Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
2.1. Ethics statement
2.2. Study population
2.3. Sample collection
2.3.1. FF and COCs Sampling
2.3.2. Cd Detection by Atomic Absorption Spectrometry
2.3.3. Statistical analyses
2.4. Electron Microscopy
3. Results
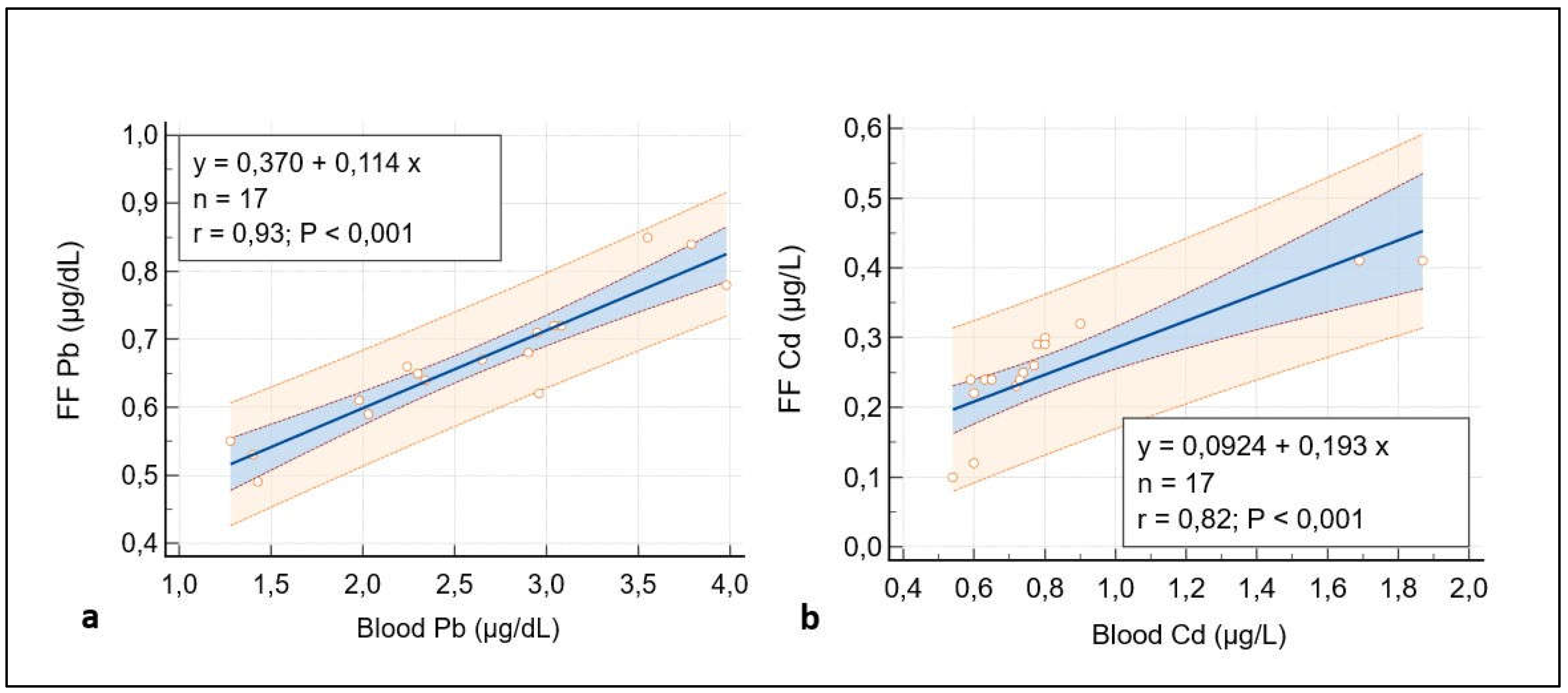
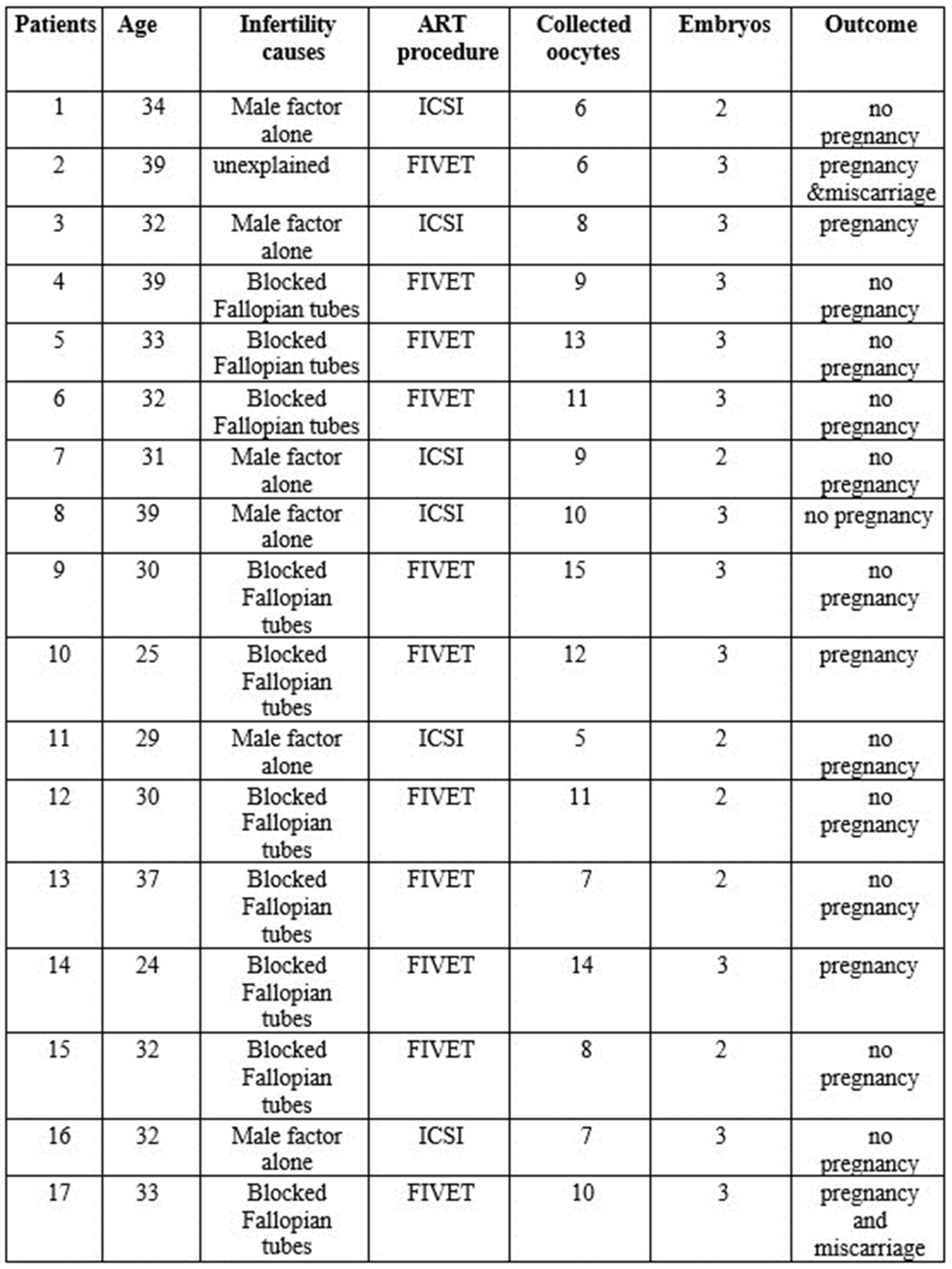
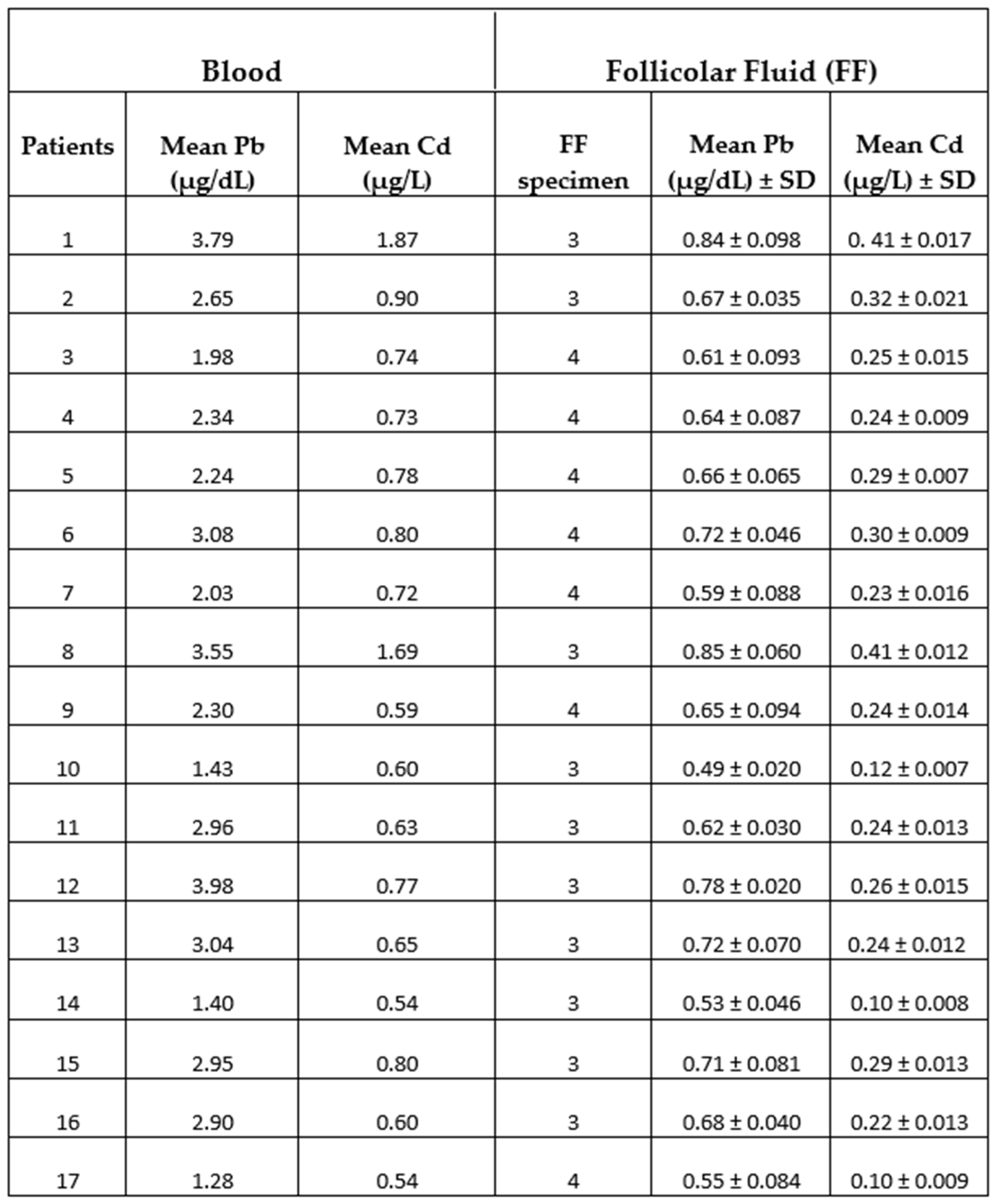
3.1. Characteristics of study population and heavy metals measurements
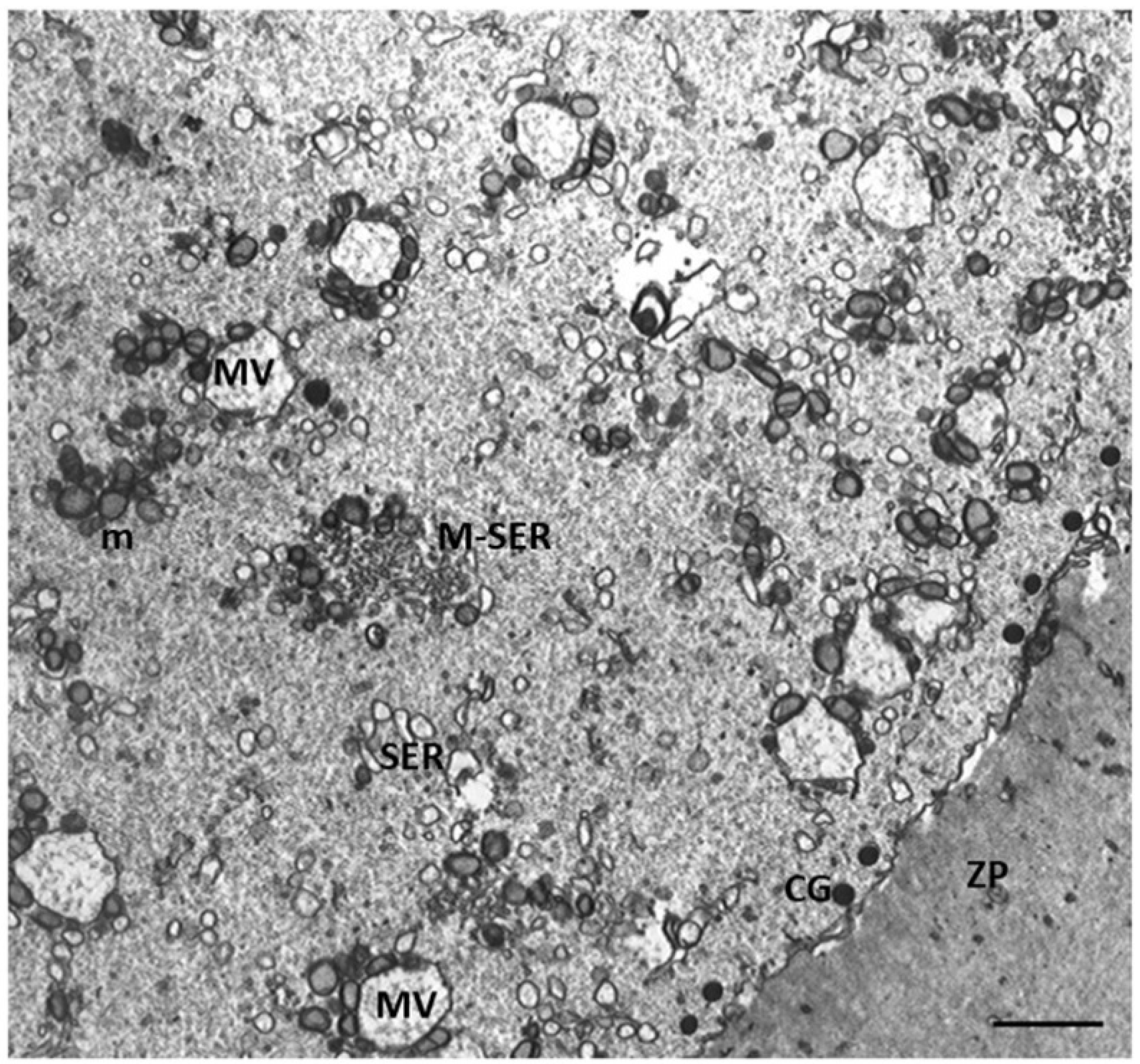
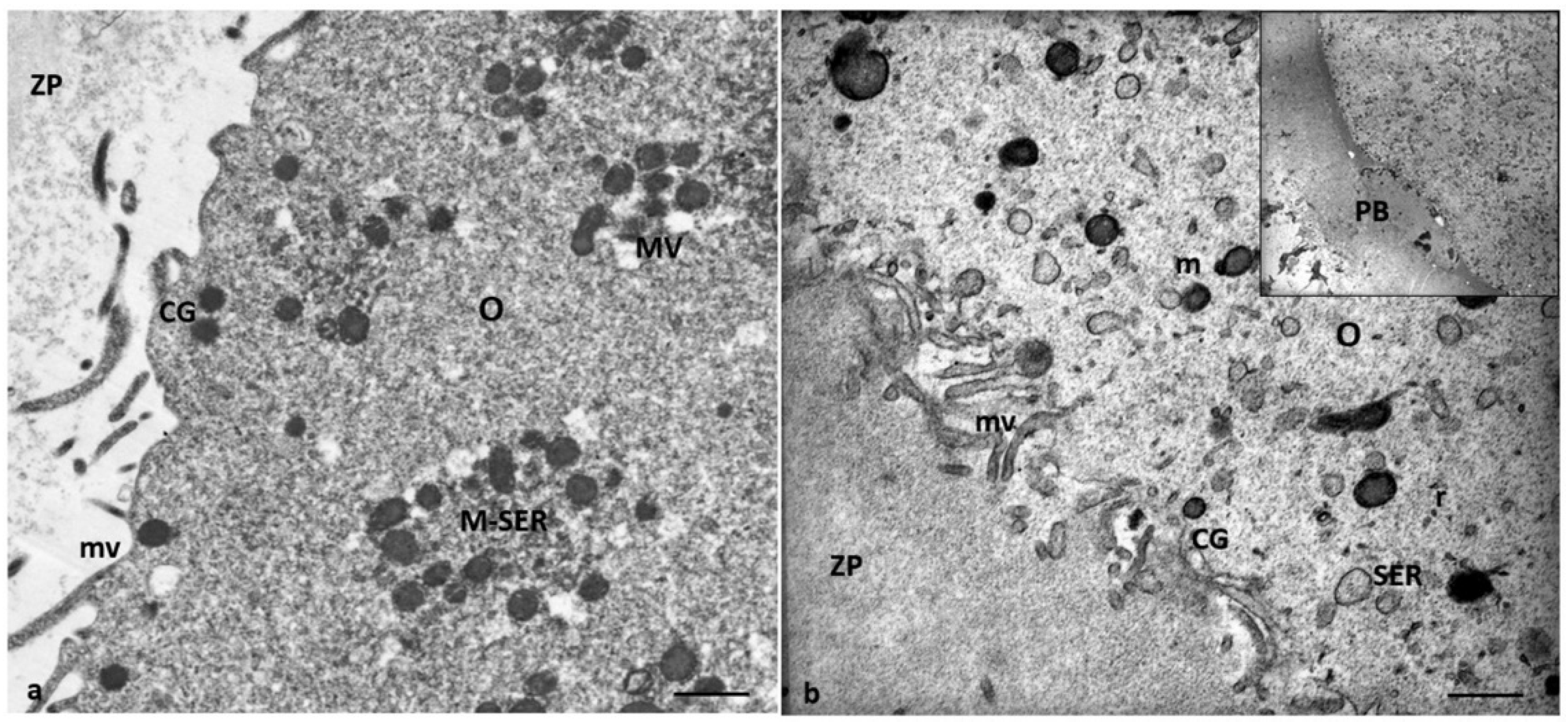
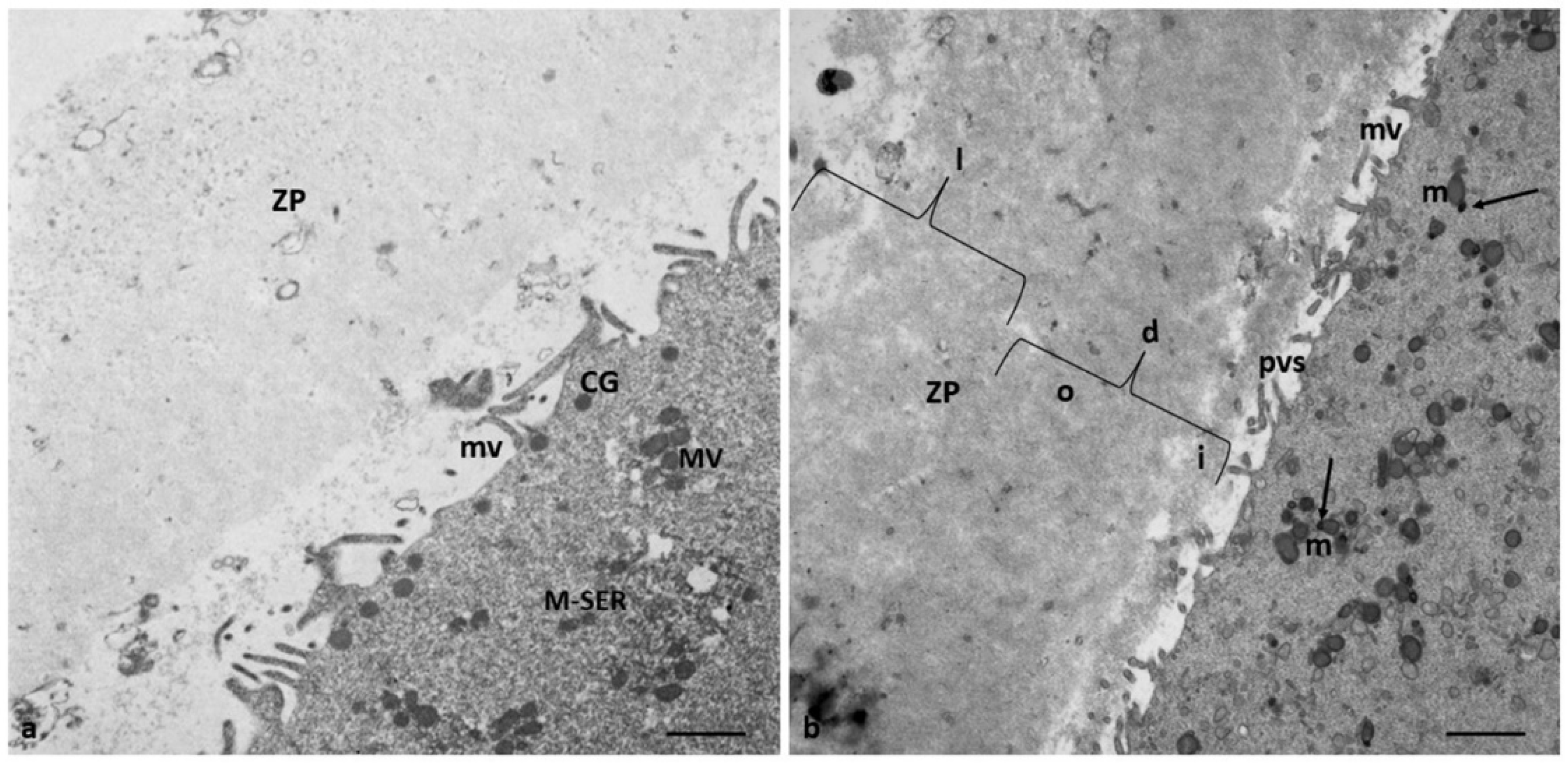
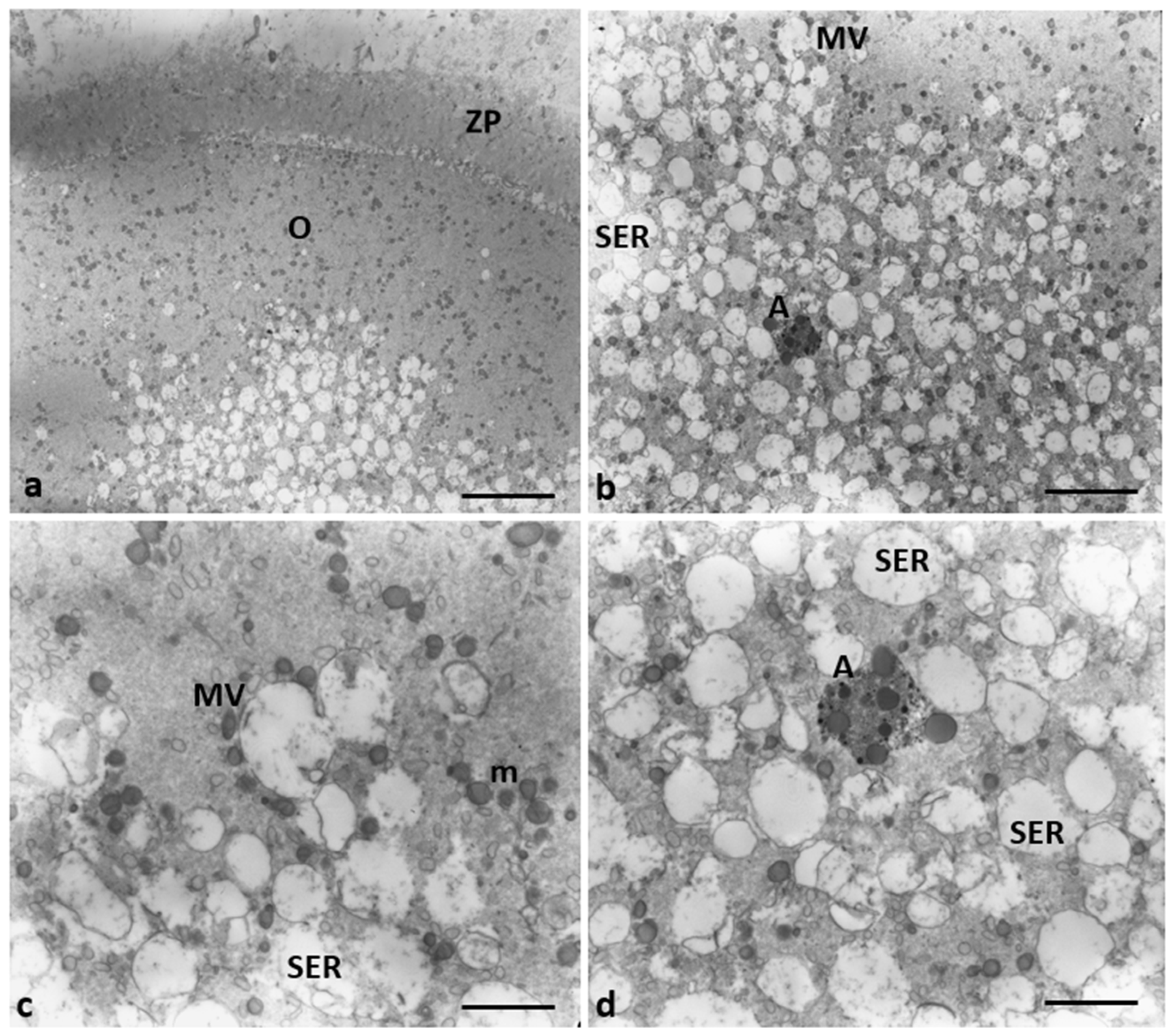
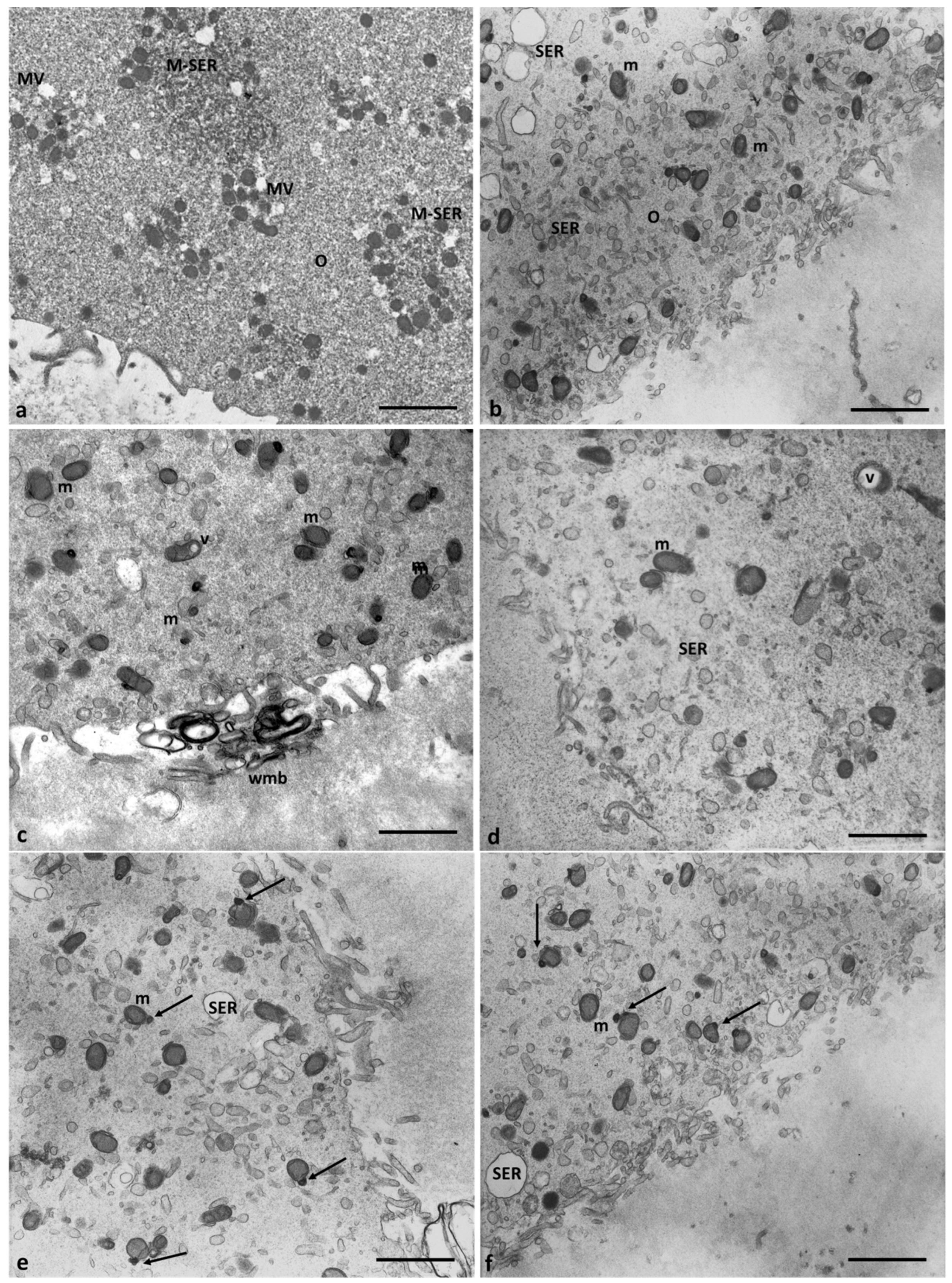
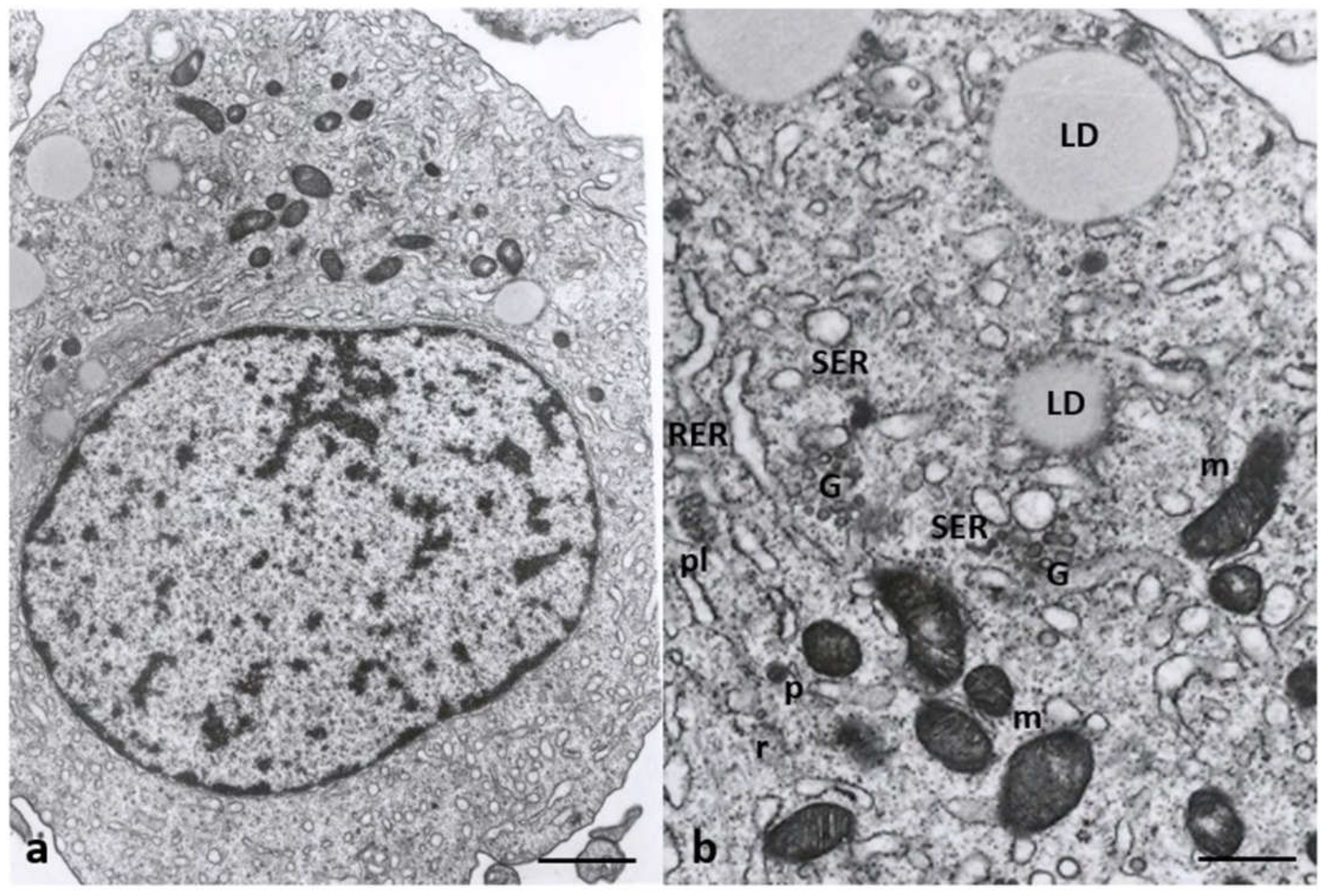
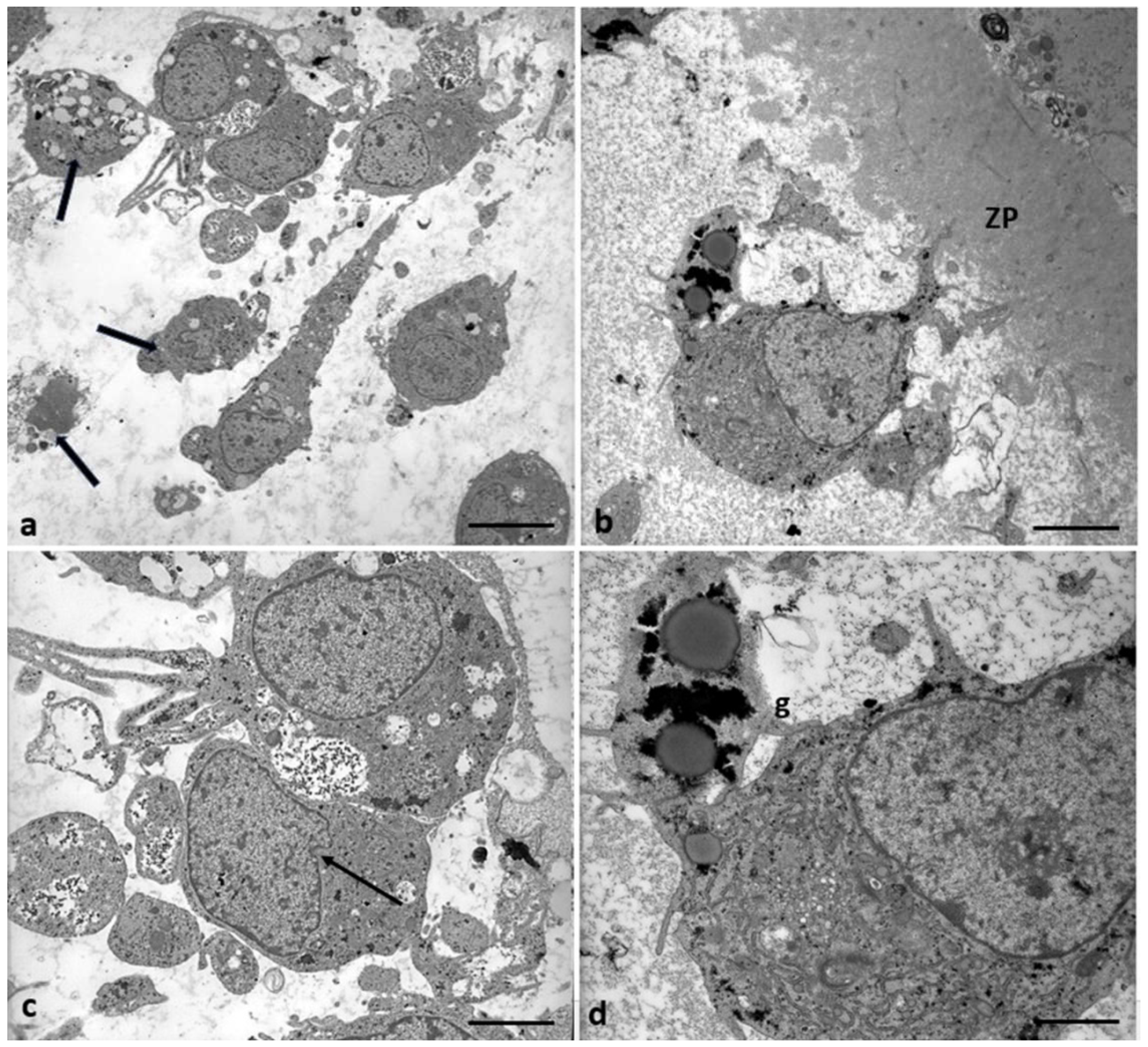
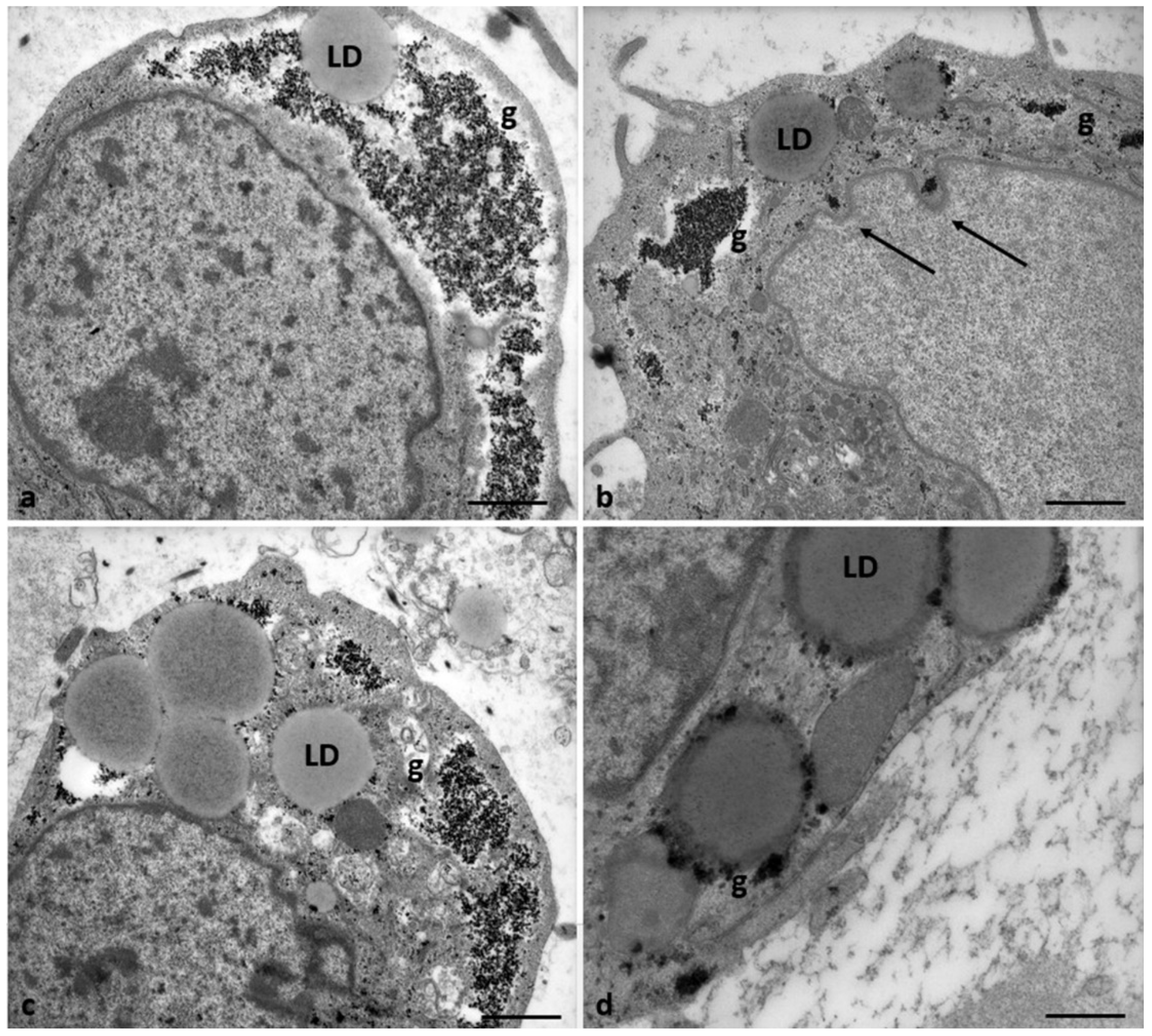
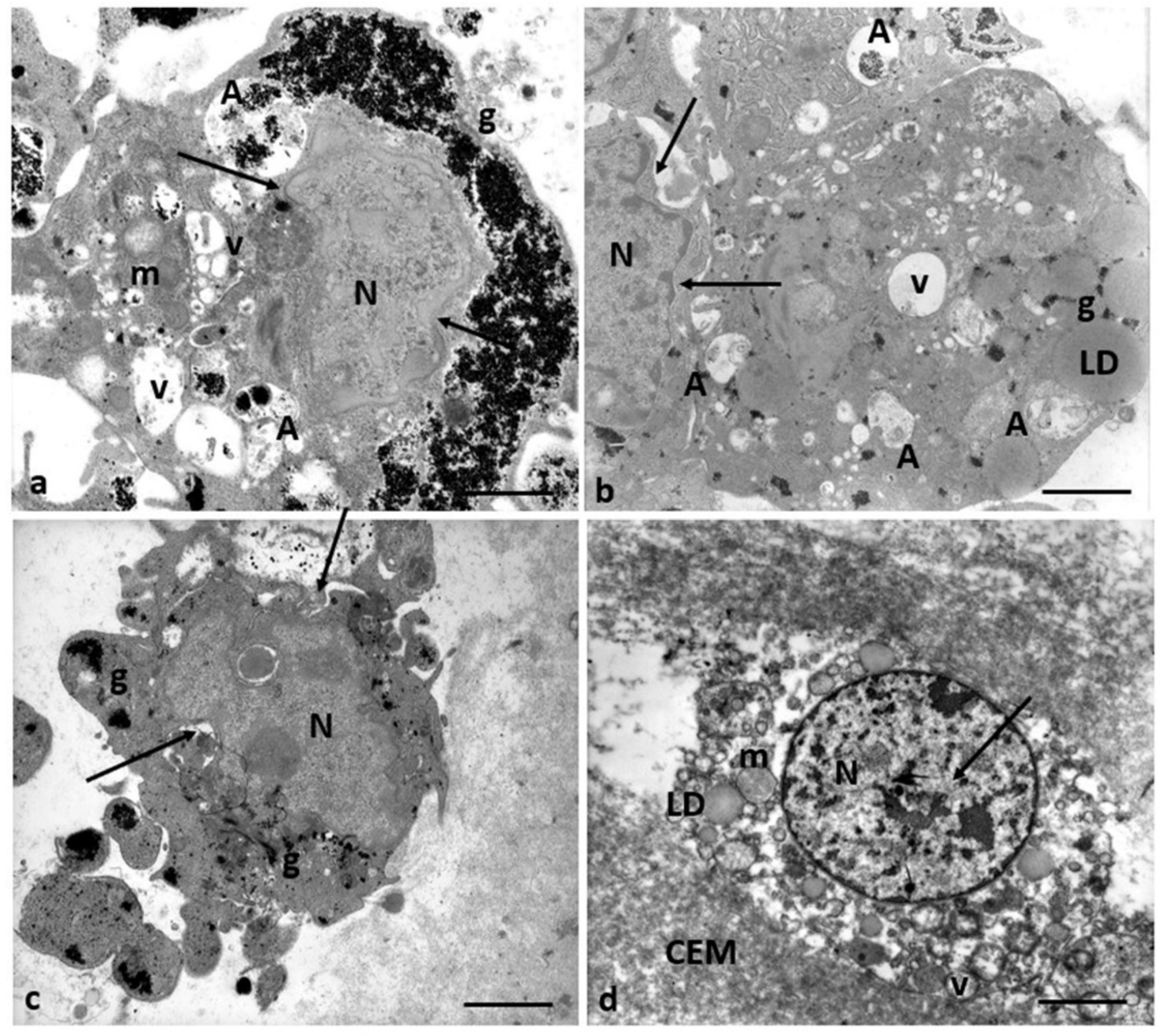
3.2. Electron microscopy
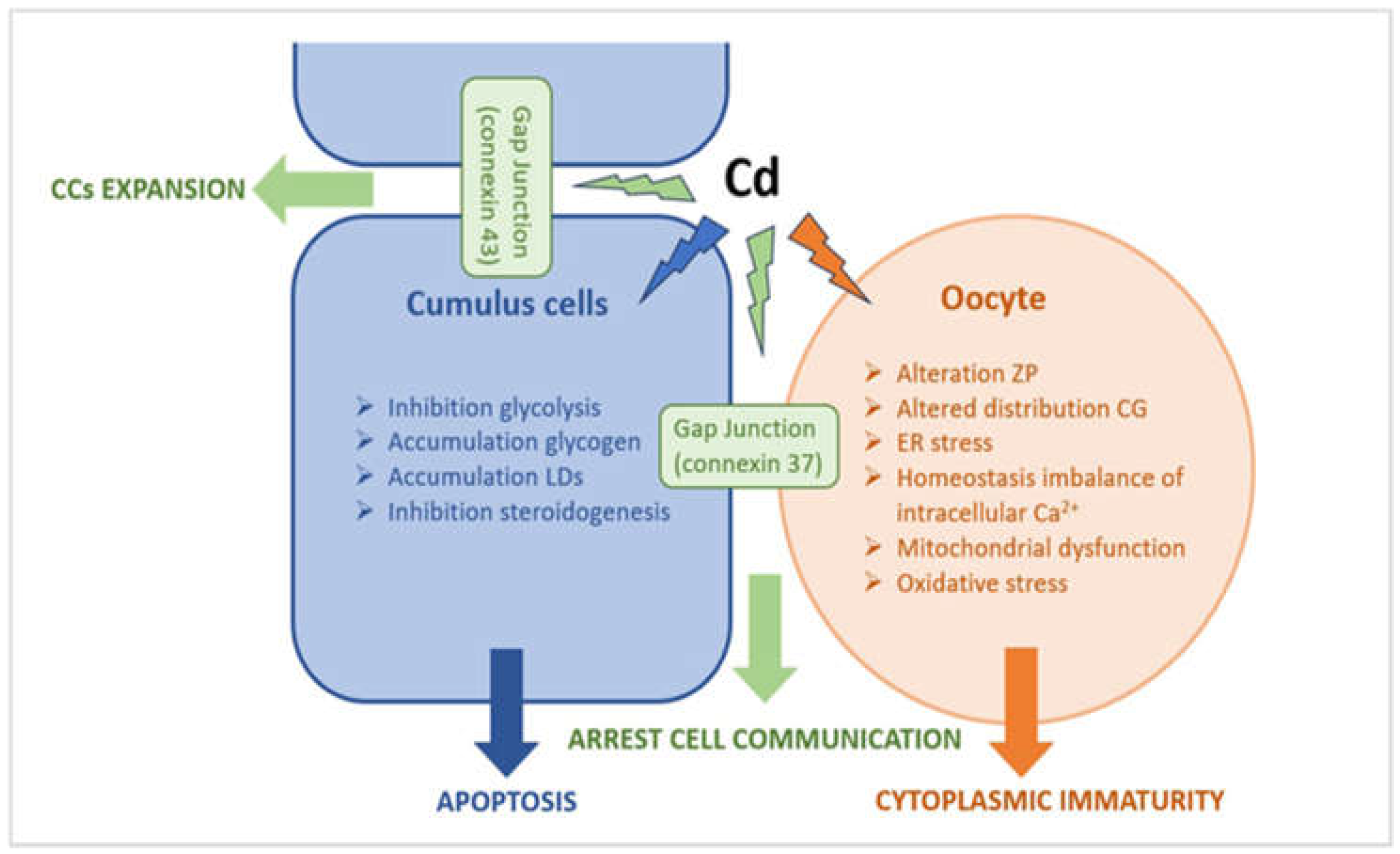
4. Discussion
4.1. Oocyte- ultrastructural changes heavy metals-dependent
4.2. Cumulus cells ultrastructural changes heavy metals-dependent.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 0469. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. ; The effects of heavy metals on human metabolism. Toxicol Mech Methods. [CrossRef]
- Caserta, D.; Maranghi, L.; Mantovani, A.; Marci, R.; Maranghi, F.; Moscarini, M. Impact of endocrine disruptor chemicals in gynaecology. Hum Reprod Update, 14. [CrossRef]
- Caserta, D.; Mantovani, A.; Marci, R.; Fazi, A.; Ciardo, F.; La Rocca, C.; Maranghi, F.; Moscarini, M. Environment and women’s reproductive health. Human Reprod Update, 2011, 17(3):418-33. [CrossRef]
- Caserta, D.; De Marco, M.P.; Besharat, A.R.; Costanzi, F. Endocrine Disruptors and Endometrial Cancer: Molecular Mechanisms of Action and Clinical Implications, a Systematic Review. Int J Mol Sci, 2956. [Google Scholar] [CrossRef]
- Ghorbani, M.R.; Ghanavati, N.; Babaenejad, T.; Nazarpour, A.; Payandeh, K. Assessment of the potential ecological and human health risks of heavy metals in Ahvaz oil field, Iran PLoS One, 2020, 15, e242703. [CrossRef]
- Bloom, M.S.; Fujimoto, V. Y.; Steuerwald, A. J.; Cheng, G.; Browne, R. W.; Parsons, P. J. Background exposure to toxic metals in women adversely influences pregnancy during in vitro fertilization (IVF). Reproductive Toxicology, 34. [CrossRef]
- Shen, L.; Liang, C.; Li, D.; Zhang, Z.; Wang, X.; Jiang, T.; Sua, X.; Yina, T.; Zoua, W.; Wang, X.; Liu, Y.; Liang, D.; Wei, Z.; Caoa, Y.; Ji, D. The association between exposure to multiple toxic metals and the risk of endometriosis: Evidence from the results of blood and follicular fluid. Science of the Total Environment. [CrossRef]
- La Llave León, O. and Pacheco, M.S. Effects of lead on reproductive health. In Book Lead Chemistry, 2020. License CC BY 3.0. [CrossRef]
- 10. Zhao, L-L.; Ru, Y-f.; Liu, M.; Tang, J-n.; Zheng, J-f.; Wu, B.; Gu, Y.; Shi, H-j. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PloS One, 8672. [CrossRef]
- Caserta, D.; Costanzi, F.; De Marco, M.P.; Di Benedetto, L.; Matteucci, E.; Assorgi, C.; Pacilli, M.C.; Besharat, A.R.; Bellati, F.; Ruscito, I. Effects of Endocrine-Disrupting Chemicals on Endometrial Receptivity and Embryo Implantation: A Systematic Review of 34 Mouse Model Studies. Int J Environ Res Public Health, 6840; 25. [Google Scholar] [CrossRef]
- Dong, F.; Lia, J. ; Leia, W-L.; Wang, F.; Wanga, Y.; Ouyanga, Y-C.; Houa, Y.; Wanga, Z-B.; Schattenc, H.; Suna, Q.Y. Chronic cadmium exposure causes oocyte meiotic arrest by disrupting spindle assembly checkpoint and maturation promoting factor. Reproductive Toxicology,.
- 13. Zhu, J-Q.; Liu, Y.; Zhang, J-H., Liu, Y-F.; Cao, J-Q.; Huang, Z-T.; Yuan, Y.; Bian, J-C., Liu, Z-P. Cadmium Exposure of Female Mice Impairs the Meiotic Maturation of Oocytes and Subsequent Embryonic Development. Toxicol Sci. [CrossRef]
- Tolunay, H.E.; Yavuz Emre Sukur, Y.E.; Ozkavukcu, S.; Seval, M.M.; Ates, C.; Turksoy, V. A; Ecemis¸T.; Atabekoglu C.M.; Ozmen B.; Berker, B.; Sonmezer M. Heavy metal and trace element concentrations in blood and follicular fluid affect ART outcome. Eur J Obstet Gynecol Reprod Biol. [CrossRef]
- Mohr, M.F.E.; Faris, F.; Bakry, S.; Hozyen, H.; Elshaer, F.M. Effect of Heavy Metals Levels in Follicular Fluid on ICSI Outcome. Egypt. Acad J Biolog Sci, 12.
- Rodríguez-Díaz, R.; Blanes-Zamora, R.; Paz-Montelongo, S.; Gómez-Rodríguez, J.; Rodríguez Fiestas, S.; González-Weller, D.; Gutiérrez, A.J.; Rubio, C.; Hardisson, A.; Niebla-Canelo, D.; Vega, S.A.; González-Dávila, E. The Influence of Follicular Fluid Metals on Assisted Reproduction Outcome. Biol Trace Elem Res, 2023. [CrossRef]
- Levay, P.E.; Huyser, C.; Fourie, F. L. R.; Rossouw, D. J. The Detection of Blood Contamination in Human Follicular Fluid. J Assist Reprod and Genet, 14. [CrossRef]
- Butt, C.D.; Blooma, M. S.; McGoughd, A.; Lenhartd, N.; Wong, R.; Mok-Lind, E.; Parsonsa, P.J.; Galushaa, A.L.; Yucelg, R. M.; Feingolda, B.J.; Brownef, R.W.; Fujimoto, V.Y. Variability of essential and non-essential trace elements in the follicular fluid of women undergoing in vitro fertilization (IVF). Ecotoxicol Environ Saf, 1117. [Google Scholar] [CrossRef]
- Miglietta, S.; Cristiano, L.; Espinola, M.S.B. , Masiello, M.G.; Micara, G., Battaglione, E., Linari, A., Palmerini, M.G., Familiari, G., Aragona, C., Bizzarri, M., Macchiarelli, G., Eds.; Nottola, S.A. Effects of Simulated Microgravity In Vitro on Human Metaphase II Oocytes: An Electron Microscopy-Based Study. Cells, 2023, 12, 1346. [Google Scholar] [CrossRef]
- Arabatzis, T. The discovery of the zeeman effect: A case study of the interplay Between theory and experiment. Stud Hist Phil Sci, 23. [CrossRef]
- Obaid Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US), 2012. PMID; 24049863, Bookshelf ID: NBK158838.
- No Author listed. Toxicological profile for Lead. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US), 2020. PMID: 36888732, Bookshelf ID: NBK589538.
- WHO guideline for clinical management of exposure to lead: executive summary. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
- Alimonti, A.; Bocca, B. , Mattei, D.; Pino, A. Biomonitoraggio della popolazione italiana per l’esposizione ai metalli: valori di riferimento 1990-2009. ISSN 1123-3117, Rapporti ISTISAN 10/22).
- Nottola, S.A.; Familiari, G.; Micara, G.; Aragona, C.; Motta, P.M. The ultrastructure of human cumulus-corona cells at the time of fertilization and early embryogenesis. A scanning and transmission electron microscopic study in an in vitro fertilization program. Arch Histol Cytol, 54. [CrossRef]
- Motta, P.M.; Nottola, S.A.; Pereda, J.; Croxatto, H.B.; Familiari, G. Ultrastructure of human cumulus oophorus: a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod, 2361; 10. [Google Scholar] [CrossRef]
- Martino, N.A.; Marzanob, G.; Mangiacottia, M.; Miedicoa, O.; Sardanellic, A.M.; Gnonic, A.; Lacalandrae, G.M.; Chiaravallea, A.E.; Cianib, E.; Bogliolof, L.; Minervinig, F.; Pizzih, F.; Dell’Aquila, M.E. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: A large animal model study. Reprod Toxic, 69. [CrossRef]
- Da Broi, M. G.; Giorgi, V. S. I.; Wang, F.; Keefe, D. L.; Albertini, D.; Navarro, P. A. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod and Genet, 35. [CrossRef]
- Biagioli, M.; Pifferi, S.; Ragghianti, M.; Bucci, S.; Rizzuto, R.; Pinton, P. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium, 43. [CrossRef]
- 30. Kitamura, M; Hiramatsu, N. The oxidative stress: endoplasmic reticulum stress axis in cadmium toxicity. Biometals. [CrossRef]
- Rajakumar, S.; Bhanupriya, N.; Ravi, C.; Nachiappan, V. Endoplasmic reticulum stress and calcium imbalance are involved in cadmium-induced lipid aberrancy in Saccharomyces Cerevisiae. Cell Stress and Chaperones, 21. [CrossRef]
- Wang, T.; Yuan, Y.; Zou, H.; Yang, J.; Zhao, S.; Ma, Y.; Wang, Y.; Bian, J.; Liu, X.; Gu, J.; Liu, Z.; Zhu, J. The ER stress regulator Bip mediates cadmium-induced autophagy and neuronal senescence. Scientific Reports, 3809; 6. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, S.; Zhang, J.; Xu, S.; Miao, Z. Cadmium induces endoplasmic reticulum stress-mediated apoptosis in pig pancreas via the increase of Th1 cells. Toxicology, 1527. [Google Scholar] [CrossRef]
- De Benedictis, M.; Gallo, A.; Migoni, D.; Papadia, P.; Roversi, P.; Santino, A. Cadmium treatment induces endoplasmic reticulum stress and unfolded protein response in Arabidopsis thaliana. Plant Physiology and Biochemistry. [CrossRef]
- Caserta, D.; Pegoraro, S.; Mallozzi, M.; Di Benedetto, L.; Colicino, E.; Lionetto, L.; Simmaco, M. Maternal exposure to endocrine disruptors and placental transmission: A pilot study. Gynecol Endocrinol, 1001; 34. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Murray, A.J. Mitochondrial—Endoplasmic reticulum interactions in the trophoblast: Stress and senescence. Placenta, 52. [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G. Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium, 69. [CrossRef]
- Ragusa, A.; Matta, M.; Cristiano, L.; Matassa, R.; Battaglione, E.; Svelato, A.; De Luca, C.; D’Avino, S.; Gulotta, A.; Ciro, M. , Rongioletti, A.; Catalano, P., Santacroce, C., Notarstefano, V., Carnevali, O., Giorgini, E., Vizza, E., Familiari, G., Eds.; Nottola, S.A. Deeply in Plasticenta: Presence of Microplastics in the Intracellular Compartment of Human Placentas. Int J Environ Res Public Health, 2022, 19, 11593. [Google Scholar] [CrossRef]
- Pu, S.; Pan, Y.; Zhang, Q.; You, T.; Yue, T.; Zhang, Y.; Wang, M. Endoplasmic Reticulum Stress and Mitochondrial Stress in Drug-Induced Liver Injury. Molecules, 3160. [Google Scholar] [CrossRef]
- Xavier, V.J.; Martinou, J.C. RNA Granules in the Mitochondria and Their Organization under Mitochondrial Stresses. Int J Mol Sci, 9502; 22. [Google Scholar] [CrossRef]
- Ghadially, F.N. Chapter 3: Mitochondria. In Ultrastructural Pathology of the Cell and Matrix, 3rd ed.; Butterworths: Oxford, UK, 1997; Volume 1, ISBN 0-407-01571-X. [Google Scholar]
- Abbott, A.L.; Ducibella, T. Calcium and the control of mammalian cortical granule exocytosis. Front Biosci, 6. [CrossRef]
- Liu, M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrinol, 9. [CrossRef]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem Biol Interact. [CrossRef]
- Tsai,P-S; Haeften,T. v.; Gadella, B.M. Preparation of the Cortical Reaction: Maturation-Dependent Migration of SNARE Proteins, Clathrin, and Complexin to the Porcine Oocyte’s Surface Blocks Membrane Traffic until Fertilization. Biol Reprod, 2011. [Google Scholar] [CrossRef]
- Familiari, G.; Heyn, R.; Relucenti, M.; Nottola, S.A.; Sathananthan, A.H. Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int Rev Cytol. [CrossRef]
- Zhou, C.; Zhang, X.; Chen, Y.; Liu, X.; Sun, Y.; Xiong, B. Glutathione alleviates the cadmium exposure-caused porcine oocyte meiotic defects via eliminating the excessive ROS. Environ Pollut, 1131. [Google Scholar] [CrossRef]
- Nottola, S.A.; Coticchio, G.; De Santis, L.; Macchiarelli, G.; Maione, M.; Bianchi, S.; Iaccarino, M.; Flamigni, C.; Borini, A. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod Biomed Online, 17. [CrossRef]
- Simoniello, P.; Filosa, S.; Scudiero, R.; Trinchella, F.; Motta, C.M. Cadmium Impairment of Reproduction in the Female Wall Lizard Podarcis sicula. Environ Toxicol, 5: 28(10). [CrossRef]
- Wan, L.; Zang, H. Cadmium toxicity. Effects on cytoskeleton, vesicular trafficking, and cell wall construction. Plant Signal Behav,. [CrossRef]
- Ge, Y.; Song, X.; Chen, L.; Hu, D.; Hua, L.; Cui, Y.; Liu, J.; An, Z.; Yin, Z.; Ning, H. Cadmium induces actin cytoskeleton alterations and dysfunction in Neuro-2a cells. Environ Toxicol, 34. [CrossRef]
- Dong, F.; Xiao, P.; Li, X.; Chang, P.; Zhang, W.; Wang, L. Cadmium triggers oxidative stress and mitochondrial injury mediated apoptosis in human extravillous trophoblast HTR-8/SVneo cells. Reprod Toxicol. [CrossRef]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod Toxicol, 25. [CrossRef]
- Yu, F.; Yan, L.; Sun, J. , Zhao, Y., Yuan, Y.; Gu, J.; Bian, J.; Zou, H.; Liu, Z. Gap junction intercellular communication mediates cadmium-induced apoptosis in hepatocytes via the Fas/FasL pathway. Environ Toxicol, 2692; 37. [Google Scholar] [CrossRef]
- Lopez, W.; Ramachandran, J.; Alsamarah, A.; Luo, Y.; Harris, A.L.; Contreras, J.E. Mechanism of gating by calcium in connexin hemichannels. Proc Natl Acad Sci USA, 7986. [Google Scholar] [CrossRef]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int J Mol Sci, 8194; 21. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. [CrossRef]
- Imanaka, S.; Shigetomi, H.; Kobayashi, H. Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance. Reprod Sci, 2022, 29, 653–667. [CrossRef]
- Sabir, S.; Akasha, M.S.H; Fiayyaz, F.; Saleemb, U.; Mehmoodb, M.H.; Rehmand, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed Pharmacother, 1088. [Google Scholar] [CrossRef]
- Oluranti, O.L.; Agboola, E.A.; Fubara, N.E.; Ajayi, M.O.; Michael, O.S. Cadmium exposure induces cardiac glucometabolic dysregulation and lipid accumulation independent of pyruvate dehydrogenase activity. Ann Med, 1109; 53. [Google Scholar] [CrossRef]
- Shen, W-J.; Azhar, S.; Kraemer, F.B. Lipid Droplets and Steroidogenic Cells. Exp Cell Res, 2016, 340, 209–214. [CrossRef]
- Knazicka, Z.; Forgacs, Z.; Lukacova, J.; Roychoudhury, S.; Massanyi, P.; Lukac, N. Endocrine disruptive effects of cadmium on steroidogenesis: Human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing. J Environ Sci Health A Tox Hazard Subst Environ Eng, 3: 50(4). [CrossRef]
- Paksy, K.; Rajczy, K.; Forgacs, Z.; Lazar, L.; Bernard, A. ; Gati. I. Kaali, G.S. Effect of Cadmium on Morphology and Steroidogenesis of Cultured Human Ovarian Granulosa Cells. J Appl Toxicol, 3: 17(5). [CrossRef]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S; Reis, F.M.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells. A review. Cell Biol Int, 1276; 42. [Google Scholar] [CrossRef]
- Huang, R.-H. , Zhou, W.-H. Granulosa Cell Biomarkers to Predict Oocyte and Embryo Quality in Assisted Reproductive Technology. Reprod Dev Med, 5. [CrossRef]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization. Cells, 2292; 10. [Google Scholar] [CrossRef]
- Xu, G.; Liu, S.; Huang, M.; Jiang, X.; Yang, M. Cadmium induces apoptosis of human granulosa cell line KGN via mitochondrial dysfunction-mediated pathways. Ecotoxicol Environ Saf, 1: 220, 1123. [Google Scholar] [CrossRef]

 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





