Submitted:
12 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
Keywords:
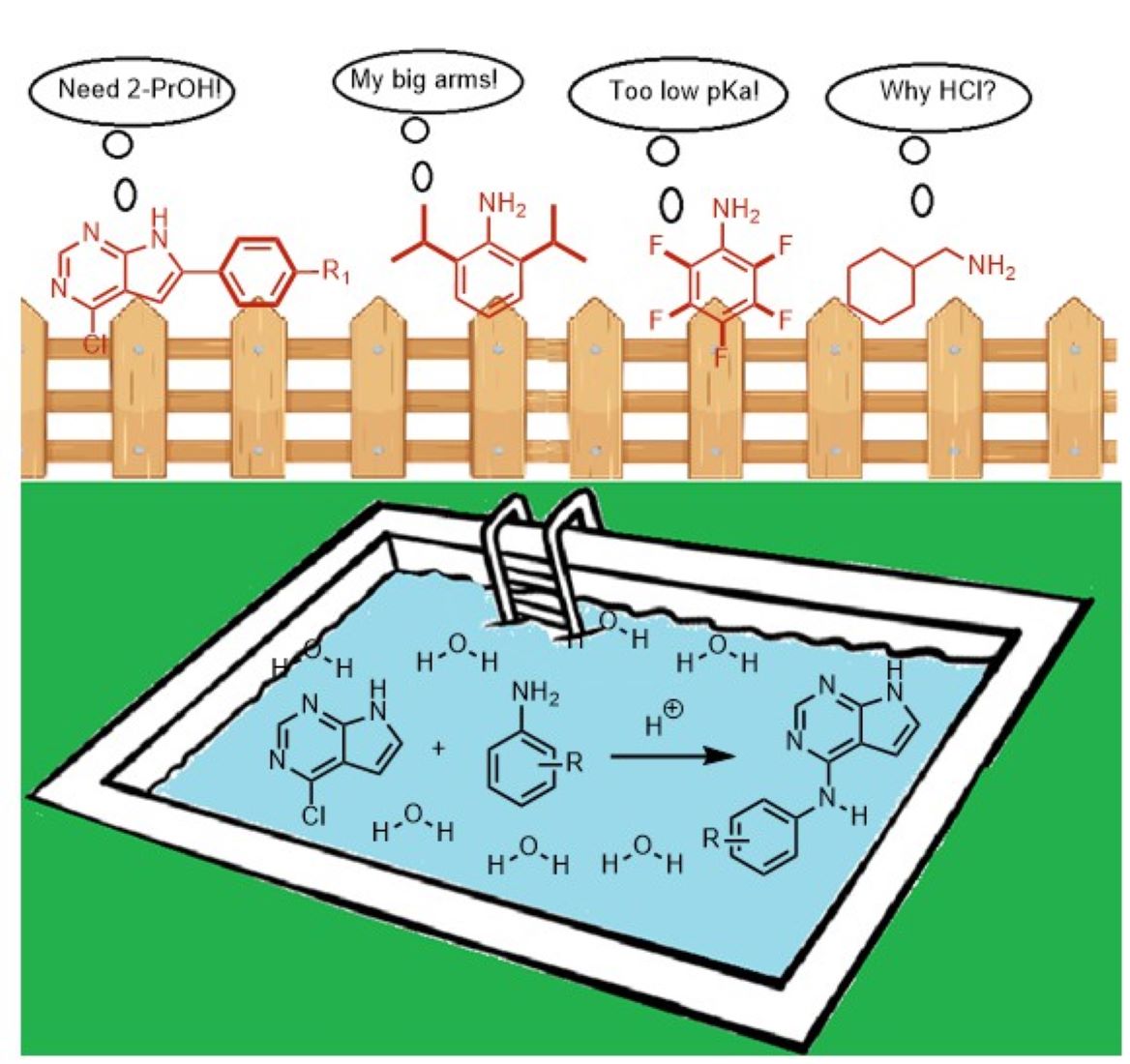
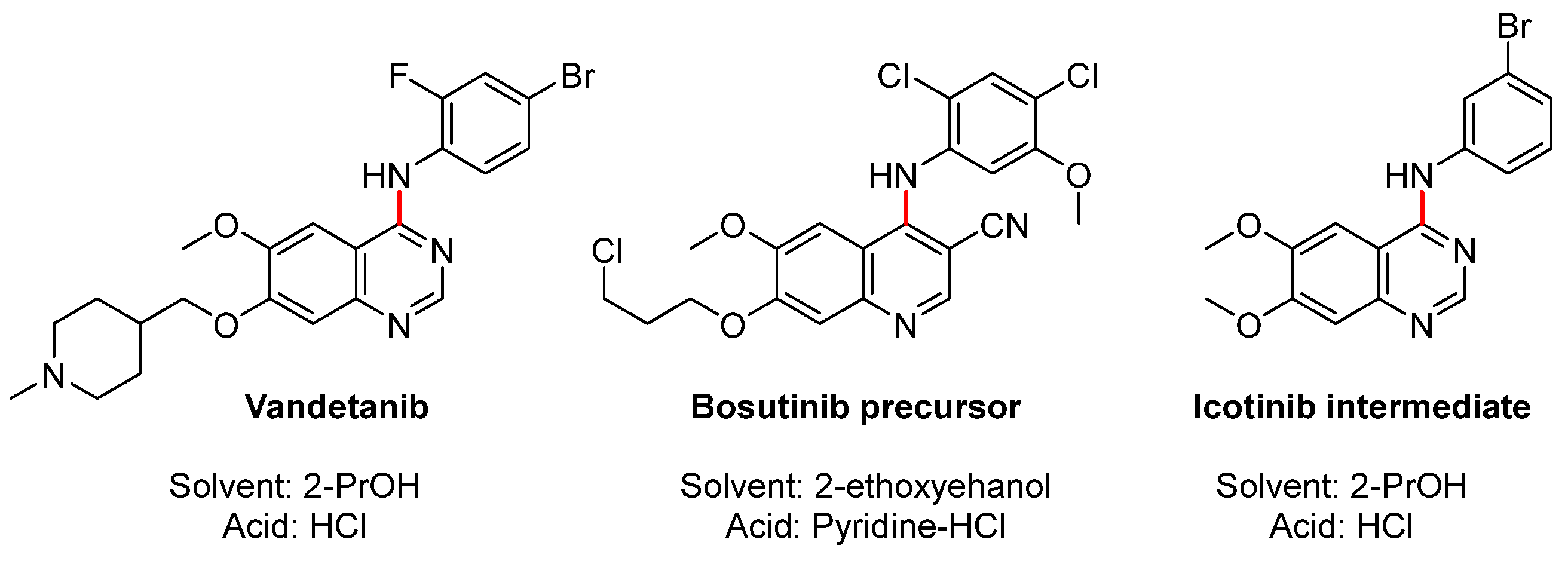
1. Introduction
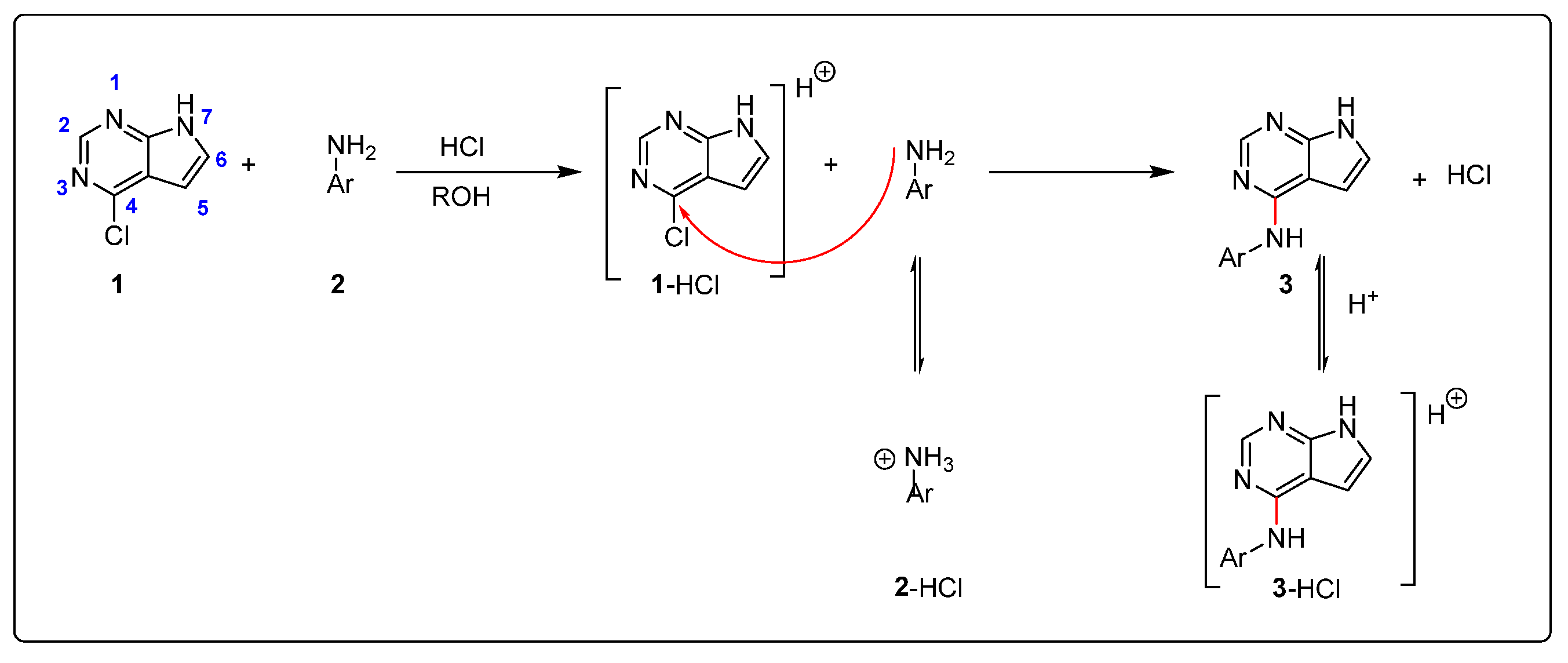
2. Results and Discussion
2.1. Initial reactions in EtOH
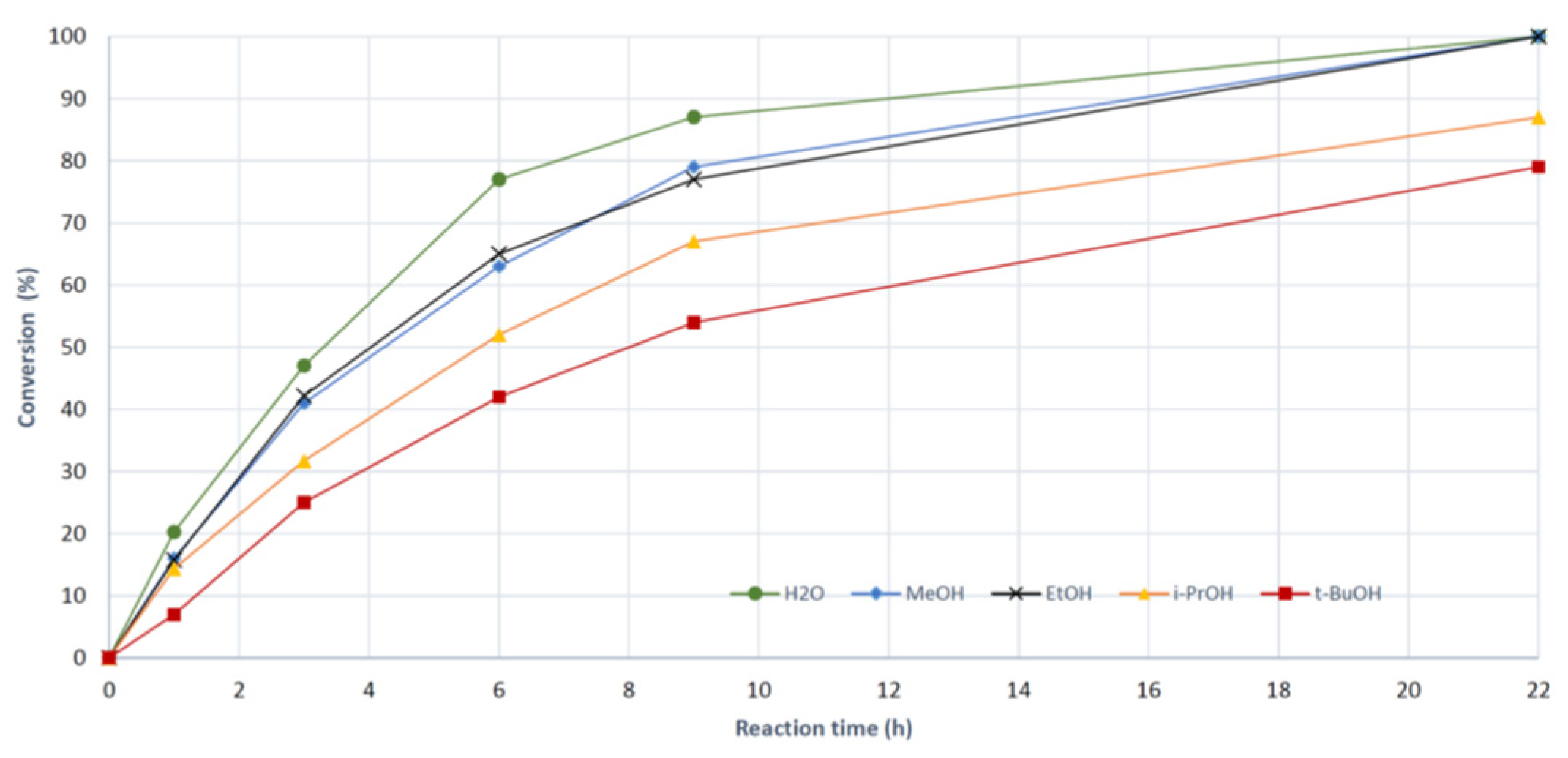
2.2. Effect of other protic solvents on the reaction
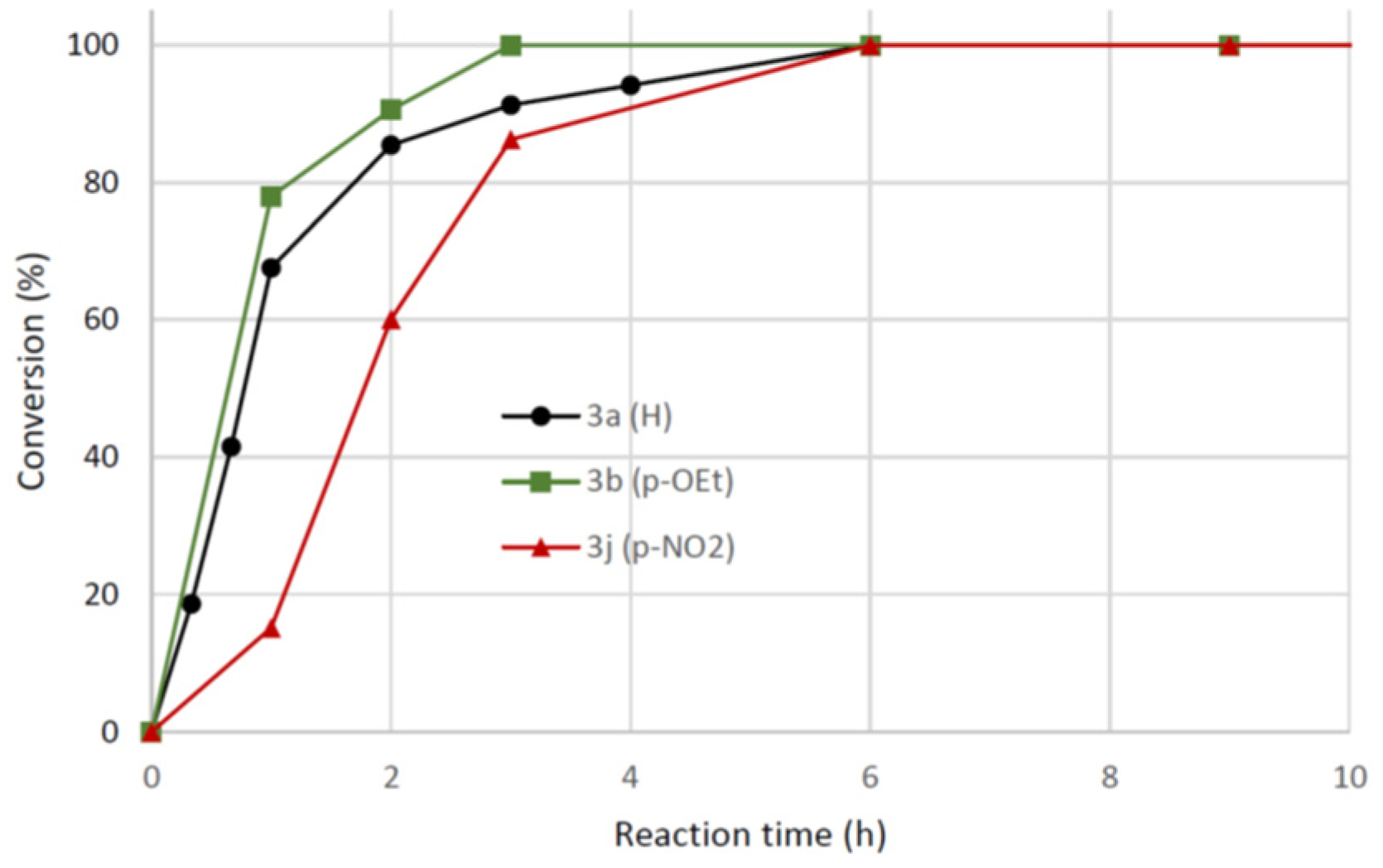
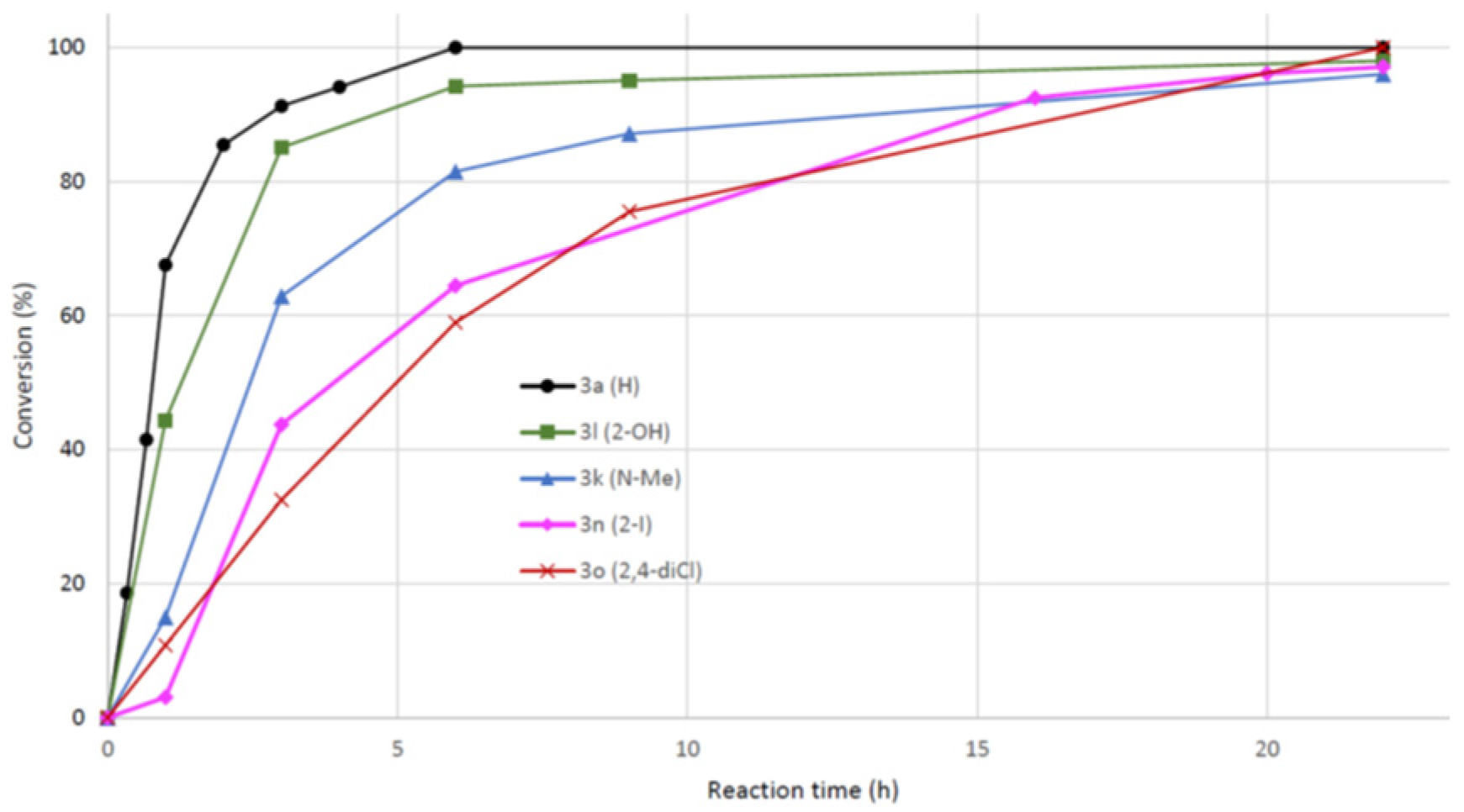
2.3. Substrate scope for amination in water
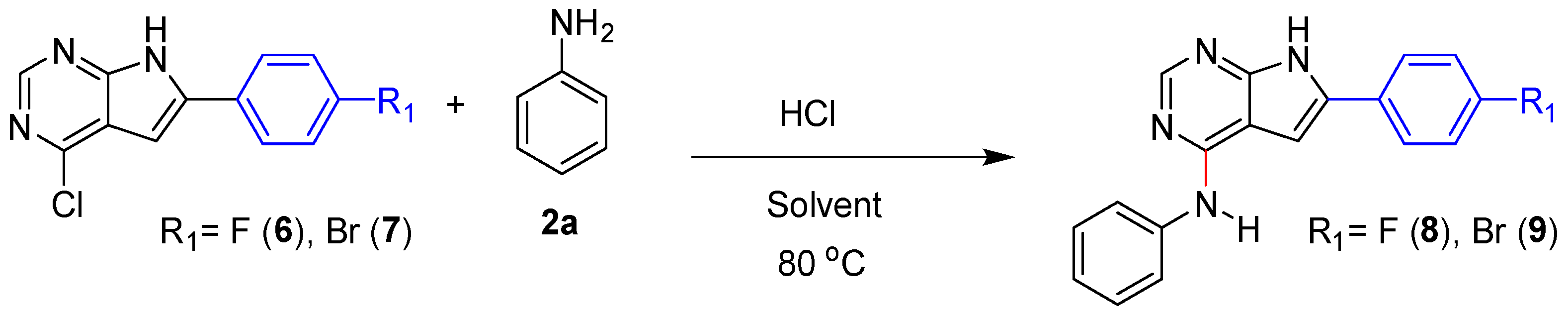
2.4. Other limitations to the use of water and HCl in amination
3. Materials and Methods
3.1. Chemicals and Analysis
3.2. General Synthetic Methods
3.2.1. General Procedure A: Test Amination (100 mg scale)
3.2.2. General Procedure B: 500 mg Scale Amination in Water
3.3. Isolated materials
3.3.1. N-Phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3a)
3.3.2. N-(4-Ethoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3b)
3.3.3. N-(4-Butylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3c)
3.3.4. N-(Benzo[d][1,3]dioxol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3d)
3.3.5. N-(4-Fluorophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3e)
3.3.6. N-(3-(Benzyloxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3f)
3.3.7. N-(3-Ethynylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3g)
3.3.8. N-(3-Chlorophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3h)
3.3.9. N-(4-Bromo-3-fluorophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3i)
3.3.10. N-(4-Nitrophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3j)
3.3.11. N-(4-Fluorophenyl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3k)
3.3.12. 2-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)phenol (3l)
3.3.13. N-(2-Iodophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amin (3n)
3.3.14. N-(2,4-Dichlorophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (3o)
3.3.15. 4-Ethoxy-7H-pyrrolo[2,3-d]pyrimidine (4)
3.3.16. 6-(4-Fluorophenyl)-N-phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (8)
3.3.17. 6-(4-Bromophenyl)-N-phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (9)
3.3.18. 4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)morpholine (12)
3.3.19. N-(Cyclohexylmethyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (13)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roughley, S. D.; Jordan, A. M., The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451-3479, . [CrossRef]
- Aarhus, T. I.; Bjørnstad, F.; Wolowczyk, C.; Larsen, K. U.; Rognstad, L.; Leithaug, T.; Unger, A.; Habenberger, P.; Wolf, A.; Bjørkøy, G.; Pridans, C.; Eickhoff, J.; Klebl, B.; Hoff, B. H.; Sundby, E., Synthesis and Development of Highly Selective Pyrrolo[2,3-d]pyrimidine CSF1R Inhibitors Targeting the Autoinhibited Form. J. Med. Chem. 2023, 66, 6959-6980, . [CrossRef]
- Walsh, K.; Sneddon, H. F.; Moody, C. J., Amination of Heteroaryl Chlorides: Palladium Catalysis or SNAr in Green Solvents? ChemSusChem 2013, 6, 1455-1460, . [CrossRef]
- Lin, Y.; Li, M.; Ji, X.; Wu, J.; Cao, S., n-Butyllithium-mediated synthesis of N-aryl tertiary amines by reactions of fluoroarenes with secondary amines at room temperature. Tetrahedron 2017, 73, 1466-1472, . [CrossRef]
- Borch Jacobsen, C.; Meldal, M.; Diness, F., Mechanism and Scope of Base-Controlled Catalyst-Free N-Arylation of Amines with Unactivated Fluorobenzenes. Chem. - Eur. J. 2017, 23, 846-851, . [CrossRef]
- Dorel, R.; Grugel, C. P.; Haydl, A. M., The Buchwald–Hartwig Amination After 25 Years. Angew. Chem. Int. Ed. 2019, 58, 17118-17129, . [CrossRef]
- Surry, D. S.; Buchwald, S. L., Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide. Chem. Sci. 2011, 2, 27-50, . [CrossRef]
- Hartwig, J. F., Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 2008, 41, 1534-1544, . [CrossRef]
- Wagaw, S.; Rennels, R. A.; Buchwald, S. L., Palladium-Catalyzed Coupling of Optically Active Amines with Aryl Bromides. J. Am. Chem. Soc 1997, 119, 8451-8458, . [CrossRef]
- Yin, J.; Buchwald, S. L., Pd-Catalyzed Intermolecular Amidation of Aryl Halides: The Discovery that Xantphos Can Be Trans-Chelating in a Palladium Complex. J. Am. Chem. Soc. 2002, 124, 6043-6048, . [CrossRef]
- Ayala-Aguilera, C. C.; Valero, T.; Lorente-Macías, Á.; Baillache, D. J.; Croke, S.; Unciti-Broceta, A., Small Molecule Kinase Inhibitor Drugs (1995–2021): Medical Indication, Pharmacology, and Synthesis. J. Med. Chem. 2022, 65, 1047-1131, . [CrossRef]
- Kurup, S.; McAllister, B.; Liskova, P.; Mistry, T.; Fanizza, A.; Stanford, D.; Slawska, J.; Keller, U.; Hoellein, A., Design, synthesis and biological activity of N4-phenylsubstituted-7H-pyrrolo[2,3-d]pyrimidin-4-amines as dual inhibitors of aurora kinase A and epidermal growth factor receptor kinase. J. Enzyme Inhib. Med. Chem. 2018, 33, 74-84, . [CrossRef]
- Guo, C.; Dong, L.; Marakovits, J.; Kephart, S., A novel method to enable SNAr reaction of aminopyrrolopyrazoles. Tetrahedron Lett. 2011, 52, 1692-1696, . [CrossRef]
- Wu, Y.; Wang, B.; Wang, J.; Qi, S.; Zou, F.; Qi, Z.; Liu, F.; Liu, Q.; Chen, C.; Hu, C.; Hu, Z.; Wang, A.; Wang, L.; Wang, W.; Ren, T.; Cai, Y.; Bai, M.; Liu, Q.; Liu, J., Discovery of 2-(4-Chloro-3-(trifluoromethyl)phenyl)-N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)acetamide (CHMFL-KIT-64) as a Novel Orally Available Potent Inhibitor against Broad-Spectrum Mutants of c-KIT Kinase for Gastrointestinal Stromal Tumors. J. Med. Chem. 2019, 62, 6083-6101, . [CrossRef]
- Lawhorn, B. G.; Philp, J.; Zhao, Y.; Louer, C.; Hammond, M.; Cheung, M.; Fries, H.; Graves, A. P.; Shewchuk, L.; Wang, L.; Cottom, J. E.; Qi, H.; Zhao, H.; Totoritis, R.; Zhang, G.; Schwartz, B.; Li, H.; Sweitzer, S.; Holt, D. A.; Gatto, G. J., Jr.; Kallander, L. S., Identification of Purines and 7-Deazapurines as Potent and Selective Type I Inhibitors of Troponin I-Interacting Kinase (TNNI3K). J. Med. Chem. 2015, 58, 7431-7448, . [CrossRef]
- Sun, L.; Cui, J.; Liang, C.; Zhou, Y.; Nematalla, A.; Wang, X.; Chen, H.; Tang, C.; Wei, J., Rational design of 4,5-disubstituted-5,7-dihydro-pyrrolo[2,3-d]pyrimidin-6-ones as a novel class of inhibitors of epidermal growth factor receptor (EGF-R) and Her2(p185erbB) tyrosine kinases. Bioorg. Med. Chem. Lett. 2002, 12, 2153-2157, . [CrossRef]
- Nozal, V.; Martínez-González, L.; Gomez-Almeria, M.; Gonzalo-Consuegra, C.; Santana, P.; Chaikuad, A.; Pérez-Cuevas, E.; Knapp, S.; Lietha, D.; Ramírez, D.; Petralla, S.; Monti, B.; Gil, C.; Martín-Requero, A.; Palomo, V.; de Lago, E.; Martinez, A., TDP-43 Modulation by Tau-Tubulin Kinase 1 Inhibitors: A New Avenue for Future Amyotrophic Lateral Sclerosis Therapy. J. Med. Chem. 2022, 65, 1585-1607, . [CrossRef]
- Staderini, M.; Bolognesi, M. L.; Menendez, J. C., Lewis Acid-Catalyzed Generation of C-C and C-N Bonds on π-Deficient Heterocyclic Substrates. Adv. Synth. Catal. 2015, 357, 185-195, . [CrossRef]
- Abou-Shehada, S.; Teasdale, M. C.; Bull, S. D.; Wade, C. E.; Williams, J. M. J., Lewis Acid Activation of Pyridines for Nucleophilic Aromatic Substitution and Conjugate Addition. ChemSusChem 2015, 8, 1083-1087, . [CrossRef]
- Hennequin, L. F.; Stokes, E. S. E.; Thomas, A. P.; Johnstone, C.; Plé, P. A.; Ogilvie, D. J.; Dukes, M.; Wedge, S. R.; Kendrew, J.; Curwen, J. O., Novel 4-Anilinoquinazolines with C-7 Basic Side Chains: Design and Structure Activity Relationship of a Series of Potent, Orally Active, VEGF Receptor Tyrosine Kinase Inhibitors. J. Med. Chem. 2002, 45, 1300-1312, . [CrossRef]
- Boschelli, D. H.; Ye, F.; Wang, Y. D.; Dutia, M.; Johnson, S. L.; Wu, B.; Miller, K.; Powell, D. W.; Yaczko, D.; Young, M.; Tischler, M.; Arndt, K.; Discafani, C.; Etienne, C.; Gibbons, J.; Grod, J.; Lucas, J.; Weber, J. M.; Boschelli, F., Optimization of 4-Phenylamino-3-quinolinecarbonitriles as Potent Inhibitors of Src Kinase Activity. J. Med. Chem. 2001, 44, 3965-3977, . [CrossRef]
- Zhang, D.; Xie, G.; Davis, C.; Cheng, Z.; Chen, H.; Wang, Y.; Kamal, M. Novel fused quinazoline derivatives useful as tyrosine kinase inhibitors. WO2003/82830, 2003.
- Rossini, E.; Bochevarov, A. D.; Knapp, E. W., Empirical Conversion of pKa Values between Different Solvents and Interpretation of the Parameters: Application to Water, Acetonitrile, Dimethyl Sulfoxide, and Methanol. ACS Omega 2018, 3, 1653-1662, . [CrossRef]
- Gross, K. C.; Seybold, P. G.; Peralta-Inga, Z.; Murray, J. S.; Politzer, P., Comparison of Quantum Chemical Parameters and Hammett Constants in Correlating pKa Values of Substituted Anilines. J. Org. Chem. 2001, 66, 6919-6925, . [CrossRef]
- Tehan, B. G.; Lloyd, E. J.; Wong, M. G.; Pitt, W. R.; Gancia, E.; Manallack, D. T., Estimation of pKa Using Semiempirical Molecular Orbital Methods. Part 2: Application to Amines, Anilines and Various Nitrogen Containing Heterocyclic Compounds. Quant. Struct.-Act. Relat. 2002, 21, 473-485, . [CrossRef]
- Broderius, S. J.; Kahl, M. D.; Hoglund, M. D., Use of joint toxic response to define the primary mode of toxic action for diverse industrial organic chemicals. Environ. Toxicol. Chemi. 1995, 14, 1591-1605, . [CrossRef]
- Eastes, J. W.; Aldridge, M. H.; Kamlet, M. J., Effects of N-alkylation and N,N-dialkylation of the pKa of anilinium and nitroanilinium ions. J. Chem. Soc. (B) 1969, 922-928, . [CrossRef]
- Pietra, F.; Bartolozzi, M.; Del Cima, F., Competition between reductive dehalogenation and nucleophilic aromatic substitution of nitro-activated iodine by amines. J. Chem. Soc. D: Chem. Commun. 1971, 1232-1232, . [CrossRef]
- Talekar, R. S.; Chen, G. S.; Lai, S.-Y.; Chern, J.-W., Nonreductive Deiodination of ortho-Iodo-Hydroxylated Arenes Using Tertiary Amines. J. Org. Chem. 2005, 70, 8590-8593, . [CrossRef]
- Choguill, H. S.; Ridd, J. H., The mechanism of protodeiodination of p-iodoaniline. J. Chem. Soc. 1961, 822-826, . [CrossRef]
- Choi, H. Y.; Chi, D. Y., A Facile Debromination Reaction: Can Bromide Now Be Used as a Protective Group in Aromatic Systems? J. Am. Chem. Soc. 2001, 123, 9202-9203, . [CrossRef]
- Gilow, H. M.; Burton, D. E., Bromination and chlorination of pyrrole and some reactive 1-substituted pyrroles. J. Org. Chem 1981, 46, 2221-2225, . [CrossRef]
- Teasdale, A.; Fenner, S.; Ray, A.; Ford, A.; Phillips, A., A Tool for the Semiquantitative Assessment of Potentially Genotoxic Impurity (PGI) Carryover into API Using Physicochemical Parameters and Process Conditions. Org. Proc. Res. Dev. 2010, 14, 943-945, . [CrossRef]
- Borukhova, S.; Noël, T.; Hessel, V., Continuous-Flow Multistep Synthesis of Cinnarizine, Cyclizine, and a Buclizine Derivative from Bulk Alcohols. ChemSusChem 2016, 9, 67-74, . [CrossRef]
- ChemBK 2,2,2-Trifluoro-1-phenyl-ethylamine: https://www.chembk.com/en/chem/2,2,2-Trifluoro-1-phenyl-ethylamine. (05.05.2023),.
- Smith, P. J.; Noble, A., A Primary Hydrogen-Deuterium Isotope Effect Study on the Carbonyl Elimination Reaction of 9-Fluorenyl Nitrate with Various Bases. Can. J. Chem. 1975, 53, 263-268, https://cdnsciencepub.com/doi/pdf/10.1139/v75-036.
- Juranic, I., Simple Method for the Estimation of pKa of Amines. Croat. Chem. Acta 2014, 87, 343-347, http://dx.doi.org/10.5562/cca2462.
- Lansbergen, B.; Meister, C. S.; McLeod, M. C., Unexpected rearrangements and a novel synthesis of 1,1-dichloro-1-alkenones from 1,1,1-trifluoroalkanones with aluminium trichloride. Beilstein J. Org. Chem. 2021, 17, 404-409, . [CrossRef]
- Jesumoroti, O. J.; Beteck, R. M.; Jordaan, A.; Warner, D. F.; Legoabe, L. J., Exploration of 4-aminopyrrolo[2,3-d]pyrimidine as antitubercular agents. Mol. Divers. 2023, 27, 753-756, . [CrossRef]
- Kaspersen, S. J.; Sørum, C.; Willassen, V.; Fuglseth, E.; Kjøbli, E.; Bjørkoy, G.; Sundby, E.; Hoff, B. H., Synthesis and in vitro EGFR (ErbB1) tyrosine kinase inhibitory activity of 4-N-substituted 6-aryl-7H-pyrrolo[2,3-d]pyrimidine-4-amines. Eur. J. Med. Chem. 2011, 46, 6002-6014, . [CrossRef]
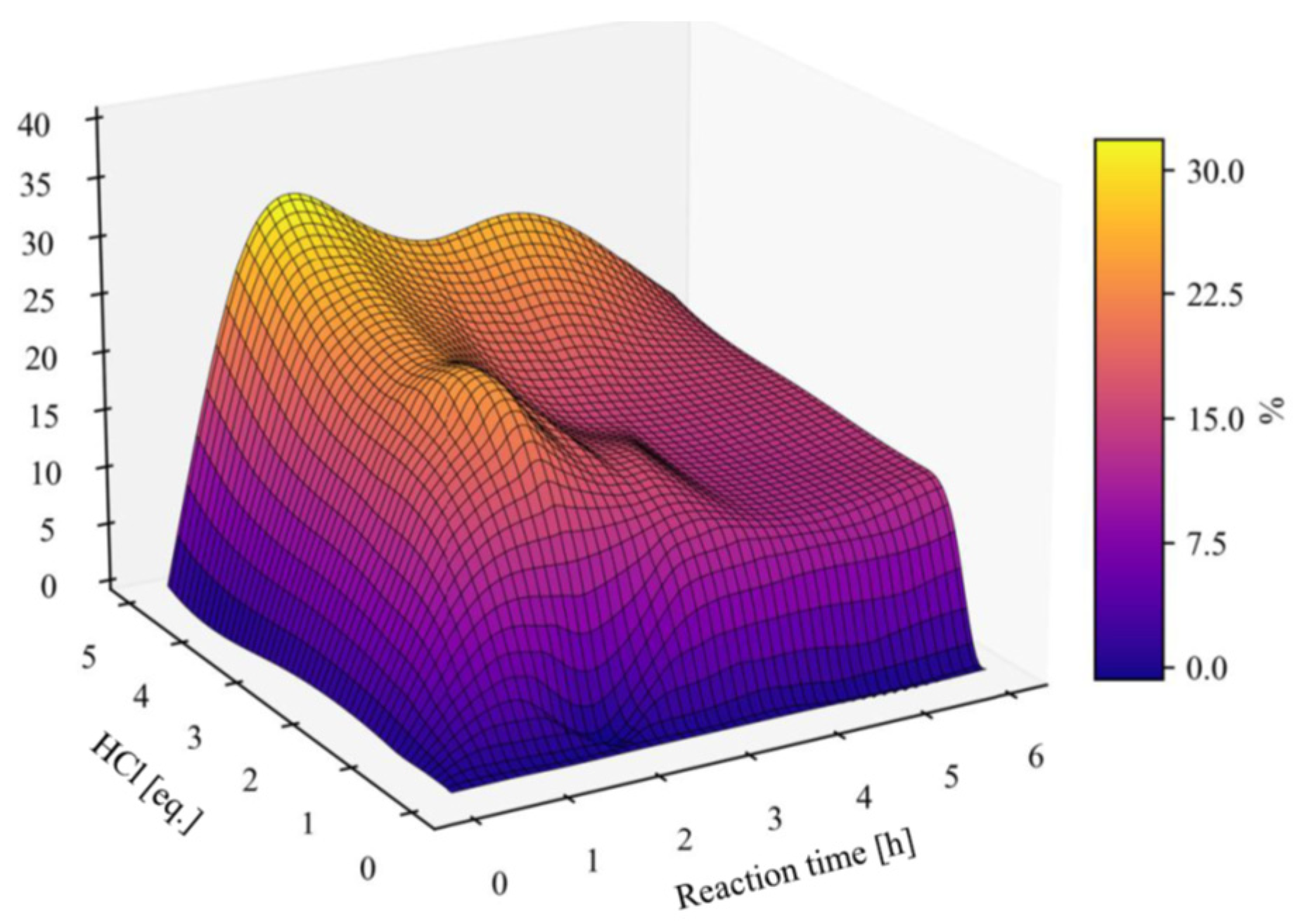
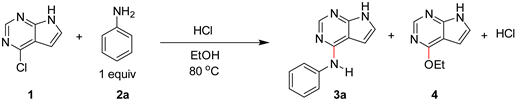
| Mole % after 6 h. 2 | |||||
|---|---|---|---|---|---|
| Entry | HCl (equiv) | Conv. 1 h (%) 1 | 1a | 3a | 4 |
| 1 | 0 | < 1 | 31 | 69 | <1 |
| 2 | 0.1 | 51 | < 1 | >98 | < 1 |
| 3 | 0.5 | 78 | < 1 | 90 | 10 |
| 4 | 1.0 | 82 | < 1 | 86 | 14 |
| 5 | 3 | 82 | < 1 | 85 | 15 |
| 6 | 5 | 86 | < 1 | 83 | 17 |
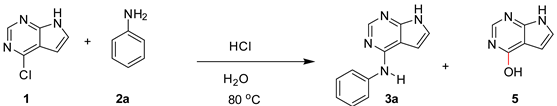
| Mole % after 6 h. 2 | |||||
|---|---|---|---|---|---|
| Entry | HCl (equiv) | Conv. 0.33 h (%) 1 | 1a | 3a | 5 3 |
| 1 | 0.1 | 19 | < 1 | >98 | <1 |
| 2 | 0.2 | 28 | < 1 | >98 | <1 |
| 3 | 0.5 | 48 | < 1 | 98 | 1 |
| 4 | 0.8 | 59 | < 1 | 98 | 1 |
| 5 | 1.0 | 54 | < 1 | 98 | 1 |
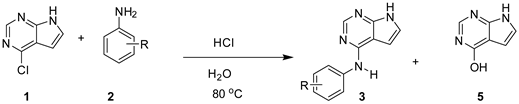
| . | Mole (%)3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Aniline | pKa1 | Conv 1 h (%)2 |
Reaction time (h) |
1 | 3 | 5 | Yield (%) | Prod. |
| 1 | 4-OEt (2b) | 5.19 | 78 | 3 | < 1 | >98 | < 1 | 94 | 3b |
| 2 | 4-Bu (2c) | 4.95 | 66 | 6 | < 1 | >98 | < 1 | 88 | 3c |
| 3 | 3,4-methylene- dioxy (2d) |
4.46 | 74 | 3 | < 1 | >98 | < 1 | 85 | 3d |
| 4 | 4-F (2e) | 4.65 | 77 | 6 | < 1 | >98 | < 1 | 92 | 3e |
| 5 | H (2a) | 4.58 | 68 | 6 | < 1 | >98 | < 1 | 91 | 3a |
| 6 | 3-OBn (2f) | ca 4.2 | 86 | 6 | < 1 | >98 | < 1 | 87 | 3f |
| 7 | 3-ethyne (2g) | 3.82 | 81 | 6 | < 1 | >98 | < 1 | 83 | 3g |
| 8 | 3-Cl (2h) | 3.34 | 80 | 6 | < 1 | >98 | < 1 | 81 | 3h |
| 9 | 4-Br-3-F (2i) | 2.73 | 84 | 3 | < 1 | >98 | < 1 | 91 | 3i |
| 10 | 4-NO2 (2j) | 1.02 | 15 | 6 | < 1 | 97 | 3 | 88 | 3j |
| 11 | N-Me-4-F (2k) | ca 4.9 | 15 | 22 | 4 | 96 | < 1 | 88 | 3k |
| 12 | 2-OH (2l) | 4.84 | 44 | 22 | 2 | 96 | 2 | 89 | 3l |
| 13 | 2,6-(i-Pr)2 (2m) | 4.51 | 0 | 22 | 83 | 0 | 17 | - | 3m |
| 14 | 2-I (2n) | 2.6 | 3 | 22 | 3 | 94 | 3 | 79 | 3n |
| 15 | 2,4-Cl (2o) | 2.0 | 11 | 22 | < 1 | 85 | 15 | 80 | 3o |
| 16 | 2,4,5-Cl (2p) | 1.09 | 0 | 22 | 73 | 15 | 12 | - | 3p |
| 17 | 2,6-Cl (2q) | 0.42 | 0 | 22 | 83 | 0 | 17 | - | 3q |
| 18 | 2-NO2 (2r) | -0.31 | 0 | 22 | 78 | 5 | 17 | - | 3r |
| 19 | 2-CF3, 4-NO2 (2s) | < 0 | 0 | 22 | 85 | 0 | 15 | - | 3s |
| 20 | 2,3,4,5,6-F (2t) | -0.28 | 0 | 22 | 82 | 3 | 15 | - | 3t |
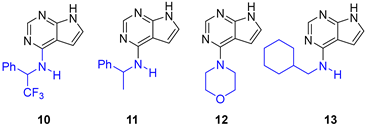
| Entry | Amine (equiv.) |
HCl (equiv.) |
Solvent | Reaction time (h) |
Conv. (%) | Product |
|---|---|---|---|---|---|---|
| 1 | 1.1 | 0.1 | H2O | 22 | low | 10 1 |
| 2 | 1.1 | 0.1 | H2O | 22 | 13 | 11 |
| 3 | 1.1 | 0.1 | H2O | 22 | 61 | 12 |
| 4 | 1.1 | 0.1 | H2O | 22 | 24 | 13 |
| 5 | 3 | 3 drops 2 | 2-PrOH | 6 | >98 | 12 |
| 6 | 3 | None | H2O | 2.5 | >98 | 12 |
| 7 | 1.5 | None | H2O | 22 | 86 | 12 |
| 8 | 3 | 3 drops 2 | 2-PrOH | 22 | 93 | 13 |
| 9 | 3 | None | H2O | 8 | >98 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





