1. Introduction
Companion animals have a great impact on human wellbeing. Since the late 1970s it is scientifically proven that being regularly in contact with pet animals can have positive effects on human’s mental and physical condition [
1]. The side effect of daily interaction with animals is lower stress level and many people recognized that they found it easier to build human relationships [
2]. Not only the ownership of a pet can cause remarkable positive changes on health and wellbeing but making brief interactions with companion animal has also a power of decreasing the human anxiety level [
3].
In the past few decades, the number of companion animals has considerably increased in the developed countries. The majority of households keep one or more animals [
4]. Several studies pointed out that the popularity of rabbits was increasing rapidly [
5,
6,
7,
8]. According to Mikuš et al. [
9] the most common companion animal species in the European Union were cats with a population of approximately 75.3 million (M) in 2020. Dogs were the second most popular with 65.5 M, followed by ornamental birds (35.6 M) and small mammals (19.4 M) [
9]. By the latest 2022 data, the order of the pet animal species did not change but the number of mammals expressively increased in the EU by more than 50%: Cat 127.1 M, Dog 104.3 M, small mammals 29.3 M [
10].
1.1. Animal Assisted Interactions
The difference between Animal Assisted Interaction (AAI) and Animal Assisted Therapy (AAT) must be clarified as these categories are confused in the public consciousness. While AAI’s primal goal is to improve the life quality of the patients through human-animal bond, in AAT the animals are integral parts of the treatment process. During AAT the animal and their handler are specially qualified, cooperating with a therapist with credential titles. The goals are previously determined, continuously monitored and the process is evaluated constantly. In contrast, AAI is a less definite human-animal interaction, although the animals and their handlers are also trained, the activities are freer, playing or even just petting an animal can fulfil the goal and may educate the participants about the animal itself [
11].
The first recorded use of animals in therapy was in 1792 in England by William Tuke who began to make efforts to improve the living conditions of mentally ill people. In the lunatic asylum under the control of Tuke, small animals such as rabbits and poultry belonged to the gardens [
12,
13]. To improve the health of people in general, farm animals were used in the earliest decades, while nowadays the most popular therapy animals are dogs [
14]. In the last 30 years other species have also been reported such as companion horses [
15,
16], rabbits [
17,
18,
19], cats [
20], birds [
16], guinea pigs [
21] and reptiles [
22]. The use of animals for assisted interventions is an expanding area as they appear in acute and hospice care, kindergartens and elementary schools.
1.2. Rabbits in Animal Assisted Interactions
Rabbits as therapy animals had been used in veterans’ homes, schools and kindergartens, residential care homes and nursing homes [
13,
23,
24]. Rabbits as partner animals in AAI can function well as an alternative for a dog or a cat but they need to get through a careful screening process. There are several criteria that must be met in order to use rabbits in AAI programs according to Granger and Kogan [
25] and Mallon and Cow [
26] suggesting that nowadays (2023) are still relevant. These animals need to be trained:
- (i)
for being transported and for the use of animal transport boxes/cages;
- (ii)
to accept stranger’s hands-on interaction and to cooperate while being hold in their lap for 2 minutes;
- (iii)
for being stroked or petting by more people at the same time;
- (iv)
to allow being petted in different body parts and areas including mouth area, teeth, ears and paws by strangers.
Animals need to be tolerable:
- (i)
with disabled persons within their wheelchair, crutch, walkers and walking aids for medical purposes etc.;
- (ii)
to sudden noise, sound and possibly shouting as well.
The rabbits satisfying the above requirements could be involved in various ways in AAI, therefore they can easily become the children’s favorites [
26]. Petting the animal is ideal for developing fine motor skills, learning pet care from an early age, sensitizing children and teaching children responsible pet ownership [
25].
AAI could not exist without animals. Therefore, it is of high importance to ensure the health and well-being of the animals in all aspects of AAI. As AAI is beneficial to people through the human-animal bond, it is significantly important not to affect the animals negatively. Whereas the needs and the advantages of people involved in AAI are becoming more and more evident, the needs and the advantages of animals are not clearly measurable. In AAI the well-being of animals can be hurt and harmed in many areas, there is risk of zoonoses, not only from animals to human but also from humans to animals, and the physical and mental stress which therapy animals suffer from (even for a short time) can cause a negative effect on the well-being of the animal [
14]. Menna et.al. (2019) does not advise to use exotic and special pet animals – and rabbits are also included in this category – for AAI purpose. They justify their opinion by saying that these species are not closely related to human on ethological level [
27]. Human-rabbit interactions were measured due to questionaries by Dobos et.al. in 2023. Depending on the rabbits’ pre-life, the condition of housing and even by their feeding, differences were found amicability of the rabbits in dimensions of “Aggression towards the owner” and “Aggression towards strangers” [
28]. (In order to involve rabbits into AAI it is essential for kits in the sensitive period (right after the birth) to get accustomed to (the touch of) human hand as the early handling has a positive impact on stress tolerance throughout the lifetime of the rabbit. Consequently, to ensure the rabbits’ well-being, it is beneficial for therapy rabbit breeders to familiarize kits with human hands and to have prior knowledge of the personality of the rabbit [
29] (Suba-Bokodi, 2022).
1.3. Considering transportation stress
Transportation of animals – regardless of the species – is a stressful experience [
30,
31,
32], therefore, commercial animals’ conveyance process is regulated by law and must be documented. Specific provisions relating to carrying companion animals are universalized and gives ordinary wellbeing instructions [
33].
1.3.1. Transporting rabbits
Generally, rabbit transport-related publications are focused on slaughterhouse delivers in order to improve meat quality [
34,
35,
36]. European Food Safety Authority (EFSA), panel on Animal Health and Welfare (AHAW) 2022 gave a scientific opinion about rabbits’ container transport’s welfare consequences [
37] in order to reduce the animals suffer of trauma. As a result of inappropriate animal handling during transportation typical injuries occur such as hemorrhages, bruises and broken bones. The EFSA and the above publications common recommendations are the followings:
- (i)
careful rabbit handling,
- (ii)
to prevent the transfer of urine and faeces solid floors of crate are recommended,
- (iii)
to avoid heat or cold stress, rabbits should travel in their thermal comfort zone. To fulfill this criterion the temperature inside the van should be kept permanent, between 10°C to 20°C. Heat stress is the most critical risk as rabbits have limited ability in decreasing body temperature through evaporation, they do not pant and sweat. To avoid hyperthermia, to keep the temperature under 20OC with a relative humidity of 70-75% during transport is strongly advised. To equip the vehicle with adequate ventilation system or well-functioning air conditioning is the most efficacious way for preventing heat stress.
- (iv)
Prolonged hunger (more than 12 hours feed withdraw) causes weight loss and pernicious to their welfare. In thermal comfort zone the total time of food and water delimitation should not exceed 12 hours.
- (v)
the crates’ height should be enough to let the rabbits sitting in natural position.
Buil et al.l. highlights that the critical point of commercial rabbits’ transport to slaughter is the condition of transport while the time of the journey is a less stressful aspect. Therefore, the details of transportation are needed to be well planned and executed in advance [
35].
1.3.2. Transporting dogs
The effects of carriage on dogs are well documented such as the methodology of observing the dogs’ behavior during transportation [
38,
39,
40]. Specific data, such as stress hormone (cortisol) examination is also available [
41,
42,
43,
44].
Herbel et.al. [
45] examined the stress response of beagle dogs where the animals were undertaken to repeated short-distance road transports. Our present study was carried out by analogy with the methodology of Herbel et.al. [
45]. At that study 18 dogs participated in the experiment and were divided into 3 groups, 6 each. The first week the animals were getting accustomed to the carrier. Group 1 had been transported once on week 2, 3 and 4 while Groups 2 and 3 served as controls in a non-moving vehicle and in the stable, respectively, in week 2. On week 6 all the 18 dogs were transported for two hours. During transports the animals had no visual contact with the other dogs in the car and could not see the outside environment. The driver of the car and a second person accompanying the transports had handled the dogs during the four-week accustomization phase before the study and were, thus, familiar to the dogs. The same two persons were also present during control experiments.
1.4. Non-invasive techniques for analyzing hormonal indicators of stress
The sympathetic-adrenal medullary (SAM) and the hypothalamic-pituitary-adrenal cortex (HPA) systems – the adrenal axes – are activated by environmental stimulus. Mammals produces hormones (catecholamines and glucocorticoids) in order to ensure the body to get readily available energy in an emergency action. By monitoring catecholamines and glucocorticoids the animals’ received short-term stress is becoming measurable. The plasma’s glucocorticoid level is rising by the activation of the HPA axis, consequently analyzing blood’s stress hormones level is available [
46,
47]. The most common method for assessing hormone levels is a blood test, but this method has many limitations, especially in the diagnostic process of non-domestic animals [
48]. As the procedure of sampling the animals need to be undertaken of capturing, clamping and venipuncture that is a highly stressful situation for them and increases the hormones levels [
46,
49].
An alternative method for blood sampling is measuring glucocorticoid levels in faeces, urine, saliva or hair in various animals in order to quantify stress. Samples that collected by non-invasive way are stress-free methods, therefore, it is highly considerable to fulfill animal welfare criteria [
46,
48]. Metabolites of glucocorticoids (GCM) appear in faeces, reflect the accumulation of glucocorticoids over several hours and can be collected without disturbing the animal. Thereby GCM is used in rabbits as an indicator of stress provided in a non-invasive way [
49], although, further bacterial metabolism of the collected faeces cortisol/corticosterone metabolites may appear, the sampling and storing condition are critical [
50]. After defecation the faeces samples need to be collected and frozen within 30 minutes and must be kept under -20°C until analysis. [
50].
According to Benedek et al. [
51] 24 hours after the pressure of stress the decomposition of hormone cortisol appears on the faeces.
We do suppose that transportation negatively affect the rabbits’ anxiety level but they can be accustomed to short lasting trips by regularly undertaking them to the procedure. We also suppose that different types of feed or treats offered to the rabbits during the transportation reduce their anxiety level.
2. Materials and Methods
2.1. Data of the involved rabbits
The study was conducted with 18 dwarf rabbits selected for tameness during seven generations. In this examination ten bucks and eight does were involved but the gender effect on transportation stress were not taken into consideration. The rabbits were between the age of 12 to 18 months, were measured twice: two days before and two days after the examination period. The data’s presented at
Table 1.
2.2. Housing and feeding
In the rabbit shed each rabbit was housed individually in a pet rabbit cage (95 x 57 x 46 cm) having a corrosion resistant metal coated wire mesh (on the top) and a dark plastic bottom. The metal wire mash and plastic bottom could be separated from each other easily to clean the cage properly and easily every day. The spacious rabbit cage provided all the necessary things rabbits need in their cage like a feeder, a hay container, a hanging water bottle, a separate sleeping area and a rabbit litter box filled with wood pellets (compressed wood shavings and sawdust from untreated wood). To be a house-trained animal rabbits used the litter box to urinate and defecate. All the accessories were made of plastic except the stainless steel feeder.
As for feeding, the rabbits received a complete diet (Versele-laga complete cuni adult rabbit) that is an all-in-one pellet to avoid selective feeding behavior and to ensure consumption of all the essential nutrients for optimal health. This feed does not contain a coccidiostat or other additives. The analytical data of the feed was as follows: protein 14.0%, fat content 3.0%, crude fibre 20.0%, crude ash 7.0%, Calcium 0.6%, Phosphorus 0.4%. Ingredients: derivatives of vegetable origin (timothy 10% grasses and herbs), Vegetable protein extracts, Vegetables (carrot 4%), Seeds (linseed 2%), Minerals, Fructo-oligosaccharides (0.3%) Marigold, Yucca. Nutritional additives: Vitamin A 10000 IU, Vitamin D3 1200 IU, Vitamin E 80 mg, Vitamin C 100 mg, E1 (iron) 100 mg, E2 (iodine) 2 mg, E4 (copper) 10 mg, E5 (manganese) 75 mg, E6 (zinc) 70 mg, E8 (selenium) 0.2 mg. Technological additives: Antioxidants. The fiber-rich pellet helped to achieve the optimal intestinal function. Besides the pellets, the rabbits were given ad libitum hay and a 35-40g fresh, raw carrot stick and a 30-35g apple piece daily, both from organic farming as a treat and vitamin complementary. Feeding was conducted three times a day: at 6 AM and at 6PM the pellet and at 11.30AM the carrot and apple were given. Rabbits had free access to the hay from the hay container and water from the nipple drinker the entire day. The cages were enriched with gnawing sticks and supplementary mineral blocks as environmental enrichment. Every second afternoon in good weather, rabbits were grazing for at least two hours in their mobile open air hutches.
Veterinary checkups including physical examination like dental health condition check and assessment of the appearance of Spilopsyllus cuniculi and Psoroptes were conducted on all rabbits prior to the transportation examination. It is important to add that all rabbits were vaccinated against myxomatosis and RHD and none of the them were infected with parasites and zoonosis disease.
2.3. The transportation
For two weeks every second day rabbits were taken for a 30-minute long transport at 8.30AM imitating an assisted animal’s duty that is used on Monday, Wednesday and Friday and need to be transported to the intervention’s spot. They were transported in rabbit-sized cages, made of strong plastic and consisting of two parts that are held together with a special fastening system made up of a clasp on the back and two hooks in front. It comes with a plasticized metal door with a safety clip. The upper part has an ergonomic handle to ease carrying and ensure a sturdy grip. Side grids make sure there is air circulating inside. According to the carrier direction of use it is suitable for pets weighing up to 3kg. The approximate size of the box is 24.5 x 28 x 41.5cm. Transportation dates (day/month/year) were the followings: 24.10.2022, 26.10.2022, 28.10.2022, 31.10.2022, 02.11.2022, 04.11.2022. The transportation was carried out by an air-conditioned minibus (Renault Traffic Combi, 2021 model, France) temperature at 19 degrees, with back rear seats removed. Each carrier was stabilized on the floor to avoid side slip. Twelve rabbits were transported in the first week and six in the second. While travelling in the car rabbits were not able to see each other and the outside environment during the experimental stages. Each trip was preceded by a 10-minute familiarization experience when rabbits were permitted to get used to their cages in the non-moving and shaded car. The 30-minute (25km) public road drive consisted of approximately a 10-minute city traffic and a 20-minute two-line main road. The same route was driven each occasion. The owner of the rabbits and a second person – the car driver –were present during the transportations. The methodology was based on the study of Herbel et.al. [
45] examination of dogs’ short-term transportation.
The rabbits were considered to belong to seven different groups presented at
Table 1 by alphabetical order. The group of:
Subjects that had been transported six times (n=2) while wood pellets (used also for housing) were on the ground of the carrier.
Subjects that had been transported six times (n=2) while wood pellets (used also for housing) were on the ground and a handful hay (given ad libitum while housing) – was put on the carrier.
Subjects that had been transported six times (n=2) while wood pellets (used also for housing) were on the ground, a handful hay (given ad libitum while housing) – and their daily portion of carrot and apple (approximately 70-75g) was also put on the carrier.
Subjects that had been transported three times (n=2) while wood pellets (used also for housing) were on the ground of the carrier.
Subjects that had been transported three times (n=2) while wood pellets (used also for housing) were on the ground and a handful hay (given ad libitum while housing) – was put on the carrier.
Subjects that had been transported three times (n=2) while wood pellets (used also for housing) were on the ground, a handful hay (given ad libitum while housing) – and their daily portion of carrot and apple (approximately 70-75g) was also put on the carrier.
Subjects of the control group (n=6) that had not been transported.
2.4. Collecting, storing and transporting the faeces samples to laboratory
The total sample number of this research was 126. All samples of faeces were collected from all rabbits. The first samples were collected two days prior to the examination period in order to get information of the individuals normal cortisol level. Sampling dates (day/month/year) were the followings: 22.10.2022, 25.10.2022, 27.10.2022, 29.10.2022, 01.11.2022, 03.11.2022, 05.11.2022. The samples were collected as follows:
The plastic bottom of the animal’s individual cage and the litter box were cleaned and sterilized by a biocidal product with a spectrum of bactericidal, fungicidal and virucidal effects (SteriClean Farm, active ingredient: Sodium hypochlorite solution (0.05%) a daily at 7.30AM.
After restoring all equipment in the cage, the litter box was filled with wood pellets again.
At 8:30AM if faeces appeared in litter box, they were removed immediately.
After 8.30AM from the first faeces of the animals, samples were collected using sterile gloves to each individual. Every 15 minute the conductor of the examination checked the litter boxes and collected the new samples.
All the samples were labeled (with the rabbit number and sampling date) and immediately frozen down.
The samples were kept at -21oC for approximately 10 weeks until it was transported to the laboratory.
The samples were transported in a cold box, that is suitable for vaccine carries, in frozen condition, on 09.01.2023 to the Department of Diagnostic Laboratory, University of Veterinary Medicine Budapest, Hungary where the laboratory examination had been carried out and GCM had been determined.
The rabbits’ faeces cortisol level was analyzed using generalized linear model (GLM) analysis taking into account that the same rabbits were evaluated repeatedly. Statistical analysis was performed using SAS 9.4 software using the PROC MIXED procedure.
3. Results
Table 2 presents the faeces cortisol level of the individuals: the first column shows the rabbit number used for the examination, while the second one shows the different groups created by the various feed they received during the transportation, in alphabetical order (detailed at chapter 2.3. – Material and Methods, The Transportation). From the 3rd to the 9th columns the measured cortisol values are presented – provided by the laboratory – on a daily basis in ug/g.
Table 3 presents the effect of transportation to the rabbits faeces cortisol concentration. As p <0.001 repeated transportation causes significant differences in stress hormone presence in rabbits faeces samples compared to the animals who had not taken under the process of transportation.
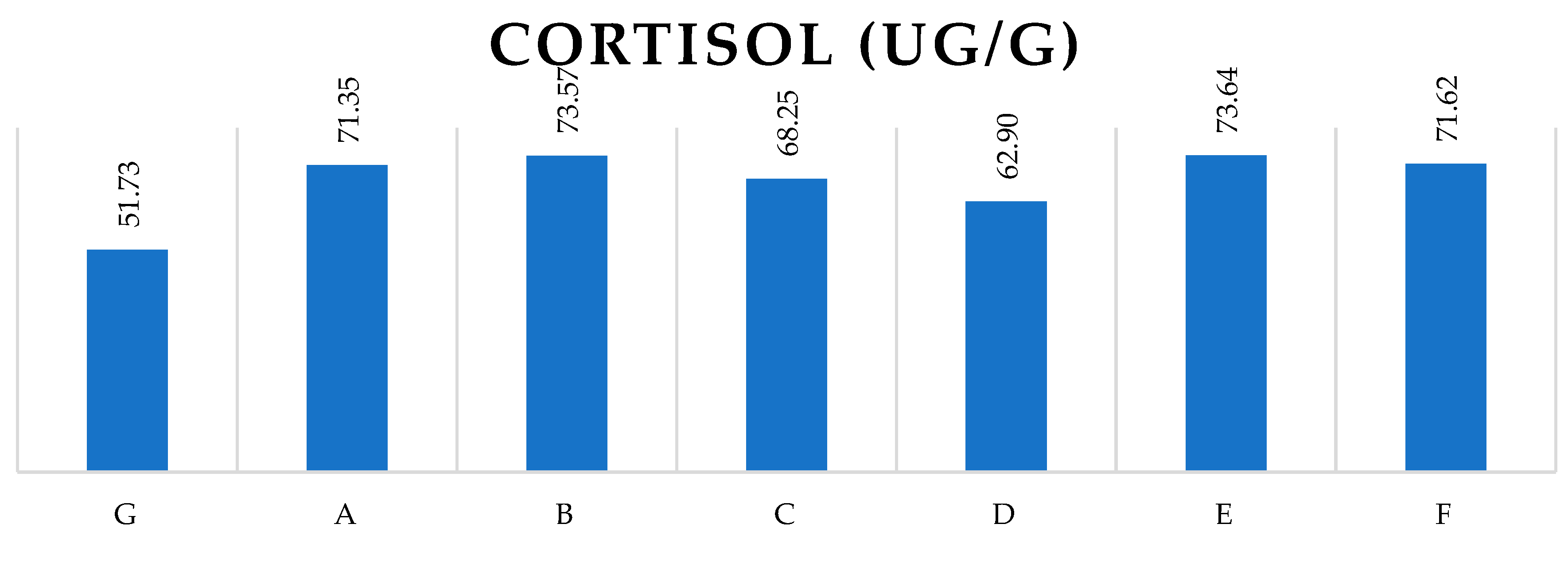
Figure 1 represents the impact of different additional feed during the transports based on the animals’ faeces cortisol level (ug/g). None of the feed (hay or hay with carrot) had demonstrably positive impact on the rabbits measured cortisol level.
where:
G – Control rabbits
A – received only wood pellet and transported 3 times
B – received wood pellet and hay, transported 3 times
C – received wood pellet, hay, and additional treats, transported 3 times
D – received only wood pellet and transported 6 times
E – received wood pellet and hay, transported 6 times
F – received wood pellet, hay, and additional treats, transported 6 times
Based on this result of our present study, there is no correspondence between the different additional feed during the transportation and faeces cortisol concentration, so hereafter we continue the analysis of the results only according to transportation and control groups. The detailed statistical data of the feeding’s effect is presented in
Appendix A.
Figure 2.
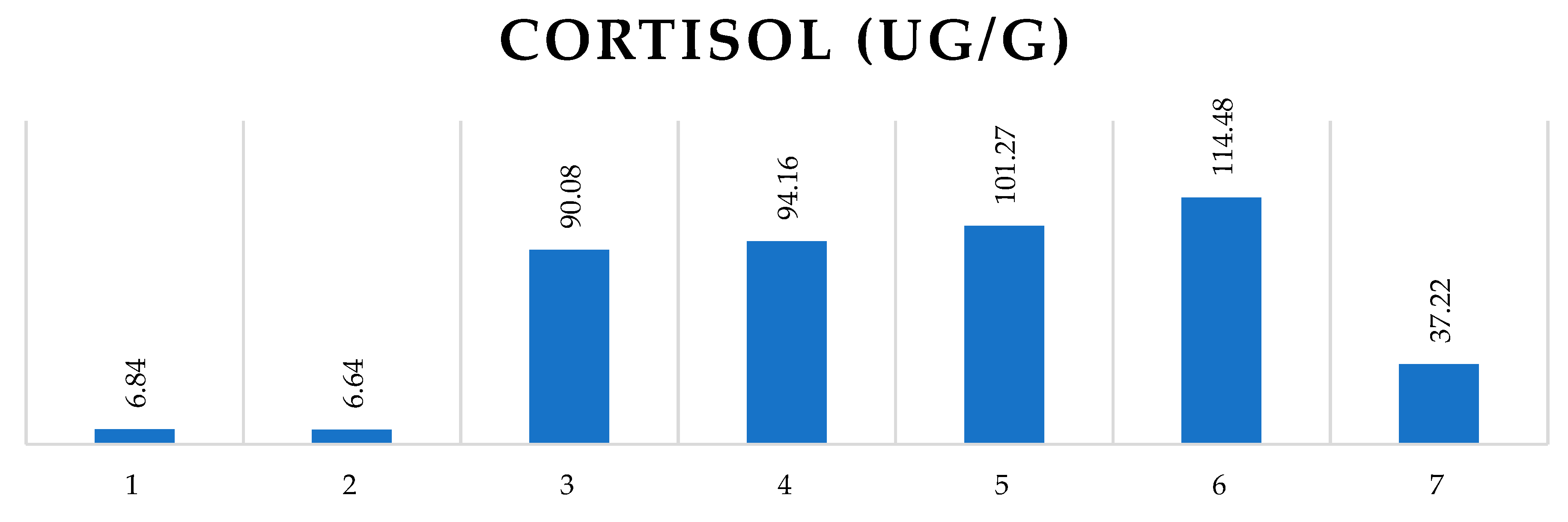
The effect of elapsed days to the rabbits’ stress hormone, cortisol (in ug/g) in faeces samples.
Figure 2.
The effect of elapsed days to the rabbits’ stress hormone, cortisol (in ug/g) in faeces samples.
where
1 – cortisol level (ug/g) of the rabbits’ faeces sample before the transport begun (day 23/10)
2 – cortisol level (ug/g) of the rabbits’ faeces sample after the first transport (day 24/10)
3 – cortisol level (ug/g) of the rabbits’ faeces sample after the second transport (day 26/10)
4 – cortisol level (ug/g) of the rabbits’ faeces sample after the third transport (day 28/10)
5 – cortisol level (ug/g) of the rabbits’ faeces sample after the 4th transport (day 31/10)
6 – cortisol level (ug/g) of the rabbits’ faeces sample after the 5th transport (day 02/11)
7 – cortisol level (ug/g) of the rabbits’ faeces sample after the 6th transport (day 04/11)
The detailed statistical data of elapsed days effect is the subject of
Appendix B.
The difference is significant (p<0.001) between the following days: 1-6; 2-5; 2-6 and 6-7. Must admit that comparison of days 1-5 (p=0.0053); 2-3 (p=0.0022) and 2-4 (p=0.0011) the p values are slightly below the significant level.
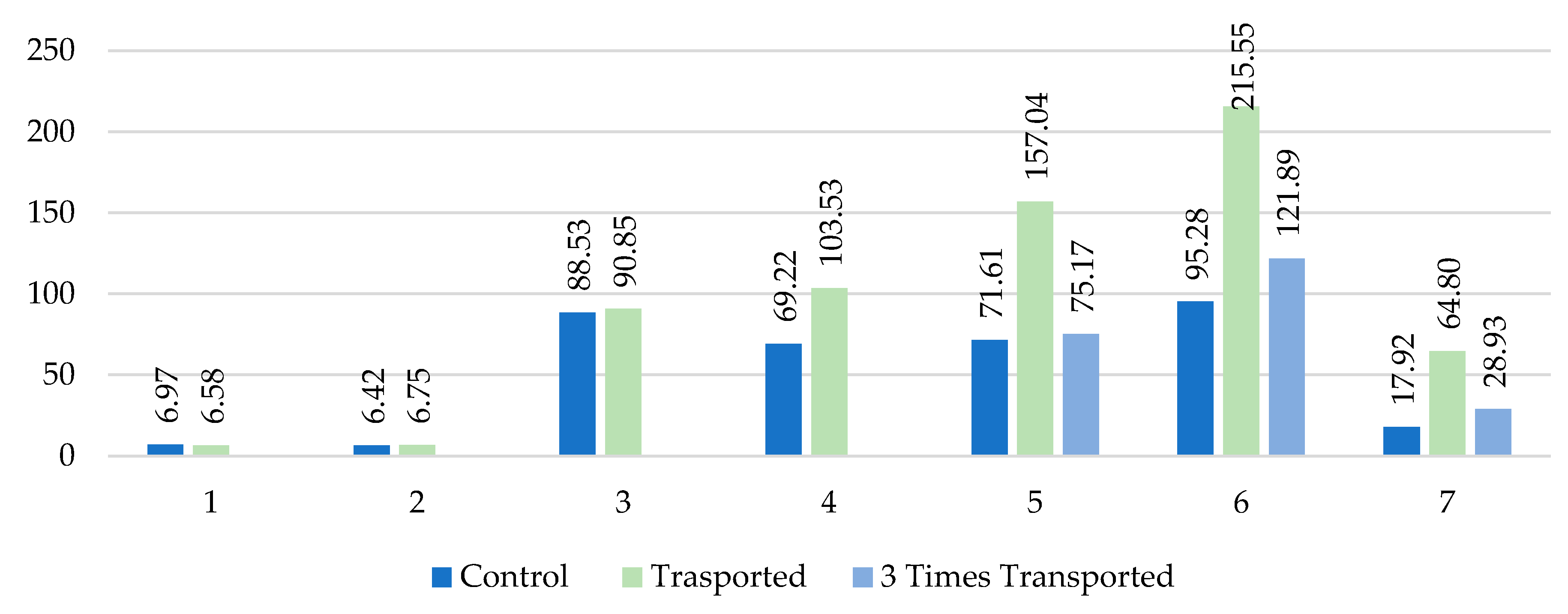
Figure 3 shows the mean of the rabbits’ faeces cortisol (ug/g) measured after the occasion sessions on a daily basis.
where
1 – cortisol level (ug/g) of the rabbits’ faeces sample before the transport begun (day 23/10)
2 – cortisol level (ug/g) of the rabbits’ faeces sample after the first transport (day 24/10)
3 – cortisol level (ug/g) of the rabbits’ faeces sample after the second transport (day 26/10)
4 – cortisol level (ug/g) of the rabbits’ faeces sample after the third transport (day 28/10)
5 – cortisol level (ug/g) of the rabbits’ faeces sample after the 4th transport (day 31/10)
6 – cortisol level (ug/g) of the rabbits’ faeces sample after the 5th transport (day 02/11)
7 – cortisol level (ug/g) of the rabbits’ faeces sample after the 6th transport (day 04/11)
The sample collection – detailed in
Table 2 – started on 23/10/2022 a day before the first transportation was carried out. There was no significant difference between the groups of the faeces cortisol level. The mean of faeces cortisol level at the transported rabbits group established at 6.58 ug/g, while 6.97 ug/g was on the control group. The total mean of all animals who participated in the study was 6.71 ug/g.
The first week’s transportations were carried out 3 times: 24, 26 and 28 October, 2022. The measured faeces cortisol levels after the first occasion was statistically the same as the first samples that had been collected before the transportation begun. From the second occasion (from 26/10) all the faeces cortisol concentration was higher compared to the starting date. There is no significant difference between the groups faeces cortisol after the first and second occasions. For the first occasion the transported rabbits’ cortisol level was 4,89% higher than the control group’s. This difference decreased to 2,56% by the second occasion. For the third occasion the control rabbits’ cortisol mean decreased from 88.53 ug/g to 69.22 ug/g while the transported ones increased by 12.68ug/g.
During the first week 12 rabbits had been transported, by the second week (31/10, 02/11 and 04/11) 6 of them remained at their housing (Group of “three times transported” rabbits, marked by light blue columns) and the other 6 rabbits were continuously transported. In each occasion the continuously transported ones had higher cortisol levels in faeces samples compared to the other two groups: the control and the non-transported ones. The transported rabbits’ faeces cortisol concentration was 157,04 ug/g on 31/10 that increased the following day by 72% and reached 215 ug/g level. On 04/11 the GCM concentration reduced to one third (64,8 ug/g) compared to the previous session’s result.
4. Discussion
The outcome of this research have proved insight into the rabbits’ anxiety level during transportation. We failed to reject the hypothesis that transportation negatively affects the rabbits’ anxiety level, however, rabbits may get accustomed to it by regular short-term transport journeys as because at the last day of the experimental period significant reduction percept.
In order to get information about the rabbits start up anxiety level, sampling from all the rabbits had been carried out a day before the transportation started. The total mean of faeces cortisol was 6.71 ug/g at the beginning and it was 6.58 ug/g after the first transport. After a day off the examination was continued, the rabbits were taken to the same session and the faeces samples were collected again. Their faeces cortisol concentration rose to 90.85 ug/g. It must be noted that after the second occasion, not only the transported rabbits’ faeces cortisol concentration increased more than tenfold, but the control groups’ also, although there was no significant difference between the control and the transported animals’ faeces cortisol concentration level.
The reason of the significant rise between the days and not the groups had been investigated. The circumstances of housing did not change during the study. All the animals were kept individually on their own cage for at least 6 weeks before the study had been carried out. No equipment was changed and the daily routine was made exactly the same way as the animals were getting used to previously. Ad libitum hay and water was available for them and portioned pellet food was offered twice a day just as before the study. The animals were taken care by their owner who was handling them during the transportation and was present entirely. No environmental changes occurred; the animals were kept under thermoneutral conditions. Before the experimental period the attendance of the rabbits’ owner always meant a joyful time for the animals as they were treated carefully and offered feed as a daily treatment. Therefore, the rabbits’ attitude towards the owner was delightful due to proper handling techniques.
In contrast, as the owner undertook some of the animals to a stressful event, this attitude toward the owner might have been changed. Because of the significant rise on faeces cortisol concentration in both groups (the control and the transported) we suppose that animals may share their stressful experiences but in a different way as human do, in a way that human senses are not able to express or detect. Previous studies proved that in different stressful situations lambs showed coherence emotional reactivity by alerting their behavioral and physiological responses [
52]. Stressful events cause catecholamine and glucocorticoid production throw the activation of the sympathetic system and hypothalamic-pituitary-adrenalin axis [
53]. However, there is little known about how stressful experience influence the animals’ relationship with each other, but it is well established that humans’ develop stronger. To survive the cooperation between the animals may improve social interoperability and animals are to share negative experiences [
54].
In our present study the control group’s faeces cortisol level was risen from the second day of transportation (26/10) till the last occasion (04/11) to average 81,16 ug/g when it reduced by more than 80% and established at level 17,92 ug/g for the last examined day.
On the bases of these data the conclusion is drawn that one occasion of 30 minutes road transport in individual boxes under thermoneutral condition did not make an essential impact on rabbits and as there is no significant difference between the transported and the control groups, we suppose that the animals were able to cope with the stress caused by the second transport. On the 3rd, 4th, 5th and 6th occasions (on 28/10 31/10 02/11) the difference did appear between the transported and control group’s faeces cortisol level but was not significant. Similarly to the control group results, transported rabbits faeces samples’ cortisol considerably reduced for the last examined day from 215.55 ug/g to 64,8 ug/g. We can conclude that transportation negatively affects the rabbits’ anxiety level but based on the large-scale reduction appeared by the 6th session we assume that rabbits might be trained for short term transport, they can get used to it by regularly undertaking them to the procedures while circumspectly handling is ensured.
To avoid the exhaustion of the rabbits that is used for assisted/therapy purpose we suggest that trainers who are responsible for the animals and ensure their welfare during the interventions, must take into consideration that transportation itself causes a high stressful experience. Presumably rabbits can get used to the transportations because the faeces cortisol hormone appearance showed a significant degradation by the last sampling date in our study, but the two-week period training was not enough for the rabbits, therefore future studies are needed.
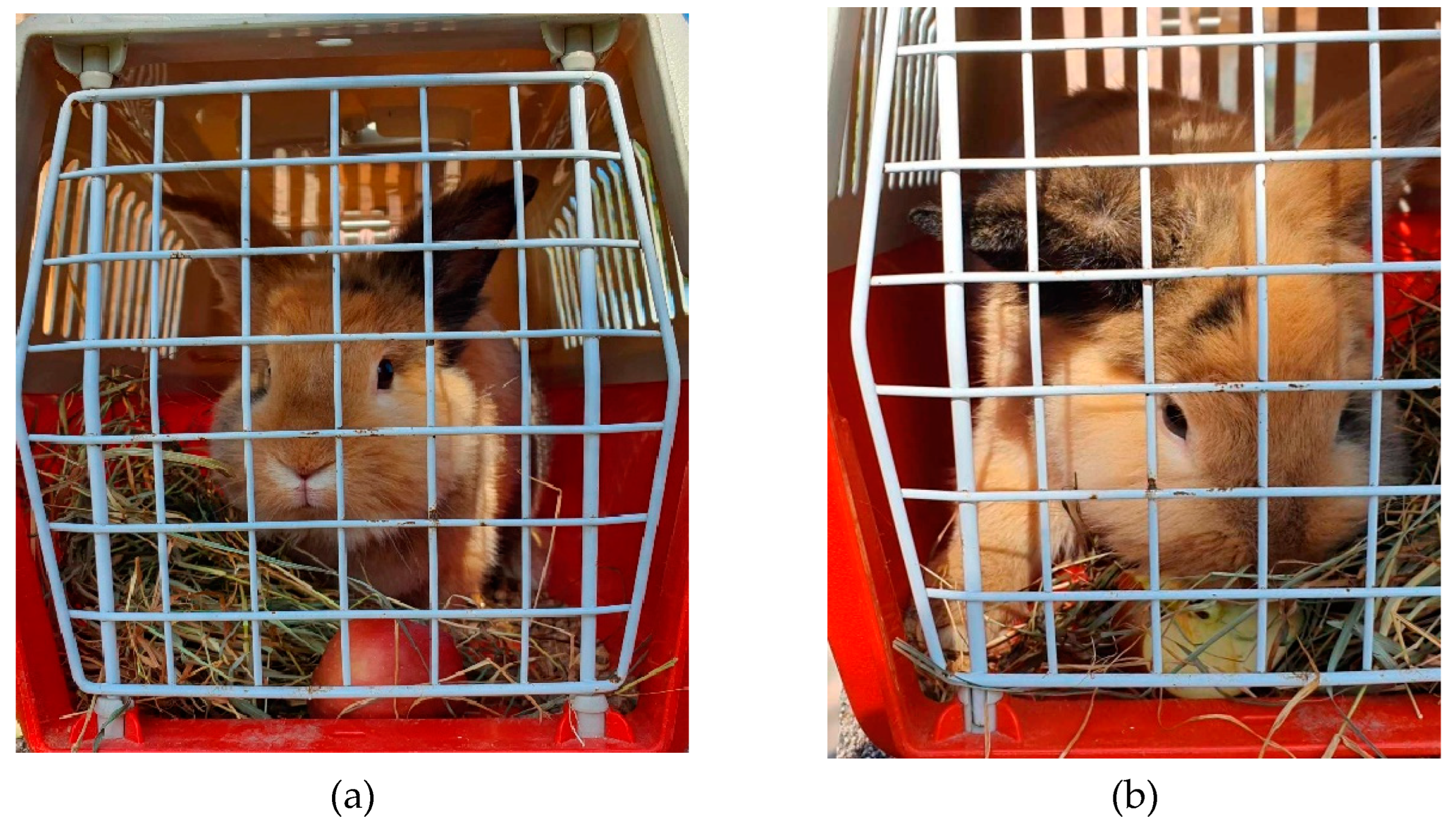
We supposed that different types of feed or treats offered to the rabbits during the transportation reduce their anxiety level. According to the examined faeces cortisol levels collected after the transportation of the rabbits our hypothesis is failed to be proven. We can not establish a correlation between the stress level of the transported animals and the offered hay or other various supplementary feed, so our hypothesis must be rejected. Although we have to note that some of the rabbits ate while they were at their carrier cages (
Figure 3 a and b) that is why we do suggest offering them additional feed as treats and hay.
Figure 4.
Rabbit at the individual carrier cage eating treats.
Figure 4.
Rabbit at the individual carrier cage eating treats.
5. Conclusions
Transportation itself put pressure on the animals in addition to the stress caused by Animal Assisted Interventions. Generally, rules of transportation are regulated and adjusted to animal species, but these orders mainly apply to a single visit to the slaughterhouse. However, animals must be transported to AAI sessions regularly, even several times a week. About the transport of companion animals there are only a few available studies, therefore data obtained on dogs can be the guide. But as rabbits are prey animal species, may react differently to transportation. During our study, the rabbits were transported every second day for two weeks, a total of 6 times for 30 minutes each time. After the transports, faeces samples were collected and in order to determine its cortisol concentration and get information about the animals’ experienced stress in a non-invasive way. In our present study none of the feeds (hay, carrot and apple) had effect on the animals’ stress, we cannot establish a correlation between the stress level of the transported animals and the offered hay or other various supplementary feed. But we must note that some of the rabbits ate while they were at their carrier cages, that is why we do suggest offering them additional treats and hay to get a chance to occupy themselves during the transport.
We can conclude that repeated transportation negatively affects the rabbits’ anxiety level but based on the large-scale reduction appeared by the last, the 6th session we assume that rabbits might be trained for short term transport. To avoid the exhaustion of the rabbits that is used for assisted/therapy purpose we suggest that trainers who are responsible for the animals and ensure their welfare during the interventions, must take into consideration that transportation itself causes a high stressful experience. Presumably rabbits can get used to the transportations because the faeces cortisol hormone appearance showed a significant degradation by the last sampling date in our study, but the two-week period training was not enough for the rabbits, therefore future studies are needed.
Institutional Review Board Statement
This research was approved by the Committee on the Ethics of Animal Experiments of the Hungarian University of Agriculture and Life Sciences Kaposvár Campus (permit number: MATE KC MÁB/2-3/2019). The authors declare that all experiments were performed in accordance with approved guidelines and regulations.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Differences of Least Squares Means according to feeding
| Effect |
feed |
feed |
Estimate |
Standard Error |
DF |
t Value |
Pr > |t| |
Adjustment |
Adj P |
| feed |
0 |
1 |
26.0033 |
26.8007 |
112 |
0.97 |
0.3340 |
Tukey-Kramer |
0.9593 |
| feed |
0 |
2 |
-17.4130 |
26.8007 |
112 |
-0.65 |
0.5172 |
Tukey-Kramer |
0.9949 |
| feed |
0 |
3 |
29.1042 |
26.8007 |
112 |
1.09 |
0.2798 |
Tukey-Kramer |
0.9310 |
| feed |
0 |
4 |
11.6410 |
21.5198 |
112 |
0.54 |
0.5896 |
Tukey-Kramer |
0.9981 |
| feed |
0 |
5 |
0.9066 |
21.5198 |
112 |
0.04 |
0.9665 |
Tukey-Kramer |
1.0000 |
| feed |
0 |
6 |
2.9218 |
21.5198 |
112 |
0.14 |
0.8922 |
Tukey-Kramer |
1.0000 |
| feed |
1 |
2 |
-43.4163 |
23.8131 |
112 |
-1.82 |
0.0709 |
Tukey-Kramer |
0.5354 |
| feed |
1 |
3 |
3.1009 |
23.8131 |
112 |
0.13 |
0.8966 |
Tukey-Kramer |
1.0000 |
| feed |
1 |
4 |
-14.3623 |
25.5367 |
112 |
-0.56 |
0.5750 |
Tukey-Kramer |
0.9977 |
| feed |
1 |
5 |
-25.0967 |
25.5367 |
112 |
-0.98 |
0.3278 |
Tukey-Kramer |
0.9568 |
| feed |
1 |
6 |
-23.0815 |
25.5367 |
112 |
-0.90 |
0.3680 |
Tukey-Kramer |
0.9713 |
| feed |
2 |
3 |
46.5172 |
23.8131 |
112 |
1.95 |
0.0533 |
Tukey-Kramer |
0.4500 |
| feed |
2 |
4 |
29.0540 |
25.5367 |
112 |
1.14 |
0.2577 |
Tukey-Kramer |
0.9150 |
| feed |
2 |
5 |
18.3196 |
25.5367 |
112 |
0.72 |
0.4746 |
Tukey-Kramer |
0.9913 |
| feed |
2 |
6 |
20.3348 |
25.5367 |
112 |
0.80 |
0.4275 |
Tukey-Kramer |
0.9849 |
| feed |
3 |
4 |
-17.4632 |
25.5367 |
112 |
-0.68 |
0.4955 |
Tukey-Kramer |
0.9933 |
| feed |
3 |
5 |
-28.1976 |
25.5367 |
112 |
-1.10 |
0.2719 |
Tukey-Kramer |
0.9256 |
| feed |
3 |
6 |
-26.1824 |
25.5367 |
112 |
-1.03 |
0.3074 |
Tukey-Kramer |
0.9471 |
| feed |
4 |
5 |
-10.7344 |
23.8131 |
112 |
-0.45 |
0.6530 |
Tukey-Kramer |
0.9993 |
| feed |
4 |
6 |
-8.7192 |
23.8131 |
112 |
-0.37 |
0.7149 |
Tukey-Kramer |
0.9998 |
| feed |
5 |
6 |
2.0152 |
23.8131 |
112 |
0.08 |
0.9327 |
Tukey-Kramer |
1.000 |
where Colum 2 numbers represents the various feed intake and number of transporting:
0 – Control rabbits (Group G)
1 – received only wood pellet and transported 3 times (Group A)
2 – received wood pellet and hay, transported 3 times (Group B)
3 – received wood pellet, hay, and additional treats, transported 3 times (Group C)
4 – received only wood pellet and transported 6 times (Group D)
5 – received wood pellet and hay, transported 6 times (Group E)
6 – received wood pellet, hay, and additional treats, transported 6 times (Group F)
Appendix B
| Effect |
day |
day |
Estimate |
Standard Error |
DF |
t Value |
Pr > |t| |
Adjustment |
Adj P |
| day |
1 |
2 |
29.3944 |
22.6172 |
118 |
1.30 |
0.1963 |
Tukey-Kramer |
0.8509 |
| day |
1 |
3 |
-54.0385 |
22.6172 |
118 |
-2.39 |
0.0185 |
Tukey-Kramer |
0.2124 |
| day |
1 |
4 |
-58.1184 |
22.6172 |
118 |
-2.57 |
0.0114 |
Tukey-Kramer |
0.1450 |
| day |
1 |
5 |
-79.8332 |
21.3713 |
118 |
-3.74 |
0.0003 |
Tukey-Kramer |
0.0053 |
| day |
1 |
6 |
-122.80 |
21.3713 |
118 |
-5.75 |
<.0001 |
Tukey-Kramer |
<.0001* |
| day |
1 |
7 |
-15.7794 |
21.3713 |
118 |
-0.74 |
0.4618 |
Tukey-Kramer |
0.9899 |
| day |
2 |
3 |
-83.4329 |
20.9395 |
118 |
-3.98 |
0.0001 |
Tukey-Kramer |
0.0022 |
| day |
2 |
4 |
-87.5128 |
20.9395 |
118 |
-4.18 |
<.0001 |
Tukey-Kramer |
0.0011 |
| day |
2 |
5 |
-109.23 |
21.3713 |
118 |
-5.11 |
<.0001 |
Tukey-Kramer |
<.0001* |
| day |
2 |
6 |
-152.20 |
21.3713 |
118 |
-7.12 |
<.0001 |
Tukey-Kramer |
<.0001* |
| day |
2 |
7 |
-45.1738 |
21.3713 |
118 |
-2.11 |
0.0366 |
Tukey-Kramer |
0.3515 |
| day |
3 |
4 |
-4.0799 |
20.9395 |
118 |
-0.19 |
0.8459 |
Tukey-Kramer |
1.0000 |
| day |
3 |
5 |
-25.7946 |
21.3713 |
118 |
-1.21 |
0.2299 |
Tukey-Kramer |
0.8902 |
| day |
3 |
6 |
-68.7634 |
21.3713 |
118 |
-3.22 |
0.0017 |
Tukey-Kramer |
0.0271 |
| day |
3 |
7 |
38.2591 |
21.3713 |
118 |
1.79 |
0.0760 |
Tukey-Kramer |
0.5573 |
| day |
4 |
5 |
-21.7147 |
21.3713 |
118 |
-1.02 |
0.3117 |
Tukey-Kramer |
0.9494 |
| day |
4 |
6 |
-64.6835 |
21.3713 |
118 |
-3.03 |
0.0030 |
Tukey-Kramer |
0.0465 |
| day |
4 |
7 |
42.3390 |
21.3713 |
118 |
1.98 |
0.0499 |
Tukey-Kramer |
0.4321 |
| day |
5 |
6 |
-42.9688 |
20.9395 |
118 |
-2.05 |
0.0424 |
Tukey-Kramer |
0.3881 |
| day |
5 |
7 |
64.0537 |
20.9395 |
118 |
3.06 |
0.0027 |
Tukey-Kramer |
0.0425 |
| day |
6 |
7 |
107.02 |
20.9395 |
118 |
5.11 |
<.0001 |
Tukey-Kramer |
<.0001* |
where: day
1 – cortisol level (ug/g) of the rabbits’ faeces sample before the transport begun (day 23/10)
2 – cortisol level (ug/g) of the rabbits’ faeces sample after the first transport (day 24/10)
3 – cortisol level (ug/g) of the rabbits’ faeces sample after the second transport (day 26/10)
4 – cortisol level (ug/g) of the rabbits’ faeces sample after the third transport (day 28/10)
5 – cortisol level (ug/g) of the rabbits’ faeces sample after the 4th transport (day 31/10)
6 – cortisol level (ug/g) of the rabbits’ faeces sample after the 5th transport (day 02/11)
7 – cortisol level (ug/g) of the rabbits’ faeces sample after the 6th transport (day 04/11)
*the difference is significant (p<0.001)
References
- Wells, D.L. The State of Research on Human–Animal Relations: Implications for Human Health. Anthrozoös 2019, 32, 169–181. [Google Scholar] [CrossRef]
- Bánszky, N.; Kardos, E.; Rózsa, L.; Gerevich, J. Az állatok által asszisztált terápiák pszichiátriai vonatkozásai. Psychiatr. Hung. 2012, 27, 180–190. [Google Scholar]
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Front. Psychol. Psychol. Clin. Settings 2012, 3, 234. [Google Scholar] [CrossRef]
- Enders-Slegers, M.-J.; Hediger, K. Pet ownership and human-animal interaction in an aging population: Rewards and challenges. Anthrozoös 2019, 32, 255–265. [Google Scholar] [CrossRef]
- Oglesbee, B.L. Rabbits. In Exotic Animal Laboratory Diagnosis, 1st ed.; Heatley, J.J., Russell, K.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; Chapter 5; pp. 63–80. [Google Scholar] [CrossRef]
- Welch, T.; Coe, J.B.; Niel, L.; McCobb, E. A survey exploring factors associated with 2890 companion-rabbit owners’ knowledge of rabbit care and the neuter status of their companion rabbit. Prev. Vet. Med. 2017, 137 Pt A, 13–23. [Google Scholar] [CrossRef]
- Gosling, E.M.; Vázquez-Diosdado, J.A.; Harvey, N.D. The Status of Pet Rabbit Breeding and Online Sales in the UK: A Glimpse into an Otherwise Elusive Industry. Animals 2018, 8, 199. [Google Scholar] [CrossRef]
- Rioja-Lang, F.; Bacon, H.; Connor, M.; Dwyer, C.M. Rabbit welfare: Determining priority welfare issues for pet rabbits using a modified Delphi method. Vet. Rec. Open 2019, 6, e000363. [Google Scholar] [CrossRef]
- Mikuš, T.; Ostović, M.; Sabolek, I.; Matković, K.; Pavičić, Ž.; Mikuš, O.; Mesić, Ž. Opinions towards Companion Animals and Their Welfare: A Survey of Croatian Veterinary Students. Animals 2020, 10, 199. [Google Scholar] [CrossRef]
- Statista.com. Available online: https://www.statista.com/statistics/453880/pet-population-europe-by-animal/ (accessed on 12 September 2023).
- Chandler, C. Animal-Assisted Therapy in Counseling, 3rd ed.; Routledge, Taylor and Francis: New York, NY, USA, 2017; pp. 4–43. ISBN 9781317374978. [Google Scholar]
- McCulloch, M.J.; Katcher, A.H.; Beck, A.M. Animal-Facilitated Therapy: Overview and Future Direction in New Perspectives on Our Lives with Companion Animals; University of Pennsylvania Press: Philadelphia, PA, USA, 1983; pp. 410–426. [Google Scholar]
- Morrison, M.L. Health Benefits of Animal-Assisted Interventions. Complement. Health Pract. Rev. 2007, 12, 51–62. [Google Scholar] [CrossRef]
- Fine, A.H.; Beck, A.M.; Zenithson, N. The State of Animal-Assisted Interventions: Addressing the Contemporary Issues That Will Shape the Future. Int. J. Environ. Res. Public Health 2019, 16, 3997. [Google Scholar] [CrossRef]
- Lönker, N.S.; Fechner, K.; Abd El Wahed, A. Horses as a Crucial Part of One Health. Vet. Sci. 2020, 7, 28. [Google Scholar] [CrossRef]
- Koukourikos, K.; Georgopoulou, A.; Kourkouta, L.; Tsaloglidou, A. Benefits of Animal Assisted Therapy in Mental Health. Int. J. Caring Sci. 2019, 12, 1898–1905. [Google Scholar]
- Loukaki, K.; Koukoutsakis, P.; Kostomitsopoulos, N. Animal welfare issues on the use of rabbits in an animal assisted therapy program for children. J. Hell. Vet. Med. Soc. 2010, 61, 220–225. [Google Scholar] [CrossRef]
- Molnár, M.; Iváncsik, R.; DiBlasio, B.; Nagy, I. Examining the Effects of Rabbit-Assisted Interventions in the Classroom Environment. Animals 2020, 10, 26. [Google Scholar] [CrossRef]
- Iváncsik, R.; Molnár, M. Criterias for the selection of rabbits suitable for animal-assisted work with the visually impaired (preliminary study). Acta Agrar. Kaposváriensis 2021, 25, 3–15. [Google Scholar] [CrossRef]
- Krause-Parello, C.A.; Gulick, E.E.; Basin, B. Loneliness, Depression, and Physical Activity in Older Adults: The Therapeutic Role of Human–Animal Interactions. Anthrozoös 2019, 32, 239–254. [Google Scholar] [CrossRef]
- Gut, W.; Crump, L.; Zinsstag, J.; Hattendorf, J.; Hediger, K. The effect of human interaction on guinea pig behavior in animal-assisted therapy. J. Vet. Behav. 2018, 25, 56–64. [Google Scholar] [CrossRef]
- Murry, F.R.; Todd, M.A. Positive Behavioral Impact of Reptile-Assisted Support on the Internalizing and Externalizing Behaviors of Female Children with Emotional Disturbance. Anthrozoös 2012, 25, 415–425. [Google Scholar] [CrossRef]
- Molnár, M.; Rudolf, Z.; Szalai, K.; Takács, I. A nyulakkal folytatott állatasszisztált pedagógiai foglalkozások hatásmechanizmusainak etológiai megközelítése (pilotvizsgálat). In Állatasszisztált Pedagógai és Terápia; Taláncs, I., Ed.; Kaposvári Egyetem Pedagógiai Kar: Dombóvár, Hungary, 2015; pp. 79–116. [Google Scholar]
- Urichuk, L.J.; Anderson, D. Current Use of Animal-Assisted Therapy. In Improving Mental Health Through Animal-Assisted Therapy; Chimo Project: Alberta, QC, Canada, 2003; pp. 12–31. ISBN 0-9732944-0-X. [Google Scholar]
- Granger, B.P.; Kogan, L. Animal-Assisted Therapy in Specialized Settings. In Handbook on Animal-Assisted Therapy: Theoretical Foundations and Guidelines for Practice; Academic Press: London, UK, 2020; Chapter 10; pp. 213–236. [Google Scholar]
- Mallon, G.P. Cow as co-therapist: Utilization of farm animals as therapeutic aides with children in residential treatment. Child Adolesc. Soc. Work J. 1994, 11, 455–474. [Google Scholar] [CrossRef]
- Menna, L.F.; Santaniello, A.; Todisco, M.; Amato, A.; Borrelli, L.; Scandurra, C.; Fioretti, A. The Human–Animal Relationship as the Focus of Animal-Assisted Interventions: A One Health Approach. Int. J. Environ. Res. Public Health 2019, 16, 3660. [Google Scholar] [CrossRef]
- Dobos, P.; Kulik, L.N.; Pongrácz, P. The amicable rabbit–interactions between pet rabbits and their caregivers based on a questionnaire survey. Appl. Anim. Behav. Sci. 2023, 260, 105869. [Google Scholar] [CrossRef]
- Suba-Bokodi, É.; Nagy, I.; Molnár, M. Changes in the Stress Tolerances of Dwarf Rabbits in Animal-Assisted Interentions. Appl. Sci. 2022, 12, 6979. [Google Scholar] [CrossRef]
- Isbrandt, R.; Wiegard, M.; Meemken, D.; Langkabel, N. Impact of Procedures and Human-Animal Interactions during Transport and Slaughter on Animal Welfare of Pigs: A Systematic Literature Review. Animals 2022, 12, 3391. [Google Scholar] [CrossRef]
- Vogt, A.; König von Borstel, U.; Waiblinger, S.; Palme, R.; Barth, K. Fecal cortisol metabolites reflect transport stress in 3-month-old dairy calves pre- and postweaning: A pilot study. J. Dairy Sci. 2022, 106, 2124–2136. [Google Scholar] [CrossRef]
- Prejsnar, S.; Ormian, M.; Topczewska, J. The Influence of Transport on the Quality of Poultry Meat. Postępy Tech. Przetwórstwa Spożywczego 2021, 2, 129–133. [Google Scholar]
- Vitale Shreve, K.R.; Udell, M.A.R. What’s inside your cat’s head? A review of cat (Felis silvestris catus) cognition research past, present and future. Anim. Cogn. 2015, 18, 1195–1206. [Google Scholar] [CrossRef]
- Jolley, P.D. Rabbit transport and its effects on meat quality. Appl. Anim. Behav. Sci. 1990, 28, 119–134. [Google Scholar] [CrossRef]
- Buil, T.; Maria, G.A.; Villarroel, M.; Liste, G.; Lopez, M. Critical points in the transport of commercial rabbits to slaughter in Spain that could compromise animals’ welfare. World Rabbit Sci. 2004, 12, 269–279. [Google Scholar] [CrossRef]
- Verga, M.; Luzi, F.; Petracci, M.; Cavani, C. Welfare aspects in rabbit rearing and transport. Ital. J. Anim. Sci. 2009, 8 (Suppl. 1), 191–204. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Herskin, M.; et al. Welfare of domestic birds and rabbits transported in containers. Eur. Food Saf. Auth. Panel Anim. Health Welf. EFSA J. 2022, 20, 7441. [Google Scholar]
- Broom, D.M. The effects of land transport on animal welfare. Rev. Sci. Tech. Off. Int. Épizooties 2005, 24, 683. [Google Scholar] [CrossRef]
- Mariti, C.; Ricci, E.; Mengoli, M.; Zilocchi, M.; Sighieri, C.; Gazzano, A. Survey of travel-related problems in dogs. Vet. Rec. 2012, 3, 299–305. [Google Scholar] [CrossRef]
- Tateo, A.; Costa, L.N.; Padalino, B. The welfare of dogs and cats during transport in Europe: A literature review. Ital. J. Anim. Sci. 2022, 21, 539–550. [Google Scholar] [CrossRef]
- Bergeron, R.; Scott, S.L.; Émond, J.-P.; Mercier, F.; Cook, N.J.; Schaefer, A.L. Physiology and behavior of dogs during air transport. Can. J. Vet. Res. 2002, 66, 211–216. [Google Scholar]
- Radisavljević, K.; Vučinić, M.; Becskei, Z.; Stanojković, A.; Ostović, M. Comparison of stress level indicators in blood of free-roaming dogs after transportation and housing in the new environment. J. Appl. Anim. Res. 2017, 45, 52–55. [Google Scholar] [CrossRef]
- D’Angelo, D.; d’Ingeo, S.; Ciani, F.; Visone, M.; Sacchettino, L.; Avallone, L.; Quaranta, A. Cortisol Levels of Shelter Dogs in Animal Assisted Interventions in a Prison: An Exploratory Study. Animals 2021, 11, 345. [Google Scholar] [CrossRef]
- Carvalho, I.R.; Nunes, T.; Sousa, L.; Almeida, V. The combined use of salivary cortisol concentrations, heart rate, and respiratory rate for the welfare assessment of dogs involved in AAI programs. J. Vet. Behav. 2020, 36, 26–33. [Google Scholar] [CrossRef]
- Herbel, J.; Aurich, J.; Gautier, C.; Melchert, M.; Aurich, C. Stress Response of Beagle Dogs to Repeated Short-Distance Road Transport. Animals 2020, 10, 2114. [Google Scholar] [CrossRef]
- Queyras, A.; Carosi, M. Non-invasive techniques for analysing hormonal indicators of stress. Ann. Ist. Super. Sanità 2004, 40, 211–221. [Google Scholar]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul Med. 2014, 87, 3–6. [Google Scholar] [CrossRef]
- Chmurska-Gąsowska, M.; Sowińska, N.; Pałka, S.; Kmiecik, M.; Lenarczyk-Knapik, J.; Migdał, Ł. Non-Invasive Measurement of Thyroid Hormones in Domestic Rabbits. Animals 2021, 11, 1194. [Google Scholar] [CrossRef]
- Buijs, S.; Keeling, L.J.; Rettenbacher, S.; Maertens, L.; Tuyttens, F.A.M. Glucocorticoid metabolites in rabbit faeces—Influence of environmental enrichment and cage size. Physiol. Behav. 2011, 104, 469–473. [Google Scholar] [CrossRef]
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf. 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Benedek, I.; Altbcker, V.; Molnár, T. Stress reactivity near birth affects nest building timing and offspring number and survival in the European rabbit (Oryctolagus cuniculus). PLoS ONE 2021, 16, e0246258. [Google Scholar] [CrossRef]
- Deiss, V.; Temple, D.; Ligout, S.; Racine, C.; Bouix, J.; Terlouw, C.; Boissy, A. Can emotional reactivity predict stress responses at slaughter in sheep? Appl. Anim. Behav. Sci. 2009, 119, 193–202. [Google Scholar] [CrossRef]
- Hefnawy, A.; Helal, M.; Sabek, A.; Shousha, S. Clinical, behavioral and biochemical alterations due to shearing stress in Ossimi sheep. Vet. Med. Sci. 2018, 80, 1281–1286. [Google Scholar] [CrossRef]
- Keshavarzi, H.; Lee, C.; Dyall, T.R.; Johnson, M.; Campbell, D.L.M. Shared stressful experiences affect social proximity in Merino sheep. Biol. Lett. 2023, 19, 20220396. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).