2. Theoretical model
The consideration in this article is based on the TDSE solution for a helium atom in a given external field. In the electric dipole approximation, the TDSE has the form
where
is the wave function,
and
are the radius vectors of electrons in a helium atom,
is the Hamiltonian of an atom in the absence of a field,
is the dipole moment operator, and
is the external (in this case, UV) field. Here and below, atomic units are used. In what follows, we will assume that the UV field is linearly polarized along the
z axis and has the form
where
is the unit vector along the
z axis,
and
are the amplitude and frequency of the UV field, respectively, and
is the trapezoidal envelope:
where
is the UV field oscillation period. In accordance with Equations (2) and (3), the UV field pulse has a constant amplitude in a time interval lasting
Nosc field cycles, while turning on and off the UV field is characterized by a linear increase (decrease) in its amplitude over 3 field cycles. In what follows, the values
Nosc = 10, 30, 50, 70, and 100 are considered (most of the figures are given for
Nosc = 30). Note that the absolute duration of the UV field envelope is inversely proportional to its carrier frequency Ω.
As mentioned in the Introduction, the results of the calculations presented in this article were obtained using two approaches to solving the TDSE for a helium atom irradiated with a femtosecond pulse of an ultraviolet field.
The first approach is based on the expansion of the atomic wave function over a limited (sufficiently large) basis of eigenfunctions corresponding to bound states and continuum states into which electric dipole transitions from the considered bound states are allowed. This expansion has the form
where
is the spatial part of the wave function of the
kth stationary state,
is the temporal factor in the same wave function, which we will further call the excitation amplitude of the state
,
, and
is the excitation amplitude of the continuum state
with energy
and orbital momentum
l, . In further calculations, the expansion of the wave function takes into account the first 10 bound states of the helium atom (
Kmax=10), corresponding to the values of the principal quantum number
n = 1,2,3,4 and the projection of the orbital momentum of the atom onto the field polarization direction
m = 0 (electric dipole transitions from ground state into states with
m ≠ 0 in a linearly polarized field are prohibited by selection rules). Namely, states
,
,
,
,
,
,
,
,
, and
are taken into account (for brevity, here and below, the value of the projection of the orbital momentum of the atom
m = 0 is not given in the designation of states). Among the continuum states, those with orbital momentum from 0 to
Lmax = 4 (s-, p-, d-, f-, and g-waves) are taken into account. This set of states makes it possible to quite accurately take into account one-, two-, three-, four-, and five-photon transitions from the ground to lower bound states
and
(transitions to higher-lying states are described with less accuracy). Note that the time dependences
take into account oscillations of the phase of the wave function at the natural frequencies of stationary states, which in the atomic system of units are equal to
, where
is the energy of the
kth stationary state in the absence of a field (state energies and other constants used are given in
Appendix A). Accordingly, depending on the ratio of the values of
and
, even in a weak UV field limit, the excitation amplitudes can be fast or slow functions of time on the scale of the UV field cycle.
Further, the excitation amplitudes of bound states are decomposed into harmonics of the UV field:
where
is the Fourier component of the excitation amplitude of the
kth bound stationary state.
To take into account the ionization of an atom from the bound states under consideration, the approximation of adiabatic exclusion of continuum states is used [
38], which, primarily, corresponds to not taking into account transitions between continuum states. In addition, reverse transitions from continuum states to bound states are also not taken into account. These approximations make it possible to express the excitation amplitudes of continuum states
through the Fourier components of the excitation amplitudes of bound states
and, ultimately, to write a closed system of linear differential equations for
, which takes into account the decrease in absolute values of
as a result of ionization. These equations have the form
where
is the projection of the dipole moment of the transition from state |
s〉 to state |
k〉 on the
z axis, and
is the ionization rate of the
nth Fourier component of the
kth state. Using the residue theory, the following expression can be obtained for
:
where
is the
z-projection of the dipole moment operator, and
is the Heaviside unit step function. The derivation of Equations (6) and (7) is given in
Appendix B.
Equations (6), (7) were solved numerically using the 4th order one-step explicit Runge-Kutta method using initial conditions
which imply that before exposure to the UV field the atom is in the ground state
with energy
.
This approach is a simplified and less resource-intensive implementation of the SSEA (state-specific expansion approach) method [
39,
40]. It makes it possible to quite accurately describe the component of the dipole response of an atom to an external field that dominates in the considered spectral range of below-threshold and near-threshold harmonics (see, for example, [
41]) and is caused by transitions between bound states,
In formula (9), the index bb of the induced dipole moment of an atom indicates transitions between bound states, the indices k and s number the stationary states taken into account, and the indices n and m number their Fourier components. Note that within the framework of this approach, the response of the atom due to transitions between continuum states, as well as from continuum to bound states, is not taken into account.
As usual in strong-field physics, the response of an atom to an external field will be characterized by dipole acceleration,
This approach allows one to analyze the contributions of certain stationary states to the generation of harmonics and take into account the properties of a real multielectron atom (through the energies of stationary states and the dipole moments of transitions between them) by choosing the correct basis functions. For this purpose, in this work we used the spectroscopic characteristics of a three-dimensional two-electron helium atom, calculated by the multi-configuration Hartree-Fock method (see
Appendix A), the error of which does not exceed several percent relative to experimental data [
42].
The second approach used in this article is to solve the TDSE from first principles in a two-dimensional model of a one-electron atom with an effective potential
, reproducing the binding energies of ground state
and lowest excited bound states
and
:
where
is the vector potential of the UV field,
c is the speed of light in vacuum, and
The energies of the lowest stationary states and the dipole moments of transitions between them calculated in this model are given in
Appendix A.
This approach has less accuracy in describing the dynamics of the localized part of the atomic wave function, but takes into account recombination and all possible transitions between states of the continuum. Accordingly, the dipole response of an atom to an external field, in addition to the contribution from transitions between bound states, contains components due to transitions from continuum to bound states (recombination) and transitions between continuum states. The dipole acceleration of an atom is calculated using Ehrenfest’s theorem:
where integration is carried out over the entire computational domain.
3. Calculation results
As was shown in [
43], the approaches to solving the TDSE used in this article give similar time dependences of the probability of ionization of a helium atom in a UV field with an intensity
IUV = 10
14 W/cm
2. In the first approach, based on the expansion of the wave function in terms of the basis of stationary states, the probability of ionization is determined as
whereas within the framework of the second approach, based on solving a two-dimensional one-electron TDSE from first principles, it has the form
where the integration is carried out inside a square with side A = 100 a.u., in the center of which the core of the He atom is located. In this case, the wave functions of bound states with principal quantum numbers
n = 1,2,3,4 are completely contained within the integration region.
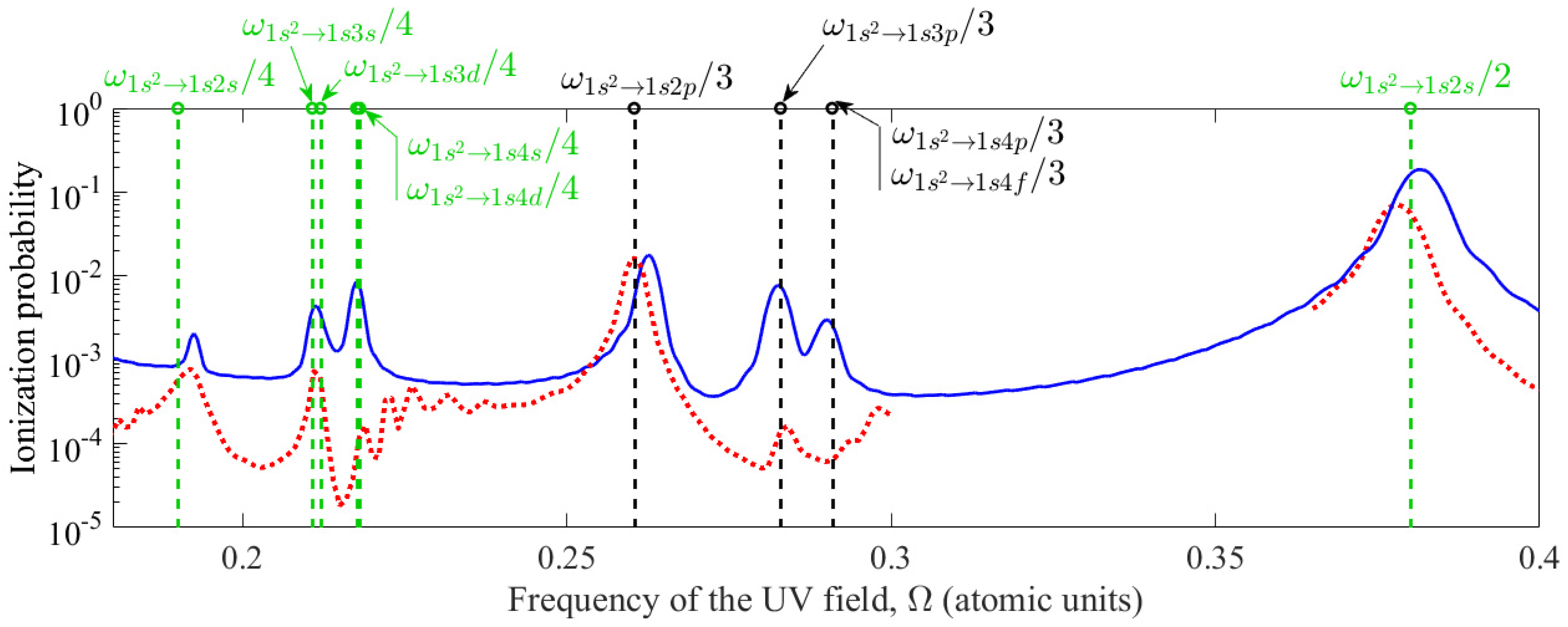
As follows from
Figure 1, the frequency dependences of the ionization probability are also qualitatively similar.
Figure 1 shows the probability of ionization of an atom at the end of a UV field pulse with a peak intensity
IUV = 10
14 W/cm
2 and a constant-amplitude interval duration equal to 30 UV field cycles (
Nosc = 30), as a function of its frequency. The frequency of the UV field is scanned in a range that covers resonances with two-, three-, and four-photon transitions from the ground to excited bound states. The blue curve corresponds to the solution obtained based on expansion (4), and the red curve based on the two-dimensional one-electron model.
Figure 1.
Dependence of the probability of ionization of a helium atom at time t = 50T on the frequency of the UV field with a peak intensity IUV = 1014 W/cm2. The blue solid curve corresponds to the solution obtained by expanding the atomic wave function in stationary states, , and the red dotted curve corresponds to the solution of the two-dimensional one-electron TDSE from first principles, . Vertical dashed lines indicate the frequencies of multiphoton transitions between unperturbed bound states of the atom. In this case, multiphoton resonances of odd order are marked in black, and resonances of even order are marked in green.
Figure 1.
Dependence of the probability of ionization of a helium atom at time t = 50T on the frequency of the UV field with a peak intensity IUV = 1014 W/cm2. The blue solid curve corresponds to the solution obtained by expanding the atomic wave function in stationary states, , and the red dotted curve corresponds to the solution of the two-dimensional one-electron TDSE from first principles, . Vertical dashed lines indicate the frequencies of multiphoton transitions between unperturbed bound states of the atom. In this case, multiphoton resonances of odd order are marked in black, and resonances of even order are marked in green.
As follows from
Figure 1, multiphoton resonances are reproduced in both models, but their frequencies are slightly different due to (a) the neglect of highly excited bound states and the use of the adiabatic continuum exclusion approximation when expanding the wave function in stationary states and (b) the difference in the dipole moments of the transitions, as well as energies of highly excited bound states, in a two-dimensional one-electron model from the corresponding parameters of a real helium atom. The best agreement in the probability of atomic ionization is achieved for a three-photon transition from state
to state
, as well as two- and four-photon transitions to state
, which is explained by the highest accuracy of both models in describing these transitions. The accuracy of the approach based on the expansion of the wave function in stationary states is due to the low probability of transitions from ground state
to disregarded intermediate states with
n ≥ 5 during three-photon excitation of state
or two/four-photon excitation of state
in a real helium atom. At the same time, the two-dimensional model of the helium atom is constructed in such a way as to reproduce the energies of states
,
, and
with few percent accuracy. The dipole moments of transitions between them are reproduced with lower, but still reasonable accuracy of tens of percent (see
Appendix A). Taken together with the fact that the three-photon transition to state
predominantly occurs through state
, while the two- and four-photon transitions to state
occur through state
, this determines the accuracy of the two-dimensional model in describing these transitions.
Let us consider in more detail the region of resonances with three-photon transitions from state
to the p- and f-states taken into account.
Figure 2 shows the dependence of the probability of ionization of an atom at the end of a UV field pulse on its frequency and peak intensity, calculated by expanding the atomic wave function over stationary states. Similar to
Figure 1, this figure is given for UV field pulses with a constant amplitude interval duration equal to 30 carrier cycles (
Nosc = 30). Note that the blue curve in
Figure 1 in the frequency range 0.25<Ω<0.3 represents a cross-section of
Figure 2 for an intensity value of 10
14 W/cm
2.
Figure 2.
Probability of ionization of a helium atom (in a logarithmic scale) after exposure to a UV field pulse depending on its intensity and frequency in the vicinity of three-photon resonances. The dotted lines indicate the frequencies of three-photon transitions between unperturbed atomic states; here black color corresponds to the transition |1s2〉 - |1s2p〉, green is for the transition |1s2〉 - |1s3p〉, whereas blue is for the transitions |1s2〉 - |1s4p〉 and |1s2〉 - |1s4f〉 (on the scale of the figure they coincide). The solid black curve indicates the frequency of the three-photon transition |1s2〉 - |1s2p〉 in the UV field.
Figure 2.
Probability of ionization of a helium atom (in a logarithmic scale) after exposure to a UV field pulse depending on its intensity and frequency in the vicinity of three-photon resonances. The dotted lines indicate the frequencies of three-photon transitions between unperturbed atomic states; here black color corresponds to the transition |1s2〉 - |1s2p〉, green is for the transition |1s2〉 - |1s3p〉, whereas blue is for the transitions |1s2〉 - |1s4p〉 and |1s2〉 - |1s4f〉 (on the scale of the figure they coincide). The solid black curve indicates the frequency of the three-photon transition |1s2〉 - |1s2p〉 in the UV field.
As follows from
Figure 2, (i) the highest probability of ionization is achieved in the vicinity of the frequencies of three-photon transitions, (ii) the absolute maximum corresponds to the vicinity of the transition |1
s2〉 - |1
s2
p〉 frequency; with increasing field intensity, (iii) the probability of ionization increases and (iv) the resonances broaden and shift in frequency.
Next, we will focus on the resonance with the three-photon transition |1
s2〉 - |1
s2
p〉, which is characterized by the highest probability of atomic ionization and is most accurately described within the framework of the models used. For each fixed intensity of the UV field, we determine the resonance frequency using the method of multiphoton photoionization spectroscopy [
44], based on the position of the maximum probability of atomic ionization in the vicinity of the frequency of the three-photon transition between the unperturbed states |1
s2〉 and |1
s2
p〉. The dependence of the transition frequency on the UV field intensity obtained in this way is shown in
Figure 2 by a black solid curve.
An increase in the probability of atomic ionization with increasing UV field intensity is accompanied by real and virtual excitation of bound states. As a consequence, the response of the atom to the UV field (dipole acceleration) is enriched in harmonics of the fundamental frequency.
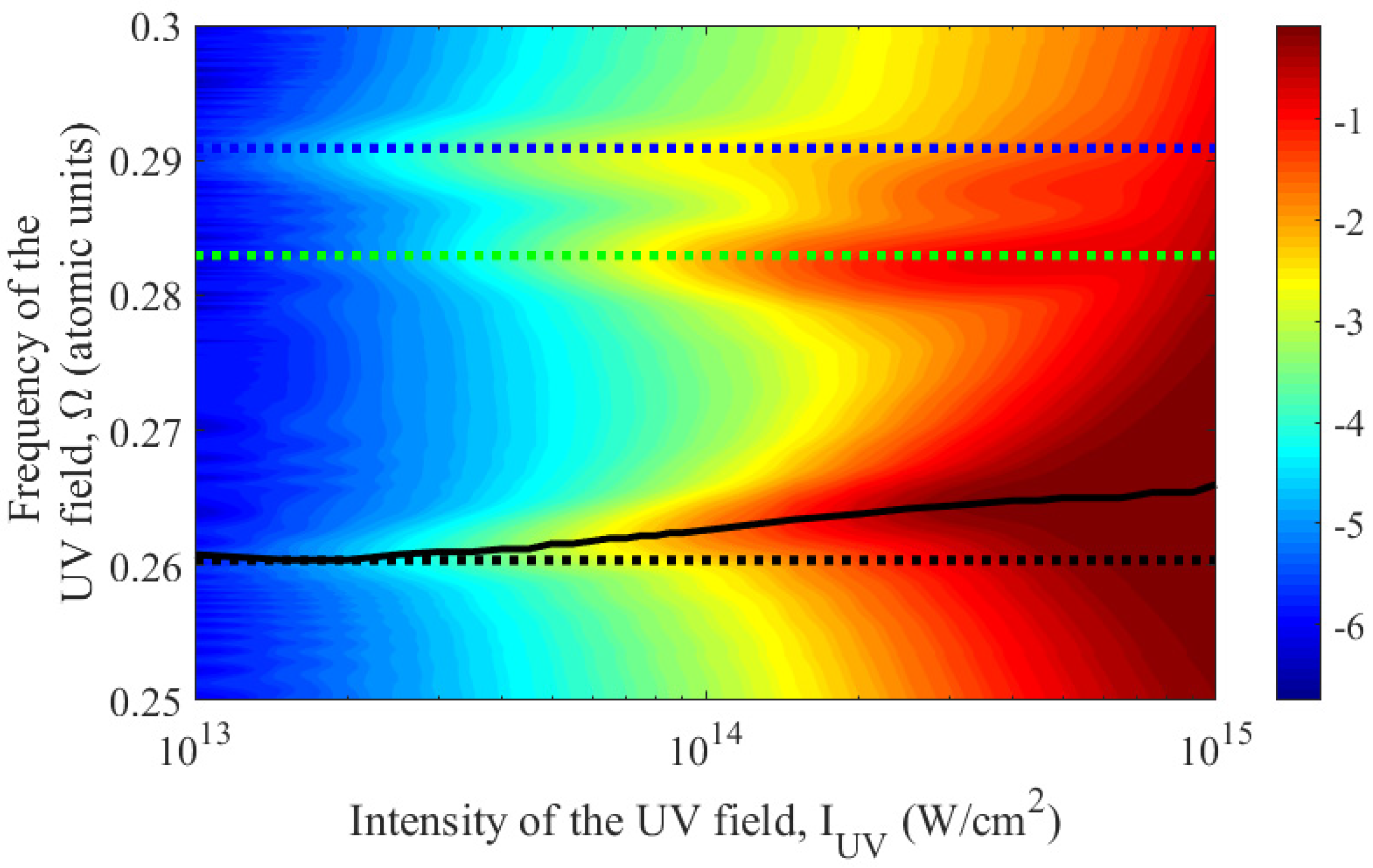
Figure 3 shows the dependence of the energy concentrated in the spectral component of the dipole acceleration of an atom (10) at the frequency of the 3rd harmonic of the UV field (the method for calculating this quantity is described in Appendix С) on its frequency and intensity. Calculations were made for the duration of the interval of constant amplitude in the UV field envelope equal to 30 carrier cycles (
Nosc = 30).
Figure 3.
Dependence (in a logarithmic scale) of the third harmonic energy in the spectrum of the dipole acceleration of a helium atom on the intensity and frequency of the UV field in the vicinity of three-photon resonances. The dotted lines indicate the frequencies of three-photon transitions between unperturbed states of the atom; here black color corresponds to the |1s2〉 - |1s2p〉 transition, green is for the |1s2〉 - |1s3p〉 transition, whereas blue is for the |1s2〉 - |1s4p〉 and |1s2〉 - |1s4f〉 transitions (on the scale of the figure they coincide). The solid black curve indicates the frequency of the three-photon transition |1s2〉 - |1s2p〉 in the UV field. The cyan dashed curve corresponds to the dependence of the UV field frequency on its intensity, which maximizes the third harmonic energy in the dipole acceleration spectrum.
Figure 3.
Dependence (in a logarithmic scale) of the third harmonic energy in the spectrum of the dipole acceleration of a helium atom on the intensity and frequency of the UV field in the vicinity of three-photon resonances. The dotted lines indicate the frequencies of three-photon transitions between unperturbed states of the atom; here black color corresponds to the |1s2〉 - |1s2p〉 transition, green is for the |1s2〉 - |1s3p〉 transition, whereas blue is for the |1s2〉 - |1s4p〉 and |1s2〉 - |1s4f〉 transitions (on the scale of the figure they coincide). The solid black curve indicates the frequency of the three-photon transition |1s2〉 - |1s2p〉 in the UV field. The cyan dashed curve corresponds to the dependence of the UV field frequency on its intensity, which maximizes the third harmonic energy in the dipole acceleration spectrum.
Two remarks should be made here. The energy (time-integrated square of the amplitude) of the 3rd harmonic in the dipole acceleration spectrum was chosen as the parameter under study, rather than its peak power spectral density or peak intensity, because under certain conditions, the spectral and time dependences of the harmonic signal may have several maxima of comparable amplitude. In particular, this case is observed under conditions of Rabi oscillations between the resonant states of a multiphoton transition [
45]. The second remark concerns the free-induction decay. Under conditions of three-photon resonance, the excited state |1
s2
p〉 of the atom, coupled by a dipole-allowed transition with the ground state |1
s2〉, is effectively populated. As a result, after the end of the UV field pulse, the atom continues to emit at the frequency of transition |1
s2〉 – |1
s2
p〉, close to the third harmonic frequency (however, slightly different from it in the absence of a field). This radiation corresponds to free-induction decay. In order to separate it from the third harmonic radiation, the dipole acceleration of the atom, calculated by formula (10) or (13), is multiplied by a temporary mask, which is nonzero only in the presence of a UV field (for more details, see Appendix С).
Similar to
Figure 2, the dotted lines in
Figure 3 indicate the frequencies of three-photon transitions to unperturbed states of the atom, and the solid black curve indicates the resonance frequency of the three-photon transition |1
s2〉 – |1
s2
p〉 |in the UV field, determined by the maximum probability of ionization. The UV field frequency that maximizes the 3rd harmonic energy in the dipole acceleration spectrum for a given field intensity is plotted in
Figure 3 by the dashed cyan curve. As follows from
Figure 3, the maximum energy of the 3rd harmonic in the dipole acceleration spectrum is achieved at a UV field intensity
IUV = 2×10
14 W/cm
2 and a frequency Ω = 0.2644 a.u., which is in close proximity to the resonant frequency, Ω
res = 0.2638 a.u., of three-photon transition to the |1
s2
p〉 state in the presence of a UV field of given intensity (in this case, the detuning from resonance is about 1/3 of the half-width of the resonance curve in the frequency dependence of the probability of ionization of an atom). For the specified optimal parameters, the probability of ionization at the end of the UV field pulse is approximately 24%.
As the UV field intensity decreases, the third harmonic energy (
Figure 3) and, in particular, the probability of atomic ionization (
Figure 2), quickly decrease. In this case, the frequency that maximizes the harmonic energy practically coincides with the frequency of the resonant three-photon transition |1
s2〉 – |1
s2
p〉 in the UV field. At the same time, for a UV field intensity exceeding the optimal value
IUV = 2×10
14 W/cm
2, the value of the maximum achievable energy of the third harmonic decreases, while the detuning of the optimal (for a given intensity) frequency of the UV field from the frequency of the three-photon resonance with the |1
s2〉 – |1
s2
p〉 transition increases. With a further increase in the intensity of the UV field, the frequency that maximizes the energy of the third harmonic in the spectrum of the dipole acceleration of the atom shifts to the region of resonances with the transitions |1
s2〉 – |1
s3
p〉, |1
s2〉 – |1
s4
p〉, and |1
s2〉 – |1
s4
f〉, while the harmonic energy continues to decrease.
To explain this dependence, let us analyze additional figures.
Figure 4 shows the frequency dependence of the power spectral density (the square of the modulus of the spectral amplitude, see Appendix С) of the dipole acceleration of an atom under conditions that maximize the energy of the 3rd harmonic for
Nosc = 30. The red curve shows the solution of Equations (6), (7), corresponding to the expansion of the wave function in terms of the basis of stationary states (4). In this case, the third harmonic energy reaches a maximum at
IUV = 2×10
14 W/cm
2 and Ω=0.2644 a.u, see
Figure 3. The black curve is obtained based on model (11), (12). In this case, the optimum shifts towards higher UV field intensity and the maximum is achieved at
IUV = 4×10
14 W/cm
2 and Ω = 0.2730 a.u. The increase in the optimal intensity value in the two-dimensional model is most likely due to the lower probability of multiphoton excitation and ionization of the atom, see
Figure 1. Similar to
Figure 3, in this case the optimal frequency of the UV field, Ω = 0.2730 a.u., turns out to be close to the resonance frequency of the |1
s2〉 – |1
s2
p〉 transition in the UV field, which for
IUV = 4×10
14 W/cm
2 in the two-dimensional model is Ω
res = 0.2720 a.u. Several conclusions can be drawn from
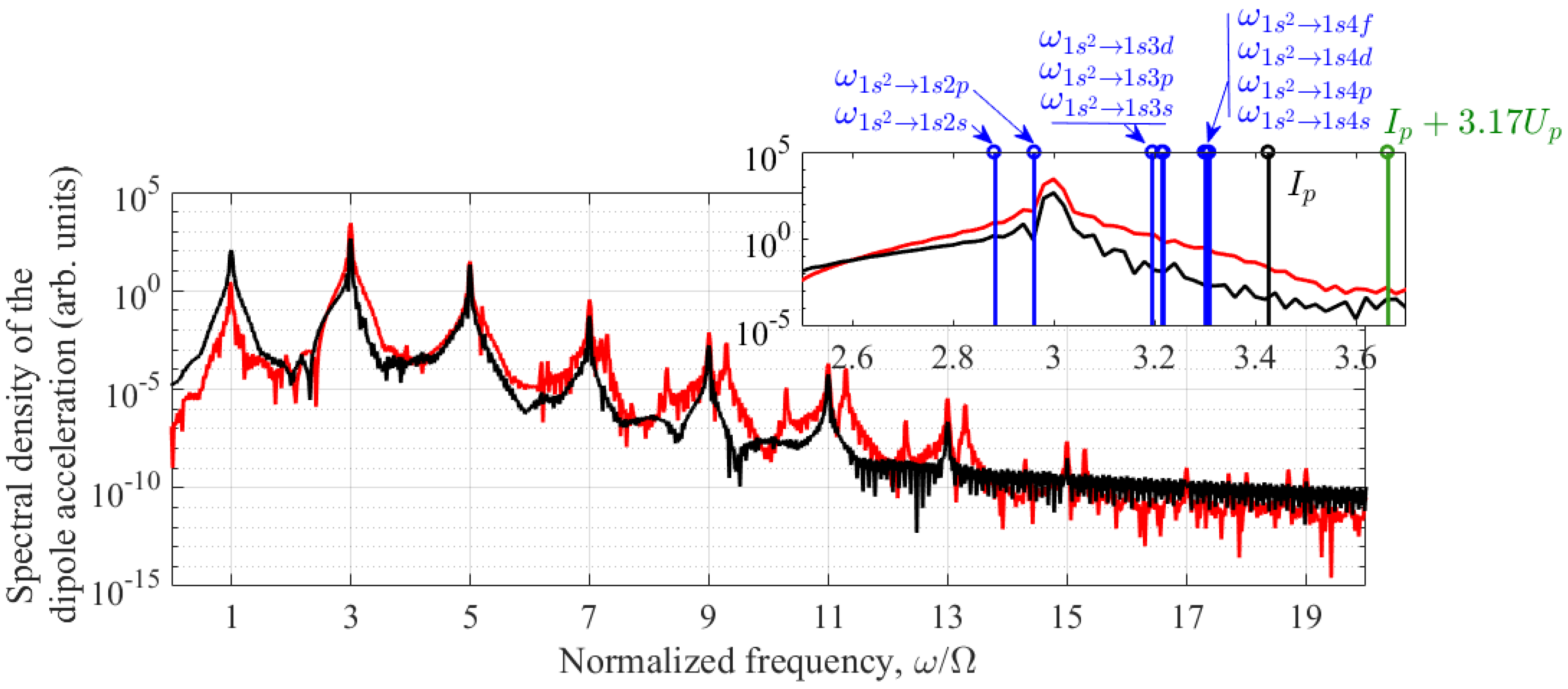
Figure 4. Firstly, the dipole acceleration of an atom is multi-frequency. In addition to the fundamental frequency of the UV field and its third harmonic, the atomic response contains harmonics of higher orders, up to the 13th one. Secondly, for the chosen UV field parameters, the 3rd harmonic in the dipole acceleration spectrum is dominant. In the solution of Equations (6), (7), it is approximately 90 times more intense than the 5th harmonic, three orders of magnitude more intense than the signal at the fundamental frequency of the UV field, and 4 orders of magnitude more intense than the 7th harmonic. Thus, the contribution of the 3rd harmonic to the spectrum of dipole acceleration is decisive. Third, as shown in the inset of
Figure 4, under the conditions considered, the 3rd harmonic is the only below-threshold harmonic; its frequency is comparable to the frequencies of single-photon transitions to all excited bound states of the atom and to the threshold of its photoionization. The fifth and higher harmonics in the case under consideration are above-threshold. Fourthly, as shown in the inset to
Figure 4, due to the small ponderomotive energy of a free electron in the UV field,
Up ≈ 0.02 a.u. << Ω, the contribution of the Corkum’s mechanism of harmonic generation [
14,
15] under the conditions under consideration is insignificant. In addition,
Ip+
Up ≈
Ip (where
Ip = 0.9036 a.u. is the ionization potential of an unperturbed atom), and the shift of the energy boundary of the continuum due to the dynamic Stark effect [
46] does not play a noticeable role. The fifth and final conclusion from
Figure 5 is the qualitative agreement between the dipole acceleration spectra at the 3rd, 5th, and, with less accuracy, the 7th harmonics, calculated (i) using a stationary-state basis and (ii) in a two-dimensional one-electron model of the helium atom, with appropriate (optimal in each model) UV field parameters. The differences in the shape of the spectral lines of higher harmonics are apparently due to the limited basis of the stationary states taken into account in expansion (4).
Figure 4.
Frequency dependence of the power spectral density of dipole acceleration under conditions that maximize the third harmonic energy. The red curve corresponds to the solution of the system of Equations (6), (7) at
Nosc = 30,
IUV = 2×10
14 W/cm
2, and Ω = 0.2644 a.u. The black curve was obtained based on model (11), (12) with
Nosc = 30,
IUV = 4×10
14 W/cm
2, and Ω = 0.2730 a.u. The inset shows the vicinity of the third harmonic frequency; the vertical blue lines indicate the frequencies of single-photon (both dipole-allowed and dipole-forbidden) transitions into the unperturbed bound states taken into account, the vertical black line indicates the ionization potential
Ip of the atom in the absence of a UV field, and the vertical green line indicates the plateau boundary in the spectrum of harmonics due to the Corkum’s mechanism,
Ip + 3.17×
Up, where
Up is the ponderomotive energy of the electron (see [
14,
15]). The frequency values in the inset to the figure correspond to model (6), (7) (see
Appendix A, formula (A1)).
Figure 4.
Frequency dependence of the power spectral density of dipole acceleration under conditions that maximize the third harmonic energy. The red curve corresponds to the solution of the system of Equations (6), (7) at
Nosc = 30,
IUV = 2×10
14 W/cm
2, and Ω = 0.2644 a.u. The black curve was obtained based on model (11), (12) with
Nosc = 30,
IUV = 4×10
14 W/cm
2, and Ω = 0.2730 a.u. The inset shows the vicinity of the third harmonic frequency; the vertical blue lines indicate the frequencies of single-photon (both dipole-allowed and dipole-forbidden) transitions into the unperturbed bound states taken into account, the vertical black line indicates the ionization potential
Ip of the atom in the absence of a UV field, and the vertical green line indicates the plateau boundary in the spectrum of harmonics due to the Corkum’s mechanism,
Ip + 3.17×
Up, where
Up is the ponderomotive energy of the electron (see [
14,
15]). The frequency values in the inset to the figure correspond to model (6), (7) (see
Appendix A, formula (A1)).
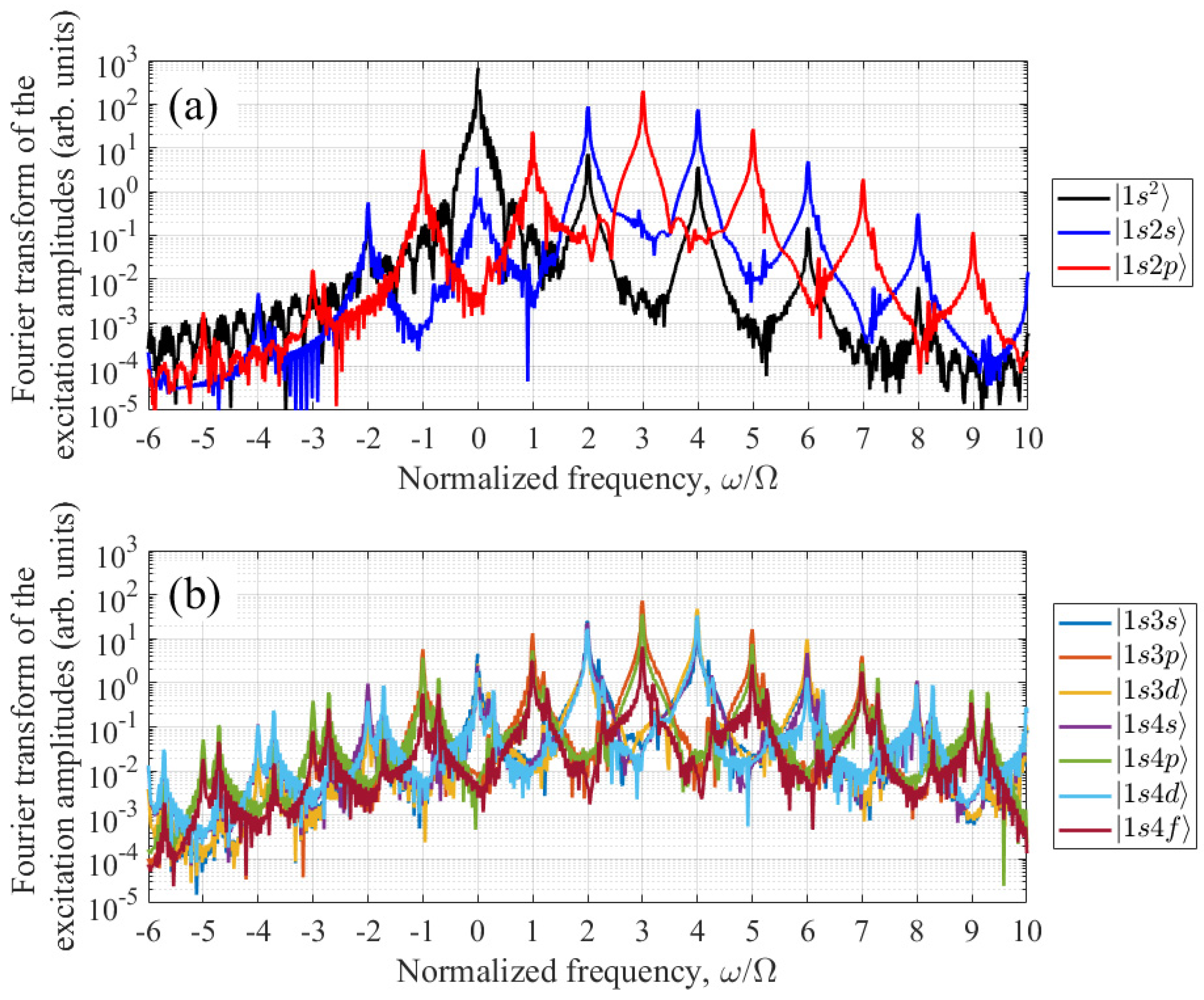
Next, we will analyze the role of various stationary states in the generation of harmonics. When using the expansion of the wave function in stationary states and the adiabatic continuum exclusion approximation, the dipole acceleration of the atom is determined by the excitation amplitudes of bound states (9), (10). The excitation amplitude spectra of the states taken into account for
Nosc = 30 (see (3)) and optimal UV field parameters,
IUV = 2×10
14 W/cm
2 and Ω = 0.2644 a.u., are shown in
Figure 5. The horizontal axis shows the excitation frequency of the atom, measured from the unperturbed frequency of the ground state |1
s2〉. As follows from Figure 5, the states |1
s2〉, |1
s2
s〉, and |1
s2
p〉, shown in
Figure 5a, have the largest excitation amplitudes, while the remaining states,
Figure 5b, are excited noticeably weaker. In accordance with Equation (9), the complex amplitude of the dipole moment of an atom at the 3rd harmonic frequency is determined by the sum of the products
over all considered states
s and
k of opposite parity and over the Fourier components of the excitation amplitudes of these states
n and
n+3. As can be seen from
Figure 5a, in the conditions under consideration, of the even parity states, the 0th Fourier component of the excitation amplitude of the state |1s
2〉,
, has the largest amplitude, and of the odd parity states, this is the case for the 3rd Fourier component of the excitation amplitude of the state |1
s2
p〉,
, oscillating at a frequency close to the frequency of the single-photon transition |1
s2〉 - |1
s2
p〉. Accordingly, the dominant contribution to the amplitude of the 3rd harmonic of the UV field frequency in the spectrum of the induced dipole moment of the atom is made by the product
. In this case, the dipole acceleration of the atom at the third harmonic frequency is proportional to
, while its energy turns out to be proportional to
, where integration is carried out over the time interval at which the UV field is different from zero (see Appendix С). On the other hand, due to the dominant contribution of the Fourier components
a1,0 and
a3,3 to the excitation amplitudes of the states |1
s2〉 and |1
s2
p〉, respectively, the approximate equality is valid:
, where
denotes the time-averaged product of the populations of the states |1
s2〉 and |1
s2
p〉, while
is the duration of the UV field pulse. The resulting equality is easy to interpret, namely, the energy of the third harmonic reaches its maximum under the conditions of the most effective excitation of the coherent superposition of states |1
s2〉 and |1
s2
p〉.
Figure 5.
Absolute values of the Fourier transform of the excitation amplitudes of bound states in a UV field with
Nosc = 30,
IUV = 2×10
14 W/cm
2, and Ω = 0.2644 a.u. In (a), the black, blue, and red curves show the excitation amplitudes of the |1
s2〉, |1
s2
s〉, and |1
s2
p〉 states, respectively. In (b), the excitation amplitudes of the remaining states taken into account, |1
s3
s〉, |1
s3
p〉, |1
s3
d〉, |1
s4
s〉, |1
s4
p〉, |1
s4
d〉, and |1
s4
f〉, are shown in different colors. All values are calculated for a time interval within which the UV field is nonzero (similar to formula (C3) in
Appendix C).
Figure 5.
Absolute values of the Fourier transform of the excitation amplitudes of bound states in a UV field with
Nosc = 30,
IUV = 2×10
14 W/cm
2, and Ω = 0.2644 a.u. In (a), the black, blue, and red curves show the excitation amplitudes of the |1
s2〉, |1
s2
s〉, and |1
s2
p〉 states, respectively. In (b), the excitation amplitudes of the remaining states taken into account, |1
s3
s〉, |1
s3
p〉, |1
s3
d〉, |1
s4
s〉, |1
s4
p〉, |1
s4
d〉, and |1
s4
f〉, are shown in different colors. All values are calculated for a time interval within which the UV field is nonzero (similar to formula (C3) in
Appendix C).
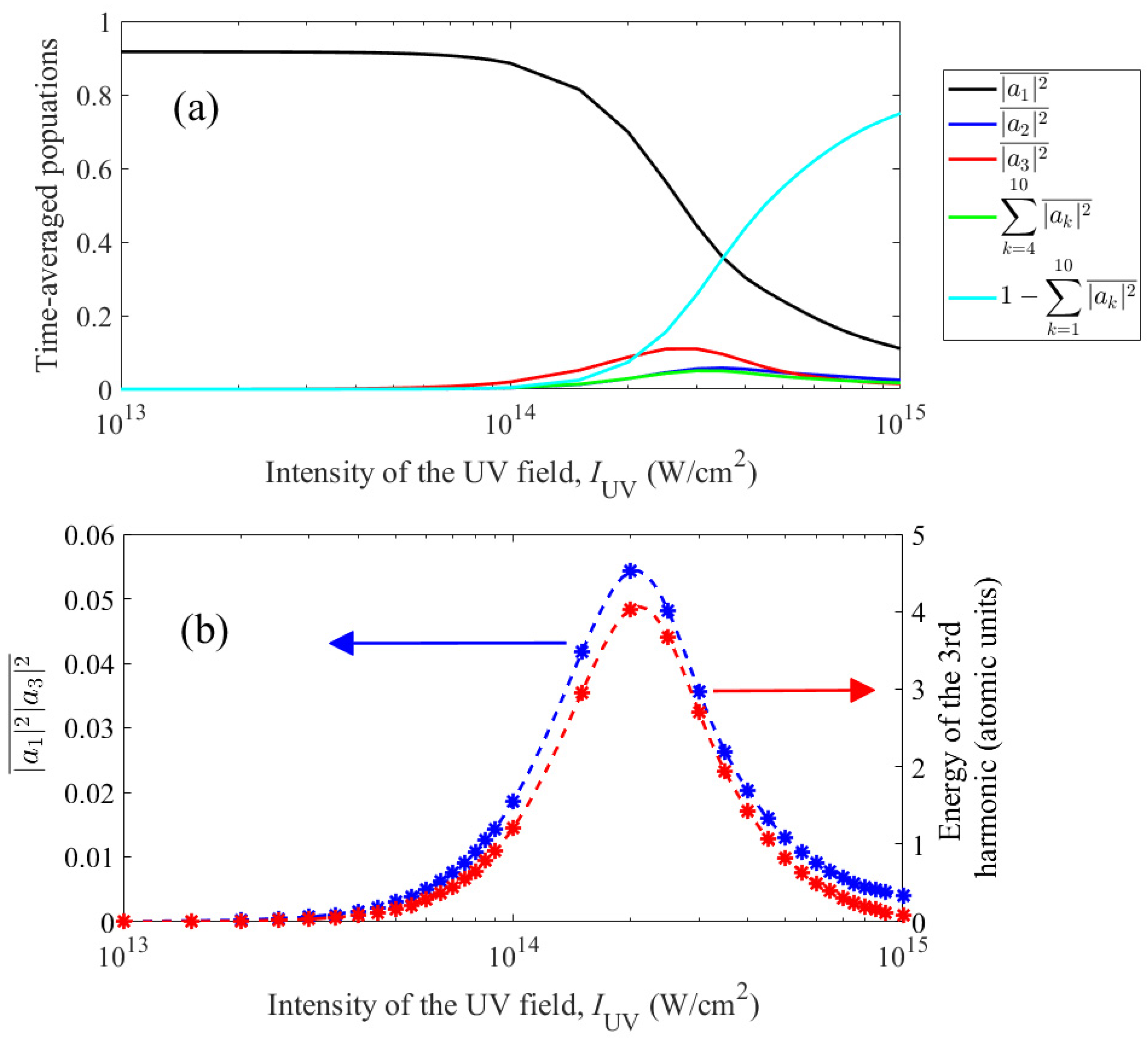
This statement is illustrated by
Figure 6. The upper part of the figure (
Figure 6a) shows the time-averaged populations of bound states,
, and the total population of continuum states,
, as a function of the UV field intensity. In
Figure 6,
Nosc = 30 (see (3)), and the frequency of the UV field for each intensity is chosen equal to the frequency of the three-photon transition |1
s2〉 – |1
s2
p〉 in the field (taking into account the dynamic Stark effect, see the black solid curve in
Figure 2). Lower part of the figure (
Figure 6b), shows (i) the time-averaged product of the populations of the states |1s
2〉 and |1s2p〉,
, and (ii) the energy of the third harmonic in the spectrum of the dipole acceleration of the atom as a function of the intensity of the UV field. As can be seen from
Figure 6b, up to the dimension factor, these two dependences practically coincide. Thus, to maximize the third harmonic energy, it is necessary to most effectively excite the |1
s2
p〉 state without excessively depleting the |1
s2〉 state.
Figure 6.
(a) Dependences of the populations of the states |1s2〉 (black curve), |1s2s〉 (blue curve), |1s2p〉 (red curve), the total population of the remaining bound states taken into account with n = 3 and n = 4 (green curve), as well as the total population of continuum states (cyan curve), averaged over the duration of the UV field pulse, on the intensity of the UV field under conditions of resonant three-photon excitation of the |1s2p〉 state. (b) Left axis, blue color: dependence of the product of the populations of the |1s2〉 and |1s2p〉 states averaged over the duration of the UV field pulse on the intensity of the UV field under conditions of three-photon excitation of the |1s2p〉 state; right axis, red color: similar dependence of the energy of the third harmonic of the UV field in the dipole acceleration spectrum. The asterisks indicate the calculated values, the dashed lines are obtained by interpolating them with a spline.
Figure 6.
(a) Dependences of the populations of the states |1s2〉 (black curve), |1s2s〉 (blue curve), |1s2p〉 (red curve), the total population of the remaining bound states taken into account with n = 3 and n = 4 (green curve), as well as the total population of continuum states (cyan curve), averaged over the duration of the UV field pulse, on the intensity of the UV field under conditions of resonant three-photon excitation of the |1s2p〉 state. (b) Left axis, blue color: dependence of the product of the populations of the |1s2〉 and |1s2p〉 states averaged over the duration of the UV field pulse on the intensity of the UV field under conditions of three-photon excitation of the |1s2p〉 state; right axis, red color: similar dependence of the energy of the third harmonic of the UV field in the dipole acceleration spectrum. The asterisks indicate the calculated values, the dashed lines are obtained by interpolating them with a spline.
This conclusion allows us to explain the dependence of the frequency that maximizes the energy of the third harmonic in the spectrum of the dipole acceleration of an atom on the intensity of the UV field in
Figure 3. In a relatively weak field, the depletion of the |1
s2〉 state is not significant (see
Figure 6a), and the optimum is achieved at maximum excitation of the |1
s2
p〉 state, i.e. when adjusting the frequency of the UV field to three-photon resonance. At the optimal UV field intensity,
IUV = 2×10
14 W/cm
2, a balance is achieved between excitation of the |1
s2
p〉 state and depletion of the |1
s2〉 state, in this case the optimal frequency of the UV field remains in the immediate vicinity of the resonant transition frequency. With a further increase in the intensity of the UV field during resonant excitation of the |1
s2〉 – |1
s2
p〉 transition, both the populations of bound states and the third harmonic energy decrease as a result of ionization of the atom (see
Figure 6a). Accordingly, the optimal frequency of the UV field in
Figure 3 moves away from the resonant one, which makes it possible to reduce the probability of ionization and, thereby, maintain a noticeable population of the |1
s2〉 and |1
s2
p〉 states.
Next, using the expansion of the wave function in terms of the basis of stationary states, we study the dependence of the conditions that maximize the energy of the third harmonic in the spectrum of the dipole acceleration of an atom on the duration of the constant amplitude interval in the UV field envelope (parameter
Nosc, see (3)).
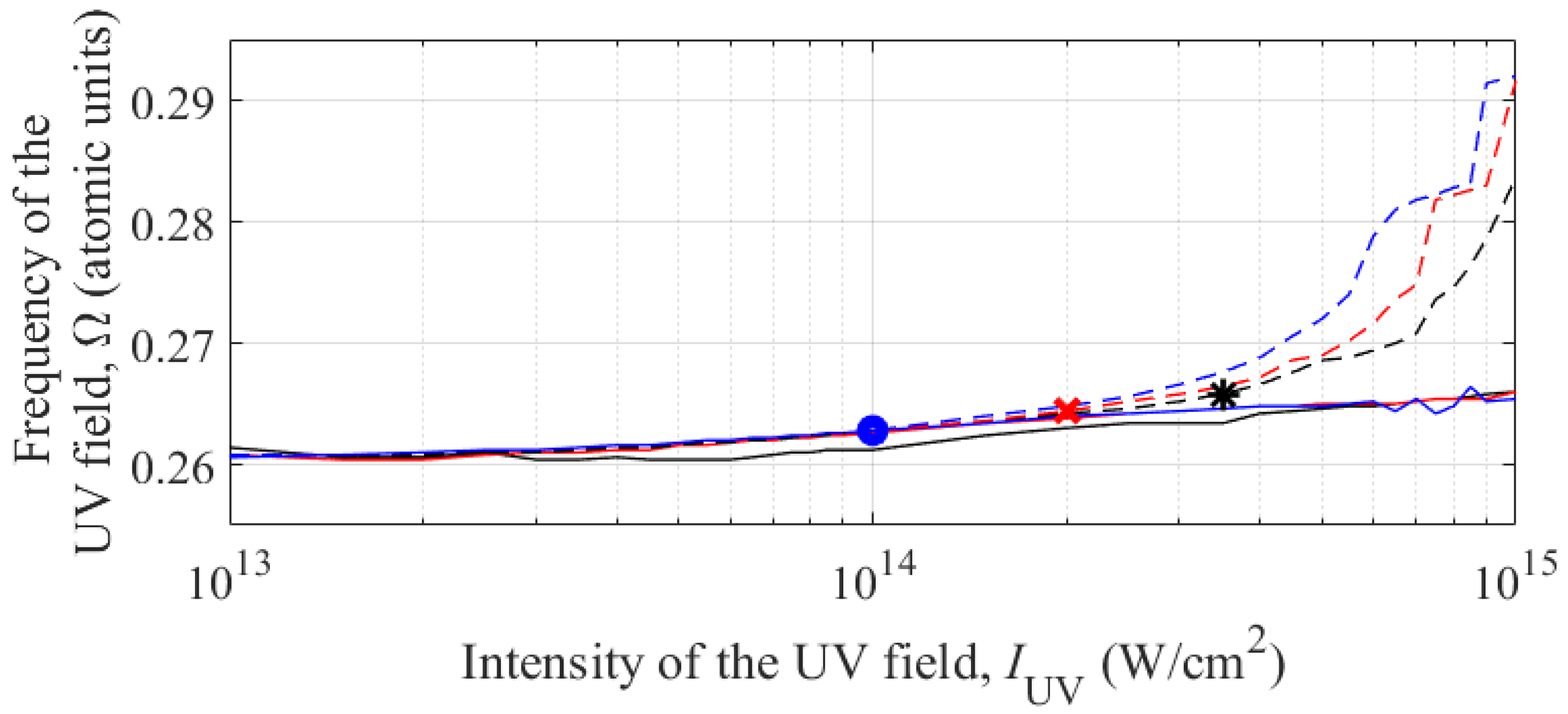
Figure 7 for three values of
Nosc = 10, 30, and 100 shows the dependences of (i) the frequencies of the resonant three-photon transition |1
s2〉 – |1
s2
p〉 and (ii) the frequencies of the UV field that maximize the energy of the third harmonic in the dipole acceleration spectrum on the intensity of the UV field.
Figure 7.
Dependence of the frequency of the three-photon transition |1s2〉 – |1s2p〉 on the intensity of the UV field (solid curves); dependence of the UV field frequency, which maximizes the energy of the third harmonic in the spectrum of the dipole acceleration of an atom, on the field intensity (dashed curves). Black color corresponds to Nosc = 10, red color is for Nosc = 30, and blue color is for Nosc = 100. The black asterisk, red cross, and blue circle indicate combinations of UV field intensity and frequency at which the absolute maximum energy of the third harmonic in the dipole acceleration spectrum is achieved for Nosc = 10, 30, and 100, respectively.
Figure 7.
Dependence of the frequency of the three-photon transition |1s2〉 – |1s2p〉 on the intensity of the UV field (solid curves); dependence of the UV field frequency, which maximizes the energy of the third harmonic in the spectrum of the dipole acceleration of an atom, on the field intensity (dashed curves). Black color corresponds to Nosc = 10, red color is for Nosc = 30, and blue color is for Nosc = 100. The black asterisk, red cross, and blue circle indicate combinations of UV field intensity and frequency at which the absolute maximum energy of the third harmonic in the dipole acceleration spectrum is achieved for Nosc = 10, 30, and 100, respectively.
The resonance frequencies of the three-photon transition |1
s2〉 – |1
s2
p〉 for different durations of the UV field envelope practically coincide, which is due to the short establishing time of the dynamic Stark effect [
46]. Some difference in the transition frequency is observed for the shortest pulse (
Nosc = 10) and is explained by the fact that the total duration of the intervals of turning on and off the UV field (3 + 3 = 6 carrier cycles) in this case is comparable to the duration of the constant amplitude interval (10 carrier cycles). Accordingly, the time-average intensity of the UV field turns out to be noticeably lower than the peak intensity, and the Stark effect at the same peak intensity is weaker than for UV field pulses with a longer duration of the constant amplitude interval. At the same time, jumps in the position of the resonance frequency in the longest pulse (
Nosc = 100) with an intensity of the order of 10
15 W/cm
2 are explained by the extremely high, exceeding 99.99%, probability of single ionization of the atom and the unreliability of the algorithm for determining the resonance frequency under such conditions.
The dependences of the UV field frequencies that maximize the third harmonic energy differ more significantly for different durations of the UV field envelope. At a fixed intensity, with increasing duration of the UV field pulse, the optimal detuning from the resonance frequency increases. At the same time, the optimal intensity of the UV field decreases with increasing envelope duration, namely, for
Nosc =10, 30, and 100 the optimal values are
IUV = 3.5×10
14 W/cm
2, 2×10
14 W/cm
2, and 10
14 W/cm
2, respectively. To interpret these data, let us turn to
Figure 8, which shows the time dependences of the populations of states |1
s2〉, |1
s2
s〉, and |1
s2
p〉, as well as the total population of more highly excited bound states and the total population of states of the continuum (a,c) and electron dipole acceleration as a function of time (b,d).
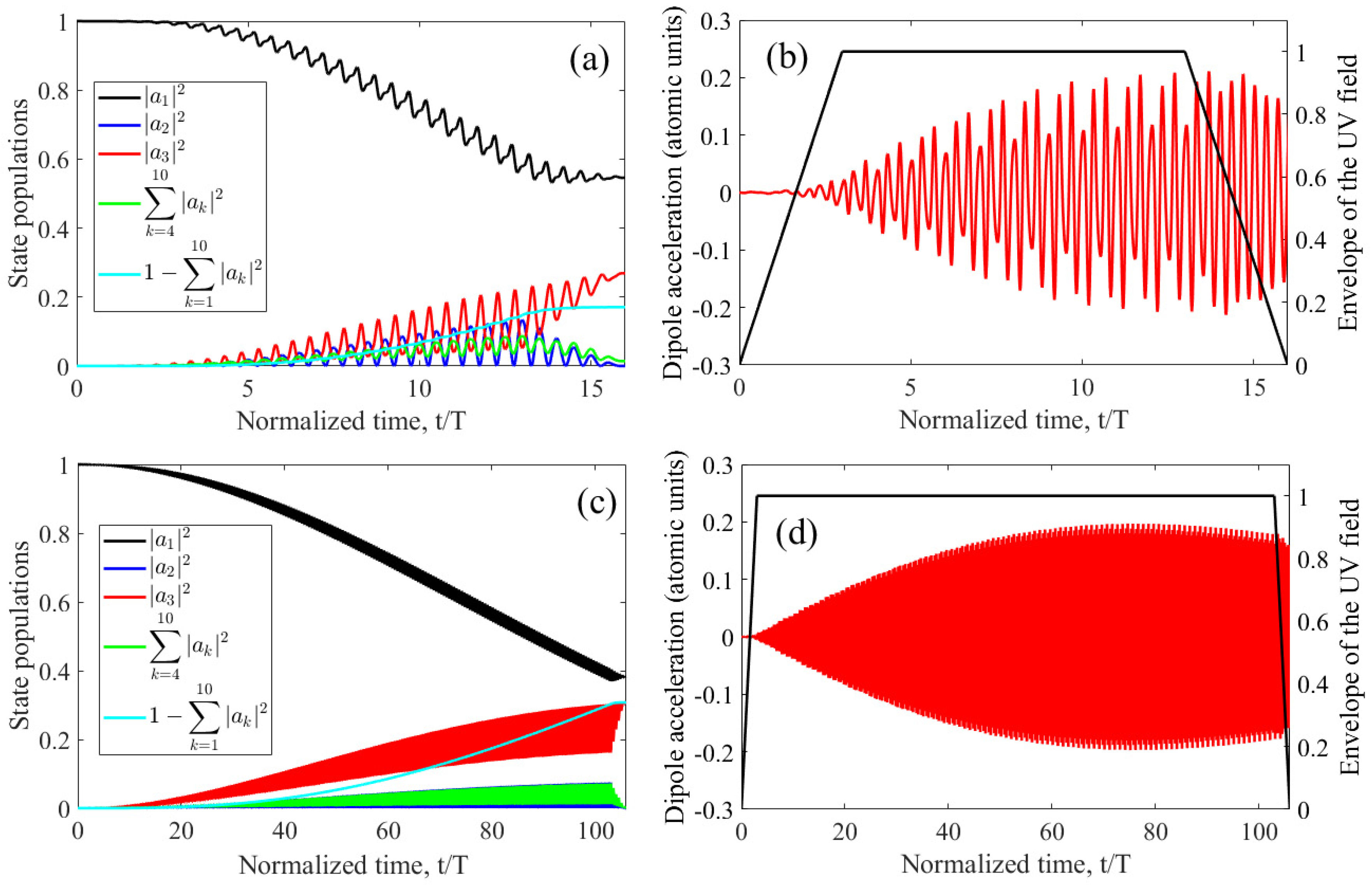
Figure 8a,b correspond to the UV field pulse of the shortest duration considered,
Nosc = 10, while
Figure 8c,d are plotted for the case of
Nosc = 100.
Figure 8.
(a,c) Time dependences of the populations of states|1s2〉 (black curve), |1s2s〉 (blue curve), |1s2p〉 (red curve), the total population of the remaining bound states taken into account (green curve), and the total population of continuum states (cyan curve). (b,d) Time dependences of the dipole acceleration (left axis, red curve) and the envelope (in amplitude) of the UV pulse (right axis, black broken curve). Figures (a) and (b) are plotted for Nosc = 10, IUV = 3.5x1014 W/cm2, and Ω = 0.2658, Figures (c) and (d) for Nosc = 100, IUV = 1014W/cm2, and Ω = 0.2628.
Figure 8.
(a,c) Time dependences of the populations of states|1s2〉 (black curve), |1s2s〉 (blue curve), |1s2p〉 (red curve), the total population of the remaining bound states taken into account (green curve), and the total population of continuum states (cyan curve). (b,d) Time dependences of the dipole acceleration (left axis, red curve) and the envelope (in amplitude) of the UV pulse (right axis, black broken curve). Figures (a) and (b) are plotted for Nosc = 10, IUV = 3.5x1014 W/cm2, and Ω = 0.2658, Figures (c) and (d) for Nosc = 100, IUV = 1014W/cm2, and Ω = 0.2628.
As can be seen from a comparison of
Figure 8b and
Figure 8d, for the considered UV field parameters, which are optimal for each envelope duration, (i) the time dependences of the dipole acceleration in the interval of constant amplitude of the UV field are similar to each other, and (ii) the peak amplitude of the dipole acceleration is approximately the same. Similar conclusions follow from
Figure 8a and
Figure 8c, namely, with an optimal choice of UV field parameters, the dynamics of excitation of stationary states weakly depends on the duration of the field envelope, and only the time scale of the process changes significantly. Under optimal conditions, at the end of the UV pulse, the atom goes into the resonant state |1
s2
p〉 with a noticeable probability of about 20-30%, ionizes with a comparable probability and remains in the ground state |1
s2〉 with a probability of about 40-50%. Accordingly, the optimal parameters of the UV field are those that, for different envelope durations, provide the same (optimal) character of excitation of the atom during its interaction with the field. Thus, an increase in the optimal intensity of the UV field with a decrease in the duration of its envelope is caused by the need to provide the same (optimal) degree of excitation of the atom in a shorter interaction time.
At the same time, at a fixed intensity of the UV field, the excitation and ionization of the atom increase or decrease as the frequency of the UV field approaches or moves away from the frequency of the three-photon resonance with the |1
s2〉 - |1
s2
p〉 transition. Accordingly, with increasing duration of the UV field envelope at a fixed intensity, the optimal detuning of the field frequency from resonance increases, and with decreasing duration, vice versa, it decreases. Note that some differences in the degree of excitation and ionization of an atom for different durations of the envelope (and, accordingly, different intensities) of the UV field (see
Figure 8a, c) are due to differences in the dependences of (i) excitation probabilities and (ii) probabilities of ionization of an atom on the strength of the UV field.
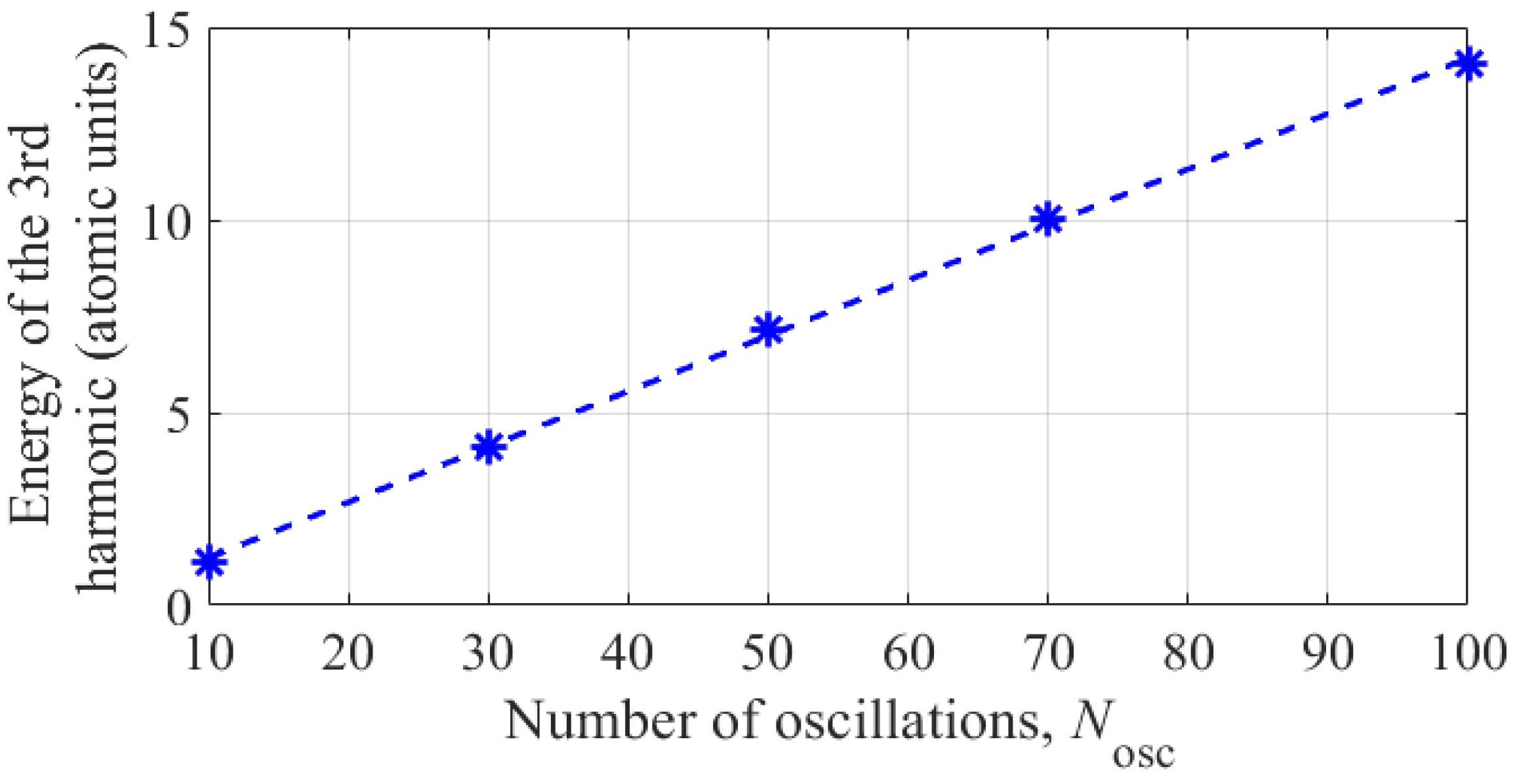
In conclusion of this section, we note that the energy concentrated in the third harmonic of the dipole acceleration of an atom varies approximately in proportion to the duration of the constant amplitude interval in the UV field envelope, see
Figure 9. This means that in the conditions under consideration, the average power concentrated in the third harmonic in the spectrum of dipole acceleration of the atom does not depend on the duration of the UV field envelope. Note, however, that with the shortening of the UV field pulse envelope, to achieve the same atomic response power at the third harmonic frequency, an increasing (proportional to the optimal intensity value, see
Figure 7) UV radiation power is required.
Figure 9.
Dependence of the energy concentrated in the third harmonic of the fundamental frequency in the dipole acceleration spectrum on the duration (in units of the oscillation period) of the constant amplitude interval in the UV field envelope (3). Asterisks are the results of numerical calculation based on system of Equations (6), (7); the energy corresponding to different Nosc is 1.098 a.u. for Nosc = 10, 4.122 a.u. for Nosc = 30, 7.13 a.u. for Nosc = 50, 10.05 a.u. for Nosc = 70, and 14.03 a.u. for Nosc = 100. The dashed line is the result of linear interpolation.
Figure 9.
Dependence of the energy concentrated in the third harmonic of the fundamental frequency in the dipole acceleration spectrum on the duration (in units of the oscillation period) of the constant amplitude interval in the UV field envelope (3). Asterisks are the results of numerical calculation based on system of Equations (6), (7); the energy corresponding to different Nosc is 1.098 a.u. for Nosc = 10, 4.122 a.u. for Nosc = 30, 7.13 a.u. for Nosc = 50, 10.05 a.u. for Nosc = 70, and 14.03 a.u. for Nosc = 100. The dashed line is the result of linear interpolation.