicrobial, anti-inflammatory, and wound healing activities were also performed.
2. Materials and Methods
2.1. Materials
κ-Carrageenan [Gelcarin GP 812, Irish Moss – CAS:9000-07-1] was provided by Phytotechnology laboratories, Prod No:C257, Lot: 04F25709B and acrylamide was obtained from Sigma (St. Louis, Mo., USA), Cetrimide (C
17H
38BrN) was provided from QualiChem’s Fine Chem Pvt. Ltd., India (
Figure 1). The utilized chemicals and reagents were high quality and used without other purification.
2.2. Preparation of κ-CAR/PAAm/CI Films
In this study, a straightforward methodology was employed to prepare healing films based on κ-Carrageenan/polyacrylamide (PAAm). Initially, 2 g of κ-Carrageenan were dissolved in 100 mL of distilled water. Once fully dissolved, 5 g of acrylamide (AAm) were added and heated to 80°C for 2 hours, resulting in a viscous solution named κ-CAR/PAAm. Defined amounts of κ-CAR/PAAm were mixed with a 1% (w/w) aqueous solution of cetrimide, as specified in
Table 1. Subsequently, 30 wt % of glycerol was introduced into the solution through 2 hours of stirring. Following this, 15 mL of the solution was poured into 10 cm Petri dishes and kept in an oven at 40°C for 24 hours. Afterward, the films were immersed in distilled water at 60 degrees Celsius for 3 hours. After that, the films were washed many times with hot water, soaked in water for 24h, and then dried in an oven at 60 degrees Celsius. This treatment effectively eliminated any unreacted species. Furthermore, the degree of conversion, determined according to a reference method, was measured to be 90.1±2.3%, contributing to the hydrophobic characteristics of the films [
28].
2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
The main functional groups present in pure κ-carrageenan, κ-CAR/PAAm/CI0, and κ-CAR/PAAm/CI2 films were identified using FT-IR spectroscopy performed with a Bruker Unicom infrared spectrophotometer (Germany), and the spectra were recorded within 400-4000 cm⁻¹ wave-length region.
2.4. Thermal Gravimetric Analysis (TGA)
The thermal stability of pure κ-CAR, CAR/PAAm/CI0, κ-CAR/PAAm/CI1, and κ-CAR/PAAm/CI2 films around the sterilization temperature was investigated by thermal gravimetric analysis (TGA) using a Shimadzu TGA-30 instrument (Japan) under a nitrogen atmosphere. The analysis covered a temperature range from room temperature to 600°C, with a heating rate of 10°C/min.
2.5. Mechanical Properties
The mechanical properties of the κ-CAR/PAAm/CI0, and κ-CAR/PAAm/CI2 samples were assessed through tensile testing using Hounsfield tensile testing equipment (model H10 KS). Dumbbell-shaped specimens measuring 50 mm in length with a 4 mm neck width were employed for the tests, which were conducted at room temperature. The stretching speed of the film was set at 10 mm/min, and a 20 kN load cell was utilized.
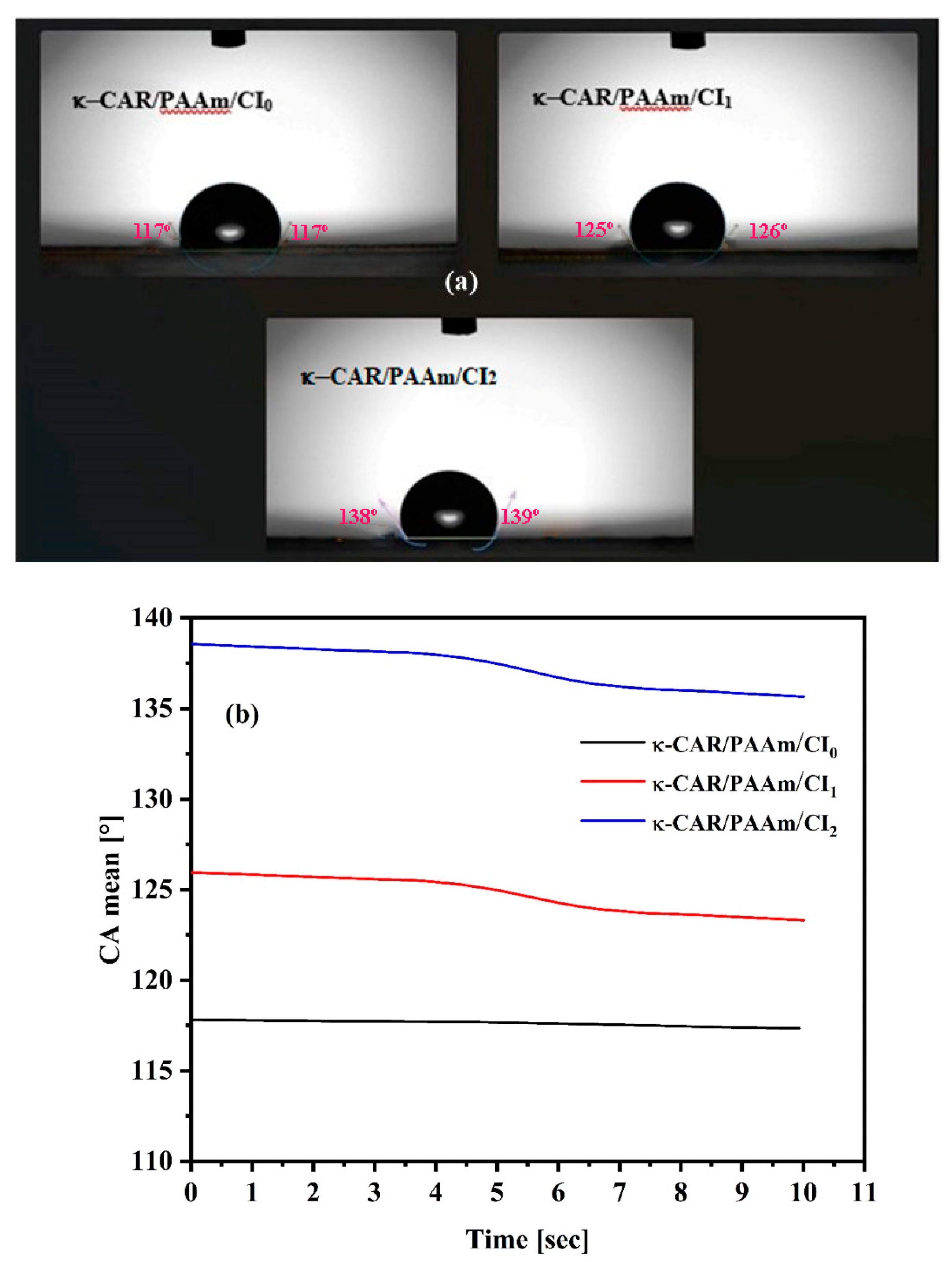
2.6. Contact Angle Measurements
The hydrophobicity or wettability of the κ-CAR/PAAm/CI
0 and κ-CAR/PAAm/CI
2 films was examined by measuring the contact angle at room temperature. A VCA Video Contact Angle System (model Krüss DSA25B, Germany) was employed for this purpose. A digital micro syringe was utilized to place a water droplet on the film surface which fixed on a well-leveled smooth platform [
29]. To ensure accuracy, triple measurements were taken for each film at different locations.
2.7. Antimicrobial Activity
The antimicrobial activity of κ-CAR/PAAm/CI
2 films was investigated using the agar well-diffusion method [
30]. In this approach, a microbial inoculum volume was evenly spread across the surface of an agar medium. The antimicrobial efficacy of the films was evaluated against three strains of bacteria, including one gram-positive Staphylococcus aureus (S. aureus), and two gram-negative Pseudomonas aeruginosa (P. aeruginosa), and Escherichia coli (E. coli). This test allowed us to assess the ability of the κ-CAR/PAAm/CI
2 films to inhibit the growth of both gram-positive and gram-negative bacteria, providing valuable insights into their potential as antimicrobial wound dressings.
2.8. Anti-inflammatory Activity
The anti-inflammatory activities of κ-CAR/PAAm/CI
0, κ-CAR/PAAm/CI
1, and κ-CAR/PAAm/CI
2 was investigated using the following protocol. Collected fresh blood from healthy volunteers was put in heparinized tubes and centrifuged at 3000 rpm for 10 minutes. The red blood pellets were dissolved in normal saline and the resulting volume was measured. A 40% v/v suspension was prepared by reconstituting the dissolved red blood pellets with an isotonic buffer solution (10 mM sodium phosphate buffer, pH 7.4) containing NaH
2PO
4, Na
2HPO
4, and NaCl. The samples were dissolved in distilled water to create a hypotonic solution. Duplicate pairs of centrifuge tubes were prepared for each dose of the extract (ranging from 100 to 1000 μg/mL) in both hypotonic and isotonic solutions. Control tubes containing distilled water or 200 μg/mL of indomethacin were also prepared. To each tube, 0.1 mL of erythrocyte suspension, which was mixed slowly. The mixture was incubated at 37°C for an hour and then centrifuged for 3 minutes at 1300 rpm. The supernatant was estimated at 540 nm by a spectrophotometer (Milton Roy). The hemolysis percent was calculated by considering that the amount of hemolysis produced in the presence of distilled water is 100%. The hemolysis inhibition percentage of the extract was calculated accordingly.
where OD
1 is the absorbance of the test sample in isotonic solution, OD
2 is the absorbance of the test sample in hypotonic solution and OD
3 is the absorbance of the control sample in hypotonic solution.
2.9. In-vivo Wound Healing
2.9.1. Excision wound mode
Animals were randomly assigned into groups for evaluation of wound healing activity. Male mice weighing between 50-60 g was utilized to evaluate the effectiveness of the biofilms, κ-CAR/PAAm/CI
0 and κ-CAR/PAAm/CI
2 in wound healing through in vivo testing in comparison to an untreated control group. The mice received standard pellet diets, water, and were kept in carefully controlled environments with 12-hour light and dark cycles. The mouse’s dorsal fur was shaved with an electric trimmer, and a wound with a diameter and depth of 100 mm
2 and 1 mm was made with sterilized surgical scissors. Biofilms of 1.5 cm x 1.5 cm sheets were applied to the wounded areas. Wound healing progress was evaluated using naked eye observation of wound contraction, epithelialization, and wound morphology. Photographs were taken at each measurement interval [
31].
2.9.2. Measurement of wound contraction
Wound size was measured at immediately and after 7th, 14th, and 21st days post-operation. The evaluation of wound healing was performed using 12 mice, divided equally into three groups. The first group was used as a control, only shaving and no dressing. The second group was treated with κ-CAR/PAAm/CI
0 films, while the third group was treated with κ-CAR/PAAm/CI
2 films. The evaluated surface area was used to calculate the percentage of wound contraction, taking initial size of the wound (100 mm
2) as 100% as in the following equation:
2.10. Statistically analysis
All results were statistically analyzed using the ONE-WAY ANOVA. Differences among average values were analyzed by Duncan’s multiple range test using IBM SPSS software version 24 as a statistical resource at P < 0.05.
3. Results and discussion
3.1. Synthetic route of κ-CAR/PAAm/CI films
κ-CAR/PAAm double-network hydrogel system was excessively produced due to the distinctive properties [
32]. Yu
et al. prepared the system using Zr
4+ ions to cross-link the first network under UV-irradiation in the presence of α-ketoglutaric acid photoinitiator and N, N′-methylene bis-acrylamide crosslinker [
33]. Deng
et al. prepared the system using K
+ ions to cross-link the first network and an ammonium persulfate initiator [
34]. In this study, κ-CAR/PAAm was prepared through a physical cross-linking method without using any initiator and crosslinker. When mixing carrageenan with acrylamide and heating the solution to 80 degrees Celsius, the thermal energy provided by the elevated temperature could have been sufficient to initiate the polymerization of acrylamide without the need for a chemical initiator as described in previous studies [
35,
36,
37]. At 80 degrees Celsius, the acrylamide monomer can be able to diffuse into the carrageenan solution and react with the carrageenan chains to form a copolymer. The carrageenan chains in the copolymer may be crosslinked with each other by the AAm. After casting and cooling, the carrageenan-acrylamide copolymer may be highly entangled, making it difficult for water molecules to penetrate the film. To ensure that no residue of AAm monomer remained in the copolymer matrix, the films were soaked for three hours in water at 60
oC, washed many times, and soaked overnight in water.
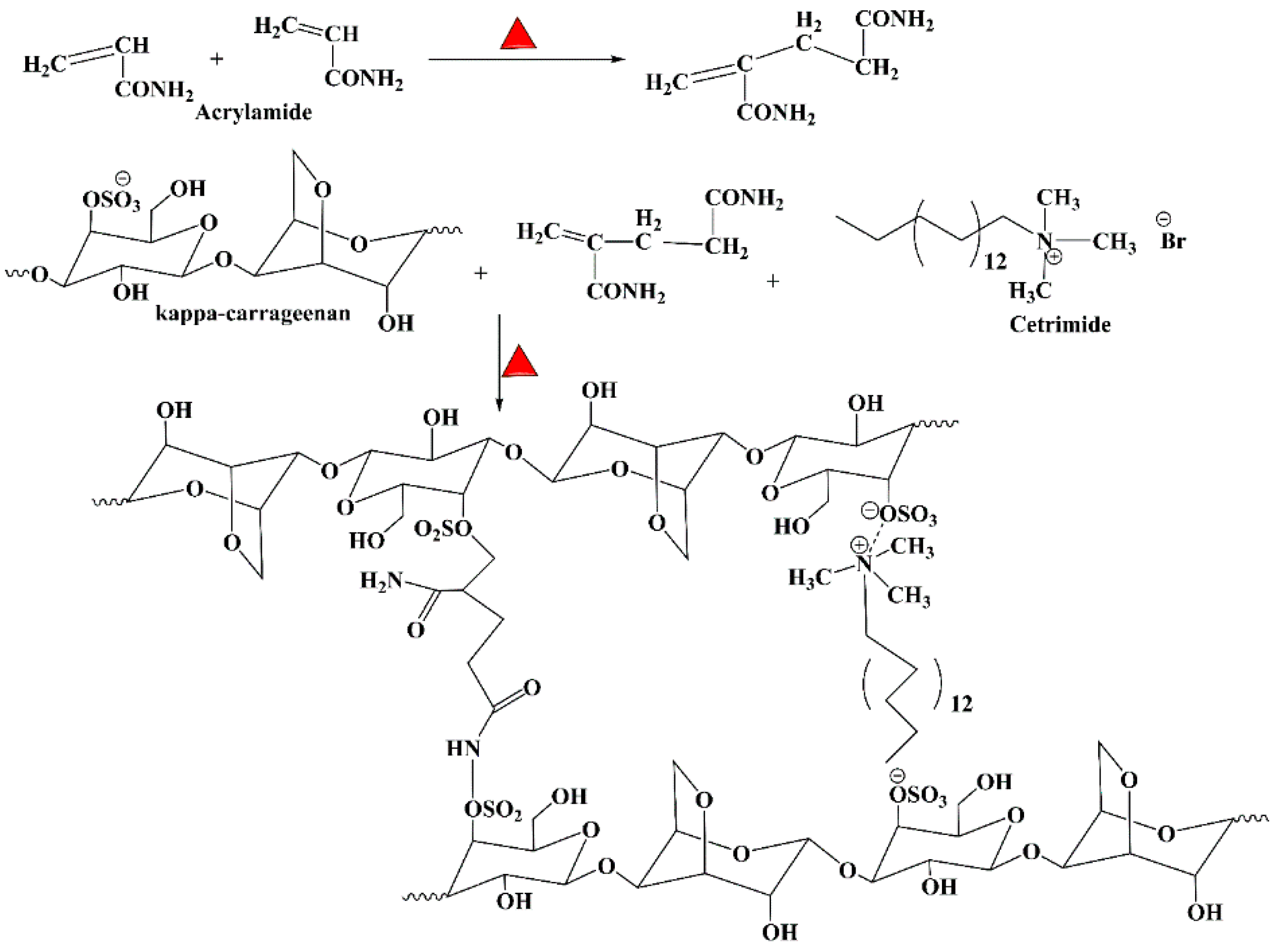
Figure 2 shows the possible synthesis route for κ-CAR/PAAm/CI by the formation of sulfonamide linkage, and Michael addition between CAR and AAm. The same mechanism was reported in the polymerization route of chitosan/acrylic acid copolymer [
27]. Moreover, cetrimide was linked with κ-CAR through electrostatic interaction between the positively charged amino group of cetrimide and the negatively charged sulfate group of κ-CAR. This mechanism is consistent with that was explained before by Cao
et al., where they synthesized complex hydrogel beads composed of hydrolyzed polyacrylamide (HPAM} and chitosan through electrostatic interaction [
28]. Moreover, chitosan/ κ-carrageenan hydrogel was greenly prepared through electrostatic interaction between positively charged amino groups on chitosan with negatively charged sulfate groups on κ-CAR without any toxic agent [
38].
3.2. Spectral Analysis
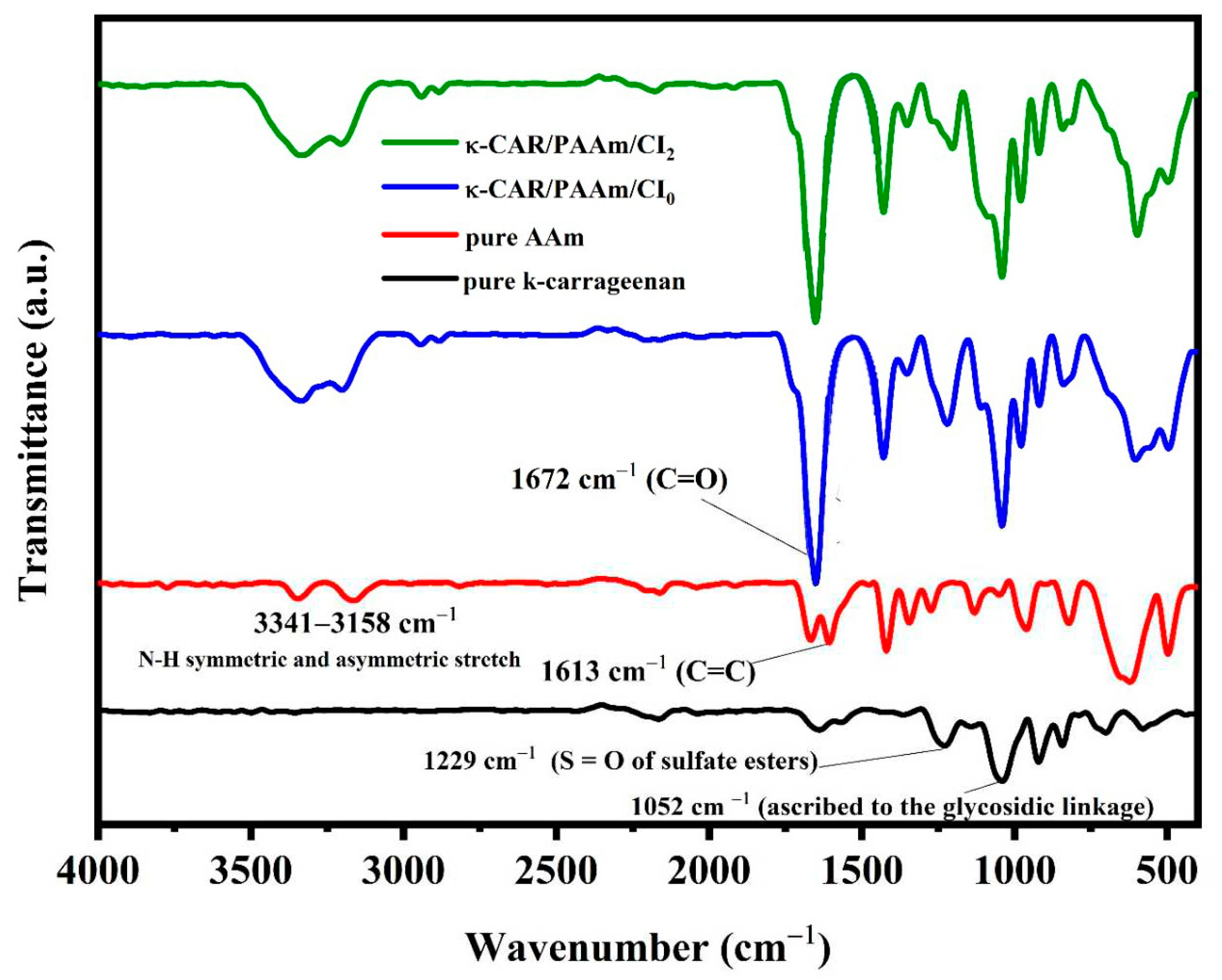
The FT-IR spectrum of pure κ-carrageenan exhibited characteristic absorption peaks that include –OH stretching vibration at 3280 cm
–1, –CH at 2920 cm
–1, the polymer bound water at 1624 cm
–1, O=S=O at 1237 cm
–1, glycoside bond at 1044 cm
–1, ether group in 3,6-anhydrogalactose of carrageenan at 918 cm
–1, and sulfate ester bonding in galactose at 849 cm
–1 (
Figure 3) [
39]. The FT-IR spectrum of pure AAm features a prominent strong peak at 1613 cm
–1 attributed to the vibration of C=C in pure AAm. Additionally, the hydrogen (H) peaks associated with C=C-H bonds at approximately 980 cm
–1, which are clear in pure AAm, exhibited reduced intensity. The FT-IR spectrum of both κ-CAR/PAAm/CI
0 and κ-CAR/PAAm/CI
2 is shown in
Figure 3. Remarkably, the C=C peak at 1613 cm
–1, indicative of AAm, is conspicuously absent in these spectra, strongly suggesting that acrylamide (AAm) has undergone full and successful polymerization into polyacrylamide (PAAm) in these samples. The FT-IR spectrum of κ-CAR/PAAm/CI
0 showed that a broad band of –OH centered at 3370 cm
–1. It must be noted that a splitting of the –OH group may reflect the presence of two different types of hydrogen bonds; intramolecular and intermolecular hydrogen bonds [
40]. Another reason for this splitting may correspond to the hydrogen bonded OH group and the non-hydrogen bonded OH group. The band of C=O of the amide group appears at 1672 cm
–1 [
41]. The peaks at 1222 and 1040 cm
–1 due to the sulfate group stretching vibrations and the anhydrogalactose unit in CAR, respectively. The pyranose ring peak appears at 603 cm
–1. The effect of CI on the chemical structure of κ- CAR/PAAm/CI
2 is obtained as shown in
Figure 3. A slight change in the spectrum of κ-CAR/PAAm/CI
2 when compared to κ-CAR/AAm/CI
0 indicated that there is no significant chemical interaction between CI and κ-CAR/PAAm. The peaks of the sulfate group, the anhydrogalactose unit, and pyranose ring stretching vibrations in κ-CAR were affected, which reflected rearrangement of these groups by the effect of CI.
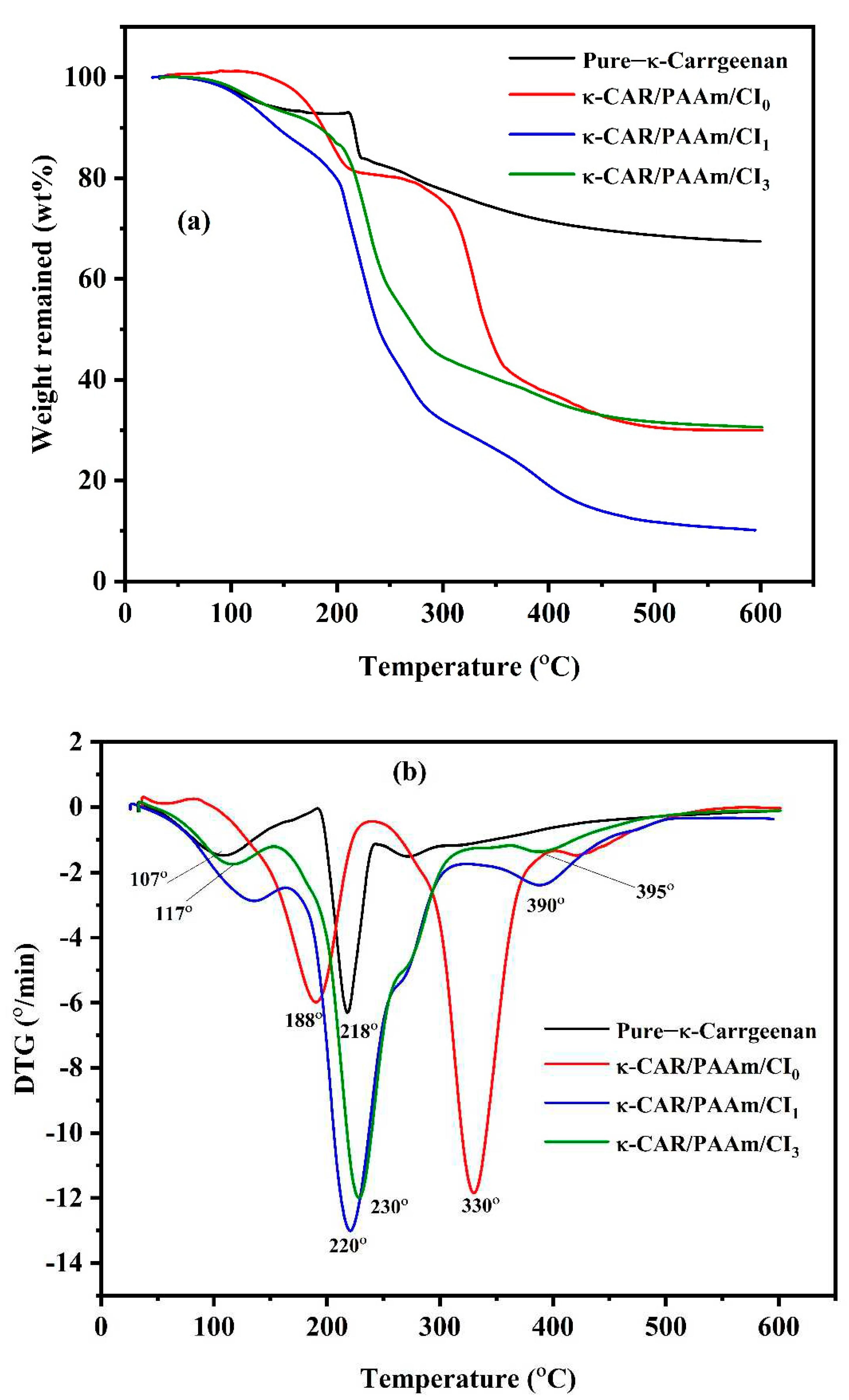
3.3. Thermogravimetric Analysis (TGA)
TGA and DTA thermograms of pure κ-CAR, κ-CAR/PAAm/CI
0, κ-CAR/PAAm/CI
1, and κ- CAR/PAAm/CI
2 are shown in
Figure 4. For pure κ-CAR, the first stage of decomposition was obtained at 120°C due to the loss of water and other volatile compounds. The main degradation of κ-CAR occurs in the range of 250°C, where a rapid weight loss is observed indicating the decomposition of the polysaccharide backbone [
42]. In light of the results obtained in our study, it is evident that κ-CAR possesses impressive thermal stability characteristics, making it a valuable material for various industrial and food-related applications [
43]. It can be observed that thermogram of κ-CAR/PAAm/CI
0 has three decomposition stages. The first stage was obtained at around 100
oC, a small amount of weight was lost owing to the loss of physically adsorbed water. The second stage occuring at 186°C may be attributed to the elimination of the –OSO
3– and –NH
2 side groups and the carbohydrate backbone fragmentation. The third stage occurs at 330°C due to the further decomposition of the backbone carbon chains. The charred residue of κ-CAR/PAAm/CI
0 is much less than pure κ-CAR which explains the high crosslink formation between κ-CAR and AAm. It can be noted that a decrease in thermal stability was obtained by the incorporation of CI in κ-CAR/PAAm/CI
1, the first stage was performed at 133°C, and the second and third stages were obtained at 220°C and 392°C. Improvement in the thermal stability was performed in κ-CAR/PAAm/CI
2 compared to κ-CAR/PAAm/CI
1. The first stage was performed at 112°C, the second and third stages were obtained at 231°C and 421°C.
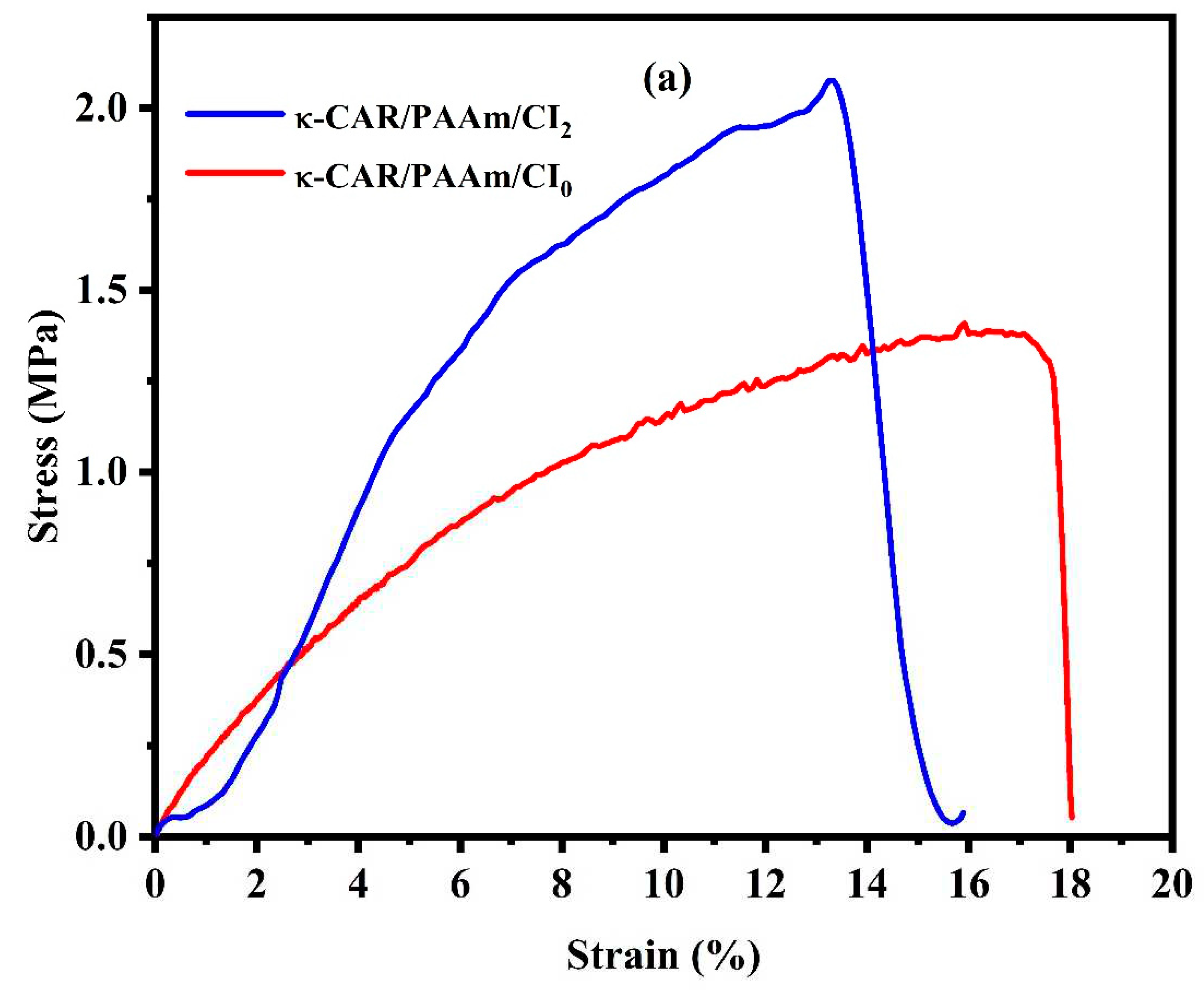
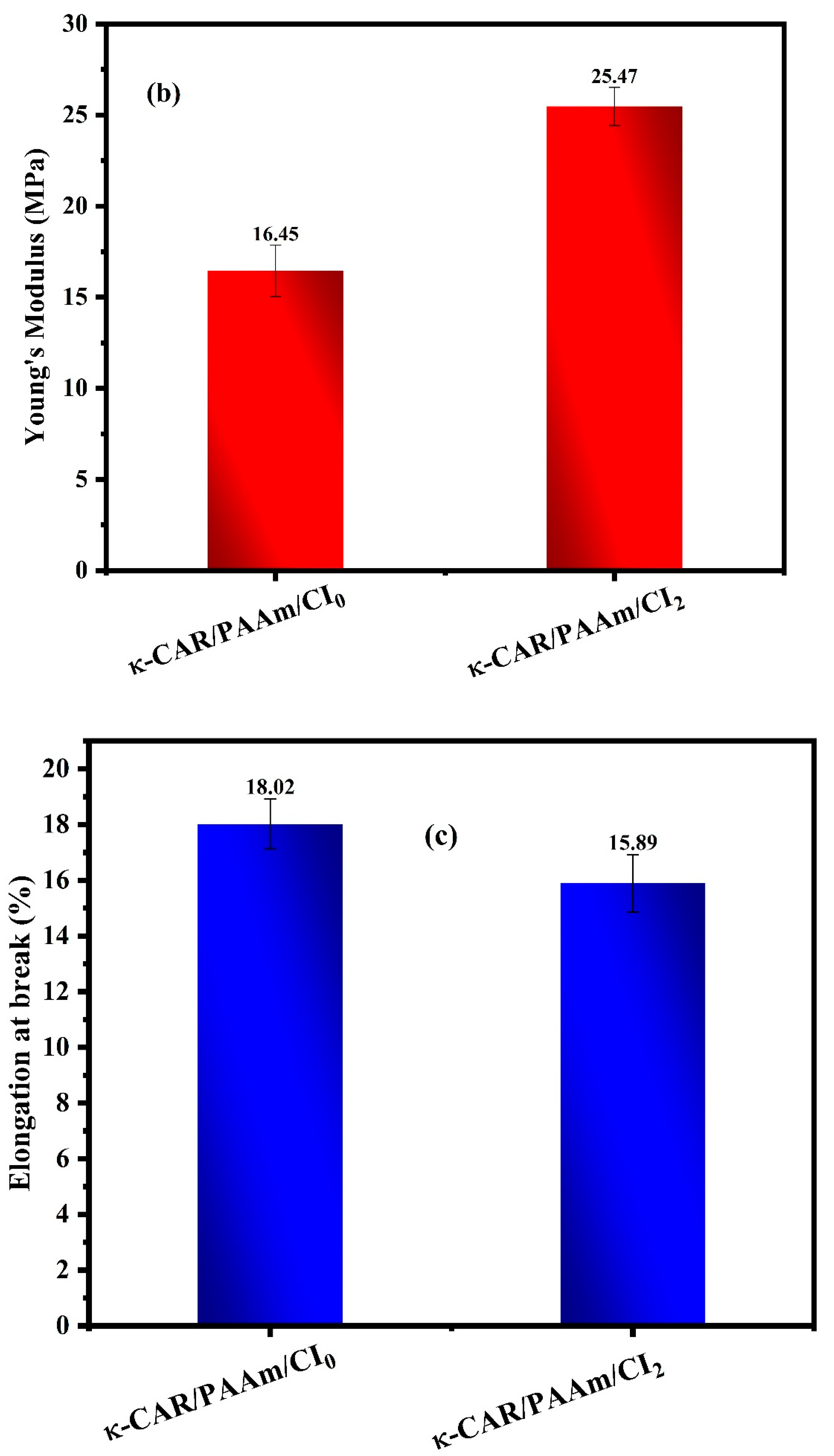
3.4. Mechanical Properties
The mechanical properties of the κ-CAR/PAAm/CI
2 film were thoroughly examined and compared with those of the κ-CAR/PAAm/CI
0 film, as visually depicted in
Figure 6 (a-c). The mechanical characteristics of wound dressings are of paramount importance due to their direct influence on wound healing outcomes. Among these properties, high tensile strength and Young’s modulus (MPa) are particularly desirable as they confer robustness and durability to the film. Notably, κ-CAR/PAAm/CI
2 displayed a significantly higher Young’s modulus (MPa) of 25.47 MPa, while κ-CAR/PAAm/CI
0 registered at 16.45 MPa. This discrepancy underscores the pivotal role of CI in enhancing the crosslinking and mechanical strength of κ-CAR/PAAm/CI
2. Conversely, the κ-CAR/PAAm/CI
2 film exhibited a percentage of elongation at a break of 15.89%, a measure of flexibility. It is crucial to recognize that the specific wound type and stage of healing can influence the ideal mechanical properties of wound dressings, and a 15.89% elongation at break may be considered suitable for wound healing and various applications in the field of wound care. These mechanical properties are vital in tailoring wound dressings to balance protection, comfort, and wound healing requirements, emphasizing the material’s suitability for various wound care applications.
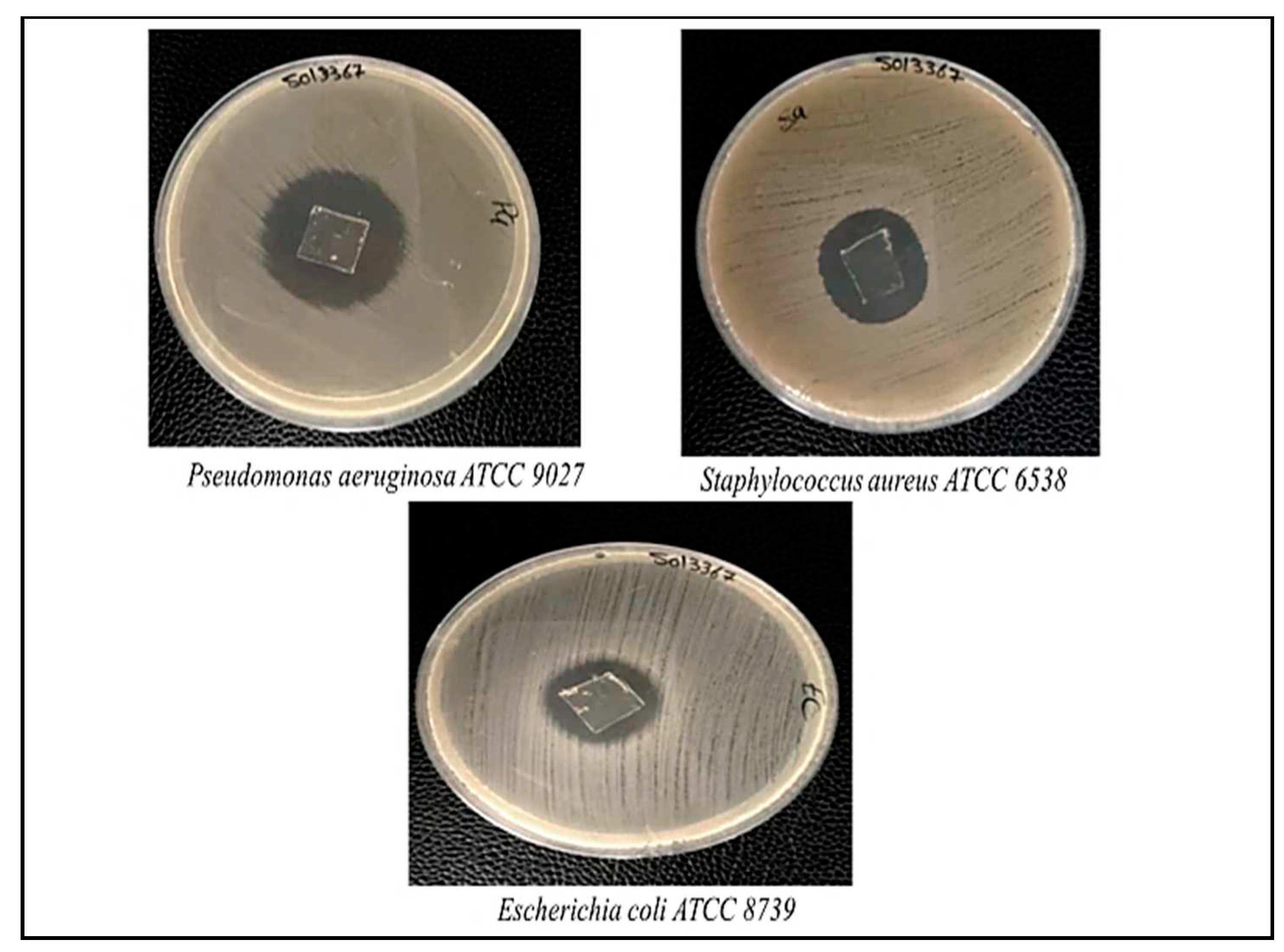
3.5. Antimicrobial Activity
The antimicrobial activity of κ-CAR/PAAm/CI
2 films against Staphylococcus aureus (S. aureus) gram-positive bacteria, Pseudomonas aeruginosa (P. aeruginosa) and Escherichia coli (E. coli) gram-negative bacteria was studied as shown in
Figure 7. It is clear that κ-CAR/PAAm/CI
2 films showed potent antibacterial properties against the investigated pathogens. The inhibition zones of S. aureus, P. aeruginosa, and E. coli were found to be 15, 31, and 13 mm, respectively. This means κ-CAR/PAAm/CI
2 films have superior antimicrobial activity against P. aeruginosa and moderate antimicrobial activity against S. aureus. While it has low antimicrobial activity against E. coli. The results in the present study are consistent with literature. The results of the antibacterial properties of κ-CAR/PAAm/CI
2 films showed considerable antimicrobial activity. This remarkable antibacterial performance can be attributed, in part, to the properties of cetrimide (CI), which serves as an essential component of the film formulation. Cetrimide is known for its inherent antimicrobial activity and has been widely utilized for its ability to inhibit the growth of both gram-positive and gram-negative bacteria [
45]. The antimicrobial mechanism of cetrimide often involves disrupting the integrity of bacterial cell membranes, thereby compromising their structural and functional integrity. The incorporation of CI into κ-CAR/PAAm films likely enhances their antimicrobial efficacy, making them promising candidates for applications where infection control and wound protection are critical.
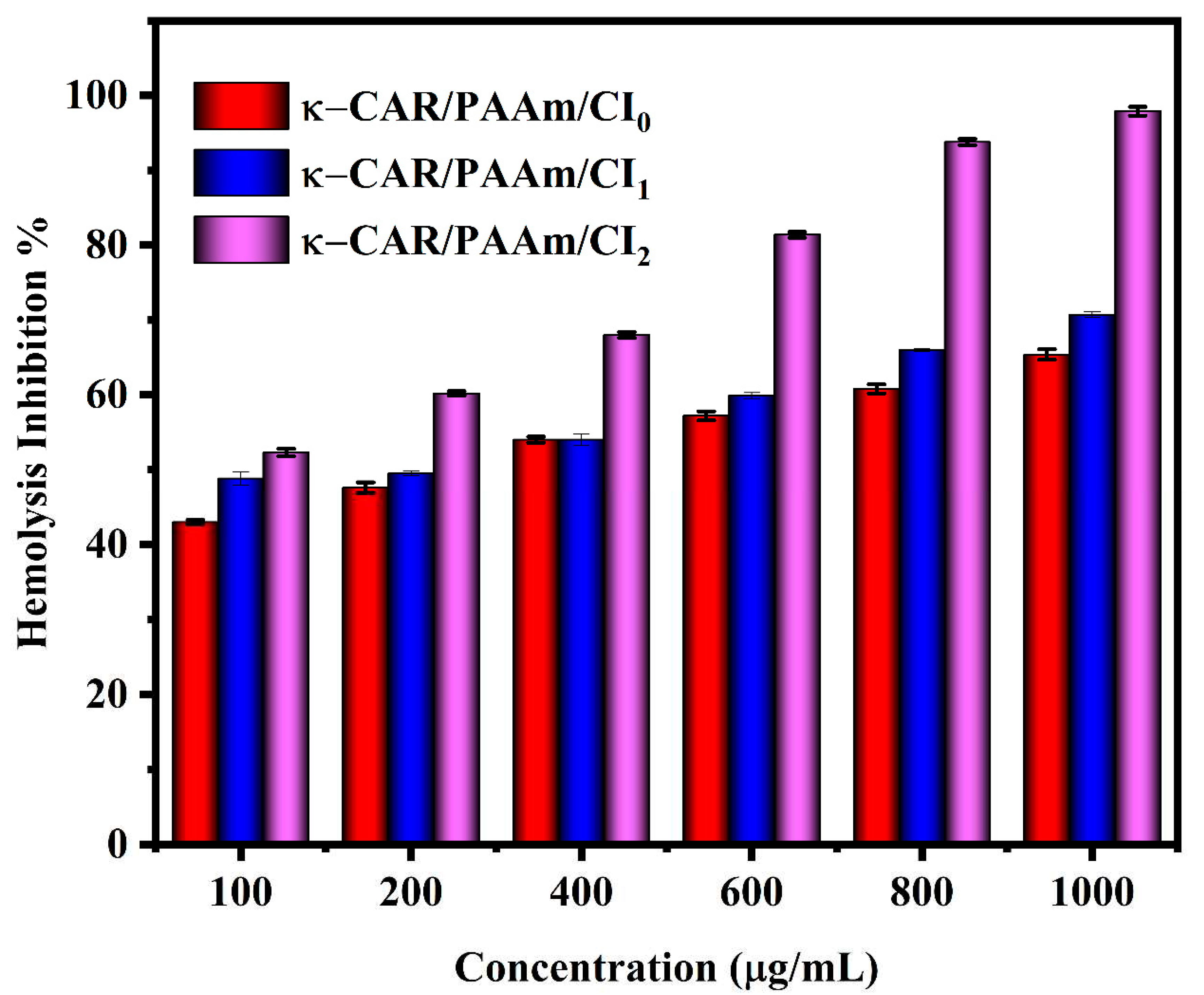
3.6. Anti-inflammatory Activity
The anti-inflammatory activity of κ-CAR/PAAm/CI
0, κ-CAR/PAAm/CI
1, and κ-CAR/PAAm/CI
2 films were investigated as shown in
Figure 8. The hemolysis curve shows that the hemolysis assay has good results and reached our expected results. The hemolysis inhibition percent at concentration 1000 µg/mL was found to be 97.9, 70.7, and 65.4% for κ-CAR/PAAm/CI
2, κ-CAR/PAAm/CI
1, and κ-CAR/PAAm/CI
0 films, respectively. It can be observed that κ-CAR/PAAm/CI
2 film exhibited the highest odema inhibition percent, followed by κ-CAR/PAAm/CI
1, and the lowest one is κ-CAR/AAm/CI
0 film. It must be noted that κ-CAR/PAAm/CI
2 film was effectively inhibiting the heat-induced hemolysis [
46].
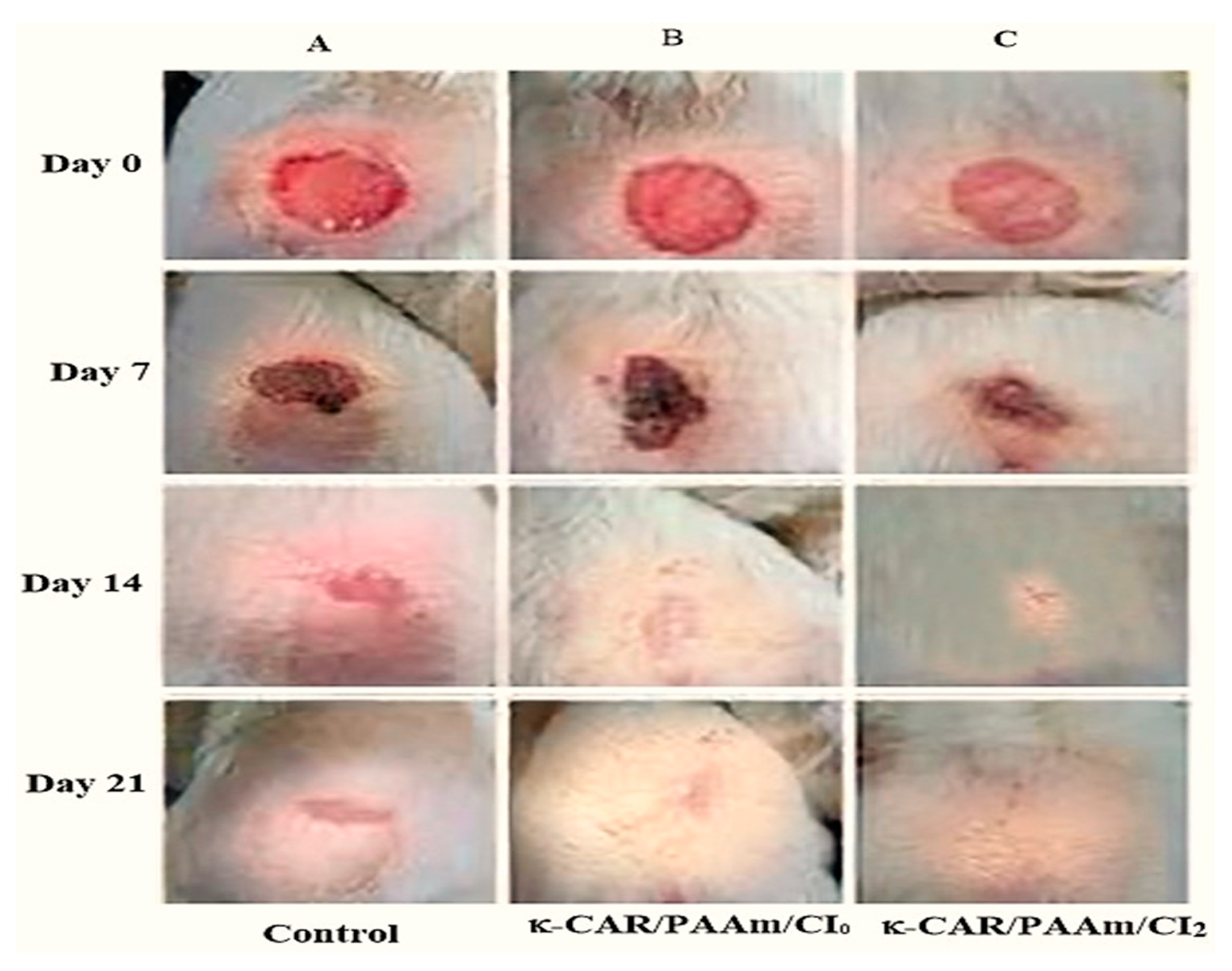
3.7. Wound Healing activity of the films
The wound contraction displays the wound healing progress at 7, 14, and 21 days, comparing groups treated with κ-CAR/PAAm/CI
0 and κ-CAR/PAAm/CI
2 films to an untreated control (
Table 2,
Figure 9). The results obtained reveal a significant enhancement in wound closure. After 7 days of treatment, the wound closure percentages were 71%, 53%, and 51% for the groups treated with κ-CAR/PAAm/CI
2, κ-CAR/PAAm/CI
0, and untreated control, respectively. By day 14, wound closure percentages improved to 98%, 90%, and 73% for the groups treated with κ-CAR/PAAm/CI
2, κ-CAR/PAAm/CI
0, and untreated control, respectively. Impressively, on day 21, groups treated with κ-CAR/PAAm/CI
2 and κ-CAR/PAAm/CI
0 films exhibited rapid healing, achieving nearly 100% wound closure, while the untreated control reached 82%. These results underscore the substantial reduction in wound area facilitated by κ-CAR/PAAm/CI
2 film. Incorporation of cetrimide; improved the film potential to expedite wound healing and shorten the epithelialization period to 19 days and 22 days in the groups treated with κ-CAR/PAAm/CI
2 and κ-CAR/PAAm/CI
0 films respectively, while in the control group it was 26 days. The presence of κ-CAR in the film, with its sulfur groups, may contribute to this effect by promoting keratosis, histological changes, and offering immunomodulatory properties [
47].
4. Conclusion
In this study κ-carrageenan/polyacrylamide/cetrimide (κ-CAR/PAAm/CI) film was prepared successfully for the first time without using an initiator and cross-linkers by manual casting method. The properties of κ-CAR/PAAm/CI films were studied in order to know the applicability to use as wound healing materials. Improvement in the thermal stability was performed in κ-CAR/PAAm/CI2 (CI: 1.5%) film compared to κ-CAR/PAAm/CI1 (CI: 0.5%). It was found that the κ-CAR/PAAm/CI0 film is hydrophobic where the contact angle is 117.95o. The contact angle increased to 126.60 o and 139.30 o of κ-CAR/PAAm/CI1 and κ-CAR/PAAm/CI2, respectively, by the effect of CI. The tensile strength and elongation percent values are considered adequate for materials used in wound care. κ-CAR/PAAm/CI2 film showed antibacterial activity against S. aureus, P. aeruginosa, and E. coli. The hemolysis inhibition percent at concentration 1000 µg/ml was found to be 97.9, 70.7, and 65.4% for κ-CAR/PAAm/CI2, κ-CAR/PAAm/CI1, and κ-CAR/PAAm/CI0 films, respectively. The in vivo wound healing study indicated that the incorporation of cetrimide into κ-CAR/PAAm film has a beneficial impact on the wound healing process and may offer a promising approach for the development of effective wound dressings. κ-CAR/PAAm/CI2 film showed higher healing activity with a wound contraction percentage of 98% after 14 days of wound treatment.
Author Contributions
Conceptualization, F.A.A and S.F.M, methodology, F.A.A and S.F.M; Investigation, F.A.A and S.F.M; writing-original draft preparation, S.F.M: writing- review and editing, F.A.A; supervision, F.A.A; project administration, F.A.A; funding acquisition, F.A.A. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was supported from the Deanship of Scientific Research, Jazan University, Saudi Arabia, project No, RUP3-4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data of this research are presented in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific research, Jazan University, Saudi Arabia supporting the project number RUP3-4.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, G.; Ceilley, R. Chronic wound healing: a review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Marioara, A.; Roxana, A.; Nicolae-Bogdan, M.; Andreea L., M.; Dana, S. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Sahana, T.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Nosrati, H.; Mohammad, K.; Zohreh, A.; Mehdi, B. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int. J. Biol. Macromol. 2021, 192, 298–322. [Google Scholar] [CrossRef]
- Riha, S.M.; Maarof, M.; Fauzi, M.B. Synergistic effect of biomaterial and stem cell for skin tissue engineering in cutaneous wound healing: A concise review. Polymers 2021, 13, 1546–1574. [Google Scholar] [CrossRef]
- Yegappan, R.; Vignesh, S.; Sivashanmugam, A.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Santosh, S. B.; Pallavi, V. M.; Kaustubh, C. P.; Rohan, C.; Prachi, B.; Prajakta, D.; Ravindra, V. A. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar]
- Neamtu, B.; Andreea, B.; Mihai, O.; Cristian, S.; Dragos, P.; Marius, Z.; Vioara, M. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117–9137. [Google Scholar] [CrossRef]
- Mokhtari, H.; Shima, T.; Fereshteh, S.; Mahshid, K.; Hamid, R.; Seeram, R.; Filippo, B. Recent advances in chemically-modified and hybrid carrageenan-based platforms for drug delivery, wound healing, and tissue engineering. Polymers 2021, 13, 1744–1765. [Google Scholar] [CrossRef]
- Natalia, P.; Saddys, R.; Rebeca, B.; Luis, B.; Sandra, F.; Francisca, L. Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: an overview of production and biotechnological applications. Biotech. 2016, 6, 146–164. [Google Scholar] [CrossRef]
- Jessie, L.; Rashin, N.; Sławomir, D.; Kazimiera, A.; Cyrille, B.; Edgar, H.; Bronwyn, H.; Marta, K.; David, A. Incorporation and antimicrobial activity of nisin Z within carrageenan/chitosan multilayers. Sci. Rep. 2021, 11, 1–15. [Google Scholar]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Wang, M.; Huihua, H.; Xiaofeng, M.; Chaokang, H.; Xiaohong, P. Copper metal-organic framework embedded carboxymethyl chitosan-g-glutathione/polyacrylamide hydrogels for killing bacteria and promoting wound healing. Int. J. Biol. Macromol. 2021, 187, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Dan, W.; Guofei, Y.; Yanjun, W.; Yining, C.; Yanping, H.; Changkai, Y.; Nianhua, D. Tough and tissue-adhesive polyacrylamide/collagen hydrogel with dopamine-grafted oxidized sodium alginate as crosslinker for cutaneous wound healing. RSC Adv. 2018, 8, 42123–42132. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jinhua, D.; Ziqiang, Z.; Dawei, L.; Wenhao, D.; Yingke, L.; Bingqi, J.; Haoxuan, L.; Qingsheng, L.; Bingyao, D. Quarternized chitosan/ quercetin/ polyacrylamide semi-interpenetrating network hydrogel with recoverability, toughness and antibacterial properties for wound healing. Int. J. Biol. Macromol. 2023, 228, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Marek, K.; Wayne, H.; Stephen, T. B.; Claire, M.; Iza, R. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Grunlan, J.; Choi, J.; Lin, A. Antimicrobial behavior of polyelectrolyte multilayer films containing cetrimide and silver. Biomacromolecules 2005, 6, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Pinto, P.; Fitas, M.; Gonçalves, L. M.; Almeida, A. J.; Ribeiro, H. M. Safety assessment of starch-based personal care products: Nanocapsules and pickering emulsions. Toxicol. Appl. Pharmacol. 2018, 342, 14–21. [Google Scholar] [CrossRef]
- Mishra, A.; Pandey, R.; Manickam, N. Antibacterial effect and physical properties of chitosan and chlorhexidine-cetrimide-modified glass ionomer cements. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 28–33. [Google Scholar]
- Giorgi, G.M.; Natalia, C.M.; Diana, R.P.; Augusto, E.J.; Giovanna, R.D.; Carlos, E.; Sérgio, L.P. Effect of cetrimide 2% with and without photodynamic therapy to reduce Streptococcus mutans burden in dentinal carious lesions. Lasers Med Sci. 2021, 36, 1935–1940. [Google Scholar]
- Aben, O.; Jhimli, B. Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys Rev. 2021, 13, 259–272. [Google Scholar]
- Bendjeddou, A.; Abbaz, T.; Khacha, N.; Benahmed, M.; Gouasmia, A.; Villemin, D. Antibacterial activity of sulfonamide derivatives against clinical strains of bacteria. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 799–804. [Google Scholar]
- Liu, S.; Li, L. Recoverable and self-healing double network hydrogel based on κ-carrageenan. ACS Appl. Mater. Interfaces 2016, 8, 29749–29758. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zixuan, W.; Huihua, X.; Qian, W.; Chuan, L.; Bo-Ru, Y.; Xuchun, G.; Xi, X.; Kai, T.; Yi, S.; Jianmin, M.; Leslie, K. N. An intrinsically stretchable humidity sensor based on anti-drying, self-healing and transparent organohydrogels. Mater. Horiz. 2019, 6, 595–603. [Google Scholar] [CrossRef]
- Zhou, L.; Xinjie, P.; Kun, F.; Rui, Z.; Jun, F. Super tough, ultra-stretchable, and fast recoverable double network hydrogels physically crosslinked by triple non-covalent interactions. Polymer 2020, 192, 122319. [Google Scholar] [CrossRef]
- Baoxiao, C.; Boying, P.; Zhengke, W.; Qiaoling, H. Advances in chitosan-based superabsorbent hydrogels. RSC Adv. 2017, 7, 42036–42046. [Google Scholar]
- Jie, C.; Yebang, T.; Yuju, C.; Qiang, M. Fabrication and properties of superabsorbent complex gel beads composed of hydrolyzed polyacrylamide and chitosan”. J. Appl. Polym. Sci. 2010, 116, 3338–3345. [Google Scholar]
- Babak, G.; Mohamad, M.; Oromiehie, A.R.; Keramat, R.; Elhame, R.R.; Jafar, M. Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT 2007, 40, 1191–1197. [Google Scholar]
- Soliman, E.A.; Khalil, A. A.; Deraz, S. F.; El-Fawal, G.; Abd Elrahman, S. Synthesis, characterization and antibacterial activity of biodegradable films prepared from Schiff bases of zein. J Food Sci Technol. 2014, 51, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Bezawit, A. A.; Tiruzer, B.; Kefyalew, A. G.; Assefa, B. A. Evaluation of Wound Healing Activity of 80% Hydromethanolic Crude Extract and Solvent Fractions of the Leaves of Urtica simensis in Mice. J. Exp. Pharmacol. 2022, 4, 221–241. [Google Scholar]
- Liu, S.; Hongbin, Z.; Wei, Y. Simultaneously improved strength and toughness in κ-carrageenan/polyacrylamide double network hydrogel via synergistic interaction. Carbohydr. polym. 2020, 230, 115596. [Google Scholar] [CrossRef] [PubMed]
- Hai, C. Y.; Chen, Y. L.; Miao, D.; Yihu, S.; Zi, L. W.; Qiang, Z. Improved toughness and stability of κ-carrageenan/polyacrylamide double-network hydrogels by dual cross-linking of the first network. Macromolecules 2019, 52, 629–638. [Google Scholar]
- Yi, D.; Min, H.; Sun, D.; Yi, H.; Yubao, L.; Taosheng, D.; Xiaohong, W.; Zhang, L.; Weizhong, Y. Dual physically cross-linked κ-carrageenan-based double network hydrogels with superior self-healing performance for biomedical application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar]
- Kishore, K.; Santhanalakshmi, K. N. Thermal polymerization of acrylamide by differential scanning calorimetry. ” J. Polym. Sci. 1981, 19, (10), 2367–2375. [Google Scholar] [CrossRef]
- Dessouki, A. M.; Taher, N. H.; El-Boohy, H. A. Radiation-induced graft polymerization of acrylamide onto poly (tetrafluoroethylene/Hexaflouropropylene/vinylidene fluoride) (TFB) films. Int. J. Radiat. Appl. Instrum. 1990, 36, 371–375. [Google Scholar]
- Wang, P.; Tan, K. L.; Kang, E. T. Surface modification of poly (tetrafluoroethylene) films via grafting of poly (ethylene glycol) for reduction in protein adsorption. ” J. Biomater Sci Polym Ed. 2000, 11, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Gholam, R.M.; Moslem, M.; Moslem, S.; Mohammad, S. Magnetic- and pH-responsive κ-carrageenan/chitosan complexes for controlled release of methotrexate anticancer drug. Int. J. Biol. Macromol 2017, 97, 209–217. [Google Scholar]
- Mahmood, W.; Khan, M.; Yee, T. Effects of reaction temperature on the synthesis and thermal properties of carrageenan ester. J. Phys. Sci. 2014, 25, 123. [Google Scholar]
- Hansen, P.E.; Spanget-Larsen, J. NMR and IR investigations of strong intramolecular hydrogen bonds. Molecules 2017, 22, 552. [Google Scholar] [CrossRef]
- Blume, A. Blume, A., Hübner, W.; Messner,G. Fourier transform infrared spectroscopy of 13C: O labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry 1988, 27, 8239–8249. [Google Scholar] [CrossRef]
- Elnashar, M.; Hassan, M. Novel epoxy activated hydrogels for solving lactose intolerance. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Sayed, A.; Mahmoud, G.A. Tuning of the properties of polyvinyl alcohol/ polyacrylamide film by phytic acid and gamma radiation crosslinking for food packaging applications. Polym.-Plast. Technol. Mater. 2023, 62, 866–876. [Google Scholar] [CrossRef]
- Meena, R.; Kamalesh, P.; Gaurav, M.; Arup, K. S. Synthesis of the copolymer hydrogel κ-carrageenan-graft-PAAm: Evaluation of its absorbent and adhesive properties. J. Appl. Polym. Sci. 2006, 102, 5144–5152. [Google Scholar] [CrossRef]
- Sayed, A.; Gehan, S.; Manar, A.; Ghada, A. M. Alkali-cellulose/Polyvinyl alcohol biofilms fabricated with essential clove oil as a novel scented antimicrobial packaging material. Carbohydrate Polymer Technologies and Applications 2023, 5, 1000273. [Google Scholar] [CrossRef]
- Yesmin, S.; Arkajyoti, P.; Tarannum, N.; Atiqur, R.; Sarkar, F. A.; Mir, I. I. W.; Talha, B.; Shafayet, A.S. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytoscience 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Touba, K.; Muhammad, S.; Muhammad, U. M.; Syed, A.S.; Nazish, J.; Shahzeb, K.; Zahid, H.; Arshad, M.; Mubeen, K.; Haroon, R. Self-crosslinked chitosan/κ-carrageenan-based biomimetic membranes to combat diabetic burn wound infections,” Int. J. Biol. Macromol, 2022, 197, 157–168. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).